Abstract
We report on the hydrogen production properties of the unicellular, diazotrophic cyanobacterium Cyanothece sp. strain ATCC 51142. This organism has a versatile metabolism and can grow in the presence or absence of combined nitrogen and can grow photosynthetically or mixotrophically and heterotrophically in the presence of glycerol. The strain produces a bidirectional hydrogenase (encoded by the hox genes), an uptake hydrogenase (hupLS), and nitrogenase (nifHDK). We demonstrated hydrogen production by both the hydrogenase and the nitrogenase under appropriate metabolic conditions. The highest rates of hydrogen production were produced under nitrogen-fixing conditions when cells were grown and incubated under continuous light conditions, in either the presence or absence of glycerol. Under such nitrogen-fixing conditions, we have achieved rates of 300 μmol H2/mg chloramphenicol (Chl)/hr during the first 24 h of incubation. The levels of H2 measured were dependent upon the incubation conditions, such as sparging with argon, which generated anaerobic conditions. We demonstrated that the same conditions led to high levels of H2 production and N2 fixation, indicating that low-oxygen conditions favor nitrogenase activity for both processes. The levels of hydrogen produced by the hydrogenase are much lower, typically 5 to 10 μmol H2/mg Chl/hr. Hydrogenase activity was dependent upon electron transport through photosystem II (PS II), whereas nitrogenase activity was more dependent on PS I, as well as on respiration. Although cells do not double under the incubation conditions when sparged with argon to provide a low-oxygen environment, the cells are metabolically active, and hydrogen production can be inhibited by the addition of chloramphenicol to inhibit protein synthesis.
Cyanobacteria are among the most ancient organisms on earth and have existed for at least 2.5 billion years (21). They are oxygenic, photosynthetic microbes that contain two photosystems, photosystem II (PS II) and PS I. The acquisition of the two photosystems enabled the bacteria to split water and to release O2 into the atmosphere. Until that time, the atmosphere was reducing, and it was the enhancement of oxygen in the atmosphere from this process that allowed for the development of higher organisms. Prior to this oxidation, organisms lived in a primarily reducing and anaerobic environment, and cyanobacteria have retained regulatory and metabolic processes that enable them to function under such conditions (33). All cyanobacteria fix CO2 from the atmosphere, and some strains, both filamentous and unicellular, can also fix N2 to produce combined nitrogen in the form of ammonia.
Many photosynthetic microbes, including cyanobacteria, can produce H2. In cyanobacteria, there are two different enzymes capable of producing molecular hydrogen, nitrogenase and a bidirectional hydrogenase (13, 22, 25, 35, 36, 43). Nitrogenase reduces protons to H2 concomitantly with reduction of N2 to ammonia. The H2 produced by the nitrogenase is typically consumed rapidly by an uptake hydrogenase, an enzyme that has been found in most of the N2-fixing cyanobacteria studied to date. Indeed, mutations that impair the activity of the uptake hydrogenase can enhance net H2 photoproduction in those strains (8, 36, 43). In addition, many cyanobacteria contain a bidirectional hydrogenase, an NiFe enzyme that belongs to the class of NAD(P)-reducing hydrogenases that are homologous to the HoxFUYH complex found in Ralstonia eutropha (13, 35, 36, 43). The bidirectional hydrogenase is not found in all cyanobacteria but can be found in non-nitrogen fixers, such as Synechocystis sp. PCC 6803, as well as in those that can fix N2. The bidirectional hydrogenase has been studied in a number of cyanobacteria, including Synechocystis sp. strain PCC 6803, especially after the genome sequence of many strains was completed (8, 12, 13, 35, 36, 43).
A great deal of effort has been invested in the study of hydrogen production in many cyanobacteria (2, 11, 30). This has included research on heterocystous cyanobacteria, such as Anabaena and Nostoc strains, as well as on a variety of non-nitrogen-fixing unicellular cyanobacteria. The amount of hydrogen produced varies over orders of magnitude, and while no single model system has currently been identified (11), efforts are being made to identify new and better systems (1). Unfortunately, few unicellular diazotrophic cyanobacteria have been studied for hydrogen production, and such strains have some rather valuable assets for net hydrogen production by a photosynthetic microbe (16, 30, 42).
We have been studying a unicellular, diazotrophic cyanobacterium, Cyanothece sp. strain ATCC 51142, a benthic strain isolated off the U.S. Gulf Coast (23). This organism has very robust metabolic properties, including the ability to grow photoautotrophically, mixotrophically, and heterotrophically (on glycerol) either with combined nitrogen in the medium or under N2-fixing conditions (26-29). We have demonstrated that this strain temporally regulates the major metabolic processes such that oxygenic photosynthesis occurs in the daytime and nitrogen fixation occurs at night. In addition, this regulation is circadian, and nitrogen fixation can also take place under continuous-light (LL, for 12-h light/light periods) conditions after entrainment under 12-h light/dark (LD) cycles. The rate of nitrogen fixation is extremely high, thus providing a good basis for high rates of hydrogen production under N2-fixing conditions. This strain is also notable for the fact that it stores fixed carbon in large starch-like granules (10), especially under N2-fixing conditions. The granules form between the photosynthetic membranes in the light and are used as a substrate for respiration at night. This gives rise to a large burst of respiration that can result in the production of ATP as well as the reduction of intracellular oxygen. This storage of carbohydrates remains high during growth under continuous-light conditions and represents a large energy reserve that can power and protect nitrogenase activity under appropriate conditions. We have also documented the significant changes in photosynthesis that occur between the light and dark phases when the bacteria is grown under N2-fixing conditions (17, 18, 40, 41).
The genome sequence of this strain is now available (44), and we know that the organism contains the bidirectional hydrogenase, an uptake hydrogenase, and an MoFe nitrogenase. We have studied transcription throughout the 24-h cycle, including growth under 12-h LD conditions (32), LL conditions (38), and 6-h LD conditions (37), and have determined that the uptake hydrogenase is transcribed in tandem with the large nitrogenase cluster, whereas the bidirectional hydrogenase is normally transcribed in the light or in the dark some 6 to 12 h out of phase with the nitrogenase. The overall ability of this strain to grow rapidly under either nitrogen-sufficient or nitrogen-deficient conditions, as well as in the presence of a carbon source such as glycerol in complete darkness (DD, for dark/dark), makes this a metabolically very attractive strain for further analysis of hydrogen production. In this study, we will present data for hydrogen production under a variety of different growth conditions, including in the presence or absence of combined nitrogen. We demonstrate that this Cyanothece strain is capable of extremely high levels of net hydrogen production, especially under nitrogen-fixing conditions, even in the presence of an uptake hydrogenase. We will also demonstrate that H2 produced by hydrogenase is tied to PS II, whereas H2 produced by nitrogenase is closely aligned with PS I and respiration.
MATERIALS AND METHODS
Strain, medium, and growth conditions.
Cyanothece sp. strain ATCC 51142 was grown in ASP2 medium as previously described in 250-ml Erlenmeyer flasks containing 100 ml of medium with or without NaNO3, with shaking at 125 rpm at 30°C, under cool-white fluorescent illumination of 30 μE m−2 s−l (23). When indicated, glycerol was supplemented at a final concentration of 50 mM.
Recently, efforts have been made to optimize or improve the nutrient medium for hydrogen production in Arthrospira (Spirulina) maxima (2), Anabaena variabilis strain ATCC 29413 (3), and Synechocystis sp. strain PCC 6803 (5). We compared the different media for both macro- and micronutrients and also made use of the elegant optimization process described by Burrows et al. (5) to develop a series of changes in ASP2. We found no change in hydrogen production by modifying our main macronutrients, such as sulfur and phosphate, and addition of additional micronutrients (such as nickel) had virtually no impact. We finally determined that changing the iron composition to 10-fold more Fe, in the form of ferric ammonium citrate, was beneficial, as was a five-fold reduction of Ca+2 by five times. We varied the pH in many of the experiments and finally determined the best rates at pH 7.4. This medium with enhanced iron and reduced Ca+2 at pH 7.4 is referred to as ASP2-M, for modified ASP2.
Measurement of chlorophyll concentration.
Chlorophyll a (Chl) concentration of the cells was determined spectrophotometrically by measuring absorbance at 750, 678, and 620 nm. Cultures are usually diluted until the maximum reading is below or equal to 0.5. Chlorophyll a concentration (in μg of Chl/ml) is given by the following equation: [Chl] = 14.97(A678 − A750) − 0.615(A620 − A750).
Hydrogen measurement.
Cyanothece sp. strain ATCC 51142 cultures were first grown under continuous light (LL) for 4 to 9 days in 250-ml flasks containing 100 ml of culture, and each culture was split equally into two 66-ml clear glass bottles. Rubber stoppers were used to seal the openings of the bottles. Our system was first tested for air tightness by using pure hydrogen. We injected 3.3 ml of pure hydrogen into the bottle, mixed well, and measured the hydrogen concentration by a gas chromatograph (GC). A week later, we measured the hydrogen concentration from the same bottle again and found that the concentration remained the same, indicating that the system was airtight.
We used an identical protocol for all the preliminary experiments. Cultures were grown under the different light conditions in 250-ml Erlenmeyer flasks in air, in the presence or absence of glycerol, and then transferred at the growth concentrations to the sealed 66-ml glass bottles. In the initial experiments, the bottles were just sealed with a rubber stopper. In later experiments, a sparging gas (such as argon, CO2, or N2) was used to provide a more anaerobic environment, and the incubation bottles were sparged for 1 min. The sealed bottles were then shaken at 125 rpm at 30°C with or without light for 6, 24, 48, and 72 h. Hydrogen production was very low prior to 6 h and then increased monotonically through 24 h, whereas O2 levels decreased about 50% by 48 h (data not shown). Depending on the exact incubation conditions, hydrogen accumulation continued to increase or began to plateau. For this reason, we report the rates of hydrogen accumulation for the initial 24 h. A syringe with a needle was used to withdraw 0.5 ml of gas from the headspace of the bottles, and this was injected into a gas chromatograph (HP 5890 Series II; Hewlett Packard Ltd.) equipped with a thermal conductivity detector and a molecular sieve column (HP-Molesieve, catalog number 19095P-MS9). Nitrogen was used as a carrier gas routinely. For important conclusions, experiments were repeated at least twice with nitrogen as the carrier gas and confirmed once with argon as the carrier gas.
Previous authors have used a variety of different units to present their hydrogen production data. The unit most frequently used for cyanobacterial H2 production is based on chlorophyll (as μmol H2 mg Chl−1 h−1), and this was our default unit (11). We calculated a rate by dividing the total H2 accumulated/mg of Chl over the first 24-h period to generate the data as μmol of H2/mg of Chl/h. We also calculated the data as percent H2 produced/μg of dry weight of cells, using the standardization of A730 versus dry weight, as generated by Nedbal et al. (20). To calculate the volume (μl) of H2 produced, the peak area values for each peak in the chromatograph were divided by a calculated conversion factor of 871.64. The molarity (μmol) of the H2 produced was calculated by dividing the H2 volume (μl) by 24.4. H2 production was plotted on the basis of chlorophyll (as μmol H2 mg Chl−1). The H2 production rate was calculated by dividing the molarity of H2 production by the time period (normally 24 h).
Nitrogenase assay.
Nitrogenase activity was assayed by a modified acetylene reduction method (6, 26). Assays were performed in 66-ml clear glass bottles, except that only 12 ml of the cultures was used, and 3 ml of acetylene was injected into each bottle. The bottles were shaken under light at 30°C for 2 h. A 0.5-ml gas sample was analyzed for the percentage of acetylene reduced to ethylene in a gas chromatograph (HP 5890 Series II; Hewlett Packard, Ltd.) with a 6-ft Poropak N column and a flame ionization detector. Triplicate samples were analyzed for each set of conditions. The percentage of acetylene reduction in culture grown in ASP2 medium without combined nitrogen (ASP2−) under aerobic conditions was normalized to 1.
Photosynthetic inhibitors.
The inhibitors used in this study included dichlorophenyl dimethylurea (DCMU; 10 μM), methyl viologen (MV; 20 μM), potassium cyanide (KCN; 10 μg/ml), and chloramphenicol. The addition of these compounds from stock solutions resulted in no change in chlorophyll concentration. Since DCMU and chloramphenicol were dissolved in ethanol, we tested the hydrogen production with ethanol (0.1%) to exclude the possible effect of ethanol on hydrogen production. The results were negative.
RESULTS
Growth of Cyanothece sp. strain ATCC 51142 under different physiological conditions.
Cyanothece sp. strain ATCC 51142 is a unicellular, diazotrophic cyanobacterium with a robust metabolism. It can grow with combined nitrogen or under nitrogen-fixing conditions under 12-h LD or LL conditions, as well as with glycerol in darkness (DD). Under nitrogen-fixing conditions, one of the key attributes is the ability of the strain to store carbon in large glycogen granules that form between the photosynthetic membranes and then utilize this potential energy as a substrate for respiration in the dark. We have made hydrogen measurements under many of these different conditions, but we will concentrate on hydrogen produced by hydrogenase when cells are grown under nitrogen-replete conditions and on the production of hydrogen by nitrogenase when combined nitrogen is absent, mostly under LL conditions. A summary of the results for many different experiments under these different conditions is shown in Table 1, and the results are described in detail below.
TABLE 1.
The rate of hydrogen production in Cyanothece sp. strain ATCC 51142 under different conditions
| Condition |
Rate of H2 productionc |
||||
|---|---|---|---|---|---|
| Lighta | NO3− | Glyb | Sparging gas | μmol H2/mg Chl/h | μmol H2/mg dry wt/h |
| LL | − | + | Argon | 80.3 ± 10.5 | 0.6 ± 0.08 |
| LL | − | + | N2 | 31.8 ± 5.0 | 0.25 ± 0.04 |
| LL | − | + | CO2 | 10.9 ± 8.1 | 0.1 ± 0.06 |
| LL | − | − | Argon | 24.3 ± 4.3 | 0.2 ± 0.03 |
| LL | − | − | N2 | 2.8 ± 1.3 | 0.02 ± 0.01 |
| LL | − | − | CO2 | 2.7 ± 1.1 | 0.02 ± 0.01 |
| DD | − | − | Argon | 0 | 0 |
| DD | − | + | Argon | 0 | 0 |
| LL | + | − | Air | 2.63 | 0.02 |
| LL | + | − | Argon | 0 | 0 |
| LL | + | + | Air | 2.83 | 0.02 |
| LL | + | + | Argon | 0 | 0 |
| HL | + | − | Air | 5.0 ± 2.0 | 0.04 ± 0.02 |
| DD | + | − | Air | 2.38 | 0.02 |
| DD | + | + | Air | 1.0 | 0.01 |
| HL | + | − | Argon | 0 | 0 |
| DD | + | − | Argon | 0 | 0 |
| DD | + | + | Argon | 0 | 0 |
LL, continuous light at 30 μmol photons m−2 s−1; DD, continuous dark; HL, continuous light at 200 μmol photons m−2 s−1.
Supplementation with glycerol (50 mM).
Over the first 24 h (average of n = 3).
Hydrogen production from hydrogenase.
When cells were grown in the presence of combined nitrogen, typical hydrogen production rates were 2 to 10 μmol H2 mg Chl−1 h−1. This rate was independent of whether the cells were grown under the LL or LD condition. Since cyanobacteria assimilate ammonium preferably over nitrate, we used ammonium nitrate or ammonium chloride instead of sodium nitrate in the culture medium to grow the cells. Cyanothece sp. strain ATCC 51142 grew faster in medium containing ammonium, but hydrogen production was not increased (data not shown). We also increased the light intensity to 100 and 200 μmol photons m−2 s−1 during both the growth and incubation periods. With increased light intensity, the maximum rate of hydrogen production increased somewhat but to levels that were still ≤10 μmol H2 mg Chl−1 h−1. When nitrate-grown cells were incubated in the presence of argon, hydrogen production fell to zero (Table 1). This is likely due to the inhibition of photosystem II, as has been shown previously (9, 15, 24). We conclude that hydrogen production by hydrogenase in Cyanothece is dependent upon photosynthesis and requires an electron flow through PS II to reduce the quinone pool.
Hydrogen production from nitrogenase in Cyanothece sp. strain ATCC 51142.
During the initial stages of our study, we grew Cyanothece sp. strain ATCC 51142 under nitrogen-fixing conditions using a variety of different light regimes. We grew cells for approximately 1 week under 12-h LD and LL conditions at either 30 or 100 μmol photons m−2 s−1. The cells were then incubated in 66-ml glass bottles under different combinations of light and dark conditions. We concluded that the highest levels of hydrogen production were obtained with cells that were grown and incubated under the LL condition (data not shown). It should be noted that we did not concentrate cells prior to incubation. When cultures were grown under the LL or LD condition for shorter periods of time, we could obtain higher specific activities with these dilute cultures. However, our aim was to identify typical conditions that could be easily scaled up for high-volume production, and we standardized on LL growth and incubation conditions.
Another interesting feature became evident as we were studying hydrogen production in the presence of glycerol. When cells were adapted to growth on glycerol as described previously (28, 29), hydrogen production decreased from the levels in control cultures that were grown in the absence of glycerol. We then studied this phenomenon in more detail by taking a fresh stock of Cyanothece sp. strain ATCC 51142 from the freezer and measuring hydrogen production at various stages during subculturing in the presence and absence of glycerol (Table 2). It can be seen that hydrogen production in the absence of glycerol was very high when the cells were young but that hydrogen production was lower in cultures containing glycerol. However, once the glycerol-grown cultures adapted well for growth on glycerol (by the second subculture on glycerol), hydrogen production increased, whereas hydrogen production for the non-glycerol-containing cultures declined. This was a reproducible phenomenon, indicating that there are significant metabolic shifts in the cells growing on glycerol.
TABLE 2.
The impact of metabolic adaptation on the rate of hydrogen production in N2-fixing Cyanothece sp. strain ATCC 51142a
| Subculture stage (week no.) | Rate of H2 production (μmol H2/mg Chl/h) in the indicated medium |
|
|---|---|---|
| ASP2− | ASP2− + Glyb | |
| 1 | 80 | 30 |
| 2 | 70 | 50 |
| 3 | 50 | 80 |
| 4 | 30 | 100 |
Cultures were grown as described in Materials and Methods.
Gly, glycerol (50 mM).
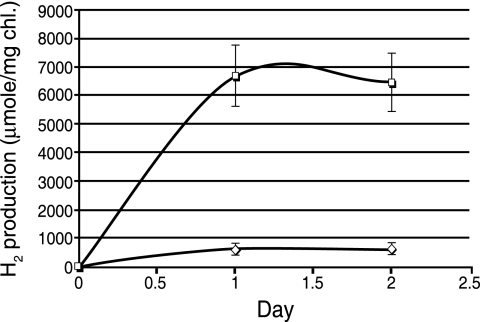
In order to determine the most consistent way to grow our cells for studying hydrogen production, we then began a series of experiments that began from colonies on an agar plate. A colony was picked from an ASP2 plate, grown in ASP2 liquid medium for ∼14 days, and then subcultured into ASP2− medium for up to 14 days. Cells were withdrawn from this culture at 7 to 14 days and incubated as described in Materials and Methods. As shown in Fig. 1, such cultures produced hydrogen at rates of 270 ± 50 μmol H2 mg Chl−1 h−1 when incubated under argon and at 33 ± 5 μmol H2 mg Chl−1 h−1 in air. These figures correspond to 2.1 and 0.254 μmol H2/mg of dry weight, respectively (Table 1). We obtained such results consistently, and this became our preferred protocol for analyzing H2 production. Nonetheless, we concentrated on analyzing cells that were adapted for growth in glycerol so that we could determine the impact of mixotrophic conditions on H2 production. Results for cells growing completely photoautotrophically will be reported separately (A. Bandyopadhyay, J. Stöckel, H. Min, L. A. Sherman, H. B. and Pakrasi, unpublished data).
FIG. 1.
H2 production by cultures of Cyanothece sp. strain ATCC 51142 grown in ASP2 without combined nitrogen (ASP2−) after subculture from a culture grown for 14 days in ASP2 medium. □, sparged with argon; ⋄, sparged with air.
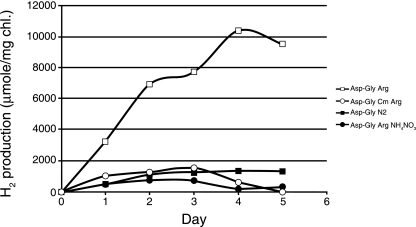
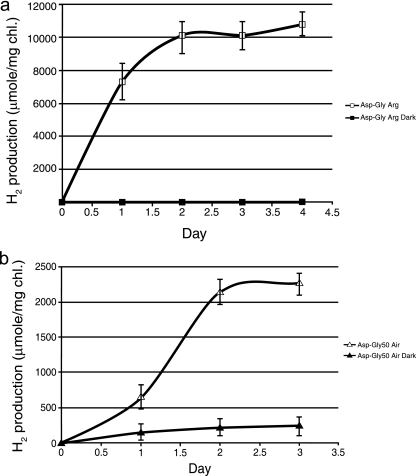
Hydrogen production in cultures of Cyanothece sp. strain ATCC 51142 grown under nitrogen-fixing conditions was significantly higher than when combined nitrogen was present (Fig. 2). Under these conditions, hydrogen production continued at a high level for at least the first 2 days before reaching a plateau by day 3. In this experiment, incubation was performed after the bottle was sparged with argon, and this reproducibly provided the highest levels of hydrogen production for cells growing in the presence of glycerol. The rates of hydrogen production on the first day could easily reach 80 to 200 μmol H2 mg Chl−1 h−1 during our normal growth regime. When we used cultures grown for 5 days instead of 7-day cultures and a 40-ml volume instead of 45 ml in the bottles, we could achieve a rate of 300 μmol H2/mg Chl/h (Fig. 3a). This implied that the headspace volume and back pressure on the nitrogenase limited the total hydrogen accumulation after a period of 2 to 4 days. Note that incubation in the dark completely obliterated H2 production, indicating an important light-dependent step. If the samples were incubated under air, the light dependence was not complete although the rates did declined by 85 to 90% (Fig. 3b). Some or all of this residual activity in the air was likely due to hydrogenase, in addition to the nitrogenase.
FIG. 2.
H2 production by cultures of Cyanothece sp. strain ATCC 51142 grown in ASP2 without combined nitrogen (ASP2−) with 50 mM glycerol and incubated under different conditions, with and without sparging with argon (Arg) and 20 μg/ml chloramphenicol (Cm), as indicated. Nitrogenase is responsible for high levels of hydrogen production in ASP2− medium under anaerobic conditions with glycerol, and de novo protein synthesis is needed.
FIG. 3.
(a) H2 production by cultures of Cyanothece sp. strain ATCC 51142 grown in ASP2 medium without combined nitrogen (ASP2−) with 50 mM glycerol, incubated under either the LL (□) or DD (▪) condition, and sparged with argon. (b) H2 production by cultures of Cyanothece sp. strain ATCC 51142 grown in ASP2− medium with 50 mM glycerol, incubated under either the LL (▵) or DD (▴) condition, and sparged with air.
We utilized other gases for sparging during incubation to study the properties of these cultures. In one set of experiments, we sparged with N2 gas instead of argon in order to provide nitrogenase with its typical substrate. We reasoned that argon was valuable by providing an anaerobic environment that prevented nitrogenase from being inactivated by oxygen. The addition of N2 to the incubation bottle would provide nitrogenase with a normal substrate, and this would decrease the overall amount of hydrogen produced. As shown in Fig. 2 and Table 1, the results were consistent with this hypothesis, and the amount of hydrogen produced dropped by 80 to 90%. We also sparged with CO2 and determined that there was an interaction between CO2 fixation by the Calvin cycle and glycerol metabolism (Table 1). The addition of CO2 seemed to prevent the breakdown of glycerol, and separate metabolic experiments support this contention (Y. Tang et al., unpublished observations).
We studied the involvement of nitrogenase further by adding NH4NO3 to the incubation bottle. Ammonium is known to inhibit nitrogenase gene expression, and we expected that there would be a drop in hydrogen produced. This was indeed the case, and hydrogen production declined by 80 to 90%. Such results raised the question of whether the cells in the incubation bottle were metabolically active and were still producing new nitrogenase. Therefore, we added 20 μg/ml chloramphenicol to the incubation bottle. Once again, hydrogen production declined significantly and eventually declined to zero after 5 days of incubation (Fig. 2). These results indicate that additional synthesis of nitrogenase enzyme during incubation was required for high levels of hydrogen production.
Hydrogen production in Cyanothece sp. strain ATCC 51142 and the cellular energy budget.
In order to understand the metabolic driving forces for the hydrogenase-generated and nitrogenase-generated hydrogen production, we performed a few simple experiments that would block certain components of the photosynthetic and respiratory mechanisms. We used 10 μM DCMU to block PS II, 20 μM methylviologen (MV) to draw off electrons from PS I, and 10 μM KCN to block respiration and electron flow on the reducing side of the cytochrome b6f complex. The results in Table 3 show that hydrogenase (cells grown with nitrate) and nitrogenase activities have different energy resource requirements. The involvement of PS II for hydrogenase activity was verified by the addition of 10 μM DCMU (a PS II inhibitor) to cells at the beginning of the incubation period. This resulted in an 80% reduction of H2 production within the first day and a 100% reduction thereafter. The addition of DCMU had a strong effect on hydrogenase activity, in the presence or absence of glycerol, whereas MV and KCN had no effect. As indicated previously, all of these experiments were performed with air in the incubation bottle since sparging with argon abolished hydrogenase activity via its action on PS II. We also measured H2 production in the presence of dithionite or glucose/glucose oxidase/catalase (14) to generate an anaerobic environment and obtained similar results for H2 production as with argon (data not shown). Therefore, we conclude that the production of H2 through hydrogenase requires electron flow through PS II.
TABLE 3.
Effects of photosynthetic inhibitors on H2 production in Cyanothece sp. strain ATTC 51142
| LL condition |
Relative H2 productiona | |||
|---|---|---|---|---|
| NO3− | Glycerol supplementation | Sparging gas(es) | Treatment | |
| + | − | Air | Control-1 | 1.0 |
| + | − | Air | DCMU | 0.2 |
| + | − | Air | MV | 1.0 |
| + | − | Air | KCN | 1.0 |
| + | + | Air | Control-2 | 1.0 |
| + | + | Air | DCMU | 0.2 |
| + | + | Air | MV | 1.0 |
| + | + | Air | KCN | 1.0 |
| − | − | Argon/air | Control-3 | 1.0 |
| − | − | Argon | DCMU | 1.0 |
| − | − | Air | DCMU | 0.8 |
| − | − | Argon | MV | 0.4 |
| − | − | Air | MV | 0.4 |
| − | − | Argon | KCN | 0.3 |
| − | − | Air | KCN | 0.3 |
| − | + | Argon/air | Control-4 | 1.0 |
| − | + | Argon | DCMU | 1.0 |
| − | + | Air | DCMU | 1.2 |
| − | + | Argon | MV | 0.4 |
| − | + | Air | MV | 0.4 |
| − | + | Air | KCN | 0.3 |
| − | + | Argon | MV+KCN | 0.3 |
| − | + | Argon | KCN | 0.3 |
H2 production rates from the untreated controls were as follows: (in μmol H2/mg Chl/h) Control-1, 2.1; Control-2, 2.0; Control-3 (argon), 31; Control-3 (air), 3.5; Control-4 (argon), 123; Control-4 (air), 18. The experiments were normalized to 1.0 for each of the six experimental conditions.
The results for H2 production from nitrogenase were complementary to those from the hydrogenase. In the presence of air, but in the absence of glycerol, DCMU reduced the activity ∼20%. However, DCMU in the presence of argon had no effect since PS II was already inhibited. Interestingly, for cultures grown in the presence of glycerol under nitrogen-fixing conditions, DCMU actually led to an increase in hydrogen production during air incubation (Table 3). We suggest that this is due to the inhibition of oxygen evolution that prevents damage to nitrogenase. Treatment with MV or KCN led to a drop of 60 to 70% in the rate of hydrogen production under all measured conditions (Table 3). We interpret this to indicate that both PS I and respiration were important as energy sources for nitrogenase-generated H2 production. Consistent with this idea, incubating N2-fixing cultures in the presence or absence of glycerol, with light (640-nm light-emitting diodes [LEDs]) absorbed primarily by phycobilisomes, generated a 20 to 40% decrease in H2 production compared to similar cultures illuminated with white light (data not shown).
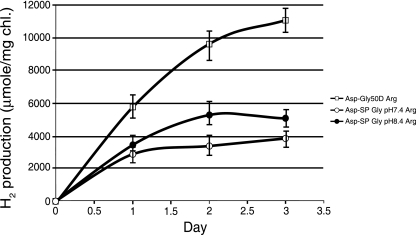
In addition, we experimented with some modifications of the normal medium. This medium is somewhat high in calcium and low in iron relative to many other media used for cyanobacteria, so we developed a modified medium with one-fifth the level of calcium and 10-fold the level of iron in the medium. We also buffered the medium at different pHs to determine if this also had an impact on the production of hydrogen. As shown in Fig. 4, the modified medium at pH 8.4 demonstrated increased hydrogen rates of some 50 to 70%. However, lowering the pH to 7.4 significantly increased the rate of hydrogen production and kept it at high levels for at least the first 2 days. The rate during the first day was 250 μmol H2/mg Chl/h, and it was still at 200 μmol/mg Chl/h if the rate was measured over a 2-day period (Fig. 4).
FIG. 4.
H2 production by cultures of Cyanothece sp. strain ATCC 51142 grown in either regular ASP2 medium without combined nitrogen (ASP2−; pH 7.9) (○) or in ASP2− medium modified (with one-fifth the amount of Ca2+ and 10-fold Fe3+) at pH 7.4 (□) or pH 8.4 (•). All samples were sparged with argon.
Nitrogenase activity.
We further tested the contention that nitrogenase is responsible for hydrogen production under nitrogen-fixing conditions by directly measuring nitrogenase activity. As shown in Table 4, nitrogenase activity increased significantly in the presence of glycerol and especially after sparging with argon. Sparging with N2 also led to increases in nitrogenase activity, but this enzyme activity was some 5-fold less than that after argon sparging. In all cases, the relative increases in nitrogenase activity and hydrogen production are similar, as can be seen in Table 4. The quantitative differences are likely due to the fact that nitrogenase activity is measured with 12-ml cultures in the incubation bottles instead of the 40 to 50 ml for the hydrogen production measurements. This will permit a greater accumulation of gas and lead to the higher ratio seen from the nitrogenase activity. We conclude that the high level of hydrogen produced under nitrogen-fixing conditions was due to production via nitrogenase.
TABLE 4.
Nitrogenase activity in Cyanothece sp. strain ATCC 51142 after incubation with different gasesa
| Glycerol supplementation | Nitrogenase condition | Nitrogenase activity |
|
|---|---|---|---|
| Absolute (μmol C2H2 reduced/mg Chl/h) | Relative (per mg Chl)b | ||
| − | Air | 0.18 | 1 |
| + | Air | 2.5 | 14 |
| − | Argon | 2.9 | 16 |
| + | Argon | 93 | 509 |
| − | N2 | 0.42 | 2.3 |
| + | N2 | 17 | 93 |
Cultures were grown in ASP2− medium under LL for 5 days, and 12 ml was added to 66-ml bottles. Some bottles were sparged with argon or N2. The nitrogenase assays were performed after the bottles were shaken under LL (30 μmol photons m−2 s−1) for 22 h. After injection of 3 ml of acetylene, the bottles were also kept under light for 2 h.
Argon and N2 readings are normalized to the value obtained in air with no glycerol.
DISCUSSION
This report represents a comprehensive study of hydrogen production in a unicellular, diazotrophic cyanobacterium. Although we have not yet begun an optimization process, the rates we report are among the highest reported for nitrogen-fixing cyanobacteria (11, 30). The rate of 300 μmol/mg Chl/h is equivalent to 16.5 ml/liter/h and would be at the high end of the range reported in a recent review by Tsygankov (39). Hydrogen production was obtained from both hydrogenase and nitrogenase under different growth conditions, and we will be interested in understanding the properties of both enzymes. A few of the topics that we must explore include the amount of hydrogenase and nitrogenase transcript and protein produced in the cell, the protection of enzymes from oxygen, and respiratory and photosynthetic electron flow. Fortunately, some of these properties have already been studied as part of the long-term analysis of Cyanothece sp. strain ATCC 51142 utilizing the same physiological conditions that appear important for H2 production (31, 32, 38, 44).
The hoxEFUYH genes and the 35-gene nif gene cluster are regulated substantially differently in Cyanothece sp. strain ATCC 51142 (32, 37, 38). The hox genes are preferentially turned on in the dark and have been categorized as a diurnally regulated operon (regulated by light or dark), whereas the nif genes encoding the nitrogenase are also transcribed in the dark but in a circadian fashion (modulated every 24 h) (37). Thus, under normal LD conditions, the cells would prefer to synthesize these oxygen-sensitive enzymes in the dark when photosynthetic O2 evolution is absent. Nonetheless, we get significant nitrogenase biosynthesis and resulting N2 fixation when cultures are grown under continuous light conditions (7, 38). More importantly, the two gene clusters are transcribed at significantly different levels. The hox genes are transcribed in the dark at a low rate under both nitrogen-sufficient and nitrogen-fixing conditions, whereas the nif genes are transcribed at a level some 200-fold higher than hox when cells are grown under nitrogen-fixing conditions (37). Interestingly, the hupLS genes, encoding the uptake hydrogenase, are transcribed similarly to the nif genes and also have high transcript levels (37) when grown under the LD condition. However, there is very low expression of hupLS when cells are grown under LL (38), and this is probably a major reason for the high H2 production during LL incubation.
We had shown previously using electron microscopy (EM) immunocytochemistry that virtually every cell in the culture had its cytoplasm filled with nitrogenase (31). In addition, proteomic results have indicated that the nitrogenase protein is found only during about 6 h of the dark period but that it represents a high percentage of all protein at this stage. Therefore, one reason for the tremendous hydrogen production potential of nitrogenase is that there is a vast amount of enzyme present in the cell. We concur with Tsygankov (39) that the cells utilize the hox genes in order to maintain the amount of reduced pyridine nucleotides at a certain level, as required by the cyanobacterium. Depending on the different conditions, the hydrogenase may either release or take up hydrogen (39). Thus, much less enzyme is needed than in the case of nitrogenase, where it is the sole enzyme for the production of reduced nitrogen in the cell when there is no combined nitrogen in the medium. Any further work to utilize Hox in Cyanothece sp. strain ATCC 51142 will require significant upregulation of the amount of protein available. This may be done by cloning the hox gene cluster behind a nitrogenase promoter, growing the cells under N2-fixing conditions, and determining the amount of hydrogen that can be produced.
It is obvious that a critical issue for the cell is the protection of the H2-producing enzymes from oxygen inactivation. Therefore, incubation of cells under a low-O2 environment provides the highest rates of hydrogen production for nitrogenase. Anaerobiosis was reported to inhibit PS II in certain systems (9, 15, 24), and our results were consistent with this pattern since there was no hydrogenase-generated hydrogen in the presence of argon or other agents that generate anaerobiosis. We hypothesize that this is due to the inability of PS II to provide electrons to NADP+ under these conditions. However, the presence of argon provided the highest rates of hydrogen production by nitrogenase under most circumstances. We found the steady rates of hydrogen produced under these circumstances to be important, and we determined that the level of nitrogenase needed to be replenished by the cell during the incubation period. Thus, although the cells are not actually doubling, they are metabolically active, and the addition of chloramphenicol to the incubation bottle quickly inhibited hydrogen production. One future experiment will involve a careful analysis of this production in order to determine a turnover rate for nitrogenase under these particular incubation conditions. It is important to note that we have studied both Synechocystis sp. strain PCC 6803 and Cyanothece sp. strain ATCC 51142 under low-oxygen conditions (using 99.9% N2 and 0.1% CO2) and determined some interesting properties (33). Synechocystis in BG-11 medium grows slowly during the first 24 h under these conditions, whereas Cyanothece growing in nitrate-sufficient medium hardly grows and evolves very little oxygen. These studies included transcriptional analyses and demonstrated that different psbA genes were inserted into PS II under these low-oxygen conditions. We will soon perform an equally detailed analysis of transcription of Cyanothece sp. strain ATCC 51142 grown under nitrate-deficient, low-oxygen conditions.
One of the more interesting and important attributes of Cyanothece is its ability to store carbohydrate in glycogen granules (27, 31). When grown under LD nitrogen-fixing conditions, the cells store the carbohydrate in large glycogen granules that form between photosynthetic membranes during the light and utilize the carbohydrate as a substrate for respiration in the dark. We had expected that the storage of carbohydrate would be an important component of optimal hydrogen production, and we utilized various LL, LD, and DD protocols for both growth and incubation conditions. If cells grown under LD conditions were incubated at the end of the light period, this allowed maximal glycogen storage and led to high rates of H2 production. However, we determined that a dark phase during incubation lowered hydrogen production significantly, presumably due to induction of HupLS. The use of anaerobic or low-oxygen conditions always improved hydrogen production, indicating that protection of the enzyme from oxygen poisoning is a key property. Under such conditions, we found that both growth and incubation under continuous light consistently gave the highest levels of hydrogen production. Under these circumstances, PS I can be used for ATP production as well as for protection against oxygen. Our transcriptional analysis of Cyanothece sp. strain ATCC 51142 under low-oxygen conditions indicated that two flavoproteins and PS I reaction center genes were upregulated when oxygen was limiting (33). It is possible that one function for PS I is to support the Mehler reaction for the reduction of O2 (19).
We were also interested in the impact of glycerol on mixotrophic and heterotrophic growth of Cyanothece sp. strain ATCC 51142. This strain is a true heterotroph and can grow for long periods on 50 mM glycerol (28, 29). Once again, we utilized a variety of different light protocols (LL, LD, and DD) and found that hydrogen production was always highest under the LL condition. This is likely due to the fact that cells store less glycogen in granules when grown with glycerol, and continuous light allows for greater energy production and less HupLS under these conditions. As we were working with cells that were adapted for growth on glycerol, we noted that the non-glycerol control levels of hydrogen production had declined (Table 2). Since we wanted to include glycerol-grown cells in our measurements, we decided to standardize on glycerol-grown cultures. It is clear that growth in the presence of glycerol generates significant metabolic changes within the cell, and this will be a topic of future transcriptomic and proteomic experiments.
There are many challenges in this field, none less daunting than identifying appropriate units with which to report the data. Various authors have used different units, and all of these approaches have one or more problems associated with them. Standardizing versus dry weight, wet weight, or protein has many pitfalls, especially since each laboratory generally uses a different protocol for these measurements. Therefore, we followed the lead of the majority of authors summarized in Dutta et al. (11) and utilized μmol H2 evolved/mg Chl/h as our standard unit. Although chlorophyll may not be the most important parameter for analyzing hydrogen evolution experiments, it is a simple and highly reproducible (± 10%) procedure that can allow comparisons across many different laboratories and types of experiments. In addition, it will be important to limit the size of the photosynthetic antennae in order to improve photosynthetic efficiency of H2 production so that this will always be an important way of reporting data in photosynthetic microbes. A recent report by Bernát et al. (4) presented genetic experiments that resulted in a smaller antenna system for PS II in Synechocystis sp. strain PCC 6803 and that had higher rates of hydrogen production.
On this basis, hydrogen production in Cyanothece sp. strain ATCC 51142 ranks extremely well. The highest results generally reported have been in A. variabilis strain PK84, a heterocystous cyanobacterial strain that lacks a functional uptake hydrogenase. This strain yielded 167.6 μmol H2/mg Chl/h under somewhat complex growth and assay conditions (10, 34), which is about half the rate that we report under our best conditions. Our results in Tables 1 and 4 indicate that the rates of hydrogen production and nitrogen fixation are proportional and consistent with the finding that the rate of H2 evolution is approximately equal to the rate of N2 fixation as measured by acetylene reduction (39). Thus, production and protection of nitrogenase, along with a plentiful supply of reductant, are the critical parameters for H2 production in Cyanothece sp. strain ATCC 51142. We feel that these early experiments in Cyanothece sp. strain ATCC 51142 have been very promising and that additional work with this well-studied strain is important to delve into the underlying processes of diazotrophic photosynthetic hydrogen production. There are still many questions that need to be answered before one even considers the applied aspects of any hydrogen evolved, and we will concentrate on understanding the basic biological processes first. We feel that a great deal of important progress can be made by molecular and genetic manipulations in this strain, and that will be among our tasks for the future.
Acknowledgments
We thank Jamie Gendron, Sarah Griffith, and Yang Zhang for technical help during this project.
The research was funded by a grant from the DOE Genomics GTL Program (DE 09-19 PO 2905402N) and, in part, by a grant from the Purdue Energy Center.
Footnotes
Published ahead of print on 7 May 2010.
REFERENCES
- 1.Allahverdiyeva, Y., H. Leino, L. Saari, D. P. Fewer, S. Shunmugam, K. Sivonen, and E.-M. Aro. 2010. Screening for biohydrogen production by cyanobacteria isolated from the Baltic Sea and Finnish lakes. Int. J. Hydrogen Energy 35:1117-1127. [Google Scholar]
- 2.Ananyev, G., D. Carrieri, and G. C. Dismukes. 2008. Optimization of metabolic capacity and flux through environmental cues to maximize hydrogen production by the cyanobacterium “Arthrospira (Spirulina) maxima.” Appl. Environ. Microbiol. 74:6102-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berberoğlu, H., J. Jay, and L. Pilon. 2008. Effect of nutrient media on photobiological hydrogen production by Anabaena variabilis ATCC 29413. Int. J. Hydrogen Energy 33:1172-1184. [Google Scholar]
- 4.Bernát, G., N. Waschewski, and M. Rogner. 2009. Towards efficient hydrogen production: the impact of antenna size and external factors on electron transport dynamics in Synechocystis PCC 6803. Photosynth. Res. 99:205-216. [DOI] [PubMed] [Google Scholar]
- 5.Burrows, E. H., F. W. R. Chaplen, and R. L. Ely. 2008. Optimization of media nutrient composition for increased photofermentative hydrogen production by Synechocystis sp. PCC 6803. Int. J. Hydrogen Energy 33:6092-6099. [Google Scholar]
- 6.Colón-López, M. S., D. M. Sherman, and L. A. Sherman. 1997. Transcriptional and translational regulation of nitrogenase in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 179:4319-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colón-López, M. S., and L. A. Sherman. 1998. Transcriptional and translational regulation of photosystem I and II genes in light-dark- and continuous-light-grown cultures of the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 180:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cournac, L., G. Guedeney, G. Peltier, and P. M. Vignais. 2004. Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex. J. Bacteriol. 186:1737-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daday, A., R. A. Platz, and G. D. Smith. 1977. Anaerobic and aerobic hydrogen gas formation by the blue-green alga Anabaena cylindrica. Appl. Environ. Microbiol. 34:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deschamps, P., C. Colleoni, Y. Nakamura, E. Suzuki, J. L. Putaux, A. Buléon, S. Haebel, G. Ritte, M. Steup, L. I. Falcón, D. Moreira, W. Löffelhardt, J. N. Raj, C. Plancke, C. d'Hulst, D. Dauvillée, and S. Ball. 2008. Metabolic symbiosis and the birth of the plant kingdom. Mol. Biol. Evol. 25:536-548. [DOI] [PubMed] [Google Scholar]
- 11.Dutta, D., D. De, S. Chaudhuri, and S. K. Bhattacharya. 2005. Hydrogen production by cyanobacteria. Microb. Cell Fact. 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghirardi, M. L., A. Dubini, J. Yu, and P. C. Maness. 2009. Photobiological hydrogen-producing systems. Chem. Soc. Rev. 38:52-61. [DOI] [PubMed] [Google Scholar]
- 13.Ghirardi, M. L., M. C. Posewitz, P. C. Maness, A. Dubini, J. Yu, and M. Seibert. 2007. Hydrogenases and hydrogen photoproduction in oxygenic photosynthetic organisms. Annu. Rev. Plant Biol. 58:71-91. [DOI] [PubMed] [Google Scholar]
- 14.Gutthann, F., M. Egert, A. Marques, and J. Appel. 2007. Inhibition of respiration and nitrate assimilation enhances photohydrogen evolution under low oxygen concentrations in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 1767:161-169. [DOI] [PubMed] [Google Scholar]
- 15.Krause, G. H., S. Köster, and S. C. Wong. 1985. Photoinhibition of photosynthesis under anaerobic conditions studied with leaves and chloroplasts of Spinacia oleracea L. Planta 165:430-438. [DOI] [PubMed] [Google Scholar]
- 16.Lopes Pinto, F. A., O. Troshina, and P. Lindblad. 2002. A brief look at three decades of research on cyanobacterial hydrogen evolution. Int. J. Hydrogen Energy 27:1209-1215. [Google Scholar]
- 17.Meunier, P. C., M. S. Colón-López, and L. A. Sherman. 1998. Photosystem II cyclic heterogeneity and photoactivation in the diazotrophic, unicellular cyanobacterium Cyanothece species ATCC 51142. Plant Physiol. 116:1551-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meunier, P. C., M. S. Colón-López, and L. A. Sherman. 1997. Temporal changes in state transitions and photosystem organization in the unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. Plant Physiol. 115:991-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milligan, A. J., I. Berman-Frank, Y. Gerchman, G. C. Dismukes, and P. G. Falkowski. 2007. Light-dependent oxygen consumption in nitrogen-fixing cyanobacteria plays a key role in nitrogenase protection. J. Phycol. 43:845-852. [Google Scholar]
- 20.Nedbal, L., M. Trtílek, J. Červený, O. Komárek, and H. B. Pakrasi. 2008. A photobioreactor system for precision cultivation of photoautotrophic microorganisms and for high-content analysis of suspension dynamics. Biotechnol. Bioeng. 100:902-910. [DOI] [PubMed] [Google Scholar]
- 21.Olson, J. M. 2006. Photosynthesis in the Archean era. Photosynth. Res. 88:109-117. [DOI] [PubMed] [Google Scholar]
- 22.Prince, R. C., and H. S. Kheshgi. 2005. The photobiological production of hydrogen: potential efficiency and effectiveness as a renewable fuel. Crit. Rev. Microbiol. 31:19-31. [DOI] [PubMed] [Google Scholar]
- 23.Reddy, K. J., J. B. Haskell, D. M. Sherman, and L. A. Sherman. 1993. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J. Bacteriol. 175:1284-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy, P. M., H. Spiller, S. L. Albrecht, and K. T. Shanmugam. 1996. Photodissimilation of fructose to H2 and CO2 by a dinitrogen-fixing cyanobacterium, Anabaena variabilis. Appl. Environ. Microbiol. 62:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rupprecht, J., B. Hankamer, J. H. Mussgnug, G. Ananyev, C. Dismukes, and O. Kruse. 2006. Perspectives and advances of biological H2 production in microorganisms. Appl. Microbiol. Biotechnol. 72:442-449. [DOI] [PubMed] [Google Scholar]
- 26.Schneegurt, M. A., D. M. Sherman, S. Nayar, and L. A. Sherman. 1994. Oscillating behavior of carbohydrate granule formation and dinitrogen fixation in the cyanobacterium Cyanothece sp. strain ATCC 51142. J. Bacteriol. 176:1586-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneegurt, M. A., D. M. Sherman, and L. A. Sherman. 1997a. Composition of the carbohydrate granules of the cyanobacterium, Cyanothece sp. strain ATCC 51142. Arch. Microbiol. 167:89-98. [PubMed] [Google Scholar]
- 28.Schneegurt, M. A., D. M. Sherman, and L. A. Sherman. 1997b. Growth, physiology, and ultrastructure of a diazotrophic cyanobacterium, Cyanothece sp. strain ATCC 51142, in mixotrophic and chemoheterotrophic cultures. J. Phycol. 33:632-642. [Google Scholar]
- 29.Schneegurt, M. A., D. L. Tucker, J. K. Ondr, D. M. Sherman, and L. A. Sherman. 2000. Metabolic rhythms of a diazotrophic cyanobacterium, Cyanothece sp. strain ATCC 51142, heterotrophically grown in continuous dark. J. Phycol. 36:107-117. [Google Scholar]
- 30.Schütz, K., T. Happe, O. Troshina, P. Lindblad, E. Leitão, P. Oliveira, and P. Tamagnini. 2004. Cyanobacterial H2 production—a comparative analysis. Planta 218:350-359. [DOI] [PubMed] [Google Scholar]
- 31.Sherman, L. A., P. Meunier, and M. S. Colón-López. 1998. Diurnal rhythms in metabolism: a day in the life of a unicellular, diazotrophic cyanobacterium. Photosynth. Res. 58:25-42. [Google Scholar]
- 32.Stöckel, J., E. A. Welsh, M. Liberton, R. Kunnvakkam, R. Aurora, and H. B. Pakrasi. 2008. Global transcriptomic analysis of Cyanothece 51142 reveals robust diurnal oscillation of central metabolic processes. Proc. Natl. Acad. Sci. U. S. A. 105:6156-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Summerfield, T. C., J. Toepel, and L. A. Sherman. 2008. Low-oxygen induction of normally cryptic psbA genes in cyanobacteria. Biochemistry 47:12939-12941. [DOI] [PubMed] [Google Scholar]
- 34.Sveshnikov, D. A., N. V. Sveshnikova, K. K. Rao, and D. O. Hall. 1997. Hydrogen metabolism of mutant forms of Anabaena variabilis in continuous cultures and under nutritional stress. FEMS Microbiol. Lett. 147:297-301. [Google Scholar]
- 35.Tamagnini, P., R. Axelsson, P. Lindberg, F. Oxelfelt, R. Wünschiers, and P. Lindblad. 2002. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol. Mol. Biol. Rev. 66:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamagnini, P., E. Leitao, P. Oliveira, D. Ferreira, F. Pinto, D. J. Harris, T. Heidorn, and P. Lindblad. 2007. Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiol. Rev. 31:692-720. [DOI] [PubMed] [Google Scholar]
- 37.Toepel, J., J. E. McDermott, T. C. Summerfield, and L. A. Sherman. 2009. Transcriptional analysis of the unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142 grown under short day/night cycles. J. Phycol. 45:610-620. [DOI] [PubMed] [Google Scholar]
- 38.Toepel, J., E. Welsh, T. C. Summerfield, H. B. Pakrasi, and L. A. Sherman. 2008. Differential transcriptional analysis of the cyanobacterium Cyanothece sp. strain ATCC 51142 during light-dark and continuous-light growth. J. Bacteriol. 190:3904-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsygankov, A. 2007. Nitrogen-fixing cyanobacteria: a review. Appl. Biochem. Microbiol. 43:250-259. [Google Scholar]
- 40.Tucker, D. L., K. Hirsh, H. Li, B. Boardman, and L. A. Sherman. 2001. The manganese stabilizing protein (MSP) and the control of O2 evolution in the unicellular, diazotrophic cyanobacterium, Cyanothece sp. ATCC 51142. Biochim. Biophys. Acta 1504:409-422. [DOI] [PubMed] [Google Scholar]
- 41.Tucker, D. L., and L. A. Sherman. 2000. Analysis of chlorophyll-protein complexes from the cyanobacterium Cyanothece sp. ATCC 51142 by non-denaturing gel electrophoresis. Biochim. Biophys. Acta 1468:150-160. [DOI] [PubMed] [Google Scholar]
- 42.van der Oost, J., B. A. Bulthuis, S. Feitz, K. Krab, and R. Kraayenhof. 1989. Fermentation metabolism of the unicellular cyanobacterium Cyanothece PCC 7822. Arch. Microbiol. 152:415-419. [Google Scholar]
- 43.Vignais, P. M., and B. Billoud. 2007. Occurrence, classification, and biological function of hydrogenases: an overview. Chem. Rev. 107:4206-4272. [DOI] [PubMed] [Google Scholar]
- 44.Welsh, E. A., M. Liberton, J. Stöckel, T. Loh, T. Elvitigala, C. Wang, A. Wollam, R. S. Fulton, S. W. Clifton, J. M. Jacobs, R. Aurora, B. K. Ghosh, L. A. Sherman, R. D. Smith, R. K. Wilson, and H. B. Pakrasi. 2008. The genome of Cyanothece 51142, a unicellular diazotrophic cyanobacterium important in the marine nitrogen cycle. Proc. Natl. Acad. Sci. U. S. A. 105:15094-15099. [DOI] [PMC free article] [PubMed] [Google Scholar]