Abstract
Existing methods for detection of food-borne pathogens and their toxins are frequently time-consuming, require specialized equipment, and involve lengthy culture procedures and/or animal testing and are thus unsuitable for a rapid response to an emergency public health situation. A series of simple and rapid affinity immunochromatography column (AICC) assays were developed to detect Clostridium botulinum neurotoxin types A, B, E, and F and Escherichia coli O157 in food matrices. Specifically, for milk, grape juice with peach juice, and bottled water, the detection limit for the botulinum neurotoxin type A complex was 0.5 ng. Use of this method with a 10-ml sample would therefore result in a detection limit of 50 pg ml−l. Thus, this assay is approximately 2 orders of magnitude more sensitive than a comparable lateral-flow assay. For botulinum neurotoxin complex types B, E, and F, the minimum detection limit was 5 ng to 50 ng. Sensitive detection of E. coli O157 was achieved, and the detection limit was 500 cells. The AICC test was also shown to be specific, rapid, and user friendly. This test takes only 15 to 30 min to complete without any specialized equipment and thus is suitable for use in the field. It has the potential to replace existing methods for presumptive detection of botulinum neurotoxin types A, B, E, and F and E. coli O157 in contaminated matrices without a requirement for preenrichment.
The majority of conventional methods used for detection and identification of pathogenic microorganisms, viruses, and/or their toxins lack the speed and sensitivity necessary for use in the field (they typically are not completed in a single day) and also require specialized equipment (20). Rapid methods, including antibody-based and nucleic acid-based assays, have revolutionized the methodology for detection of microbial pathogens and their toxins in foods (16). However, while most antibody-based and nucleic acid-based assays are rapid, specialized equipment is often required, and specific enrichment is needed to achieve the necessary sensitivity. This means that the analysis time can still be several days (16). Lateral-flow assays (LFAs) and column flow assays are tests that have considerable merit in terms of rapidity and ease of use in the field without specialized equipment (4, 5, 8, 19, 34).
Two contrasting agents were used as detection targets in this study: (i) a potent microbial toxin (Clostridium botulinum neurotoxin), including type A, B, E, and F neurotoxins; and (ii) an infectious pathogen, Escherichia coli O157. These two targets present different problems for detection; the first target is a protein toxin, and the second target is intact bacterial cells. The botulinum neurotoxin is the most potent toxin known, and as little as 30 to 100 ng has the potential to be fatal to humans (28). It is responsible for botulism, a severe neuroparalytic disease that affects humans and also animals and birds (28). There are seven antigenically distinct botulinum neurotoxins (types A to G), and a number of subtypes have also been described (9, 11, 15, 28, 36). Botulism in humans is associated principally with neurotoxin types A, B, E, and F (27, 29). Since the botulinum neurotoxins are the toxic agents and they can be produced by six physiologically distinct clostridia (28), considerable emphasis has been placed on detection of the neurotoxins rather than the bacteria. The “gold standard” method for detecting botulinum neurotoxins is the mouse bioassay due to its high levels of sensitivity and specificity. However, this technique is also problematic (33). It typically requires 24 to 48 h to yield results, is expensive, and is becoming less favored because of its use of animals (4). The alternative tests include enzyme-linked immunosorbent assays (ELISAs), lateral-flow assays (LFAs), a chemiluminescent slot blot immunoassay, surface plasmon resonance (SPR), the assay with a large immunosorbent surface area (ALISSA) test, and quantum dot immunoassays (4, 5, 7, 22, 43, 46). Lateral-flow assays are available and are convenient for toxin testing as they are easy to perform and rapid (<30 min) and no additional equipment is required. However, their poor sensitivity has limited their use (23).
E. coli O157 produces a cytotoxin (verotoxin), and an E. coli O157 infection can lead to severe bloody diarrhea, kidney failure, brain damage, and death. Enumeration, identification, and control of this pathogen are challenging due to the low infectious dose necessary to cause disease, which is between 2 and 2,000 ingested cells (41). Sources of E. coli O157 infection include ground beef and unpasteurized milk and apple juice (1), raw milk (6), and spinach and lettuce (42). Isolation of E. coli O157:H7 from water, food, and environmental samples is laborious. Culture is difficult due to the large competing microflora that either overgrows or mimics the non-sorbitol-fermenting organism E. coli O157:H7 (12). According to Tokarskyy and Marshall (41), the largest group of rapid test kits commercially available for testing for the presence of E. coli O157 in food includes immunological methods, such as latex agglutination, reverse passive latex agglutination, immunodiffusion, ELISA, immunomagnetic separation (IMS), and immunoprecipitation. The other methods that have been developed include a dipstick test device (2), a lateral-flow immunoassay (8), real-time PCR (39), and an enzyme-linked immunomagnetic chemiluminescent assay (17). However, in many cases these tests require preenrichment or have limited sensitivity.
The objective of the work described here was to develop a rapid sensitive diagnostic test for detection of botulinum neurotoxins A, B, E, and F and E. coli O157 that can be used without preenrichment.
MATERIALS AND METHODS
Neurotoxins and bacterial cultures.
Type A, B, E, and F botulinum neurotoxin complexes (300 to 550 kDa) were purchased from Metabiologics (Madison, WI). Toxins were tested to determine their identities by using neutralization with type-specific antibodies. Purity was checked by using SDS-PAGE. The potencies of the botulinum neurotoxins were determined by Metabiologics using mice and were as follows: type A, 3.6 × 107 50% minimum lethal dose (MLD50) mg−1; type B, 2 × 107 MLD50 mg−1; type E, 3 × 107 MLD50 mg−1; and type F, 3.6 × 106 MLD50 mg−1. A diverse collection of botulinum toxin-producing strains and verotoxigenic E. coli were used in the present study, along with nontoxic equivalents which were obtained from various sources (Tables 1 and 2). Clostridial cultures were grown anaerobically at 30°C in 10 ml PYGS (38) for 24 h and then pelleted by centrifugation at 3,000 × g. Supernatants were filter sterilized with Ministart 0.2-μm disposable filters (Sartorius, Germany) and stored at −20°C until used. The E. coli cultures were grown for 24 h at 30°C in 10 ml of tryptone soya broth (TSB) (Oxoid, Basingstoke, United Kingdom). Total viable counts were determined by plating on tryptone soya agar (TSA) (Oxoid).
TABLE 1.
Specificity of the AICC assay for detection of botulinum neurotoxins in culture supernatants
| Strain | Detection witha: |
|||
|---|---|---|---|---|
| Botulinum neurotoxin type A AICC assay | Botulinum neurotoxin type B AICC assay | Botulinum neurotoxin type E AICC assay | Botulinum neurotoxin type F AICC assay | |
| Proteolytic C. botulinum strains | ||||
| Type A strain Eyemouth (subtype A1) | + | + | + | − |
| Type A strains NCTC 3805 (subtype A1), ATCC 3502 (subtype A1), NCTC 9837 (subtype A2), NCTC 2012 (subtype A3), CDC 657 (subtype Ba4), and H04464 107 [subtype A5(B)] | + | + | − | − |
| Type B strains NCIMB 10657 and NCTC 7273 | − | + | − | − |
| Type F strains ATCC 25764 and NCTC 10281 | − | − | − | + |
| Nonproteolytic C. botulinum strains | ||||
| Type B strain Eklund 17B | − | + | − | − |
| Type E strains Beluga, Sebald P34, and CDC 7854 | − | − | + | − |
| Type F strain Colworth 195 | − | − | − | − |
| Other strains | ||||
| C. botulinum type C strains Colworth 3334, Colworth 3335, and Colworth 3337 | − | − | − | − |
| C. botulinum type D strain NCIMB 10619 | − | − | − | − |
| C. sporogenes NCIMB 10696 | − | − | − | − |
| C. butyricum NCIMB 7423 | − | − | − | − |
+, detection; −, no detection.
TABLE 2.
Specificity of the AICC assay for detection of E. coli O157
| E. coli serotype | Verotoxin(s)a | Strain | Detection with AICC assayb |
|---|---|---|---|
| O157 | None | PHL P1432 | + |
| O157 | None | NCTC 13125 | + |
| O157 | None | NCTC 13126 | + |
| O157 | None | NCTC 13127 | + |
| O157:H7 | None | NCTC 12900 | + |
| O157:H7 | VT1 and VT2 | ATCC 43895 | + |
| O157:H7 | VT1 and VT2 | NCTC 12079 | + |
| O26 | VT1 | PHL P0759 | − |
| O111 | VT1 and VT2 | PHL P0761 | − |
VT1, verotoxin type 1; VT2, verotoxin type 2.
+, detection; −, no detection.
Antibodies.
Polyclonal antibody Bactrace against E. coli O157:H7 isolated from a pool of sera from goats immunized with heat-killed whole cells of E. coli serotype O157 was obtained from Kirkegaard and Perry Laboratories (KPL), Gaithersburg, MD; this antibody was previously reported not to react with a range of enteric pathogens. Monoclonal antibodies against botulinum neurotoxin A designated F1-2 and F1-40, which bind to the 100-kDa heavy chain and the 50-kDa light chain, respectively, were a generous gift from Larry Stanker (United States Department of Agriculture Agricultural Research Service Western Regional Research Laboratory, Albany, CA) and were produced as previously described (37). Polyclonal antibodies against botulinum neurotoxin types B, E, and, F were obtained from Metabiologics. Finally, monoclonal antibodies against botulinum neurotoxin type B (clone C3/4 IgG1) and polyclonal antibodies against botulinum neurotoxin type E [biotinylated, horse F(ab)2 fragments] were purchased from DiaVita (Heidelberg, Germany).
Biotinylation.
Biotinylation of antibodies was performed using an EZ-Link N-hydroxysuccinimide (NHS)-LC biotinylation kit (catalog no. 21425; Pierce) according to the manufacturer's instructions. Antibodies were dissolved in phosphate-buffered saline (PBS) to obtain a final concentration of 200 μg ml−l (0.18 mM), and a sulfo-NHS-LC-biotin solution (9 mM) was added to obtain a molar ratio of biotin to antibody of 50:1. The reaction mixture was incubated on ice for 1 h, and excess salt removed by passing the protein solution through a desalting column. The supernatant fraction, containing the purified protein sample, was collected, and the protein content determined. This method was used for all antibodies unless they were supplied preconjugated with biotin.
ELISA.
Initially, an ELISA format was used so that the performance of pairs of antibodies could be assessed using the procedure described by Ferreira and Crawford (14). Different combinations of capture and detection antibodies were tested, and the sensitivity of the resulting assay was measured. Nunc Maxisorp microtiter plates (Thermo Fisher, Loughborough, United Kingdom) were coated with 100 μl per well of capture antibody to a botulinum neurotoxin or E. coli O157 at a concentration of 1 μg ml−l in PBS at 4°C overnight. The plates were then washed three times with 300 μl of PBST (10 mM phosphate buffer, 2.7 mM KCl, 140 mM NaCl, 0.05% Tween 20; pH 7.4) and blocked by adding 300 μl of 1% bovine serum albumin (BSA) in PBS (Sigma) to each well, and then they were incubated at 37°C for 1 h. The appropriate antigens (botulinum neurotoxin complex or E. coli O157 strain NCTC 13126) were serially diluted in PBS and added to wells (100 μl), and the plates were incubated at 37°C for 1 h. Each plate was washed as described above; 100 μl of the detection antibody (1 μg ml−l) was added to each well and the plate incubated at 37°C for 1 h. After another washing step, 100 μl of horseradish peroxidase (HRP)-conjugated streptavidin (New England Biolabs, Herts, United Kingdom) was added to each well, and the plate was incubated at 37°C for 1 h. After another washing step, 3,3′,5′5′-tetramethylbenzidine (TMB) (Europa Bioproducts, Ely, United Kingdom) was added to the wells (100 μl per well). The reaction was stopped with 2 M H2SO4 (25 μl per well), and the absorbance at 450 nm in the wells was measured. The detection limit was the mean plus 3 standard deviations of the absorbance value for the negative control (1% BSA in PBS). All experiments were carried out in triplicate.
Principles and AICC construction.
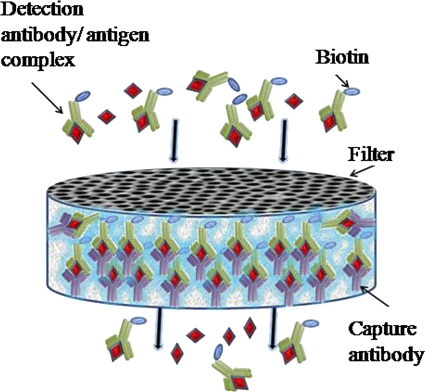
The antibodies were used in a proprietary column-based immunofiltration assay based on the antibody immunocolumn for analytical process (ABICAP) technology (DiaVita). The columns (5.5 cm high with a porous matrix equivalent to a surface area of 40 cm2) function as a flowthrough ELISA driven by gravity at a rate of 350 μl min−l; following use of capture and detection antibodies, a visible blue band is then observed after precipitation of TMB (Fig. 1). All materials used for fabrication of columns were purchased from DiaVita unless otherwise stated.
FIG. 1.
Diagram of the assay. A test sample is preincubated with a biotinylated detection antibody for 10 min, resulting in formation of an antibody-antigen complex. The antibody-coated filter captures the antigen-antibody complex from solution, while unbound antibody flows through. Then a second solution, which contains HRP-labeled streptavidin that binds to the biotin, is added. Finally, an insoluble substrate for the HRP enzyme is added, which produces a visible band following precipitation of TMB where the HRP, and thus the antigen, is present. For the E. coli O157 assay, unbound cells were run through the column to bind to the capture antibody before addition of the detection antibody.
Column construction.
Columns were constructed by using the method of Lucht et al. (24). Briefly, polyethylene filters (frits) (2.5 by 5 mm; pore size, 40 μm) were degassed in ethanol (96%, vol/vol), which was followed by three washes in 50% ethanol. The frits were then washed and degassed three times in immobilization buffer (PBS, pH 7.4). Finally, the frits were coated by adsorption with capture antibody diluted in PBS at a concentration of 20 μg ml−l for 12 h at room temperature and then saturated with PBS containing 1% (wt/vol) bovine serum albumin (BSA) (Sigma) and 0.05% 5-bromo-5-nitro-1,3-dioxane (Sigma) as a preservative. The frits were then freeze-dried overnight and stored at 4°C. Negative-control frits were treated as described above, except that antibodies were not added. Positive-control frits precoated with an anti-HRP antibody were supplied by DiaVita. Dried coated frits were assembled into columns with a negative-control frit toward the top of each column, a specific measurement frit in the middle, and a positive-control frit toward the bottom of the column. Measurement frits were separated with a spacer frit absorbed with 1% (wt/vol) BSA.
AICC assay procedure.
Botulinum neurotoxin-spiked samples (serial 10-fold dilutions with concentrations ranging from 0.1 ng ml−l to 1 μg ml−l) and the negative control PBS were preincubated with the biotinylated antibody (at concentrations of 5 μg ml−l for type A neurotoxin, 10 μg ml−l for type B and E neurotoxins, and 1 μg ml−l for type F neurotoxin) in casein buffer or food samples for 10 min. The preincubated toxin-detector-antibody conjugate (500 μl) was applied to a column at room temperature and allowed to flow through it. Next, 500 μl of streptavidin-poly-HRP (1:5,000 dilution; DiaVita) was added at room temperature. Each of these two steps took 6 min. Then 500 μl of wash buffer (0.01 M PBS, 0.1% BSA, 0.05% Tween 20) was added twice, followed by 500 μl substrate buffer (0.1 M sodium acetate-citrate buffer, pH 4.9). Finally, 500 μl of a precipitating HRP substrate (TMB) was added at room temperature, and the column was washed with 500 μl of substrate buffer. All experiments were carried out in triplicate.
E. coli O157-spiked samples (500 μl; serial 10-fold dilutions with concentrations ranging from 2 × 101 CFU ml−l to 2 × 107 CFU ml−l) and a PBS negative control were applied to columns and incubated as described above. Then 500 μl of the detection antibody (goat anti-E. coli O157:H7 HRP-labeled polyclonal antibodies [KPL] diluted in PBS-2.5% [wt/vol] casein at a concentration of 8 μg ml−l) was added, and the column was incubated as described above. This was followed by two washes with 500-μl aliquots of wash buffer and addition of 500 μl substrate buffer (0.1 M sodium acetate-citrate buffer, pH 4.9). Finally, 500 μl of the precipitating HRP substrate (TMB) was added at room temperature, and the column was washed with 500 μl of substrate buffer. All experiments were carried out in triplicate.
Analytical specificity of the AICC test systems.
Botulinum neurotoxin type A, B, E, and F AICC assays were tested for specificity for their targets using a panel of culture supernatants of C. botulinum strains that form different botulinum neurotoxins (Table 1), as well as strains of Clostridium sporogenes and Clostridium butyricum (nontarget organisms). Culture supernatants (500 μl) were added to the AICC system using the method described above. Similarly, the specificity of the E. coli O157 AICC system was assessed using a panel of E. coli strains (Table 2) comprising target organisms, as well as nontarget organisms known to cause similar clinical manifestations. For testing, colonies from plates were suspended in phosphate-buffered saline (PBS) at concentrations up to approximately 1 × 107 CFU ml−l. Finally, 500-μl samples were tested using the AICC system and the method described above.
Preparation of spiked food samples.
Commercially produced skim milk, semiskim milk, apple juice, grape juice with peach juice, and two types of bottled still water were purchased locally. Botulinum neurotoxin type A, B, E, and F complexes were diluted in the test matrices to obtain final concentrations of 0.5 ng ml−l to 1 μg ml−l before addition to the AICC systems. E. coli O157 strains were inoculated into 10 ml of TSB and incubated for 24 h at 30°C. Cells were resuspended in each of the matrices at concentrations of 103, 105, and 107 CFU ml−l before addition to the AICC systems.
RESULTS
The AICC test is based on a “sandwich” format using commercially available antibodies and columns. A capture antibody is immobilized on the column matrix; the analyte in solution is introduced into the column matrix and is captured and concentrated by the capture antibody as the supernatant flows through. Following addition of a labeled second antibody, a visual band is observed (Fig. 1).
ELISA.
Pairs of capture and detection antibodies that performed well were identified in the preliminary tests. The characteristics of the sandwich ELISAs for botulinum neurotoxin type A, B, E, and F complexes and E. coli O157 are shown in Fig. 2. Each of the botulinum neurotoxin-sourced antibodies was labeled with biotin and then used as either the capture antibody or the detection antibody to determine the optimum combination. E. coli O157 antibodies were labeled with peroxidase before determination of sensitivity. Significantly lower levels of activity were observed when various other combinations of capture and detection antibodies were used (data not shown). The detection limits of the antibody pairs selected were as follows: botulinum neurotoxin type A complex, 6.5 pg ml−l; botulinum neurotoxin type B complex, 2.3 ng ml−l; botulinum neurotoxin type E complex, 70 pg ml−l; and botulinum neurotoxin type F complex, 15.4 ng ml−l. The minimum detectable level (MDL) for E. coli O157 cells was 5 × 103 CFU ml−l.
FIG. 2.
Characterization of antibodies in the sandwich ELISA. (a) Botulinum neurotoxin type A complex assay using monoclonal antibody F1-2 as the capture antibody and F1-40 labeled with biotin as the detection antibody. (b) Botulinum neurotoxin type B complex assay using monoclonal antibody clone C3/4 IgG1 as the capture antibody and rabbit IgG-specific polyclonal antibody (Metabiologics) labeled with biotin as the detection antibody. (c) Botulinum neurotoxin type E complex assay using rabbit IgG-specific polyclonal antibody (Metabiologics) as the capture antibody and horse F(ab)2 fragments labeled with biotin as the detection antibody. (d) Botulinum neurotoxin type F complex assay using rabbit IgG-specific polyclonal antibody (Metabiologics) as the capture antibody and the same antibody labeled with biotin as the detection antibody. (e) E. coli O157 NCTC 13126 cells using Bactrace polyclonal antibody as the capture antibody and the same antibody labeled with horseradish peroxidase as the detection antibody. The symbols indicate the averages of three determinations, and the error bars indicate ±1 standard deviation.
AICC construction.
Successful detection of E. coli O157 cells and botulinum neurotoxin type A, B, E, and F complexes was accomplished with the AICC constructed. In particular; for assay buffer spiked with various concentrations of botulinum neurotoxin type A complex the limit of detection was 0.5 ng in 500 μl (Fig. 3). For botulinum neurotoxin types B and F the minimum detection limit was 50 ng in 500 μl, while for botulinum neurotoxin type E the minimum detection limit was 5 ng in 500 μl. The detection limit for the E. coli O157 AICC was 500 cells in 500 μl using HRP-labeled antibody (Fig. 3).
FIG. 3.
AICC columns used for sensitive detection of botulinum neurotoxin complex A and E. coli O157. The limit of detection for the botulinum type A neurotoxin complex AICC assay was 0.5 ng. The limit of detection for the E. coli AICC assay was 500 CFU. Each column contained a negative-control frit toward the top of the column, a specific measurement filter in the middle, and a positive-control frit toward the bottom of the column. Measurement frits were separated with a spacer frit absorbed with 1% (wt/vol) BSA.
The analytical specificity was validated using a series of target agents and nontarget contaminants with relevance for routine microbiological diagnostics. All tests were performed in triplicate. Overall, the selectivity of the system was very high. The botulinum neurotoxin type A AICC assay was positive only for supernatants from cultures of C. botulinum strains that produced type A neurotoxin. All five subtypes of type A neurotoxin, including type A1 (strains NCTC 3805, ATCC 3502, and Eyemouth), type A2 (strain NCTC 9837), type A3 (strain NCTC 2012), type A4 (strain CDC 657), and type A5 (strain H04464 107), were detected (Table 1). The botulinum neurotoxin type B AICC assay was positive for the three botulinum neurotoxin B-producing strains tested (Table 1). Furthermore, the botulinum neurotoxin type B AICC assay was also positive for all botulinum neurotoxin type A strains, although the signal was weaker. However, one of the type A strains (CDC 657) is known to also form type B neurotoxin (36). The botulinum neurotoxin type E AICC assay was positive for the three botulinum neurotoxin type E-producing strains and also for the botulinum neurotoxin type A-producing strain Eyemouth. Finally, the botulinum neurotoxin type F AICC assay was positive for the two proteolytic C. botulinum type F strains but was negative for the nonproteolytic C. botulinum neurotoxin type F strain Colworth 195. The E. coli O157 AICC assay detected all of the O157 strains tested (Table 2). Finally, no cross-reactivity was observed with two other E. coli serogroups (serogroup O111 strain PHL P0761 and serogroup O26 strain PHL P0759).
The sensitivity of the AICC assay for determination of the presence of the botulinum neurotoxin complex was also evaluated using foodstuff matrices (Table 3). All assays were carried out in triplicate using negative-control (PBS) samples for comparison. The detection limits for the botulinum neurotoxin type A, B, E, and F AICC assays with milk, grape juice with peach juice, and bottled water were consistent with those with buffer. However, botulinum neurotoxin type A, B, E, and F complexes were not detected in apple juice. For a sample volume of 500 μl, the limit of detection for E. coli O157 in the AICC assays was equivalent to that for buffer-spiked samples (500 cells). Unspiked food samples did not give positive results in any of the AICC tests.
TABLE 3.
Detection limits for botulinum neurotoxin type A, B, E, and F complexes and E. coli O157 in various matrices using the AICC system
| Matrix | Detection limit of AICC assay fora: |
||||
|---|---|---|---|---|---|
| Botulinum neurotoxin type A (ng) | Botulinum neurotoxin type B (ng) | Botulinum neurotoxin type E (ng) | Botulinum neurotoxin type F (ng) | E. coli O157 (cells) | |
| Semiskim milk | 0.5 | 50 | 5 | 50 | 500 |
| Skim milk | 0.5 | 50 | 5 | 50 | 500 |
| Apple juice | − | − | − | − | 500 |
| Grape juice with peach juice | 0.5 | 50 | − | 50 | 500 |
| Bottled water (type 1) | 0.5 | 50 | 5 | 50 | 500 |
| Bottled water (type 2) | 0.5 | 50 | 5 | 50 | 500 |
The volume for each test was 500 μl. −, no detection.
DISCUSSION
Rapid methods for sensitive and specific detection of pathogenic bacteria and their toxins are needed (33). In particular, there is need for methods that can be used easily in the field. The most prevalent group of rapid test kits commercially available for E. coli O157 (41) and botulinum neurotoxins (23) involve immunological methods. However, in terms of the detection limit, most systems targeted at E. coli O157 have various limitations (41), and the typical detection limits are approximately 103 to 105 CFU ml−l (13). The “gold standard” for detecting botulinum neurotoxins continues to be the mouse bioassay due to its exquisite sensitivity to botulinum neurotoxin (0.01 ng ml−l) (45). However, this technique can be problematic and usually requires 24 to 48 h to yield results (23). Although not as sensitive as the mouse assay, the prototype AICC test reported here offers several advantages compared to existing techniques for rapid detection of botulinum neurotoxins and E. coli O157.
The AICC assay for detecting type A botulinum neurotoxin had a limit of detection of 0.5 ng, which is comparable to the limits of detection of many other detection systems, such as an ELISA (14, 23). However, Sharma et al. (32) reported a detection limit of 60 pg/ml for a type A ELISA. Furthermore, the AICC assay is rapid and requires little technical knowledge, and in this respect it is comparable to lateral-flow devices (19). In particular, Gessler et al. (19) evaluated four lateral-flow assays (LFAs) for detection of purified botulinum neurotoxin type A. The greatest sensitivity for purified neurotoxin was observed with the rapid analyte measurement platform (RAMP) test (50 ng ml−l), with two of the tests failing to detect purified neurotoxin. All four tests detected the type A neurotoxin complex with the best sensitivity (10 ng ml−l). Furthermore, Sharma et al. (34) examined commercially available LFAs and reported sensitivities of 10 ng ml−l when type A neurotoxin complex was used. A test based on similar ABICAP technology for detection of type A neurotoxin complex has been described (4), and the limit of detection is 0.2 ng ml−l (0.45 pM). However, this value was obtained using a photometer rather than visual determination. The AICC assay described here detects 0.5 ng neurotoxin; assuming that the reaction kinetics are similar, if this amount were in 10 ml, then the visual detection limit would be 0.05 ng ml−l. Thus, this assay is more than 2 orders of magnitude more sensitive than the LFA and therefore could be a more suitable screening tool. Additionally, a photometer may improve the detection limit further.
The AICC tests for botulinum neurotoxin types B, E, and F (50, 5, and 50 ng, respectively) were not as sensitive as the test for type A botulinum neurotoxin. LFAs that detect type B neurotoxin (10 ng ml−l) and type E neurotoxin (20 ng ml−l) have been described (19, 34). If 10 ml were used in the AICC assay developed in the present study, the detection limits would be 5 ng ml−l and 0.5 ng ml−l for type B and E neurotoxin complexes, respectively. Thus, this assay is marginally more sensitive for the type B neurotoxin complex and more than 1 order of magnitude more sensitive for the type E neurotoxin complex. Additionally, larger sample volumes could be applied to the columns, which should increase the sensitivity of the AICC assay further. Moreover, there appears to be no reported ABICAP test for detection of neurotoxin type F.
The limit of detection for the E. coli O157 AICC test was 500 cells. This compares favorably to the limits of detection for other tests (21, 26, 47). Since enrichment is a prerequisite for using many immunosensors (21, 31, 35, 47), the AICC assay is a more viable alternative as no enrichment is required for this assay to achieve low detection limits. Furthermore, large sample volumes can be applied, and thus lower concentrations of cells can be detected by concentrating the analyte (18). Additionally, in preliminary experiments with the present test system positive results were obtained using a 1-liter test sample (data not shown). Another important consideration is that, unlike an ELISA system, sample components that might interfere with or inhibit the immunological reaction are washed out if they are small enough to pass through the AICC assay frits (18).
To assess the performance of antibodies in the AICC assay, experiments were carried out with other E. coli serogroups, related bacterial strains, and proteins to evaluate the specificity. It was important to test the specificity with the column format instead of an ELISA format as antibodies can perform differently in the AICC assay. In particular, the E. coli O157 AICC test was highly specific for E. coli O157, even when non-O157 E. coli serogroups were applied at a high concentration (107 CFU ml−l).
The specificity of the botulinum neurotoxin assay varied with the neurotoxin type. The high affinities and distinct epitopes (i.e., heavy chain versus light chain) of the monoclonal antibodies to botulinum neurotoxin type A used in this study allowed development of a sensitive sandwich ELISA to detect both purified type A botulinum neurotoxin and the neurotoxin complex (37). The results of AICC assays suggested that these antibodies were specific for botulinum neurotoxin type A and detected all five reported subtypes of type A neurotoxin (9, 10, 28). The botulinum neurotoxin type B, E, and F AICC assays all detected the toxin types for which they are intended. Cross-reactions were seen in some instances and might have been due to structural similarities of the toxins (36); cross-reactions have also been reported for lateral-flow devices (34). For the purposes of this detection system, cross-reactivity with related botulinum neurotoxins is not very important, as the test is designed to produce a positive or negative response for any botulinum neurotoxin and characterization of the botulinum neurotoxin type is a secondary feature; i.e., it is more important to have a fail-safe detection system.
Food components can interfere with immunologically based tests, resulting in lower sensitivity (23). The detection limits for botulinum neurotoxin type A, B, E, and F AICC assays were comparable when the toxin was added to buffer and to milk, grape juice with peach juice, and bottled water. The results provide evidence that the tests could be successfully used for regular screening or postcontamination detection of botulinum neurotoxin in the public milk supply. The potential deliberate contamination of the public milk supply has been evaluated using values based on analysis of historical data (44). It was reported that pasteurization (77°C for 15 min) resulted in a <10-fold reduction in the activity of the neurotoxin (44). Although many assays that detect botulinum neurotoxin have been described, few are sensitive in complex matrices (30). Sharma et al. (34) evaluated two LFAs for detecting botulinum neurotoxin in several food matrices, including milk, juice, infant formula, processed meats, and spices. They found that the sensitivities of the lateral-flow devices were approximately 5 ng (150 μl per test) for botulinum neurotoxin complex types A and B and approximately 10 ng for botulinum neurotoxin complex type E (34). However, spiked samples required centrifugation, organic solvent extraction, and resuspension in assay buffer before application. In addition, the slow filtration required for some food matrices was found to delay assay results with the lateral-flow devices (34). The botulinum neurotoxin type A AICC assay developed in the present study has a detection limit of 0.5 ng neurotoxin complex, and it required no preparation step for the foods tested. A simple prefiltration step could be included if it was necessary to remove large particulates. However, botulinum neurotoxin type A, B, E, and F complexes were not detected in apple juice using the AICC assay. There are several reasons for the failure to detect toxins in apple juice. As described previously, each food sample has an inimitable biochemical composition, making it difficult to be certain which matrix element interferes with the toxin complex or with the antigen-antibody interaction (32). Furthermore, it has been suggested that several components in apple juice could interfere with antibody-antigen interactions (25, 40).
Outbreaks of food-borne illness involving E. coli O157:H7 have been associated with unpasteurized apple juice, orange juice, unpasteurized milk, alfalfa sprouts, lettuce, and water (3, 6, 42). These outbreaks resulted from natural contamination events. Rapid detection tests are therefore necessary to identify the source of contaminated food. The AICC test has been shown to be effective for this purpose. E. coli O157 cells could be detected in all matrices at the lowest concentration used (500 cells). This limit of detection was the same as that obtained for a spiked buffer solution. The robustness of the AICC assay for E. coli O157 samples in these matrices may be due in part to the fact that the target antigen is presented in its natural state on the surface of a living cell, in contrast to botulinum toxins, which, as excreted proteins, are not protected to the same extent.
In summary, the present report describes a series of easy-to-use rapid AICC tests that are sensitive for detecting botulinum neurotoxins and E. coli O157 in a variety of matrices. Further work is necessary to widen the scope of the AICC assays in terms of the materials that can be tested and the sample preparation methods associated with these materials. Furthermore, as new antibodies are developed, there is potential to make the AICC test even more sensitive. The new methods are likely to be an improvement on traditional labor- and time-intensive methods.
Acknowledgments
We thank P. Miethe (Senova/fzmb) for technical advice and for supplying materials for column construction, Larry Stanker (ARS-WRRL, USDA) for supplying monoclonal antibodies against botulinum neurotoxin type A, and Sandra Stringer, June Plowman, Clare Aldus, and Andrew Carter for advice and assistance.
This work was supported by the United Kingdom Home Office (project TR01/05/109).
Footnotes
Published ahead of print on 30 April 2010.
REFERENCES
- 1.Abubakar, I., L. Irvine, C. M. Aldus, G. M. Wyatt, R. Fordham, S. Schelenz, L. Shepstone, A. Howe, M. Peck, and P. R. Hunter. 2007. A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food. Health Technol. Assess. 11:1-216. [DOI] [PubMed] [Google Scholar]
- 2.Aldus, C. F., A. van Amerongen, R. M. C. Ariens, M. W. Peck, J. H. Wichers, and G. M. Wyatt. 2003. Principles of some novel rapid dipstick methods for detection and characterization of verotoxigenic Escherichia coli. J. Appl. Microbiol. 95:380-389. [DOI] [PubMed] [Google Scholar]
- 3.Arcidiacono, S., P. Pivarnik, C. M. Mello, and A. Senecal. 2008. Cy5 labeled antimicrobial peptides for enhanced detection of Escherichia coli O157:H7. Biosens. Bioelectron. 23:1721-1727. [DOI] [PubMed] [Google Scholar]
- 4.Attree, O., V. Guglielmo-Viret, V. Gros, and P. Thullier. 2007. Development and comparison of two immunoassay formats for rapid detection of botulinum neurotoxin type A. J. Immunol. Methods 325:78-87. [DOI] [PubMed] [Google Scholar]
- 5.Bagramyan, K., J. R. Barash, S. S. Arnon, and M. Kalkum. 2008. Attomolar detection of botulinum toxin type A in complex biological matrices. PLoS One 3:e2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhat, M., J. Denny, K. MacDonald, J. Hofmann, S. Jain, and M. Lynch. 2007. Escherichia coli O157:H7 infection associated with drinking raw milk—Washington and Oregon, November-December 2005. JAMA 297:1426-1428. [PubMed] [Google Scholar]
- 7.Cadieux, B., B. Blanchfield, J. P. Smith, and J. W. Austin. 2005. A rapid chemiluminescent slot blot immunoassay for the detection and quantification of Clostridium botulinum neurotoxin type E. Int. J. Food Microbiol. 101:9-16. [DOI] [PubMed] [Google Scholar]
- 8.Capps, K. L., E. M. McLaughlin, A. W. A. Murray, C. F. Aldus, G. M. Wyatt, M. W. Peck, A. Amerongen, R. M. C. Ariens, J. H. Wichers, C. L. Baylis, D. R. A. Wareing, and F. J. Bolton. 2004. Validation of three rapid screening methods for detection of verotoxin-producing Escherichia coli in foods: interlaboratory study. J. AOAC Int. 87:68-77. [PubMed] [Google Scholar]
- 9.Carter, A. T., C. J. Paul, D. R. Mason, S. M. Twine, M. J. Alston, S. M. Logan, J. W. Austin, and M. W. Peck. 2009. Independent evolution of neurotoxin and flagellar genetic loci in proteolytic Clostridium botulinum. BMC Genomics 10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter, A. T., D. R. Mason, K. A. Grant, G. Franciosa, P. Aureli, and M. W. Peck. 2010. Further characterization of proteolytic Clostridium botulinum type A5 reveals that neurotoxin formation is unaffected by loss of the cntR (botR) promoter sigma factor binding site. J. Clin. Microbiol. 48:1012-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, Y., H. Korkeala, J. Aarnikunnas, and M. Lindström. 2007. Sequencing the botulinum neurotoxin gene and related genes in Clostridium botulinum type E strains reveals orfx3 and a novel type E neurotoxin subtype. J. Bacteriol. 189:8643-8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer, E., and A. E. Heuvelink. 2000. Methods for the detection and isolation of Shiga toxin-producing Escherichia coli. J. Appl. Microbiol. 88:133S-143S. [DOI] [PubMed] [Google Scholar]
- 13.de Boer, E., and R. R. Beumer. 1999. Methodology for detection and typing of foodborne microorganisms. Int. J. Food Microbiol. 50:119-130. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira, J. L., and R. G. Crawford. 1998. Detection of type A botulinal toxin-producing organisms subcultured from cheese using an amplified ELISA system. J. Rapid Methods Automat. Microbiol. 6:289-296. [Google Scholar]
- 15.Franciosa, G., A. Maugliani, F. Floridi, and P. Aureli. 2006. A novel type A2 neurotoxin gene cluster in Clostridium botulinum strain Mascarpone. FEMS Microbiol. Lett. 261:88-94. [DOI] [PubMed] [Google Scholar]
- 16.Ge, B., and J. Meng. 2009. Advanced technologies for pathogen and toxin detection in foods: current applications and future directions. J. Assoc. Lab. Automat. 14:235-241. [Google Scholar]
- 17.Gehring, A. G., P. L. Irwin, S. A. Reed, S. I. Tu, P. E. Andreotti, H. Akhavan-Tafti, and R. S. Handley. 2004. Enzyme-linked immunomagnetic chemiluminescent detection of Escherichia coli O157:H7. J. Immunol. Methods 293:97-106. [DOI] [PubMed] [Google Scholar]
- 18.Gessler, F., K. Hampe, and H. Bohnel. 2005. Sensitive detection of botulinum neurotoxin types C and D with an immunoaffinity chromatographic column test. Appl. Environ. Microbiol. 71:7897-7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gessler, F., S. Pagel-Wieder, M. A. Avondet, and H. Bohnel. 2007. Evaluation of lateral flow assays for the detection of botulinum neurotoxin type A and their application in laboratory diagnosis of botulism. Diagn. Microbiol. Infect. Dis. 57:243-249. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal, S. S., M. W. Mayo, J. G. Bruno, B. V. Bronk, C. A. Batt, and J. P. Chambers. 2000. A review of molecular recognition technologies for detection of biological threat agents. Biosens. Bioelectron. 15:549-578. [DOI] [PubMed] [Google Scholar]
- 21.Kamma, S., L. Tang, K. Leung, E. Ashton, N. Newman, and M. R. Suresh. 2008. A rapid two dot filter assay for the detection of E. coli O157 in water samples. J. Immunol. Methods 336:159-165. [DOI] [PubMed] [Google Scholar]
- 22.Ladd, J., A. D. Taylor, J. Homola, and S. Jiang. 2008. Detection of botulinum neurotoxins in buffer and honey using a surface plasmon resonance (SPR) sensor. Sens. Actuators B Chem. 130:129-134. [Google Scholar]
- 23.Lindström, M., and H. Korkeala. 2006. Laboratory diagnostics of botulism. Clin. Microbiol. Rev. 19:298-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucht, A., P. Formenty, H. Feldmann, M. Gotz, E. Leroy, P. Bataboukila, A. Grolla, F. Feldmann, T. Wittmann, P. Campbell, C. Atsangandoko, P. Boumandoki, E. J. Finke, P. Miethe, S. Becker, and R. Grunow. 2007. Development of an immunofiltration-based antigen-detection assay for rapid diagnosis of Ebola virus infection. J. Infect. Dis. 196:S184-S192. [DOI] [PubMed] [Google Scholar]
- 25.Nyquist-Battie, C., L. E. Frank, D. Lund, and D. V. Lim. 2004. Optimization of a fluorescence sandwich enzyme-linked immunosorbent assay for detection of Escherichia coli O157:H7 in apple juice. J. Food Prot. 67:2756-2759. [DOI] [PubMed] [Google Scholar]
- 26.Park, S., H. Kim, S. H. Paek, J. W. Hong, and Y. K. Kim. 2008. Enzyme-linked immuno-strip biosensor to detect Escherichia coli O157:H7. Ultramicroscopy 108:1348-1351. [DOI] [PubMed] [Google Scholar]
- 27.Peck, M. W. 2006. Clostridium botulinum and the safety of minimally heated, chilled foods: an emerging issue? J. Appl. Microbiol. 101:556-570. [DOI] [PubMed] [Google Scholar]
- 28.Peck, M. W. 2009. Biology and genomic analysis of Clostridium botulinum. Adv. Microb. Physiol. 55:183-265, 320. [DOI] [PubMed] [Google Scholar]
- 29.Peck, M. W., and S. C. Stringer. 2005. The safety of pasteurised in-pack chilled meat products with respect to the foodborne botulism hazard. Meat Sci. 70:461-475. [DOI] [PubMed] [Google Scholar]
- 30.Scarlatos, A., B. A. Welt, B. Y. Cooper, D. Archer, T. DeMarse, and K. V. Chau. 2005. Methods for detecting botulinum toxin with applicability to screening foods against biological terrorist attacks. J. Food Sci. 70:R121-R130. [Google Scholar]
- 31.Seo, K. H., R. E. Brackett, and J. F. Frank. 1998. Rapid detection of Escherichia coli O157:H7 using immuno-magnetic flow cytometry in ground beef, apple juice, and milk. Int. J. Food Microbiol. 44:115-123. [DOI] [PubMed] [Google Scholar]
- 32.Sharma, S. K., J. L. Ferreira, B. S. Eblen, and R. C. Whiting. 2006. Detection of type A, B, E, and F Clostridium botulinum neurotoxins in foods by using an amplified enzyme-linked immunosorbent assay with digoxigenin-labeled antibodies. Appl. Environ. Microbiol. 72:1231-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma, S. K., S. Cai, and B. R. Singh. 2009. Here a method, there a method, everywhere many methods. What should a laboratory do to validate an assay for the detection of botulinum neurotoxins. Botulinum J. 1:183-198. [Google Scholar]
- 34.Sharma, S. K., B. S. Eblen, R. L. Bull, D. H. Burr, and R. C. Whiting. 2005. Evaluation of lateral-flow Clostridium botulinum neurotoxin detection kits for food analysis. Appl. Environ. Microbiol. 71:3935-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shelton, D. R., J. A. Higgins, J. A. Van Kessel, Y. A. Pachepsky, K. Belt, and J. S. Karns. 2004. Estimation of viable Escherichia coli O157 in surface waters using enrichment in conjunction with immunological detection. J. Microbiol. Methods 58:223-231. [DOI] [PubMed] [Google Scholar]
- 36.Smith, T. J., J. Lou, I. N. Geren, C. M. Forsyth, R. Tsai, S. L. LaPorte, W. H. Tepp, M. Bradshaw, E. A. Johnson, L. A. Smith, and J. D. Marks. 2005. Sequence variation within botulinum neurotoxin serotypes impacts antibody binding and neutralization. Infect. Immun. 73:5450-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanker, L. H., P. Merrill, M. C. Scotcher, and L. W. Cheng. 2008. Development and partial characterization of high-affinity monoclonal antibodies for botulinum toxin type A and their use in analysis of milk by sandwich ELISA. J. Immunol. Methods 336:1-8. [DOI] [PubMed] [Google Scholar]
- 38.Stringer, S. C., M. D. Webb, and M. W. Peck. 2009. Contrasting effects of heat-treatment and incubation temperature on germination and outgrowth of individual spores of nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 75:2712-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi, H., B. Kimura, Y. Tanaka, J. Shinozaki, T. Suda, and T. Fujii. 2009. Real-time PCR and enrichment culture for sensitive detection and enumeration of Escherichia coli. J. Microbiol. Methods 79:124-127. [DOI] [PubMed] [Google Scholar]
- 40.Taylor, A. D., J. Ladd, Q. M. Yu, S. F. Chen, J. Homola, and S. Y. Jiang. 2006. Quantitative and simultaneous detection of four foodborne bacterial pathogens with a multi-channel SPR sensor. Biosens. Bioelectron. 22:752-758. [DOI] [PubMed] [Google Scholar]
- 41.Tokarskyy, O., and D. L. Marshall. 2008. Immunosensors for rapid detection of Escherichia coli O157:H7: perspectives for use in the meat processing industry. Food Microbiol. 25:1-12. [DOI] [PubMed] [Google Scholar]
- 42.Vugia, D., A. Cronquist, J. Hadler, M. Tobin-D'Angelo, D. Blythe, K. Smith, K. Thornton, D. Morse, P. Cieslak, T. Jones, K. Holt, J. Guzewich, O. Henao, E. Scallan, F. Angulo, P. Griffin, R. Tauxe, and E. Barzilay. 2006. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, United States, 2005. JAMA 295:2241-2243. [Google Scholar]
- 43.Warner, M. G., J. W. Grate, A. Tyler, R. M. Ozanich, K. D. Miller, J. Lou, J. D. Marks, and C. J. Bruckner-Lea. 2009. Quantum dot immunoassays in renewable surface column and 96-well plate formats for the fluorescence detection of botulinum neurotoxin using high-affinity antibodies. Biosens. Bioelectron. 25:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wein, L. M., and Y. Liu. 2005. Analyzing a bioterror attack on the food supply: the case of botulinum toxin in milk. Proc. Natl. Acad. Sci. U. S. A. 102:9984-9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wictome, M., K. Newton, K. Jameson, B. Hallis, P. Dunnigan, E. Mackay, S. Clarke, R. Taylor, J. Gaze, K. Foster, and C. Shone. 1999. Development of an in vitro bioassay for Clostridium botulinum type B neurotoxin in foods that is more sensitive than the mouse bioassay. Appl. Environ. Microbiol. 65:3787-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wictome, M., K. A. Newton, K. Jameson, P. Dunnigan, S. Clarke, J. Gaze, A. Tauk, K. A. Foster, and C. C. Shone. 1999. Development of in vitro assays for the detection of botulinum toxins in foods. FEMS Immunol. Med. Microbiol. 24:319-323. [DOI] [PubMed] [Google Scholar]
- 47.Yu, L. S. L., S. A. Reed, and M. H. Golden. 2002. Time-resolved fluorescence immunoassay (TRFIA) for the detection of Escherichia coli O157:H7 in apple cider. J. Microbiol. Methods 49:63-68. [DOI] [PubMed] [Google Scholar]