Abstract
A study of the quality of reclaimed water in treated effluent, after storage, and at three points in the distribution system of four plants in California, Florida, Massachusetts, and New York was conducted for 1 year. The plants had different treatment processes (conventional versus membrane bioreactor), production capacities, and methods for storage of the water, and the intended end uses of the water were different. The analysis focused on the occurrence of indicator bacteria (heterotrophic bacteria, coliforms, Escherichia coli, and enterococci) and opportunistic pathogens (Aeromonas spp., enteropathogenic E. coli O157:H7, Legionella spp., Mycobacterium spp., and Pseudomonas spp.), as well as algae. Using immunological methods, E. coli O157:H7 was detected in the effluent of only one system, but it was not detected at the sampling points, suggesting that its survival in the system was poor. Although all of the treatment systems effectively reduced the levels of bacteria in the effluent, bacteria regrew in the reservoir and distribution systems because of the loss of residual disinfectant and high assimilable organic carbon levels. In the systems with open reservoirs, algal growth reduced the water quality by increasing the turbidity and accumulating at the end of the distribution system. Opportunistic pathogens, notably Aeromonas, Legionella, Mycobacterium, and Pseudomonas, occurred more frequently than indicator bacteria (enterococci, coliforms, and E. coli). The Mycobacterium spp. were very diverse and occurred most frequently in membrane bioreactor systems, and Mycobacterium cookii was identified more often than the other species. The public health risk associated with these opportunistic pathogens in reclaimed water is unknown. Collectively, our results show the need to develop best management practices for reclaimed water to control bacterial regrowth and degradation of water before it is utilized at the point of use.
Freshwater is becoming increasingly scarce because of increasing populations, changing precipitation patterns, and/or degradation of existing sources of water. Reclaimed water can be used to alleviate some of the shortages. Indeed, reclaiming water for reuse has become increasingly common (39, 42). By definition, the term reclaimed water refers to effluents that have undergone a combination of physical, chemical, and biological treatments in engineered systems that utilize wastewater treatment technologies to remove suspended solids, dissolved solids, organic matter, nutrients, metals, and pathogens. When ideally practiced, the technologies used result in an effluent whose quality is commensurate with the intended use. Typically, reclaimed water contains a high level of organic matter, which potentially can react with the disinfectant (L. A Weinrich, P. K. Jjemba, E. Giraldo and M. W. LeChevallier, submitted for publication) but also provides nutrition in the form of organic carbon (40, 41). Depending on the mode of storage, some carbon may also be fixed through photosynthesis by algae, further impacting water quality.
Evaluation of the microbial quality of reclaimed water currently focuses mainly on the abundance of indicator bacteria (namely, coliforms, Escherichia coli, and enterococci) in the treated effluent (4, 13, 39, 42). However, there is increasing concern about the quality of the water at the point of use (17, 27, 31, 35; http://www.americascarcare.com/database/dms/acc1006w50.pdf; http://www.tucsonaz.gov/water/reclaimed.htm). Studies have focused on the regrowth of indicator bacteria, notably coliforms and E. coli (27). However, most of these indicator organisms are nonpathogenic, and their presence does not clearly correlate with the presence of actual opportunistic pathogens, such as Aeromonas, Legionella, and Mycobacterium, in reclaimed water (7, 16, 20, 22, 24, 27, 29). To address concerns about the quality of reclaimed water, regrowth of these pathogens in such water, in addition to regrowth of the indicator bacteria, has to be examined.
In this study the biostability of reclaimed water in the distribution systems at four plants that use different treatment processes was examined (Table 1). The treatment practices at the plants in California and Florida examined are conventional practices, whereas the plants in Massachusetts and New York examined use membrane bioreactor (MBR) technology. MBR treatment is a form of activated sludge treatment with a much smaller footprint; it combines membrane-based separation technology with a high level of biological treatment. The plants studied differ in size, geographic location, disinfection practices, and the way in which the reclaimed water is stored. The abundance of indicator bacteria in relation to the abundance of bacteria of public health concern (i.e., Aeromonas, Legionella, Mycobacterium, and Pseudomonas) was specifically determined for each distribution system. Because of its contribution to organic carbon, algal growth in reclaimed water was also determined.
TABLE 1.
Characteristics of the four reclaimed water systems studied
| Characteristic | California | Florida | Massachusetts | New York |
|---|---|---|---|---|
| Treatment process | Screening, flow equalization, trickling filters, primary and secondary clarification, flocculation, denitrification, filtration, disinfection system, and two reclamation storage reservoirs; trickling filters with tertiary sand filtration | Activated sludge with Bardenpho and secondary filtration; approx 150 collection system pump stations | Single anoxic; MBR (Zenon but switched to Torrey flat plate prior to the fall sampling) with nitrification and denitrification system; 2.57 × 107-liter equalization tank with a 13,200-liter/min submersible lift station | Anoxic and aerobic; MBR (Zenon) in the basement of a 293-unit high-rise building |
| Plant capacity | 88,960 liters/day | Average, 2.9 × 107 liters/day; full capacity, 3.4 × 107 liters/day | 950,000 liters/day but if needed can expand to 4.2 × 107 liters/day, a capacity that is attainable during high-flow seasons (i.e., full-scale activity and use of the sports stadium that it serves) | Generates 95,000 liters/day reclaimed water, 34,000 liters/day of which is for toilet flushing and 43,500 liters/day of which is for the cooling tower |
| Disinfection | Chlorine | Chlorine | Chlorine | UV and ozone |
| Storage | Open reservoir | Open reservoir (6.84 m deep with a useable storage vol of approx 2.11 × 108 liters) | 3.79 × 106-liter tank | 75,700-liter tank |
| Reuse type | Irrigation (landscaping) | Irrigation (residential, golf, schools, etc.) | Toilet flushing and urinals at stadium | Irrigation, toilet flushing, cooling tower |
| pH range | 7.07-9.60 | 7.12-8.19 | 7.32-8.94 | 6.12-7.62 |
| Conductivity (μS/cm) | 1,360-2,029 | 518-1,601 | 735-1,461 | 175-914 |
| Turbidity (NTU) | 0.67-12.7 | 0.58-16.2 | 0.23-25.2 | 0.06-2.20 |
| Water temp (°C) | 14.1-25.1 | 21.1-30.1 | 2.4-29.7 | 20.8-33.2 |
| TOC concn (mg/liter) | 12.8 ± 1.9 | 7.2 ± 0.8 | 3.8 ± 1.2 | 2.7 ± 0.5 |
| BDOC concn (mg/liter) | 6.2 ± 1.4 | 1.4 ± 0.8 | 0.6 ± 0.4 | 0.4 ± 0.2 |
| AOC concn (μg/liter) | 1,407 ± 983 | 1,141 ± 503 | 459 ± 467 | 149 ± 109 |
| Alkalinity (mg of CaCO3/liter) | 236 ± 28 | 205 ± 9 | 145 ± 16 | 33 ± 10 |
| NH3-N concn (mg/liter) | 7.8 ± 10 | 0.1 ± 0.2 | 0.04 ± 0.2 | 0.01 ± 0 |
| NO3-N concn (mg/liter) | 6.2 ± 10 | 0.7 ± 1 | 2.5 ± 1 | 24.5 ± 10 |
| P concn (mg/liter) | 11 ± 3 | 4 ± 2 | 22 ± 12 | 2 ± 0.4 |
MATERIALS AND METHODS
Sampling and physicochemical analyses.
Samples of reclaimed water were obtained from the four plants in winter 2006 and in spring, summer, and fall 2007. In each season samples were collected on four consecutive days. Samples were obtained from the plant effluent, reservoir, and three points in the distribution system (designated DS1, DS2, and DS3). DS1 was always closest to the reservoir, and DS3 was always farthest from the reservoir. Because the lengths of the distribution systems are different, the distances between DS1, DS2, and DS3 were different for the systems. For example, the shortest distance was approximately 5 m from the effluent to the storage tank in the New York high-rise building, and the longest distance was 19.3 km between the effluent and DS3 in the Florida system. The samples used for bacterial analysis were collected in sterile, 1-liter, polypropylene, wide-mouth Nalgene bottles (Nalge Nunc Corporation, Rochester, NY) which contained 0.1 ml of 2% sodium thiosulfate to quench the disinfectant (12). The samples were shipped on ice via overnight delivery to the laboratory. The water temperature, conductivity, and pH were determined onsite using a SympHony SP80PC and a portable Hach meter (HQ40d), respectively. The residual disinfectant at each sampling point was measured by measuring the free and total chlorine using standard method 4500-Cl G (12). Nitrate N (NO3-N), ammonia N (NH3-N), and phosphorus (P) contents were determined using Hach methods 8039, 8155, and 8114, respectively (18). Alkalinity was determined by using the titration method (12). Turbidity was measured with a nephelometer (Hach 2100N turbidimeter; Hach, Loveland, CO) as specified by the manufacturer using a set of standards. The sulfide concentrations in the water samples were measured by the methylene blue method (Hach method 8131) using 25-ml aliquots, 1.0 ml of sulfide 1 reagent, and 1.0 ml of sulfide 2 reagent (18). Hydrogen sulfide and acid-soluble metal sulfides reacted with N,N-dimethyl-p-phenylenediamine sulfate to form methylene blue, whose absorbance at 665 nm was measured after 5 min.
Total organic carbon (TOC) and biodegradable dissolved organic carbon (BDOC) contents were determined as described elsewhere (41). The assimilable organic carbon (AOC) content was determined as described elsewhere by Weinrich et al. (L. A Weinrich et al., submitted) using bioluminescence. Because in a recent survey of 21 reclaimed water plant effluents resulting from different treatment processes the median AOC content was 450 μg/liter (range, 45 to 3,200 μg/liter) (41), this concentration, twice this concentration, and three or more times this concentration were considered low (450 μg/liter), medium (451 to 1,350 μg/liter), and high (>1,350 μg/liter) AOC concentrations, respectively, in the present study.
Bacterial analyses.
Heterotrophic bacteria (HPCs) were enumerated by the spread plate method using R2A medium (pH 7.2) and 100-μl aliquots (12). Concentrations of coliforms, E. coli, enterococci, and Pseudomonas spp. were determined using the membrane filtration method (with a 0.45-μm-pore-size cellulose nitrate filter) and 0.1-, 10-, and/or 100-μl aliquots, whereas analyses of Aeromonas spp. also included 0.01-μl aliquots. Coliform contents were determined using m-Endo LES agar incubated at 35°C for 24 h, and presumptive positive results were confirmed based on the ability of the organisms to ferment lactose (brilliant green broth) at 35°C within 48 h (12). Fecal coliform contents were determined similarly using m-FC agar containing 1% rosolic acid per liter incubated at 44.5 ± 0.2°C for 24 h, and the presence of presumptive fecal coliforms was verified by fermentation of lactose at 44.5 ± 0.2°C for 24 ± 2 h. E. coli in the reclaimed water was enumerated on m-TEC agar using plates that were initially incubated for 2 h at 35 ± 0.5°C and then incubated at 44.5 ± 0.2°C for 22 h (12). The E. coli content was confirmed with a urea substrate (2 g urea with 10 mg phenol red/100 ml water) after incubation for 15 min and subsequent enumeration of the yellow or yellowish brown colonies under a UV lamp. E. coli ATCC 13706 was used as a positive control for analyses of coliforms and nonpathogenic E. coli. E. coli O157:H7 was detected using a nonquantitative immunomagnetic separation (IMS)-Reveal method as described by Bukhari et al. (8) by filtering 100 ml reclaimed water through a 0.45-μm-pore-size membrane filter. E. coli O157:H7 strain ATCC 35150 and E. coli ATCC 13706 were used as positive and negative controls, respectively.
The concentration of Aeromonas spp. was determined using m-Aeromonas selective agar (ASA) modified with ampicillin sodium salt and vancomycin hydrochloride (12). The ASA plates were incubated overnight at 35°C. Distinct bright yellow colonies that were 1 to 1.5 mm in diameter were considered Aeromonas colonies. Aeromonas hydrophila ATCC 7966 was used as a positive control. Enterococci were enumerated on mE agar amended with cycloheximide (Acti-Dione) and nalidixic acid, as well as 2,3,5-triphenyltetrazolium chloride after autoclaving (12). The mE agar plates were incubated at 41 ± 0.5°C for 48 h, and then filters with suspect enterococci were placed on EIA substrate plates containing 1 g esculin per liter, 0.5 g ferric citrate per liter, and 15 g agar per liter (pH 7.1 ± 0.2). The EIA plates were incubated at 41 ± 0.5°C for 20 min, and all pink or red colonies that produced a black or reddish brown precipitate on the underside of the plate were counted. Pseudomonas aeruginosa was enumerated on modified M-PA agar incubated at 41.5 ± 0.5°C for 72 h by counting all colonies that were ≤2.2 mm in diameter, were flat, and had light outer rims and brown to greenish black centers.
The concentration of Legionella spp. was determined by filtering (pore size, 0.45 μm) 100 ml of reclaimed water and submerging the filter aseptically in 10 ml phosphate buffer. The buffer was vortexed for 30 s, and a 0.1-ml aliquot was mixed with an equal volume of acid (HCl-KCl, pH 2.2) (12). The mixture was incubated at room temperature for 15 min and then neutralized with 0.1 ml of KOH-KCl base. A 0.1-ml aliquot and dilutions were then spread onto buffered charcoal yeast extract (BCYE) plates supplemented with Legionella agar enrichment (BD Difco, MD), which contains primarily cysteine, an essential amino acid for Legionella spp. A PAV supplement (Remel, KS) comprised of polymyxin B, anisomycin, and vancomycin was also added to the autoclaved medium. Legionella pneumophila ATCC 33152 was used as a positive control. The BCYE plates were incubated in a moisturized tub at 35°C, and growth was monitored for 1 week. Randomly selected presumptive Legionella spp. (a maximum of five colonies from each plate) were streaked on BCYE without cysteine (28). Failure to grow in the absence of cysteine was considered confirmation that Legionella spp. were present.
Mycobacterium spp. were enumerated by initially decontaminating an aliquot of a sample with a 0.005% cetylpyridinium chloride (CPC) solution (12). The CPC-treated sample was then filtered (pore size, 0.45 μm), and the filter was placed on a Middlebrook 7H10 agar plate and incubated for 21 days at 35°C. Randomly selected colonies (a maximum of five colonies from each plate) were subjected to acid-fast staining with carbol-fuchsin and Zeihl-Neelsen stain (Ricca Chemical Company, Arlington, TX) and counterstained with a 1% methylene blue solution (33). Cells that had a characteristic red color when they were examined with a microscope were considered cells of Mycobacterium spp.
Amplification and further identification of Mycobacterium spp.
Because Mycobacterium spp. were encountered so frequently in reclaimed water, DNA was extracted from a few isolates obtained from samples obtained in the winter and summer to identify the predominant species. Cells from individual colonies were grown in M7H9 broth (Hardy Diagnostics) at 35°C until the cultures were fully turbid (4 to 7 days). DNA was extracted from the broth as described by Blackwood et al. (5) using 5-mm glass beads. The DNA was amplified, and the heat shock protein 65 (hsp65) gene was targeted using primers Tb11 (ACCAACGATGGTGTGTCCAT) and Tb12 (CTTGTCGAACCGCATACCCT) (14, 30, 38). Amplification was done with PerfCTa SYBR green FastMix (Quanta Biosciences, Gaithersburg, MD) as specified by the manufacturer using 25-μl reaction mixtures. The PCR protocol of Devallois et al. (14) was modified as follows: 45 cycles of amplification (1 min at 94°C, 1 min at 60°C, and 1 min at 72°C), followed by a final extension at 72°C for 10 min using a Roche LightCycler 480 System II device (Roche, Indianapolis, IN). The products were subjected to melting curve analysis using the same PCR device after a 2-μl aliquot for restriction enzyme analysis was obtained. A melting temperature analysis was conducted using one cycle by heating the PCR products to 95°C for 5 s and cooling them to 65°C. Fluorescence was then detected continuously by increasing the temperature to 97°C in 7 min. Restriction enzyme digestion was performed with HaeIII (at 37°C) and BstEII (at 60°C) for 2 h. Both enzymes were obtained from Promega (Madison, WI) and were used as described by the manufacturer. The digested products were electrophoresed on a 2% 3:1 NuSieve agarose (Cambrex BioScience, Rockland, MD) gel containing 1.5 μg of ethidium bromide/ml. All gels also contained a 100-bp DNA marker (Lonza Rockland Inc., Rockland, ME). The gels were run at 100 V for 1.5 h and visualized under UV light to determine the sizes of the fragments. The patterns were analyzed with PRASITE (http://app.chuv.ch/prasite/index.html) for identification. Bands for fragments shorter than 60 bp were not taken into account as they were suspected of being primer or primer dimer bands (14, 38). Algorithms were also developed based on the presence and absence of specific restriction fragments (100, 110, 120, 140, 180, 190, and 200 bp for HaeI; 60, 100, 110, 120, 140, 180, 200, 210, and 300 bp for BstI) and DNA melting temperatures. PCR products with melting temperatures differing by 0.5°C were placed in different categories. The algorithms were analyzed by the unweighted-pair group method with arithmetic mean (UPGMA) (http://genomes.urv.cat/UPGMA) algorithm using the Jaccard coefficient with 100 bootstrap replicates. A dendrogram was constructed to evaluate the relatedness of the strains identified by the objective method as described by Sokal and Rohlf (34).
Chlorophyll content.
The chlorophyll content was determined as an indicator of the alga and cyanobacterium content in the two systems that have open reservoirs by filtering 500-ml aliquots of reclaimed water through 0.45-μm-pore-size membranes (Whatman). In some instances, more than one filter was necessary for this large volume. The filter(s) for each aliquot was ground in a glass tissue grinder (Kontes, Vineland, NJ) and dissolved in a mixture of acetone and MgCO3 (12). The acetone-MgCO3 mixture was made by adding 1 g of MgCO3 to 100 ml of distilled water and combining 90 parts of acetone with 10 parts of the saturated MgCO3 solution. The dissolved mixture was stored at 4°C in the dark for 4 h and then centrifuged at 500 × g for 20 min. The absorbance at 664 nm of the supernatant was determined after optimization by acidification (0.1 N HCl) and incubation at room temperature for 90 s. The optical densities at 664 nm (OD664) were converted to concentrations using a set of chlorophyll standards (Tokyo Chemical Industries, Tokyo, Japan) whose concentrations were 0, 0.0185, 0.034, 0.05, 0.1, and 0.32 mg/ml.
Statistical analyses.
The significance of differences between residual chlorine concentrations was analyzed using analysis of variance (ANOVA) with Excel, and the means were separated using the least significance difference (LSD) test (P < 0.05). The statistical significance of differences between the densities of bacteria at sampling points at each site was evaluated by adding 1 to each density and log10 transforming the densities. The transformed data for each sampling location (effluent, storage, and the three points in each distribution system) were compared using the nonparametric Wilcoxon rank sum test.
RESULTS
Physicochemical characteristics of the water.
The reclaimed water was generally neutral to slightly alkaline, except for a few instances when the water in the New York MBR was slightly acidic (pH 6.12) (Table 1). The water temperatures were >20°C, except for extreme cases when the temperatures in the reservoir in the Massachusetts system were close to freezing during the winter. The temperatures were highest and least variable in the New York facility, where the plant and distribution system are entirely inside an air-conditioned high-rise apartment building that is adequately heated in the winter. The turbidity was highest in the two systems with open reservoirs and typically exceeded 5 nephelometric turbidity units (NTU) (the permissible limit in many states) in the reclaimed water distribution systems; the maximum values approached five times this limit. The increases in the distribution system turbidity levels were most prominent in the facilities with open reservoirs. The conductivity, alkalinity, TOC content, and BDOC content were higher in the conventional systems (Table 1). The AOC concentrations in the systems ranged from 149 ± 109 to 1,407 ± 982 μg/liter (averages ± standard deviations), and these concentrations were consistently lower in the MBR systems than in the conventional treatment systems with open reservoirs. The concentration of ammonia nitrogen (NH4-N) was higher in the conventional systems, whereas the concentration of NO3-N was significantly high in the New York MBR system. The concentration of phosphorus was highest in the Massachusetts MBR system.
Stability of the residual disinfectant.
Three of the four systems use chlorine as a disinfectant (Table 1). However, after disinfection, the residual chlorine in the distribution system was rapidly and significantly (P < 0.05) depleted (Table 2). This depletion occurred in the open storage reservoirs in California and Florida and also in the closed tank in Massachusetts, and none of the systems was able to consistently maintain residual disinfectant at the end of the reclaimed distribution network. The residual disinfectant dissipated rapidly and significantly in every season. A similar trend was observed for the total chlorine content (data not shown).
TABLE 2.
Dissipation of free chlorine in reclaimed water distribution systems
| Location | Season | Chlorine concn (mg/liter)a |
||||
|---|---|---|---|---|---|---|
| Effluent | Storage | DS1b | DS2b | DS3b | ||
| California | Winter | 2.5 A | 0.15 E | 0.13 E | 0.09 E | 0.02 E |
| Spring | 1.43 C | 0.05 E | 0.04 E | 0.03 E | 0.06 E | |
| Summer | 1.78 B | 0.14 E | 0.11 E | 0.11 E | 0.14 E | |
| Fall | 0.75 D | 0.03 E | 0.02 E | 0.01 E | 0.01 E | |
| Florida | Winter | 1.64 I | 0.09 J | 0.03 K | <0.01 K | <0.01 K |
| Spring | 4.33 G | 0.08 J | 0.65 J | 0.20 J | 0.10 J | |
| Summer | 2.68 H | 0.10 J | 0.10 J | 0.13 J | 0.07 J | |
| Fall | 5.06 F | 0.20 J | 0.15 J | 0.14 J | 0.03 J | |
| Massachusetts | Winter | <0.01 Q | 0.14 Oc | <0.01 Q | <0.01 Q | <0.01 Q |
| Spring | <0.01 Q | 0.23 Nc | 0.10 P | 0.10 P | <0.01 Q | |
| Summer | <0.01 Q | 0.33 Lc | <0.01 Q | <0.01 Q | <0.01 Q | |
| Fall | <0.01 Q | 0.28 Mc | <0.01 Q | <0.01 Q | <0.01 Q | |
For each location numbers followed by the same letter in each row and numbers followed by the same letter in each column are not significantly different (P > 0.05). The mean concentrations are based on data for four replicates, and each replicate consisted of samples obtained on four consecutive days. Chlorination is not used at the New York site.
DS1, DS2, and DS3 are locations in the distribution system; DS1 is closest to the storage site, and DS3 is farthest from the storage site.
At the Massachusetts site, chorine is added at the storage site instead of to the effluent.
Inactivation of bacteria.
The lowest concentration of heterotrophic bacteria occurred in the trickling filter system in California, where, after chlorination, the concentration of HPCs in the effluent was 6 × 103 CFU/100 ml (Table 3). However, the concentration of HPCs increased at least 10-fold in the distribution system. Similar or even larger increases in the concentration of HPCs were observed in the other three systems irrespective of whether conventional treatment or MBR practices were used. In all four systems the indicator bacteria and opportunistic bacterial pathogens in the disinfected effluent were effectively inactivated. Thus, no or very low densities of coliforms, E. coli, enterococci, Pseudomonas spp., Aeromonas spp., or Legionella spp. were detected in the effluent from the conventional plants (the California and Florida plants) (Table 3). Chlorination was also effective against these organisms in the Massachusetts MBR reservoir, where the disinfectant is applied. Some coliforms, E. coli, and enterococci were detected in the UV- and ozone-disinfected New York effluent, but very rarely (i.e., once in summer 2007). Irrespective of the treatment technology used, higher densities of bacteria were frequently detected in the water after disinfection, suggesting that there was regrowth in the distribution system. The increases were more pronounced for Aeromonas spp., Legionella spp., and Mycobacterium spp., whose concentrations were at least 10-fold higher than the concentrations of the indicators (i.e., coliforms and E. coli) that are typically monitored for regulatory purposes. This set of organisms was also more prevalent in water, as reflected by the abundance of them expressed as a fraction of the heterotrophic bacteria compared to indicator bacteria (Table 3). The increases were statistically significant (P < 0.05) for heterotrophic bacteria at all sites and for a range of organisms in the two systems which use conventional treatment technologies. In the MBR system in Massachusetts Mycobacterium and Legionella also regrew significantly after disinfection.
TABLE 3.
Concentrations of microorganisms in treated effluents and their regrowth in the distribution systems
| Organisms | Location | Concn (CFU/100 ml) (geometric mean ± standard error)a |
||||
|---|---|---|---|---|---|---|
| Effluent | Storageb | DS1 | DS2 | DS3 | ||
| HPCs | California | 0.006 × 106 ± 0.04 × 106 B | 1.2 × 106 ± 72 × 106 AB | 17.8 × 106 ± 54.5 × 106 A | 27 × 106 ± 48 × 106 A | 103 × 106 ± 50.4 × 106 A |
| Florida | 28 × 106 ± 52 × 106 B | 126 × 106 ± 30 × 106 A | 38.7 × 106 ± 19 × 106 A | 86.3 × 106 ± 28.8 × 106 A | 70.1 × 106 ± 15.6 × 106 A | |
| Massachusetts | 1.6 × 106 ± 1.1 × 106 AB | 1.01 × 106 ± 0.6 × 106 B | 41.7 × 106 ± 6.6 × 106 A | 29.8 × 106 ± 3.2 × 106 A | 14.2 × 106 ± 32.0 × 106 A | |
| New York | 1.8 × 106 ± 4.9 × 106 B | 23.1 × 106 ± 150 × 106 AB | 105.5 × 106 ± 32.5 × 106 A | 50.6 × 106 ± 18.3 × 106 A | 1.9 × 106 ± 39 × 106 AB | |
| Total coliforms | California | <1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 6 |
| Florida | 1 ± 1 B | 11 ± 7 A | 3 ± 6 AB | 9 ± 46 AB | 7 ± 17 AB | |
| Massachusetts | 2 ± 1 | <1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | |
| New York | 3 ± 23 | <1 | <1 | 1 ± 1 | <1 | |
| Fecal coliforms | California | 1 ± 1 | <1 | <1 | <1 | <1 |
| Florida | 1 ± 1 B | 12 ± 9 A | 1 ± 3 B | 4 ± 4 B | 4 ± 5 B | |
| Massachusetts | <1 | <1 | <1 | <1 | <1 | |
| New York | 2 ± 6 | <1 | <1 | 1 ± 1 | <1 | |
| E. coli | California | 1 ± 1 | 1 ± 1 | 1 ± 1 | <1 | 1 ± 1 |
| Florida | 1 ± 1 | 7 ± 8 | 2 ± 2 | 2 ± 3 | 4 ± 2 | |
| Massachusetts | <1 | <1 | <1 | 1 ± 1 | 1 ± 1 | |
| New York | 3 ± 10 | 1 ± 1 | <1 | 1 ± 1 | 1 ± 1 | |
| Enterococci | California | <1 | <1 | <1 | 1 ± 1 | <1 |
| Florida | 1 ± 0 | 9 ± 26 | 3 ± 9 | 27 ± 36 | 10 ± 18 | |
| Massachusetts | <1 | <1 | <1 | <1 | <1 | |
| New York | 3 ± 34 | <1 | <1 | <1 | <1 | |
| Pseudomonas spp. | California | <1 | 2 ± 2 | 2 ± 5 | 3 ± 4 | 6 ± 12 |
| Florida | 1 ± 1 C | 8 ± 4 AB | 2 ± 2 BC | 9 ± 10 A | 4 ± 2 AB | |
| Massachusetts | 1 ± 1 | <1 | 2 ± 5 | 2 ± 3 | 2 ± 5 | |
| New York | 1 ± 1 | 2 ± 1 | 1 ± 1 | 1 ± 3 | 6 ± 130 | |
| Aeromonas spp. | California | 1 × 102 ± 1 × 102 | 2 × 102 ± 7 × 102 | 6 × 102 ± 3 × 102 | 20 × 102 ± 200 × 102 | 66 × 102 ± 900 × 102 |
| Florida | 1 × 102 ± 1 × 102 | 210 × 102 ± 480 × 102 | 91 × 102 ± 170 × 102 | 120 × 102 ± 700 × 102 | 300 × 102 ± 410 × 102 | |
| Massachusetts | 1 × 102 ± 1 × 102 | <1 × 102 | 6 × 102 ± 46 × 102 | 5 × 102 ± 57 × 102 | 1 × 102 ± 33 × 102 | |
| New York | 1 × 102 ± 2 × 102 | 1 × 102 ± 1 × 102 | 10 × 102 ± 18 × 102 | 32 × 102 ± 42 × 102 | 1 × 102 ± 1 × 102 | |
| Mycobacterium spp. | California | 1 ± 1 B | 5 ± 17 AB | 22 ± 15 A | 35 ± 46 A | 30 ± 125 A |
| Florida | 11 ± 20 | 65 ± 220 | 59 ± 290 | 73 ± 610 | 107 ± 800 | |
| Massachusetts | 170 ± 190 AB | 2 ± 1 B | 57 ± 25 A | 320 ± 130 A | 120 ± 80 A | |
| New York | 6 ± 150 | 50 ± 80 | 42 ± 110 | 16 ± 14 | 31 ± 29 | |
| Legionella spp. | California | <0.3 × 103 B | 2.2 × 103 ± 4.1 × 103 A | 2.3 × 103 ± 2.0 × 103 AB | 0.9 × 103 ± 1.5 × 103 AB | 1.9 × 103 ± 1.6 × 103 AB |
| Florida | <0.3 × 103 B | 3.0 × 103 ± 70 × 103 A | 2.7 × 103 ± 13 × 103 A | 3.5 × 103 ± 16 × 103 A | 8 × 103 ± 52 × 103 A | |
| Massachusetts | 0.4 × 103 ± 0.2 × 103 A | <0.3 × 103 B | 1.3 × 103 ± 2.8 × 103 A | 0.7 × 103 ± 2.0 × 103 A | 0.4 × 103 ± 0.7 × 103 A | |
| New York | 0.6 × 103 ± 2.1 × 103 | 0.7 × 103 ± 0.6 × 103 | 0.5 × 103 ± 0.6 × 103 | 0.5 × 103 ± 0.6 × 103 | 0.5 × 103 ± 0.4 × 103 | |
The data are averages for the four seasons. Numbers followed by different letters in each row are significantly different (P ≤ 0.05). In rows with no letters there was no difference between the values. At each location, one sample was collected from the effluent and the reservoir once during each season (4 samples per site per year), whereas one sample was collected from each distribution system site on four consecutive days during each season (16 samples per site per year).
Point of disinfection for the Massachusetts site. For all other sites, disinfection occurred at the effluent stage.
Organic carbon and occurrence of bacteria.
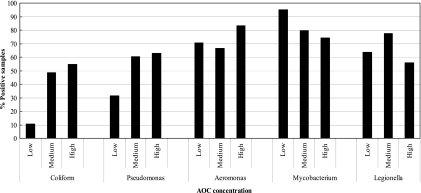
The frequency with which coliforms and Pseudomonas spp. occurred in reclaimed water systems increased with increasing AOC concentration (Fig. 1). The occurrence of Aeromonas, Mycobacterium, and Legionella was high (>55% of the samples) irrespective of whether the AOC concentration in the reclaimed water was 450 μg/liter or more.
FIG. 1.
Frequency of occurrence of various organisms with different AOC concentrations. The low, medium, and high AOC concentrations are equivalent to 450 μg/liter, 451 to 1,350 μg/liter, and >1,350 μg/liter, respectively.
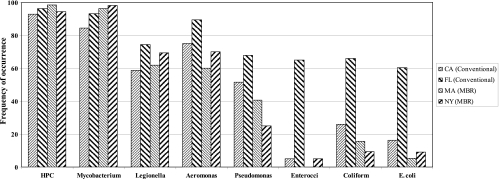
Of the three types of indicator bacteria, enterococci were detected least frequently in the reclaimed water (Fig. 2). In comparison, opportunistic pathogens, notably Aeromonas, Legionella, Mycobacterium, and Pseudomonas, occurred more frequently in the reclaimed water systems. Thus, there were numerous instances in which potentially opportunistic pathogens were present in the reclaimed water distribution system in the absence of indicator organisms. For example, in the New York MBR system, the routinely monitored coliforms were detected in only 10% of the samples throughout the year, whereas Mycobacterium spp. were detected in 97% of the samples. Likewise, in the conventional Florida system, coliforms and Mycobacterium spp. were detected in 66% and 95% of the samples, respectively. The presence of Legionella spp. and the presence of Mycobacterium spp. in reclaimed distribution systems were significantly (R2 = 0.2; P < 0.01) correlated.
FIG. 2.
Frequency of occurrence of opportunistic pathogens and indicator bacteria in reclaimed water.
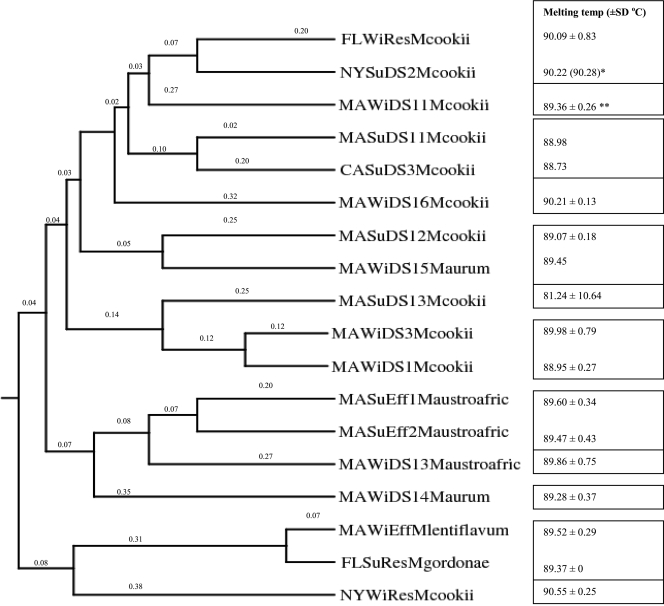
Because of the high frequency of occurrence of Mycobacterium in the distribution systems (range, 84% to 98% of the samples), a few randomly selected isolates were subjected to further characterization using restriction enzyme fragment length polymorphism (RFLP) and DNA melting temperature analyses. This subsample was quite diverse; the isolates belonged to 12 clusters (Fig. 3). The species identified most frequently was Mycobacterium cookii. The other taxa identified included Mycobacterium gordonae, Mycobacterium lentiflavum type 4, Mycobacterium astroafricanum type 1, and Mycobacterium aurum type 1. Mycobacterium spp. do not appear to be distribution system specific as M. cookii was detected in all four systems.
FIG. 3.
Diversity of Mycobacterium spp. in distribution systems for samples obtained in winter 2006 and summer 2007. FLWiResMcookii, M. cookii in Florida reservoir in winter; NYSuDS2Mcookii, M. cookii in New York DS2 in summer; CASuDS3Mcookii and CAWi DS3Mcookii, M. cookii in California DS3 in summer and winter, respectively; MAWiDS11Mcookii and MAWiDS12Mcookii, M. cookii in Massachusetts DS1 colonies 1 and 2 in winter, respectively; CAWiDS1Mcookii, M. cookii in California DS1 in winter; MASuDS12Mcookii and MASuDS13, M. cookii in Massachusetts DS1 colonies 2 and 3 in summer; MAWiDS1Mcookii and MAWiDS3Mcookii, M. cookii in Massachusetts DS1 and DS3 in winter, respectively; MASuDS11Mcookii, M. cookii in Massachusetts DS1 colony 1 in summer; MAWiDS15Maurum and MAWiDS16Mcookii, M. aurum and M. cookii, respectively, in Massachusetts DS1 colonies 5 and 6 in winter; MASuEff1Maustroafric and MASuEff2Maustroafric, M. austroafricanum in Massachusetts effluent colonies 1 and 2, respectively, in summer; MAWiDS13Maustroafric and MAWiDS14Maurum, M. austroafricanum and M. aurum, respectively, in Massachusetts DS1 colonies 3 and 4 in winter; MAWiEffMlentiflavum, M. lentiflavum in Massachusetts effluent in winter; FLSuResMgordonae, M. gordonae in Florida reservoir in summer; NYWiResMcookii, M. cookii in New York storage tank in winter. The numbers on the dendrogram are similarity matrix distances. The dendrogram has a cophenetic correlation coefficient of 0.79. One asterisk indicates that the value is identical to the value for CAWiDS3Mcookii with a melting temperature of 90.28°C. Two asterisks indicate that the value is identical to the values for MAWiDS12Mcookii with a melting temperature of 89.16 ± 0.40°C and CAWiDS1Mcookii with a melting temperature of 89.13 ± 0.40°C.
Occurrence of E. coli O157:H7.
The presence of E. coli O157:H7 was determined using the Reveal test system. This organism was found in reclaimed water only twice in the Florida effluent obtained during the spring and fall. It was not detected in the open reservoir or the distribution system, suggesting that this pathogen may not survive or grow in this environment. E. coli O157:H7 was not detected in any section of the other three systems.
Algal growth and hydrogen sulfide in reclaimed water.
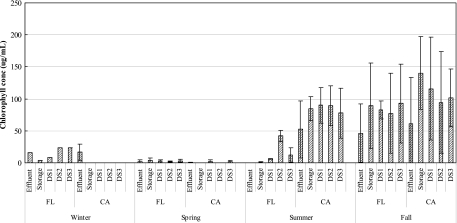
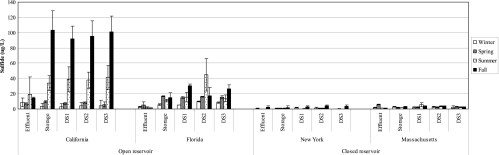
The standard curve for the chlorophyll content analysis had a correlation coefficient (R2) of 0.9947. In the open reservoirs, the levels of algae and cyanobacteria were greater during the warmer months; the concentrations increased in the summer and reached the maximum values in the fall (Fig. 4). Algal cells persisted throughout the distribution system and were not confined to the open reservoir. The chlorophyll level in the distribution system was significantly correlated with the AOC content (R2 = 0.2112; P < 0.01) and the BDOC content (R2 = 0.4033; P < 0.01), suggesting that algal growth from the reservoir contributed to increases in the level of biodegradable carbon in the distribution system. The levels of hydrogen sulfide in the reclaimed water were several times higher in the two conventional plants than in the MBR systems (Fig. 5).
FIG. 4.
Chlorophyll content as an indicator of algal growth in the two systems with open reservoirs. DS1, DS2, and DS3 are locations in the distribution system.
FIG. 5.
Hydrogen sulfide content in reclaimed water as an indicator of the intensity of odor. DS1, DS2, and DS3 are locations in the distribution system.
DISCUSSION
The loss of residual disinfectant in the systems was accompanied by an increase in the level of bacteria, indicating that it is necessary to maintain a sufficient and stable residual level of disinfectant. The rapid dissipation of free chlorine may have been a result of its ability to react with organic matter, as reflected by the high organic carbon concentration compared to the concentrations typically encountered in potable water. AOC is readily available for consumption by microorganisms, which in turn can enhance the regrowth of bacteria in the reclaimed water. Falkinham et al. (15) reported that there was a strong positive correlation between Mycobacterium and AOC levels (17 to 234 μg/liter) in potable water systems that had only a fraction of the AOC levels encountered in the reclaimed water systems. In the present study, high levels of AOC (>1,350 μg/liter) were associated with increased occurrence of coliforms and Pseudomonas spp. in the reclaimed water. Both coliforms and Pseudomonas spp. grow extremely fast. Even at the lowest levels of AOC in reclaimed water, Mycobacterium and Legionella, both of which have slightly lower growth rates than coliforms, Aeromonas spp., and Pseudomonas spp., occurred very frequently. Mycobacterium and Legionella survive quite well in biofilms (25). Unlike most indicator bacteria, some species of Pseudomonas, Aeromonas, Legionella, and Mycobacterium are opportunistic pathogens, and these organisms regrew to a greater concentration in the distribution system than the indicator bacteria. Regrowth is undesirable as it may cause odor and esthetic problems in reclaimed water, degrading the water quality in the distribution system. The present study shows that the treatment systems investigated (conventional and MBR) are typically capable of generating a high-quality effluent with very low densities of indicator microorganisms. Coliforms were rare in these systems, but opportunistic pathogens (Aeromonas, Legionella, Mycobacterium, and Pseudomonas) were detected quite frequently. Thus, while indicator organisms are effective for assessing the efficacy of treatment processes, they may not be representative of the possible risk from regrowth of opportunistic pathogens in the distribution system.
The occurrence of Mycobacterium spp. and the occurrence of Legionella spp. in the distribution system were significantly correlated. Members of both of these genera are known to associate with ciliated protozoa and amoebae (2, 21, 23), and it has been hypothesized that their intracellular survival provides a training ground for enhancing their pathogenicity to humans (10, 11, 26). The Legionella-protozoan and Mycobacterium-protozoan symbiotic relationships can effectively protect the intracellular bacteria against disinfectants (24, 37). The presence of L. pneumophila and Mycobacterium spp. can pose a hazard, particularly when the water is used for irrigation of lawns with sprinklers as the aerosols generated can expose the general public to these pathogens (1, 9, 36). Inhalation of airborne droplets or drop nuclei that contain Legionella spp. is believed to be the most common mode of transmission (3). Such droplets can also be generated by cooling towers or decorative water fountains where reclaimed water is used.
The accumulation of algal cells in a distribution system can significantly impact water quality. Furthermore, decaying algal cells release organic carbon, which in turn increases the demand for disinfectant and increases turbidity. Algal cellular fractions can be a significant source of biodegradable carbon, especially in the presence of an oxidant such as chlorine (6, 19, 32). All of these factors stimulate bacterial growth, resulting in a loss of oxygen and creating anoxic conditions, which in turn favor anaerobes, such as sulfur-reducing bacteria, as documented for the systems with open reservoirs in which there were also high hydrogen sulfide concentrations. Hydrogen sulfide is an indicator of foul odors, a common complaint of reclaimed water end users (http://www.americascarcare.com/database/dms/acc1006w50.pdf). The accumulation of algal cells can be controlled by regular flushing of the reclaimed water systems, use of algaecides, or removal of the algae using fine-mesh screens.
In summary, trickling filters with tertiary sand filtration, activated sludge with secondary filtration, and membrane bioreactor processes effectively removed the indicator bacteria. However, the bacteria regrew in reclaimed water after disinfection because of the rapid dissipation of the disinfectant in the system and the high levels of organic carbon, which created a high demand for disinfectant. The regrowth and frequency of occurrence were even greater for opportunistic pathogens, such as Aeromonas, Mycobacterium, and Legionella, than for routine indicator bacteria. The use of indicator bacteria to monitor water quality may not reflect all of the subsequent risks that can occur downstream of the treatment process, particularly in reclaimed water distribution systems where nutrient and temperature profiles are more favorable for microbial survival and regrowth. A full assessment of the public health risks associated with opportunistic pathogens in reclaimed water needs to be conducted.
Acknowledgments
We are very grateful to the four utilities for their participation and cooperation. In addition, the advice and help provided by the Project Advisory Committee (Katie Benko, Bureau of Reclamation; Uzi Daniel, West Basin Water District; Sharon C. Long, University of Wisconsin—Madison; Craig Riley, Washington State Department of Health; Terri Slifko, County Sanitation Districts of Los Angeles; Anthony Andrade, Southwest Florida Water Management District; Rich Mills, California State Water Resources Control Board) and Burnett King (WateReuse Foundation) are greatly appreciated.
This study was funded by the WateReuse Foundation (grant WRF-05-002), the Bureau of Reclamation, the Southwest Florida Water Management District, the California State Water Resources Control Board, and the utility subsidiaries of American Water, Voorhees, NJ.
Footnotes
Published ahead of print on 7 May 2010.
REFERENCES
- 1.Addiss, D. G., J. P. Davis, M. LaVentura, P. J. Wand, M. A. Hutchinson, and R. M. McKinney. 1989. Community-acquired Legionnaires' disease associated with a cooling tower: evidence for longer-distance transport of Legionella pneumophila. Am. J. Epidemiol. 130:557-568. [DOI] [PubMed] [Google Scholar]
- 2.Adékambi, T., S. B. Salah, M. Khlif, D. Raoult, and M. Drancourt. 2006. Survival of environmental mycobacteria in Acanthamoeba polyphaga. Appl. Environ. Microbiol. 72:5974-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong, T. W., and C. N. Haas. 2007. Quantitative microbial risk assessment model for Legionnaires' disease: assessment of human exposure for selected spa outbreaks. J. Occup. Environ. Hyg. 4:634-646. [DOI] [PubMed] [Google Scholar]
- 4.Asano, T., F. L. Burton, H. L. Leverenz, R. Tsuchihashi, and G. Tchobanoglous. 2007. Water reuse: issues, technologies, and applications. McGraw Hill, New York, NY.
- 5.Blackwood, K. S., C. He, J. Gunton, C. Y. Turenne, J. Wolfe, and A. M. Kabani. 2000. Evaluation of recA sequences for identification of Mycobacterium species. J. Clin. Microbiol. 38:2846-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouteleux, C., S. Saby, D. Tozza, J. Cazzard, V. Lahoussine, P. Hartemann, and L. Mathieu. 2005. Escherichia coli behavior in the presence of organic matter released by algae exposed to water treatment chemicals. Appl. Environ. Microbiol. 71:734-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandi, G., M. Sisti, F. Giardini, G. F. Schiavano, and A. Albano. 1999. Survival ability of cytotoxic strains of motile Aeromonas spp. in different types of water. Lett. Appl. Microbiol. 29:211-215. [DOI] [PubMed] [Google Scholar]
- 8.Bukhari, Z., J. R. Weihe, and M. W. LeChevallier. 2007. Rapid detection of Escherichia coli O157:H7 in water. J. Am. Water Works Assoc. 99:157-167. [Google Scholar]
- 9.Castellani Pastoris, M., L. Ciceroni, R. Lo Monaco, P. Goldoni, B. Mentore, G. Flego, L. Cattani, S. Ciarrocchi, A. Pinto, and P. Visca. 1997. Molecular epidemiology of an outbreak of Legionnaires' disease associated with a cooling tower in Genova-Sestri Ponente, Italy. Eur. J. Clin. Microbiol. Infect. Dis. 16:883-892. [DOI] [PubMed] [Google Scholar]
- 10.Cirillo, J. D., S. Falkow, and L. S. Tompkins. 1994. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Appl. Environ. Microbiol. 62:3254-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cirillo, J. D., S. L. Cirillo, L. Yan, L. E. Bermudez, S. Falkow, and L. S. Tompkins. 1999. Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 67:4427-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 2005. Standard methods for the examination of water and wastewater, 20th ed. APHA, AWWA, and WEF Publishing, Washington, DC.
- 13.Costán-Longares, A., M. Montemayor, A. Payán, J. Méndez, J. Jofre, R. Mujeriego, and F. Lucena. 2008. Microbial indicators and pathogens: removal, relationships and predictive capabilities in water reclamation facilities. Water Res. 42:4439-4448. [DOI] [PubMed] [Google Scholar]
- 14.Devallois, A., K. Goh, and N. Rastogi. 1997. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J. Clin. Microbiol. 35:2969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falkinham, J. O., C. D. Norton, and M. W. LeChevallier. 2001. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 67:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavriel, A. A., J. P. Landre, and A. J. Lamb. 1998. Incidence of mesophilic Aeromonas within a public drinking water supply in north-east Scotland. J. Appl. Microbiol. 84:383-392. [DOI] [PubMed] [Google Scholar]
- 17.Geldreich, E. E. 1996. Microbial quality of water supply in distribution systems. CRC Press, Boca Raton, FL.
- 18.Hach Company. 1997. DR/4000 spectrophotometer handbook. Hach Company, Loveland, CO.
- 19.Hammes, F. A., and T. Egli. 2005. New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ. Sci. Technol. 39:3289-3294. [DOI] [PubMed] [Google Scholar]
- 20.Harwood, V. J., A. D. Levine, T. M. Scott, V. Chivukula, J. Lukasik, S. R. Farrah, and J. B. Rose. 2005. Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl. Environ. Microbiol. 71:3163-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilvington, S., and J. Price. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J. Appl. Bacteriol. 68:519-525. [DOI] [PubMed] [Google Scholar]
- 22.Landre, J. P. B., A. A. Gavriel, and A. J. Lamb. 1998. False-positive coliform reaction mediated by Aeromonas in the Colilert defined substrate technology system. Lett. Appl. Microbiol. 26:352-354. [DOI] [PubMed] [Google Scholar]
- 23.La Scola, B., R. J. Birtles, G. Greub, T. J. Harrison, R. M. Ratcliff, and D. Raoult. 2004. Legionella drancourtii sp. nov., a strictly intracellular amoebal pathogen. Int. J. Syst. Evol. Microbiol. 54:699-703. [DOI] [PubMed] [Google Scholar]
- 24.Le Dantec, C., J.-P. Duguet, A. Montiel, N. Dumountier, S. Dubron, and V. Vincent. 2002. Chlorine disinfection of atypical mycobacteria isolated from a water distribution system. Appl. Environ. Microbiol. 68:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehtola, M. J., E. Torvinen, J. Kusnetsov, T. Pitkänen, L. Maunula, C.-H. von Bonsdorff, P. J. Martikainen, S. A. Wilks, C. W. Keevil, and I. T. Miettinen. 2007. Survival of Mycobacterium avium, Legionella pneumophila, Escherichia coli, and caliciviruses in drinking water-associated biofilms grown under high-shear turbulent flow. Appl. Environ. Microbiol. 73:2854-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molmeret, M., M. Horn, M. Wagner, M. Santic, and Y. Abu Kwaik. 2005. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 71:20-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narasimhan, R., J. Brereton, M. Abbaszadegan, H. Ryu, P. Butterfield, K. Thompson, and H. Werth. 2005. Characterizing microbial water quality in reclaimed water distribution systems. AwwaRF report 91072F. AWWA Research Foundation, Denver, CO.
- 28.National Health Service. 2007. Identification of Legionella species. National standard method BSOP ID 18. Standards Unit, Evaluation and Standards Laboratory Centre for Infections, United Kingdom. http://www.hpa-standardmethods.org.uk/documents/bsopid/pdf/bsopid18.pdf.
- 29.New Hampshire Department of Environmental Services. 2003. Fecal coliform as an indicator organism. Environmental fact sheet WD-WEB-18. New Hampshire Department of Environmental Services, Concord, NH. http://des.nh.gov/organization/commissioner/pip/factsheets/wwt/documents/web-18.pdf.
- 30.Pourahmad, F., K. D. Thompson, A. Adams, R. H. Richards. 2009. Comparative evaluation of polymerase chain reaction-restriction enzyme analysis (PRA) and sequencing of heat shock protein 65 (hsp65) gene for identification of aquatic mycobacteria. J. Microbiol. Methods 76:28-135. [DOI] [PubMed] [Google Scholar]
- 31.Ryu, H., A. Alum, and M. Abbaszadegan. 2005. Microbial characterization and population changes in nonpotable reclaimed water distribution systems. Environ. Sci. Technol. 39:8600-8605. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt, W., B. Hambsch, and H. Petzoldt. 1998. Classification of algogenic organic matter concerning its contribution to the bacterial regrowth potential and by-products formation. Water Sci. Technol. 37:91-96. [Google Scholar]
- 33.Seeley, H. W., P. J. Vandemark, and J. J. Lee. 1991. Microbes in action—a laboratory manual of microbiology. W. H. Freeman and Company, New York, NY.
- 34.Sokal, R. R., and F. J. Rohlf. 1962. The comparison of dendrograms by objective methods. Taxon 11:33-40. [Google Scholar]
- 35.Speight, V. 2008. Water-distribution systems: the next frontier. Bridge 38:31-37. http://www.nae.edu/Publications/TheBridge/Archives/V38N2/Water-DistributionSystemsTheNextFrontier.aspx. [Google Scholar]
- 36.Steele, T. W., J. Lanser, and N. Sangster. 1990. Isolation of Legionella longbeachae serogroup 1 from potting mixes. Appl. Environ. Microbiol. 56:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor, R. H., J. O. Falkinham, C. D. Norton, and M. W. LeChevallier. 2000. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol. 66:1702-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Böttger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Environmental Protection Agency. 2004. Guidelines for water reuse. Publication EPA625/R-04/108. U.S. Environmental Protection Agency, Washington, DC. http://www.epa.gov/NRMRL/pubs/625r04108/625r04108.pdf.
- 40.van der Kooij, D., A. Visser, and W. A. M. Hijnen. 1982. Determining the concentration of easily assimilable organic carbon in drinking water. J. Am. Water Works Assoc. 74:540-545. [Google Scholar]
- 41.Weinrich, L. A., E. Giraldo, and M. W. LeChevallier. 2009. Development and application of a bioluminescence AOC test in reclaimed waters. Appl. Environ. Microbiol. 75:7385-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. 2006. Guidelines for the safe use of wastewater, excreta and greywater, vol. 2. Wastewater use in agriculture. World Health Organization, Geneva, Switzerland.