Abstract
The l-tryptophan degradation product indole is a purported extracellular signaling molecule that influences biofilm formation in various bacteria. Here we analyzed the mechanisms of indole production in Fusobacterium nucleatum and the effects of tryptophan and indole on F. nucleatum planktonic and biofilm cells. The amino acid sequence deduced from the fn1943 gene in F. nucleatum ATCC 25586 was 28% identical to that deduced from tnaA in Escherichia coli, which encodes tryptophanase catalyzing the β-elimination of l-tryptophan to produce indole. The fn1943 gene was cotranscribed with the downstream gene fn1944, which is a homolog of tnaB encoding low-affinity tryptophan permease. The transcript started at position −68 or −153 from the first nucleotide of the fn1943 translation initiation codon. Real-time quantitative PCR showed that much more F. nucleatum fn1943 transcripts were obtained from log-phase cells than from stationary-phase cells. Indole production by the purified recombinant protein encoded by fn1943 was examined using high-performance liquid chromatography. The Km and kcat of the enzyme were 0.26 ± 0.03 mM and 0.74 ± 0.04 s−1, respectively. F. nucleatum biofilm formation and the biofilm supernatant concentration of indole increased dose dependently with increasing tryptophan concentrations. Exogenous indole also increased F. nucleatum biofilm formation in a dose-dependent manner. Even at very high concentrations, tryptophan did not affect fn1943 expression, whereas similar indole concentrations decreased expression. Thus, exogenous tryptophan and indole were suggested to increase F. nucleatum biofilms.
Fusobacterium nucleatum is a Gram-negative, anaerobic, nonmotile, non-spore-forming, spindle-shaped or fusiform rod bacterium that has been implicated as a causative agent of various periodontal (4, 11, 39), orofacial (5, 26), brain (8), liver (2), abdominal (6), blood (33), and gynecological (38) abscesses and infections in humans. The oral cavity is the primary colonization site of F. nucleatum, which is one of the most abundant anaerobes in subgingival plaque. F. nucleatum is an important central species in oral biofilm development, bridging early and late plaque biofilm colonizers by coaggregating with a wide array of microorganisms in the oral cavity (29). However, the mechanism driving F. nucleatum progression from planktonic to biofilm cells is poorly understood.
Indole has long been known as a chemorepellent with a nasty odor (54) and more recently has been shown to control the expression of several genes, including those associated with amino acid metabolism (55), plasmid maintenance (7), multidrug exporters (24), pathogenicity islands (1), and polysaccharide production (41). In addition, indole acts as a cell-to-cell signaling molecule that mediates biofilm formation in various bacteria carrying the gene for tryptophanase (also known as tryptophan indole-lyase; EC 4.1.99.1) (34, 37, 41, 54). Tryptophanase has been isolated from several bacterial species, including Escherichia coli (44), Bacillus alvei (25), Aeromonas liquefaciens (10), Proteus rettgeri (58), Proteus vulgaris (27), Haemophilus influenzae (36), and Porphyromonas gingivalis (61). It consists of four identical monomers, each containing one molecule of pyridoxal 5′-phosphate (PLP). The PLP molecule forms an aldimine bond with a lysine residue (31) and catalyzes the reversible hydrolytic cleavage of l-tryptophan to produce indole and ammonium pyruvate via an α,β-elimination mechanism (equation 1) (50):
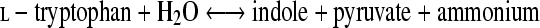 |
(1) |
The tryptophanase (tna) operon has been extensively studied in E. coli (57) and consists of a promoter-leader regulatory region (13) followed by tnaA encoding tryptophanase and tnaB encoding low-affinity tryptophan permease (Fig. 1) (18). Transcription of these two structural genes is regulated by both the catabolite repression and tryptophan-induced inhibition of Rho factor-dependent transcriptional termination in the leader region of the operon (tnaL) (51). The tnaL transcript contains a coding region for a 24-residue leader peptide (tnaC) followed by a Rho factor-binding site. Tryptophan induction requires the attempted translation of tnaC. The last sense codon of tnaC (a proline codon) is translated by tRNA2Pro The translating ribosome retaining uncleaved TnaC-tRNA2Pro stalls at the tnaC stop codon to block access of Rho factor to the rut site in the tna transcript, thereby preventing transcriptional termination in the tnaL region of the operon (19-22).
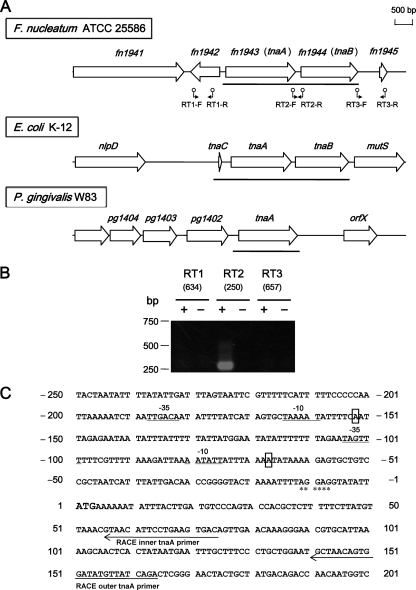
FIG. 1.
Genetic organization and transcriptional analyses of the tnaA region of F. nucleatum ATCC 25586. (A) Gene arrangements of the tnaA region in F. nucleatum ATCC 25586, E. coli K-12, and P. gingivalis W83. The DNA sequence of each region was obtained from the database. Large and small arrows indicate ORF positions and RT-PCR primers, respectively. Solid bars indicate tna region transcripts. (B) RT-PCR analysis of tnaA gene expression. Total RNA was prepared from a culture in the exponential growth phase. PCR amplicons were applied to 1.2% agarose gel electrophoresis. The expected size (bp) of each PCR amplicon is shown in parentheses. Lanes marked + or − are standard RT-PCR amplifications or negative controls containing no cDNA, respectively. Positions of the DNA size standards (in bp) are indicated on the left. (C) Determination of the transcription start site by RLM-RACE. The coding region starting with ATG (large boldface) is shown with the upstream promoter region. Identified transcriptional start sites are boxed. Positions of the tnaA-specific outer and inner primers used for RLM-RACE are indicated by arrows. The consensus promoter sequences (23) are shown directly below the −10 and −35 regions. A Shine-Dalgarno sequence is marked with asterisks (49).
We recently reported the molecular basis for indole production in P. gingivalis W83 (61). Although the enzymatic properties of tryptophanase in P. gingivalis were somewhat similar to those in E. coli, the tnaA regions completely differed in both genetic organization and transcriptional mechanism between the two bacteria. Unlike the flanking region of E. coli tnaA, no genes homologous to tnaB or tnaC were identified in the P. gingivalis tnaA region or elsewhere in the genome (61). Studies of the indole production mechanism have now been extended to F. nucleatum, a representative indole producer in oral microorganisms. In the present study, we identified and examined the expression of the tryptophanase-encoding gene in F. nucleatum and characterized the tryptophanase enzyme activity. The effects of exogenous tryptophan and indole on F. nucleatum biofilms and tnaA expression were also investigated.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
F. nucleatum subsp. nucleatum ATCC 25586 obtained from RIKEN BioResource Center (Tsukuba, Japan) was grown anaerobically at 37°C in Columbia broth (Difco, Detroit, MI). E. coli strains DH5α (Invitrogen, Carlsbad, CA) and Rosetta (Novagen, Madison, WI), used for DNA manipulation and protein purification, respectively, were grown aerobically in 2× TY broth (Difco) with 100 μg ml−1 ampicillin or 20 μg ml−1 chloramphenicol.
Extraction of RNA.
Unless otherwise specified, total RNA was extracted from an F. nucleatum ATCC 25586 culture brought to exponential growth using FastPrep Blue tubes (Bio101, Vista, CA), as described previously (61). Contaminating DNA in the samples was eliminated by digestion with RNase-free DNase (Takara Bio, Otsu, Japan).
RT-PCR.
Reverse transcriptase (RT)-mediated PCR was performed as previously described (60), with minor modifications. Briefly, RNA (5 ng μl−1) was reverse transcribed into single-stranded cDNA with 0.05 ng μl−1 random hexadeoxyribonucleotide primers (Takara Bio) using PrimeScript Reverse Transcriptase (Takara Bio) according to the manufacturer's instructions. The gene-specific primers used for PCR are listed in Table 1. The reaction mixtures used as negative controls contained no reverse transcriptase, allowing us to evaluate the existence of contaminating genomic DNA in the samples.
TABLE 1.
Oligonucleotide primers used in this study
| Purpose | Primer | Sequence (5′ to 3′)a |
|---|---|---|
| RT-PCR | RT-1-F | CTCAATCTTCCCCACATATC |
| RT-1-R | AGGAAAAACCTTAGGTCCATCTTT | |
| RT-2-F | ATAATTTACCTATGAGACACTTCC | |
| RT-2-R | CCAATACAAGCTAAGATGAAG | |
| RT-3-F | TGCACTTCTTGGAGA | |
| RT-3-R | GAGACAGTTCGGTAT | |
| RLM-RACE | ||
| RACE outer adapter primer | GCGAGCACAGAATTAATACGACT | |
| RACE inner adapter primer | CGCGGATCCGAACACTGCGTTTGCTGGCTTTGATG | |
| RACE outer tnaA primer | AATTCATTATAGTGAGTTGCTTT | |
| RACE inner tnaA primer | CTGTCACTTCAGGAATGTTAC | |
| Real-time PCR | 071609-tnaA-F2 | TGAACAAAGGGAACGTGCATT |
| 071609-tnaA-R2 | ATTCCAGCAGGGAAAGCAAA | |
| 081909-fn0654-Real-F | TGCTAAGACTGTTGTATGGAATGGA | |
| 081909-fn0654-Real-R | ACATACTCCTATTGTTCCTTTTGCAA | |
| 081909-fn2054-Real-F | CCCTGCTTCTGTTGACTTAACAAC | |
| 081909-fn2054-Real-R | AGGTTTCTTCTGCCATCTTGGA | |
| Purification of Fn1943 | 091708-fn-F1 | AAGGATCCAAAAAATATTTACTTGATGTCCC |
| 110608-fn-R1 | AAGTCGACCTATTTTTCTTCATTTGGATAAGG |
Underlined nucleotides indicate the positions of restriction endonuclease sites incorporated to facilitate cloning.
RLM-RACE.
RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) was used to determine the transcription start site of tnaA in F. nucleatum ATCC 25586 with total RNA. Rapid amplification of the 5′ cDNA ends was performed using a FirstChoice RLM-RACE kit (Ambion, Austin, TX) according to the manufacturer's instructions. Briefly, a 45-base RNA adapter was ligated to the RNA population using T4 RNA ligase. This RNA population was then used as a template for a random-primed reverse transcription reaction. The cDNA product was used as a template for PCR using a tnaA-specific outer primer (RACE outer tnaA primer) and a reverse primer to the adapter (RACE outer adaptor primer). The PCR conditions were as follows: 94°C for 3 min; 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s; and 72°C for 5 min. The identity of the product was confirmed using inner gene-specific primers (RACE inner tnaA primer and RACE inner adaptor primer). E. coli was transformed with the amplicon ligated into an intermediate-copy-number vector, pMCL210 (42). Plasmids isolated from 11 colonies were sequenced, and sequences were analyzed using Vector NTI software (Invitrogen).
Real-time quantitative PCR analysis.
Overnight cultures of F. nucleatum ATCC 25586 were diluted (1/50) in fresh Columbia broth and then incubated for 4, 8, 12, or 24 h. Cell growth was monitored by determining the optical density at 595 nm (OD595), and the cells collected at each incubation point were used for RNA extraction.
To evaluate the effect of tryptophan or indole on tnaA expression in F. nucleatum ATCC 25586, either material was added to the cell culture that had been incubated for 8 h, and the cells were incubated for another 1 h. RNA obtained from the cultures was reverse transcribed into single-stranded cDNA with random primers, as described above. Real-time quantitative PCR amplification, detection, and analysis were performed using the Thermal cycler Dice RealTime system (Takara Bio) with Power SYBR green PCR master mix (Applied Biosystems, Foster City, CA). A reaction mixture for real-time PCR (25 μl) contained 1× Power SYBR green PCR master mix, 22.5 pmol of each forward and reverse primer, and 2.5 μl of cDNA template. The reaction conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. At the end of each run, a dissociation protocol (95°C for 15 s, 60°C for 30 s, and 95°C for 15 s) was performed to ensure that nonspecific PCR products were absent. Two constitutive housekeeping genes, the phosphoglycerate kinase gene fn0654 and glucose-6-phosphate isomerase gene fn2054, were used as internal standards to normalize for the amount of total RNA in each sample (47). The primers used in real-time quantitative PCR (Table 1) were designed using Primer Express software (version 3.0; Applied Biosystems).
To estimate the initial amounts of template in each sample, serial real-time PCRs were performed using the purified genomic DNA of F. nucleatum ATCC 25586. For each gene, a standard curve was plotted using the log of the initial quantity of template against the threshold cycle (i.e., the cycle at which the fluorescence rose above the background level). Data were obtained from four independent experiments.
Purification of recombinant tryptophanase.
The recombinant tryptophanase of F. nucleatum ATCC 25586 was purified using the expression vector pGEX-6P-1 (GE Healthcare, Piscataway, NJ) as previously described (59). The coding sequence of fn1943 was PCR amplified with KOD DNA polymerase (Toyobo, Osaka, Japan) from the genomic DNA of F. nucleatum ATCC 25586 using the primers listed in Table 1. The amplicons were purified and ligated into the pGEX-6P-1 vector via the BamHI and SalI restriction sites juxtaposing the fn1943 gene, which is downstream from the coding sequence for glutathionine S-transferase (GST) and a PreScission protease cleavage site. E. coli Rosetta cells were transformed with the resulting plasmid, pGER100-3, after the correctness of the amplification of the 1,635-bp insert was verified by sequencing.
Production cultures (500 ml) were inoculated 1:100 with an overnight culture and grown to an OD595 of 0.7 at 30°C. The cell culture was incubated at 30°C for 1.5 h with 0.2 mM isopropyl-β-d-thiogalactoside (IPTG) to induce protein expression, and the cells were harvested by centrifugation at 4°C, resuspended in 12 ml of phosphate-buffered saline (PBS), and lysed by ultrasonication. Cell debris was sedimented by centrifugation, and the portion of the GST-fusion protein that remained in the supernatant was absorbed onto a glutathione-Sepharose 4B affinity matrix (GE Healthcare), and cleaved with PreScission protease (GE Healthcare) according to the manufacturer's protocol. The protein concentrations were determined as described previously (45), and sample purity was analyzed by SDS-PAGE.
HPLC analysis.
Indole production in an enzymatic reaction was evaluated using reversed-phase high-performance liquid chromatography (HPLC) as previously described (30), with minor modifications. The reaction mixture contained the following reagents in a final volume of 100 μl: 167 mM potassium phosphate buffer (pH 7.5), 0.165 mM PLP, 0.2 mM reduced glutathione, 0.25 mg ml−1 bovine serum albumin (BSA), and 2 mM l-tryptophan with or without 100 μg ml−1 of the purified enzyme of F. nucleatum ATCC 25586. After layering with 100 μl of toluene, the mixture was incubated for 2 h at 37°C. A toluene aliquot (20 μl) from the sample was injected onto a C18 reversed-phase column (4.6 mm by 250 mm) (TSK-Gel ODS-80Ts; Tosoh, Tokyo, Japan). The mobile-phase solvent, 50% (vol/vol) methanol-water, was pumped through the column at a flow rate of 1 ml min−1 at 40°C. Excitation and emission wavelengths of 285 and 320 nm, respectively, were used.
Enzyme assay.
l-Tryptophan degradation by purified tryptophanase was examined by measuring indole formation, as previously reported (40), with minor modifications. Briefly, after layering the reaction mixture (200 mM potassium buffer [pH 7.5], 0.165 mM PLP, 0.2 mM reduced glutathione, 0.25 mg ml−1 BSA, 10 μg ml−1 purified tryptophanase, and l-tryptophan) with 100 μl toluene, the reaction mixture was prewarmed for 5 min at 37°C. The reaction was initiated by adding a substrate solution. After 10 min of incubation, the reaction was terminated by the addition of 1 ml Ehrlich's reagent, which was prepared daily by mixing five volumes of 5% (wt/vol) p-dimethylaminobenzaldehyde in 95% (vol/vol) ethanol with 12 volumes of 5% (vol/vol) H2SO4 in 1-butanol. After incubation for 20 min at room temperature, the mixture was centrifuged at 15,000 × g for 2 min to remove insoluble materials. The supernatant was examined spectrophotometrically at 568 nm. The amounts of indole in each reaction mixture were calculated from a standard curve. Kinetic parameters were computed from a Lineweaver-Burk transformation of the Michaelis-Menten equation. The kcat value was calculated from the Vmax value and the molecular weight of F. nucleatum tryptophanase. Data were obtained from three independent experiments.
The degradation of S-ethyl-l-cysteine, S-methyl-l-cysteine, l-cysteine, l-alanine, and l-serine was measured by assaying pyruvate formation, as previously described (59). The assays were performed in 100-μl reaction mixtures containing 200 mM potassium phosphate buffer (pH 7.6), 0.165 mM PLP, 1 μg of purified enzyme, and several concentrations of each substrate. After 10 min of incubation at 37°C, reactions were terminated by adding 50 μl of 4.5% (vol/vol) trichloroacetic acid. The reaction mixtures were centrifuged, and 100 μl of the supernatants was added to 300 μl of 0.67 M sodium acetate (pH 5.2) containing 0.017% (wt/vol) 3-methyl-2-benzothiazolinone hydrazone. After incubation at 50°C for 30 min, the absorbance at 335 nm (A335) was determined. The amounts of pyruvate were calculated from a standard curve prepared using crystalline sodium pyruvate. Data were obtained from three independent experiments.
Indole assay.
Overnight cultures of F. nucleatum ATCC 25586 were diluted 1/20 in Columbia broth. Aliquots (1 ml) of the diluted samples were incubated in the wells of a flat-bottom 24-well polystyrene plate (Greiner Bio-One, Tokyo, Japan) with or without 1, 3, or 6 mM tryptophan for 48 h at 37°C. After the cell culture was centrifuged at 15,000 × g for 2 min and the bacterial pellet was removed, the supernatants of the samples were mixed immediately with 140 μl of Kovac's reagent (5% [wt/vol] p-dimethylamino-benzaldehyde, 75% [wt/vol] methanol, 2.5 M HCl). The indole concentration was measured spectrophotometrically from the A540 and calculated according to the standard curve.
Biofilm formation assay.
Biofilm formation by F. nucleatum ATCC 25586 was assayed using a method described previously (35) with some modifications. Overnight cultures of F. nucleatum were diluted 1/20 in Columbia broth. Aliquots (200 μl) of the diluted samples were anaerobically incubated in the wells of a flat-bottom 96-well polyvinyl chloride (PVC) plate (BD Japan, Tokyo, Japan) with or without tryptophan (1, 3, or 6 mM) or indole (0.05, 0.1, 0.2, 0.4, 0.8, or 1.6 mM) for 48 h at 37°C. A portion (100 μl) of each cell culture was then transferred to a 96-well polystyrene plate and spectrophotometrically measured at 595 nm to assess planktonic bacterial growth. After discarding the remaining bacterial culture in the wells, bacterial cells bound to the wells were gently washed twice with PBS, air dried, and then stained with 200 μl of 0.1% (wt/vol) crystal violet for 15 min. After being washed 4 times with PBS to remove excess dye, the cell-bound dye was eluted using 200 μl of 99% methanol. Biofilm formation was quantified by measuring the A595.
Confocal laser-scanning microscopy.
Biofilms of F. nucleatum were formed on PVC sheets (5 × 5 mm) sunk in a 12-well plate containing Columbia broth with or without tryptophan (1, 3, or 6 mM) or indole (0.2 or 1.6 mM) for 48 h at 37°C. The biofilms formed on each sheet were washed twice with PBS to remove unbound cells, stained for 15 min in the dark with a drop of 5 mM SYTO9 (Invitrogen) in PBS, and rinsed twice with PBS. The sheet was then mounted on a drop of ProLong Gold (Invitrogen). Samples were observed using a confocal laser-scanning microscope (FV1000-D; Olympus, Tokyo, Japan) with an argon laser at 488 nm to excite the SYTO9 dye, and its fluorescence was gained through a 505- to 605-nm optical filter. Optical sections were collected with 2.5-μm steps through a sample depth of 70 μm. The three-dimensional structures of the biofilms were constructed using the Fluoview software (Olympus).
RESULTS
Organization of the tnaA homolog and the flanking regions in F. nucleatum.
The DNA sequences of the tnaA homolog (fn1943) and flanking regions in F. nucleatum ATCC 25586 were obtained from the whole genome sequence of the strain (28) using GenBank (http://www.ncbi.nlm.nih.gov/). The open reading frame (ORF) (1,635 bp) is longer by at least 200 bp than the tnaA genes (1,389 to 1,416 bp) from other bacteria, such as E. coli (12), P. vulgaris (27), H. influenza (36), and P. gingivalis (61). The amino acid sequence deduced from fn1943 of F. nucleatum displayed 28 to 34% identity with the tnaA sequences of the bacteria described above. In E. coli, the transcribed leader region of tnaA contains a 72-bp coding region, tnaC, for a 24-residue leader peptide (52), which is necessary for tna operon expression (20). In F. nucleatum ATCC 25586, no sequences corresponding to the leader peptide were found in the fn1943 operon transcript (Fig. 1A), which was determined as described below. The upstream ORF from fn1943, namely, fn1942, existed in the opposite strand of the region, although homologous genes were not found in the corresponding region of E. coli or P. gingivalis. In contrast, the amino acid sequence of the gene downstream from fn1943 of F. nucleatum ATCC 25586 (fn1944) was 15% identical to that of tnaB encoding low-affinity tryptophan permease in E. coli (18).
Transcriptional analyses of the fn1943 region in F. nucleatum.
Transcripts of the fn1943 region in F. nucleatum ATCC 25586 were characterized using RT-PCR. The positions of the primer pairs designed to detect intergenic and intragenic regions are shown in Fig. 1A. No PCR product corresponding to the region spanning the borders of fn1944/fn1945 were amplified (Fig. 1B), and fn1942 expression was not detected. In contrast, PCR fragments corresponding to the region spanning the borders of fn1943 and fn1944 were amplified, with amplicons of the expected lengths. However, no products were amplified using cDNA prepared without reverse transcriptase, indicating that the RT-PCR products were not derived from contaminated chromosomal DNA (Fig. 1B). These results demonstrated that the fn1943 and fn1944 genes were cotranscribed as an operon in F. nucleatum ATCC 25586.
The transcription start site of fn1943 in F. nucleatum ATCC 25586 was determined by 5′ RLM-RACE experiments using cDNA reverse transcribed from total RNA, which was extracted from F. nucleatum ATCC 25586 grown to the exponential phase. PCR fragments amplified with inner primers were subjected to DNA sequencing of the upstream region up to the transcription start site of fn1943. The junction of the adapter with F. nucleatum genomic DNA is the 5′ end of the transcript. Two forms of the fn1943 transcript were detected, starting at adenine at positions −68 (in 4 of 11 plasmids analyzed) and −153 (in 7 of 11 plasmids analyzed) from the first nucleotide of the translation initiation codon of fn1943 (Fig. 1C). Sequences similar to the consensus promoter, the −10 and −35 regions (23), were found in the regions upstream from both transcript starting positions.
Enzymatic characterization of the fn1943 product of F. nucleatum.
To characterize the product of the fn1943 gene, we purified the recombinant protein using the pGEX-6P-1 vector system (Fig. 2 A). The molecular mass of the denatured polypeptide agreed well with the predicted molecular mass (61 kDa).
FIG. 2.
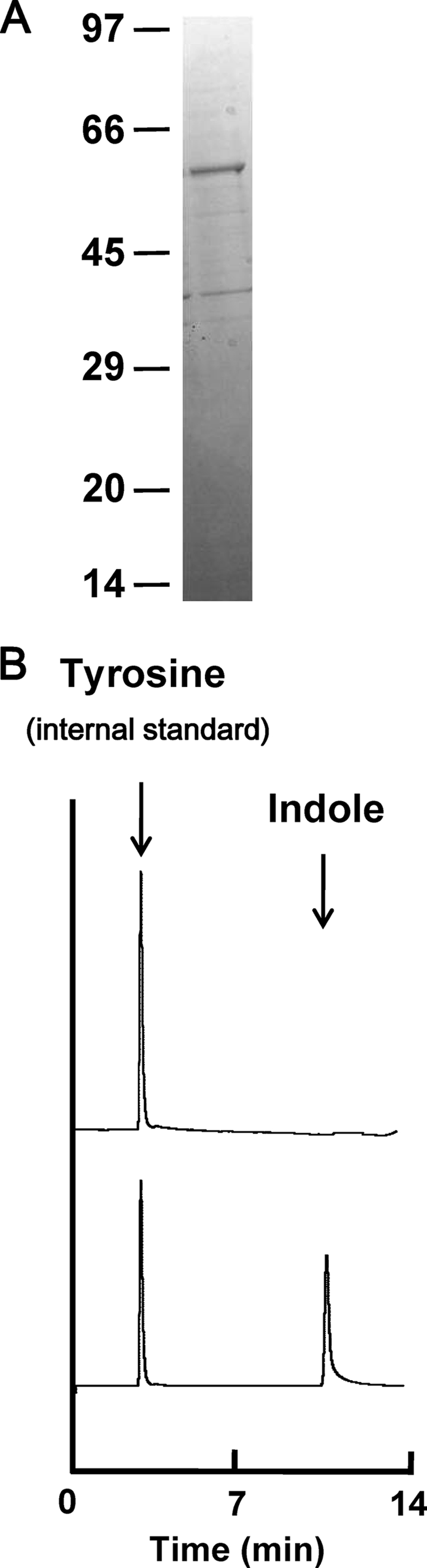
Characterization of purified protein encoded by fn1943 of F. nucleatum ATCC 25586. (A) SDS-PAGE analysis of recombinant Fn1943 of F. nucleatum ATCC 25586. Proteins were purified by affinity chromatography on glutathione-Sepharose 4B resin and digested with PreScission protease. The ∼5-μg protein sample was subjected to SDS-PAGE and stained with Coomassie brilliant blue. Positions of molecular mass markers (in kDa) are shown. (B) Reversed-phase HPLC profiles of indole production from l-tryptophan by the purified Fn1943 of F. nucleatum ATCC 25586. A 2-μl aliquot of toluene that had been layered on the reaction mixture was injected onto a column with l-tyrosine as an internal standard. Reaction mixtures were prepared with (bottom) or without (top) recombinant Fn1943. Arrows indicate the elution positions of indole and tyrosine.
HPLC analysis showed that incubation of the purified protein with l-tryptophan resulted in indole production, indicating that the fn1943 gene encodes tryptophanase (Fig. 2B). Based on this result, we designated fn1943 as tnaA. The kinetic activity of the recombinant tryptophanase was spectrophotometrically evaluated. The breakdown of substrates other than l-tryptophan was determined by assaying pyruvate production, since enzymatic degradation of the substrates invariably resulted in pyruvate formation. Our preliminary experiments showed that indole inhibited the assay using 3-methyl-2-benzothiazolinone hydrazone to detect pyruvate formation. Hence, the kinetic properties of the tryptophanase for l-tryptophan were determined by measuring the amounts of indole. The enzymatic properties of the purified enzyme are summarized in Table 2. The kcat and kcat/Km values of the enzyme for l-tryptophan were much higher than those for S-methyl-l-cysteine or S-ethyl-l-cysteine. The enzyme had no detectable elimination activity with l-alanine, l-serine, or l-cysteine as a substrate.
TABLE 2.
Steady-state kinetic parameters for TnaA from F. nucleatum ATCC 25586a
| Substrate | Km (mM) | kcat (s−1) | kcat/Km (mM−1 s−1) |
|---|---|---|---|
| l-Tryptophan | 0.26 ± 0.03 | 0.74 ± 0.04 | 2.79 ± 0.25 |
| S-Methyl-l-cysteine | 0.9 ± 0.19 | 0.11 ± 0.01 | 0.12 ± 0.002 |
| S-Ethyl-l-cysteine | 0.5 ± 0.06 | 0.4 ± 0.03 | 0.8 ± 0.04 |
Values are means ± standard deviations from three experiments. No detectable elimination activity with l-alanine, l-serine, or l-cysteine as a substrate was observed.
Effect of the growth phase on tnaA expression.
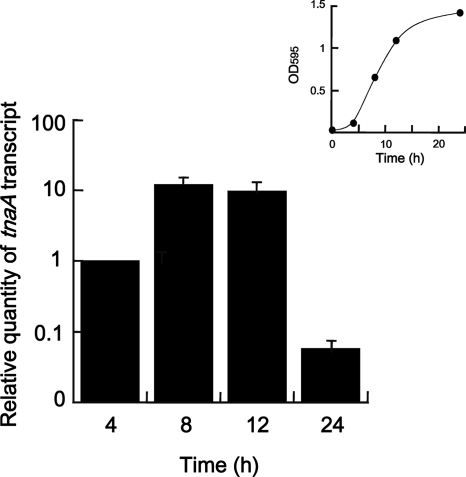
To examine the effect of the F. nucleatum growth phase on tnaA expression, real-time quantitative PCR analysis was performed. After the OD595 of cell cultures incubated for 4, 8, 12, and 24 h was measured to create a growth curve (Fig. 3), cDNA was prepared using total RNA extracted from each cell culture. The amounts of tnaA transcript in cells grown for 8 and 12 h were 12 and 9.7 times, respectively, larger than those for 4 h (early log phase). However, expression of the tnaA gene in the stationary phase (24 h) was only 5% of that in the early log phase (Fig. 3). The amounts of total cDNA were normalized using the housekeeping genes fn0654 (phosphoglycerate kinase) and fn2054 (glucose-6-phosphate isomerase; data not shown).
FIG. 3.
Expression levels of tnaA in several cell growth phases of F. nucleatum ATCC 25586. The OD595 of cells incubated for 4, 8, 12, or 24 h for RNA extraction is indicated as an inset. The amounts of tnaA cDNA in each sample were analyzed by real-time quantitative PCR and are shown as relative values, after normalization using the housekeeping gene fn0654. Data are given as the means ± standard deviations from four experiments.
Effect of tryptophan on indole and F. nucleatum biofilm formation.
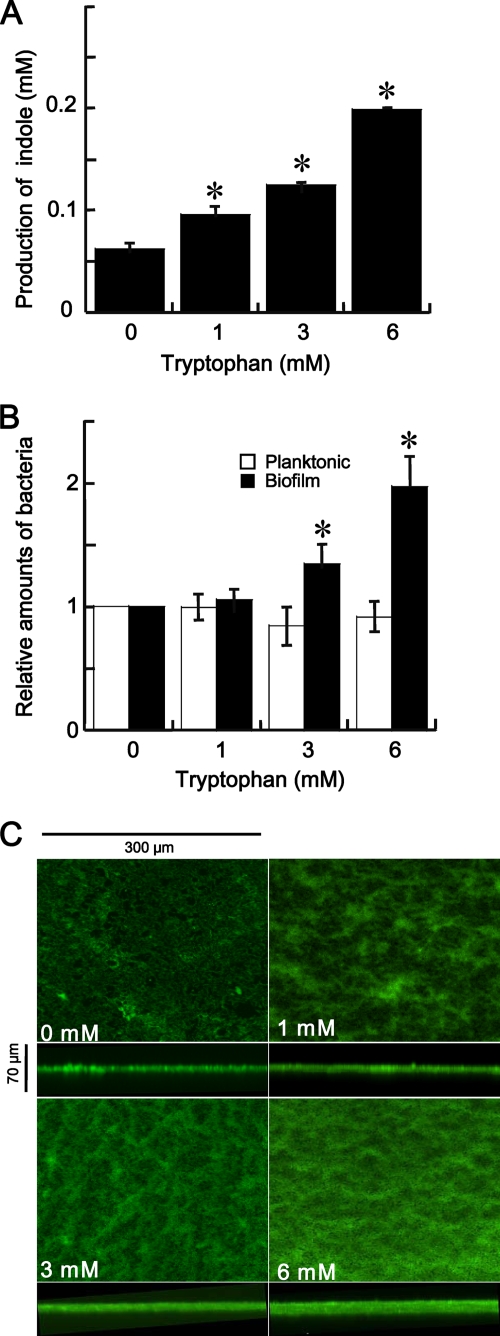
The indole concentration was measured in a culture of F. nucleatum grown with tryptophan for 48 h. Addition of tryptophan into the bacterial cell culture increased the indole concentration in culture supernatants in a dose-dependent manner, although 0.06 mM indole was detected even in cell cultures grown without tryptophan (Fig. 4 A).
FIG. 4.
Effects of tryptophan on indole production and biofilm formation by F. nucleatum ATCC 25586. (A) Indole production by F. nucleatum ATCC 25586 cells grown in the absence or presence of tryptophan; (B) relative amounts of planktonic and biofilm cells grown in the absence or presence of tryptophan. Bacteria were incubated for 48 h in 96-well PVC plates containing Columbia broth supplemented with or without 1, 3, or 6 mM tryptophan. The OD595 of the cell culture was measured to determine the amounts of planktonic cells. After cells were removed by centrifugation, the indole concentrations in the supernatants were measured. The amounts of biofilms were quantified using OD595 measurements following crystal violet staining and methanol elution. Data are given as the means ± standard deviations from three experiments. *, P < 0.01, t test. (C) Structure of F. nucleatum biofilms formed for 48 h with or without 1, 3, or 6 mM tryptophan. Biofilms formed on PVC sheets were stained with SYTO9 and observed by confocal laser-scanning microscopy. x-z reconstructions of each biofilm are shown below each x-y image.
Biofilm formation by F. nucleatum grown with tryptophan was also examined. The levels of biofilm formation on the flat bottoms of 96-well PVC plates were estimated by measuring the OD595 after staining the bacteria with crystal violet (Fig. 4B). The amount of biofilm formed by F. nucleatum also increased dose dependently with increasing tryptophan concentration. In contrast, the planktonic cells grown with or without various tryptophan concentrations were not significantly different. F. nucleatum biofilms formed on small PVC sheets in the presence or absence of tryptophan for 48 h were examined using confocal laser-scanning microscopy (Fig. 4C). The surface area of the PVC sheet that was covered with F. nucleatum biofilm was visually increased in accordance with the concentration of tryptophan added to the culture, reflecting the results obtained by the biofilm assay with crystal violet.
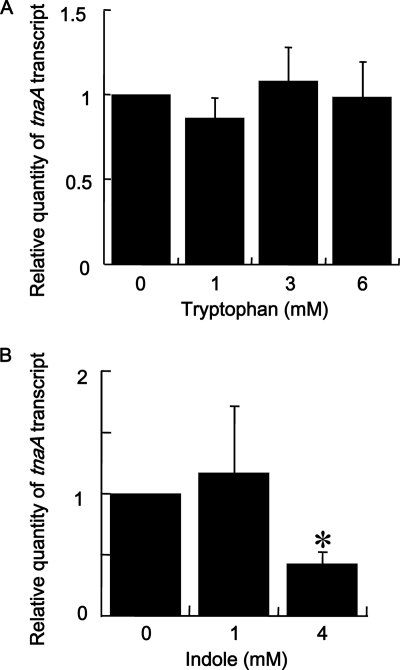
The effect of the addition of tryptophan on tnaA expression in F. nucleatum was also evaluated using real-time quantitative PCR (Fig. 5 A). Cells were incubated for 8 h without tryptophan followed by incubation for another 1 h with or without 1, 3, or 6 mM tryptophan. The amounts of tnaA transcripts were not significantly different among F. nucleatum cells grown with or without tryptophan (Fig. 5A).
FIG. 5.
Effect of tryptophan (A) and exogenous indole (B) on tnaA expression in F. nucleatum. Total RNA was extracted from F. nucleatum cells incubated for 8 h without indole and for another 1 h in the absence or presence of 1, 3, or 6 mM tryptophan or in the absence or presence of 1 or 4 mM indole. The relative amounts of tnaA in each cDNA sample were measured by real-time quantitative PCR analysis using fn0654 to normalize the total cDNA. Data are given as means ± standard deviations from four experiments. *, P < 0.01, t test.
Effect of exogenous indole on biofilm formation and tnaA expression in F. nucleatum.
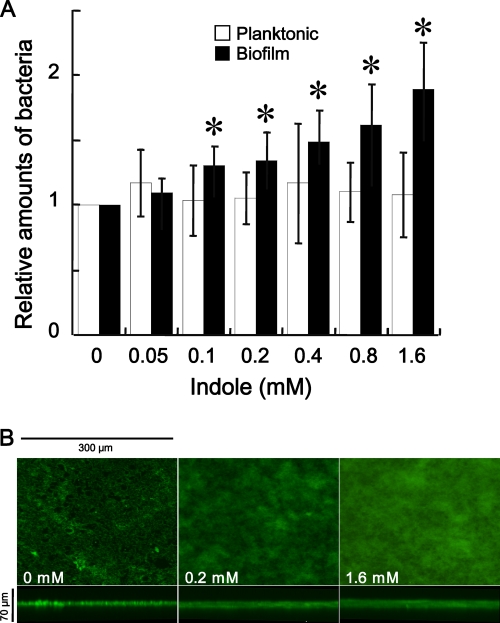
The crystal violet biofilm assay revealed that F. nucleatum biofilm formation increased dose dependently with increasing exogenous indole (Fig. 6 A). These findings were confirmed by confocal laser-scanning microscopy of biofilms formed in presence or absence of 0.2 or 1.6 mM indole (Fig. 6B). In contrast, planktonic cells grown without indole were not significantly different from those grown with any tested concentration of indole (Fig. 6A).
FIG. 6.
Effects of exogenous indole on planktonic and biofilm cells of F. nucleatum ATCC 25586. (A) Relative amounts of planktonic and biofilm cells grown in the absence or presence of exogenous indole are shown. Bacteria were incubated for 48 h in 96-well PVC plates containing Columbia broth supplemented with or without 0.05, 0.1, 0.2, 0.4, 0.8, or 1.6 mM indole. The OD595 of the cell culture was measured to determine the amounts of planktonic cells. The amounts of biofilms were quantified using OD595 measurements following crystal violet staining and methanol elution. Data are given as the means ± standard deviations from three experiments. *, P < 0.01, t test. (B) Structure of F. nucleatum biofilms formed in absence or presence of 0.2 or 1.6 mM indole. Biofilms that formed on the PVC sheets were stained with SYTO9 and observed by confocal laser microscopy. x-z reconstructions of each biofilm are shown below each x-y image.
The effect of exogenous indole on tnaA expression in F. nucleatum was also evaluated using real-time quantitative PCR (Fig. 5B). Cells were incubated for 8 h without indole followed by further incubation for 1 h with or without 1 or 4 mM indole. There was no significant difference in the amounts of tnaA between F. nucleatum cells incubated with or without 1 mM indole. However, when cells were incubated with 4 mM indole, the tnaA expression was decreased to ∼43% compared to control. Similar results were obtained regardless of whether the amounts of total cDNA were normalized using the housekeeping gene fn0654 or fn2054.
DISCUSSION
Until the tryptophanase of P. gingivalis was recently reported (61), the mechanism by which indole was produced by oral microorganisms had not been examined in much detail. This is probably because the importance of indole had not been recognized, except for its usefulness as a common diagnostic marker. However, increasing evidence suggests that indole mediates cell-to-cell signaling, similar to the N-acyl derivatives of homoserine lactone, cyclic peptide, and quinolones (9, 34, 41, 55). The goal of this study was to gain insight into indole production by F. nucleatum, a key microorganism for dental biofilm formation, and to evaluate the effects of tryptophan and indole on biofilm formation.
The tna operon of E. coli contains two major structural genes, tnaA and tnaB (12). The former gene is preceded by a 319-bp transcribed leader region, tnaL, which contains a short coding region, tnaC. In P. gingivalis, the flanking regions of tnaA do not contain tnaB or tnaC (61). As seen in the tna operon of E. coli, the tnaA gene was cotranscribed with tnaB in F. nucleatum. However, the transcription start site of the tna operon in F. nucleatum ATCC 25586 was 67 or 152 bp upstream from the initiation codon of tnaA (Fig. 1C), revealing that the region corresponding to tnaL in F. nucleatum was much shorter than that in E. coli. No sequence homologous with tnaC was found in the tnaL region of F. nucleatum ATCC 25586. Furthermore, no sequence was found that bound to the cyclic AMP (cAMP) receptor protein, which is proximal to the transcription start site of the tna operon in E. coli and associated with tnaA operon expression (13). These findings suggest that tna operon regulation in F. nucleatum is different from that in E. coli.
The Km value (0.26 ± 0.03 mM) of F. nucleatum tryptophanase for l-tryptophan was slightly lower than that of E. coli (0.32 mM) (43, 56) or B. alvei (0.27 mM) (25) but slightly higher than that of P. gingivalis (0.20 mM) (61). These findings indicate that the affinity of F. nucleatum tryptophanase to l-tryptophan was similar to those of tryptophanases of other bacteria. In contrast, the kcat and kcat/Km values (0.74 ± 0.04 s−1 and 2.79 ± 0.25 mM−1 s−1, respectively) were less than those in E. coli (6.8 s−1 and 30 mM−1 s−1, respectively) (46) and P. gingivalis (1.37 s−1 and 6.87 mM−1 s−1, respectively). Thus, the capacity of tryptophanase from F. nucleatum to produce indole was lower than that of tryptophanase from E. coli or P. gingivalis.
Neither tryptophanase from F. nucleatum nor that from P. gingivalis (61) degraded l-serine or l-cysteine, both of which were degraded by tryptophanases from E. coli (40) and P. vulgaris (62). Since the amino acid identity between tryptophanase from F. nucleatum and that from P. gingivalis was nearly identical to that between tryptophanase from F. nucleatum and those from the other bacteria described above, some specific amino acids in tryptophanase might play an important role in determining the enzyme substrate specificity. In addition to previous reports describing the amino acids associated with the active site of the enzyme (14-16, 32), further studies on which amino acids determine the substrate specificity would be interesting.
The evidence that indole is a cell-to-cell signaling molecule influencing bacterial biofilm formation is rather complicated. In E. coli, indole represses biofilm formation by inducing the quorum-sensing signal autoinducer I (34). Indole increases Vibrio cholerae biofilms by activating genes involved in Vibrio polysaccharide production, which is essential for biofilm formation in the bacteria (41). We demonstrated that F. nucleatum biofilms were increased in accordance with the amounts of indole (Fig. 6A) or tryptophan (Fig. 4B) added to the media. The latter finding is likely due to indole production from tryptophan by tryptophanase. Indeed, the indole concentration was increased by the addition of tryptophan to the bacterial cultures.
The physiological indole concentration seen in stationary-phase supernatants of E. coli is 0.3 mM (55), which is high enough to increase biofilm formation by F. nucleatum. Incubation of F. nucleatum with 6 mM tryptophan, at which F. nucleatum biofilms were significantly increased (Fig. 4B), resulted in production of approximately 0.2 mM indole (Fig. 4A). In contrast, the F. nucleatum biofilms were efficiently increased by the presence of 0.2 mM indole (Fig. 6A). Interestingly, the F. nucleatum biofilms formed in the presence of 6 mM tryptophan were quantitatively more than those in the presence of 0.2 mM indole (Fig. 4C and 6B), suggesting that the biofilm development may be influenced not only by indole but also by tryptophan, or its metabolites. However, the mechanism by which indole and tryptophan increase F. nucleatum biofilm formation is unclear. Studies on indole- or tryptophan-mediated gene regulation using microarrays (41) and two-dimensional electrophoresis (9) might provide useful information to elucidate the mechanism.
Real-time quantitative PCR analysis revealed approximately 10 and 100 times more F. nucleatum tnaA transcripts in the mid- or late-log-phase cells than in the early-log-phase or stationary-phase cells, respectively (Fig. 3). The relatively low stationary-phase expression of tnaA agrees with a previous report showing that tnaA was repressed 13-fold in 6-day-old E. coli biofilms (48). Interestingly, addition of tryptophan even at a very high concentration (6 mM), at which indole was produced at 0.23 mM in the F. nucleatum culture, did not affect tnaA expression (Fig. 5A). This might be because the F. nucleatum tnaA region contains the tnaC sequence, which is associated with the tryptophan-induced inhibition of Rho factor-dependent transcription termination (53). In contrast, the decreased tnaA level at high indole concentrations seemed to be associated with the feedback response (Fig. 5B).
Certain periodontopathogenic bacteria, including P. gingivalis, Prevotella intermedia, and F. nucleatum, are able to produce indole from l-tryptophan (17). It was reported more than 50 years ago that saliva from patients with periodontal disease produced more indole than saliva from periodontally healthy subjects (3). However, it is unclear whether the indole toxicity is directly associated with periodontal disease, whereas F. nucleatum biofilms were increased in the presence of indole (Fig. 6A). It should be noted that PVC was used as a substrate for F. nucleatum biofilms in this study. Our preliminary experiments showed that the plates and sheets made of PVC, which have been frequently employed in similar experiments, fix F. nucleatum biofilms more firmly than those of other artificial substrates, including polystyrene. However, such plates and sheets do not necessarily reflect the conditions in the oral cavity, since the scaffold on which bacterial biofilms are experimentally formed can generally influence their amount and structure. In addition, natural biofilms such as dental plaque are complex structures of multiple organisms which may behave quite differently from monoculture biofilms due to cell-to-cell signaling molecules. The function of indole as an interspecies signal that decreases E. coli and increases Pseudomonas sp. biofilms (34) is interesting, given that F. nucleatum is an important central microorganism in oral biofilms (29). In contrast, tryptophan metabolism and indole production in F. nucleatum may also be influenced by cell-to-cell signaling molecules that the other bacteria produce. Thus, more comprehensive studies are necessary to precisely understand the effect of indole on the biofilm formation by F. nucleatum and the other bacteria in the human oral cavity.
Acknowledgments
This study was supported in part by a Research Grant for Promoting Technological Seeds (Y.Y.) and by Grants-in-Aid for Scientific Research (no. 20592463, 20592181, and 21592631) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
Published ahead of print on 14 May 2010.
REFERENCES
- 1.Anyanful, A., J. M. Dolan-Livengood, T. Lewis, S. Sheth, M. N. Dezalia, M. A. Sherman, L. V. Kalman, G. M. Benian, and D. Kalman. 2005. Paralysis and killing of Caenorhabditis elegans by enteropathogenic Escherichia coli requires the bacterial tryptophanase gene. Mol. Microbiol. 57:988-1007. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, C., D. Schoonbroodt, C. Wagner, and Y. Horsmans. 2000. Liver abscesses due to Fusobacterium species. Liver 20:267-268. [DOI] [PubMed] [Google Scholar]
- 3.Berg, M., D. Y. Burrill, and L. S. Fosdick. 1946. Chemical studies in periodontal disease. III. Putrefaction of salivary proteins. J. Dent. Res. 25:231-246. [DOI] [PubMed] [Google Scholar]
- 4.Bolstad, A. I., H. B. Jensen, and V. Bakken. 1996. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin. Microbiol. Rev. 9:55-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botha, S. J., R. Senekal, P. L. Steyn, and W. J. Coetzee. 1993. Anaerobic bacteria in orofacial abscesses. J. Dent. Assoc. S. Afr. 48:445-449. [PubMed] [Google Scholar]
- 6.Brook, I., and E. H. Frazier. 2000. Aerobic and anaerobic microbiology in intra-abdominal infections associated with diverticulitis. J. Med. Microbiol. 49:827-830. [DOI] [PubMed] [Google Scholar]
- 7.Chant, E. L., and D. K. Summers. 2007. Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol. Microbiol. 63:35-43. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhry, R., B. Dhawan, B. V. Laxmi, and V. S. Mehta. 1998. The microbial spectrum of brain abscess with special reference to anaerobic bacteria. Br. J. Neurosurg. 12:127-130. [DOI] [PubMed] [Google Scholar]
- 9.Collet, A., S. Vilain, P. Cosette, G. A. Junter, T. Jouenne, R. S. Phillips, and P. Di Martino. 2007. Protein expression in Escherichia coli S17-1 biofilms: impact of indole. Antonie Van Leeuwenhoek 91:71-85. [DOI] [PubMed] [Google Scholar]
- 10.Cowell, J. L., and R. D. DeMoss. 1973. Trytophanase from Aeromonas liquefaciens. Subunit structure and aggregation of the enzyme into enzymatically active polymeric species. J. Biol. Chem. 248:6262-6269. [PubMed] [Google Scholar]
- 11.Debelian, G. J., I. Olsen, and L. Tronstad. 1998. Anaerobic bacteremia and fungemia in patients undergoing endodontic therapy: an overview. Ann. Periodontol. 3:281-287. [DOI] [PubMed] [Google Scholar]
- 12.Deeley, M. C., and C. Yanofsky. 1981. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J. Bacteriol. 147:787-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeley, M. C., and C. Yanofsky. 1982. Transcription initiation at the tryptophanase promoter of Escherichia coli K-12. J. Bacteriol. 151:942-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demidkina, T. V., M. V. Barbolina, N. G. Faleev, B. Sundararaju, P. D. Gollnick, and R. S. Phillips. 2002. Threonine-124 and phenylalanine-448 in Citrobacter freundii tyrosine phenol-lyase are necessary for activity with L-tyrosine. Biochem. J. 363:745-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demidkina, T. V., N. G. Faleev, A. I. Papisova, N. P. Bazhulina, V. V. Kulikova, P. D. Gollnick, and R. S. Phillips. 2006. Aspartic acid 214 in Citrobacter freundii tyrosine phenol-lyase ensures sufficient C-H-acidity of the external aldimine intermediate and proper orientation of the cofactor at the active site. Biochim. Biophys. Acta 1764:1268-1276. [DOI] [PubMed] [Google Scholar]
- 16.Demidkina, T. V., L. N. Zakomirdina, V. V. Kulikova, I. S. Dementieva, N. G. Faleev, L. Ronda, A. Mozzarelli, P. D. Gollnick, and R. S. Phillips. 2003. Role of aspartate-133 and histidine-458 in the mechanism of tryptophan indole-lyase from Proteus vulgaris. Biochemistry 42:11161-11169. [DOI] [PubMed] [Google Scholar]
- 17.Duerden, B. I., J. G. Collee, R. Brown, A. G. Deacon, and W. P. Holbrook. 1980. A scheme for the identification of clinical isolates of Gram-negative anaerobic bacilli by conventional bacteriological tests. J. Med. Microbiol. 13:231-245. [DOI] [PubMed] [Google Scholar]
- 18.Edwards, R. M., and M. D. Yudkin. 1982. Location of the gene for the low-affinity tryptophan-specific permease of Escherichia coli. Biochem. J. 204:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong, F., K. Ito, Y. Nakamura, and C. Yanofsky. 2001. The mechanism of tryptophan induction of tryptophanase operon expression: tryptophan inhibits release factor-mediated cleavage of TnaC-peptidyl-tRNAPro. Proc. Natl. Acad. Sci. U. S. A. 98:8997-9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong, F., and C. Yanofsky. 2002. Analysis of tryptophanase operon expression in vitro: accumulation of TnaC-peptidyl-tRNA in a release factor 2-depleted S-30 extract prevents Rho factor action, simulating induction. J. Biol. Chem. 277:17095-17100. [DOI] [PubMed] [Google Scholar]
- 21.Gong, F., and C. Yanofsky. 2002. Instruction of translating ribosome by nascent peptide. Science 297:1864-1867. [DOI] [PubMed] [Google Scholar]
- 22.Gong, F., and C. Yanofsky. 2001. Reproducing tna operon regulation in vitro in an S-30 system. Tryptophan induction inhibits cleavage of TnaC peptidyl-tRNA. J. Biol. Chem. 276:1974-1983. [DOI] [PubMed] [Google Scholar]
- 23.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirakawa, H., Y. Inazumi, T. Masaki, T. Hirata, and A. Yamaguchi. 2005. Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 55:1113-1126. [DOI] [PubMed] [Google Scholar]
- 25.Hoch, J. A., F. J. Simpson, and R. D. DeMoss. 1966. Purification and some properties of tryptophanase from Bacillus alvei. Biochemistry 5:2229-2237. [DOI] [PubMed] [Google Scholar]
- 26.Hockensmith, M. L., D. L. Mellman, and E. L. Aronsen. 1999. Fusobacterium nucleatum empyema necessitans. Clin. Infect. Dis. 29:1596-1598. [DOI] [PubMed] [Google Scholar]
- 27.Kamath, A. V., and C. Yanofsky. 1992. Characterization of the tryptophanase operon of Proteus vulgaris. Cloning, nucleotide sequence, amino acid homology, and in vitro synthesis of the leader peptide and regulatory analysis. J. Biol. Chem. 267:19978-19985. [PubMed] [Google Scholar]
- 28.Kapatral, V., I. Anderson, N. Ivanova, G. Reznik, T. Los, A. Lykidis, A. Bhattacharyya, A. Bartman, W. Gardner, G. Grechkin, L. Zhu, O. Vasieva, L. Chu, Y. Kogan, O. Chaga, E. Goltsman, A. Bernal, N. Larsen, M. D'Souza, T. Walunas, G. Pusch, R. Haselkorn, M. Fonstein, N. Kyrpides, and R. Overbeek. 2002. Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. J. Bacteriol. 184:2005-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolenbrander, P. E., R. N. Andersen, D. S. Blehert, P. G. Egland, J. S. Foster, and R. J. Palmer, Jr. 2002. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 66:486-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krstulovic, A. M., and C. Matzura. 1979. Rapid assay for tryptophanase using reversed-phase high-performance liquid chromatography. J. Chromatogr. 176:217-224. [DOI] [PubMed] [Google Scholar]
- 31.Ku, S. Y., P. Yip, and P. L. Howell. 2006. Structure of Escherichia coli tryptophanase. Acta Crystallogr. D Biol. Crystallogr. 62:814-823. [DOI] [PubMed] [Google Scholar]
- 32.Kulikova, V. V., L. N. Zakomirdina, I. S. Dementieva, R. S. Phillips, P. D. Gollnick, T. V. Demidkina, and N. G. Faleev. 2006. Tryptophanase from Proteus vulgaris: the conformational rearrangement in the active site, induced by the mutation of tyrosine 72 to phenylalanine, and its mechanistic consequences. Biochim. Biophys. Acta 1764:750-757. [DOI] [PubMed] [Google Scholar]
- 33.Lark, R. L., S. A. McNeil, K. VanderHyde, Z. Noorani, J. Uberti, and C. Chenoweth. 2001. Risk factors for anaerobic bloodstream infections in bone marrow transplant recipients. Clin. Infect. Dis. 33:338-343. [DOI] [PubMed] [Google Scholar]
- 34.Lee, J., A. Jayaraman, and T. K. Wood. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 182:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin, K., G. Morlin, A. Smith, A. Nordyke, A. Eisenstark, and M. Golomb. 1998. The tryptophanase gene cluster of Haemophilus influenzae type b: evidence for horizontal gene transfer. J. Bacteriol. 180:107-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martino, P. D., R. Fursy, L. Bret, B. Sundararaju, and R. S. Phillips. 2003. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can. J. Microbiol. 49:443-449. [DOI] [PubMed] [Google Scholar]
- 38.Martius, J., and D. A. Eschenbach. 1990. The role of bacterial vaginosis as a cause of amniotic fluid infection, chorioamnionitis and prematurity—a review. Arch. Gynecol. Obstet. 247:1-13. [DOI] [PubMed] [Google Scholar]
- 39.Moore, W. E., and L. V. Moore. 1994. The bacteria of periodontal diseases. Periodontol. 2000 5:66-77. [DOI] [PubMed] [Google Scholar]
- 40.Morino, Y., and E. E. Snell. 1970. Tryptophanase (Escherichia coli B). Methods Enzymol. 17A:439-446. [Google Scholar]
- 41.Mueller, R. S., S. Beyhan, S. G. Saini, F. H. Yildiz, and D. H. Bartlett. 2009. Indole acts as an extracellular cue regulating gene expression in Vibrio cholerae. J. Bacteriol. 191:3504-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano, Y., Y. Yoshida, Y. Yamashita, and T. Koga. 1995. Construction of a series of pACYC-derived plasmid vectors. Gene 162:157-158. [DOI] [PubMed] [Google Scholar]
- 43.Newton, W. A., Y. Morino, and E. E. Snell. 1965. Properties of crystalline tryptophanase. J. Biol. Chem. 240:1211-1218. [PubMed] [Google Scholar]
- 44.Newton, W. A., and E. E. Snell. 1964. Catalytic properties of tryptophanase, a multifunctional pyridoxal phosphate enzyme. Proc. Natl. Acad. Sci. U. S. A. 51:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pace, C. N., F. Vajdos, L. Fee, G. Grimsley, and T. Gray. 1995. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 4:2411-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips, R. S., and P. D. Gollnick. 1989. Evidence that cysteine 298 is in the active site of tryptophan indole-lyase. J. Biol. Chem. 264:10627-10632. [PubMed] [Google Scholar]
- 47.Qi, M., K. E. Nelson, S. C. Daugherty, W. C. Nelson, I. R. Hance, M. Morrison, and C. W. Forsberg. 2005. Novel molecular features of the fibrolytic intestinal bacterium Fibrobacter intestinalis not shared with Fibrobacter succinogenes as determined by suppressive subtractive hybridization. J. Bacteriol. 187:3739-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren, D., L. A. Bedzyk, S. M. Thomas, R. W. Ye, and T. K. Wood. 2004. Gene expression in Escherichia coli biofilms. Appl. Microbiol. Biotechnol. 64:515-524. [DOI] [PubMed] [Google Scholar]
- 49.Shine, J., and L. Dalgarno. 1974. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. U. S. A. 71:1342-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snell, E. E. 1975. Tryptophanase: structure, catalytic activities, and mechanism of action. Adv. Enzymol. Relat. Areas Mol. Biol. 42:287-333. [DOI] [PubMed] [Google Scholar]
- 51.Stewart, V., R. Landick, and C. Yanofsky. 1986. Rho-dependent transcription termination in the tryptophanase operon leader region of Escherichia coli K-12. J. Bacteriol. 166:217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart, V., and C. Yanofsky. 1985. Evidence for transcription antitermination control of tryptophanase operon expression in Escherichia coli K-12. J. Bacteriol. 164:731-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart, V., and C. Yanofsky. 1986. Role of leader peptide synthesis in tryptophanase operon expression in Escherichia coli K-12. J. Bacteriol. 167:383-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tso, W. W., and J. Adler. 1974. Negative chemotaxis in Escherichia coli. J. Bacteriol. 118:560-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, D., X. Ding, and P. N. Rather. 2001. Indole can act as an extracellular signal in Escherichia coli. J. Bacteriol. 183:4210-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe, T., and E. E. Snell. 1977. The interaction of Escherichia coli tryptophanase with various amino and their analogs. Active site mapping. J. Biochem. 82:733-745. [DOI] [PubMed] [Google Scholar]
- 57.Yanofsky, C. 2007. RNA-based regulation of genes of tryptophan synthesis and degradation, in bacteria. RNA 13:1141-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshida, H., H. Kumagai, and H. Yamada. 1974. Crystalline holotryptophanase from Proteus rettgeri. FEBS Lett. 48:56-59. [DOI] [PubMed] [Google Scholar]
- 59.Yoshida, Y., Y. Nakano, A. Amano, M. Yoshimura, H. Fukamachi, T. Oho, and T. Koga. 2002. lcd from Streptoccus anginosus encodes a C-S lyase with α,β-elimination activity that degrades l-cysteine. Microbiology 148:3961-3970. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida, Y., M. Negishi, and Y. Nakano. 2003. Homocysteine biosynthesis pathways of Streptococcus anginosus. FEMS Microbiol. Lett. 221:277-284. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida, Y., T. Sasaki, S. Ito, H. Tamura, K. Kunimatsu, and H. Kato. 2009. Identification and molecular characterization of tryptophanase encoded by tnaA in Porphyromonas gingivalis. Microbiology 155:968-978. [DOI] [PubMed] [Google Scholar]
- 62.Zakomirdina, L. N., V. V. Kulikova, O. I. Gogoleva, I. S. Dementieva, N. G. Faleev, and T. V. Demidkina. 2002. Tryptophan indole-lyase from Proteus vulgaris: kinetic and spectral properties. Biochemistry (Mosc.) 67:1189-1196. [DOI] [PubMed] [Google Scholar]