Abstract
Actinobacteria, particularly bifidobacteria, are widely observed to be underrepresented in metagenomic studies of microbial communities. We have compared human fecal microbiota clone libraries based on 16S rRNA and cpn60 PCR products. Taxonomic profiles were similar except that the cpn60 libraries contained large numbers of bifidobacterial sequences.
Bifidobacteria are Gram-positive, high G+C members of the phylum Actinobacteria that are a focus of study for their role in intestinal metabolism as well as their potential as probiotics. Culture-based studies of mammalian intestinal and fecal microbiota consistently indicate the presence of large numbers of bifidobacteria, with viable counts of 108 to 1010 CFU/g in feces (3, 15, 16, 34).
The advent of sequence-based methods for studying microbial communities has led to characterizations of intestinal microbiota based on sequencing of cloned PCR amplicons derived from the 16S rRNA gene or, more recently, the direct sequencing of 16S rRNA PCR products using pyrosequencing methods. The results of these studies almost invariably provide a description of the distal intestinal or fecal microbiota as a population in which Firmicutes and Bacteroidetes dominate, accounting for as much as 98% of the observed sequences (7, 23, 24). These descriptions conflict with the results of investigations in which bifidobacteria are cultured from samples or detected using taxon-specific methods, such as fluorescent in situ hybridization (FISH) (22, 36) or taxon-specific PCR (34, 35). There are a few exceptions to the problem of “missing bifidobacteria” in metagenomic studies, and these have employed modified PCR protocols involving higher annealing temperatures (30) or “universal” 16S rRNA primers known to be more sensitive for Actinobacteria (2). PCR primer bias also has been observed as a problem in amplification of Actinobacteria sequences from environmental samples (13).
An alternative gene target for metagenomic studies of microbial communities is the cpn60 gene that encodes the universal 60-kDa chaperonin protein (also known as GroEL or Hsp60) (19). This target has been exploited in microbial ecology studies involving clone libraries of PCR products (6, 10, 11, 17, 18, 20, 28), pyrosequencing of PCR amplicons (31), and quantitative real-time PCR (4, 9). Recently, we published a modification of the cpn60 universal primer PCR protocol which includes the use of a cocktail of degenerate primers optimized to give proportional amplifications of sequences across a broad range of G+C contents (21). This approach has subsequently been used in the characterization of human vaginal microbiota (31) and feline fecal microbiota (6). Although in the feline study a large proportion of sequences observed corresponded to bifidobacteria, there is no corresponding 16S rRNA-based study of the samples for comparison. Here, we have applied a cpn60-based approach to the characterization of human fecal microbiota and conducted a direct comparison to 16S rRNA clone libraries generated from the same samples.
Fecal samples were collected from healthy adult volunteers (aged 18 to 65 years) enrolled in a study of the effects of whole chickpea or raffinose on intestinal health (14). Total genomic DNA was extracted from fecal samples as described previously (18) and pooled according to diet protocol (control [diet A], raffinose [diet B], and chickpea [diet C]) such that 12 individuals were represented in each diet library. cpn60 PCR was conducted as described previously (21). For 16S rRNA amplification, universal bacterial primers F1 (5′-GAGTTTGATCCTGGCTCAG-3′) and R2 (5′-GWATTACCGCGGCKGCTG-3′) (8) were used to amplify the region corresponding to nucleotides 11 to 536 of the Escherichia coli 16S rRNA gene. PCR amplifications were performed with 50-μl reaction mixtures containing 10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris HCl (pH 8.75), 0.1% Triton X-100, 0.1 mg/ml bovine serum albumin (BSA), 2 mM MgSO4, 0.2 mM deoxynucleoside triphosphates (dNTPs), 0.4 μM concentrations of each primer, and 1 U of Taq DNA polymerase (UBI Life Sciences, Calgary, AB, Canada) using an Eppendorf Mastercycler EP. The amplification program was 3 min at 95°C followed by 40 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s and a final extension of 10 min at 72°C. The resulting PCR products from each template pool were purified and ligated into cloning vector pGEM-T Easy (Promega). Ligation reactions were used to transform E. coli JM109-competent cells, and recombinants (672 per library) were picked, placed in 96-well plates, and sequenced with the T7 sequencing primer.
Sequences were processed and warehoused using Another Portal for Examining DNA (APED) (25) as described previously (6). For taxonomic identification, cpn60 nucleotide and translated peptide sequences were compared to the reference cpn60 sequence database within cpnDB (19) (http://cpndb.cbr.nrc.ca) using FASTA (29) and BLASTp (1). 16S rRNA sequences were identified using the Ribosomal Database Project (RDP) Classifier provided by the RDP (http://rdp.cme.msu.edu). Additionally, unique sequences identified from the cpn60 and 16S rRNA libraries were subjected to phylogenetic analysis to support their taxonomic identification (data not shown). A total of 1,763 cpn60 clones (618 from library A, 554 from library B, 591 from library C) and 1,314 16S rRNA clones (459 from library A, 440 from library B, 415 from library C) were included in the analysis. The number of cloned sequences available for each library was influenced by sequencing success rates, as only full-length, high-quality insertion sequences were included in the analysis. To compensate for library size variation, all comparisons were done based on the proportional representation of sequences in each library.
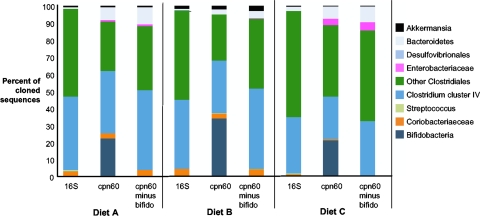
Figure 1 shows the proportional representation of the major taxonomic groups in the clone libraries. Differences in taxonomic profiles between the different diets are discussed in detail elsewhere (14). Firmicutes (including Streptococcus, Clostridium, Faecalibacterium, and Eubacterium) accounted for 93 to 96% of the sequences in the 16S rRNA clone libraries. Bacteroidetes comprised only 1 to 2% of the 16S rRNA libraries. This profile is similar to those previously reported for human fecal microbiota, for which the Firmicutes and Bacteroidetes combined account for up to 98% of sequences (24). Bacteroidetes were somewhat more abundant in the cpn60 libraries, where they accounted for 3 to 8% of the clones. The major difference between the libraries based on the two gene targets was in the Actinobacteria (including Coriobacterineae and Bifidobacterium). While sequences from the Coriobacterineae family constituted 0.5 to 4% of each of the libraries regardless of the gene target, Bifidobacterium sequences were detected in 16S rRNA libraries from diets B (0.5% of clones) and C (0.2% of clones) only. In the cpn60 libraries, Bifidobacterium sequences accounted for 22%, 34%, and 21% of libraries from diets A, B, and C, respectively. When bifidobacterial sequences are removed from each of the cpn60 libraries, their taxonomic profiles are very similar to those of the corresponding 16S rRNA libraries.
FIG. 1.
Proportional representation of major taxonomic groups in the PCR product clone libraries generated from three diets, based on either 16S rRNA or cpn60. Each pair of libraries was generated from the same sample pool and DNA extract, as described in the text. cpn60 libraries are shown with and without inclusion of bifidobacterial sequences.
Real-time PCR was used to quantify Clostridium cluster IV and Bifidobacterium spp. in the genomic DNA extracts used to create the PCR product libraries (26, 27). Similar quantities of the two groups were detected in all samples: 108.8 to 109.3 copies/g of feces for Clostridium cluster IV and 108.8 to 109.8 copies/g for Bifidobacterium spp. (14). Clostridium cluster IV is a group within the Firmicutes that includes Faecalibacterium prausnitzii, C. orbiscindens, C. leptum, and C. methylpentosum (5, 12, 32). This group accounted for 47%, 40%, and 33% of the cloned 16S rRNA sequences in libraries A, B, and C, respectively, and 47%, 47%, 32% of the corresponding nonbifidobacterial cpn60 sequences. However, despite similar abundances indicated by taxon-specific PCRs, only 0%, 0.5%, and 0.2% of 16S rRNA clones were from bifidobacteria, illustrating a gross underrepresentation of these organisms in the 16S rRNA clone libraries.
In a recent study of human intestinal microbiota in lean and obese twins that utilized deep pyrosequencing of multiple regions of the 16S rRNA gene, sequencing of full-length 16S rRNA amplicons, and shotgun metagenome sequencing, Turnbaugh et al. (33) found that in almost all samples, <10% of sequences were from Actinobacteria. However, they also determined that 75% of the obesity-enriched genes were from Actinobacteria. Given this observation and the acknowledged importance of bifidobacteria in health and nutrition, any assessment of intestinal or fecal microbiota would ideally be conducted with a method that allows detection and monitoring of this taxon. The potential biases of PCR primers must be carefully considered. None of the other targets that are routinely exploited for bacterial speciation, such as rpoB and gyrB, have been applied in metagenomic studies of microbial communities, so it is unknown if they would offer improved representation of bifidobacteria. Our experiment, in which we compared directly the microbial population profiles generated from three samples, demonstrates that the cpn60 target offers a useful alternative or complement to 16S rRNA and alleviates the “missing bifidobacteria” problem.
Nucleotide sequence accession numbers.
The 1,763 cpn60 clones and 1,314 16S rRNA clones analyzed were deposited in GenBank under accession numbers GQ178291 to GQ179638.
Acknowledgments
This study was supported by grants from Saskatchewan Pulse Growers and Pulse Canada.
Footnotes
Published ahead of print on 30 April 2010.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, A. F., M. Lindberg, H. Jakobsson, F. Backhed, P. Nyren, and L. Engstrand. 2008. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One 3:e2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benno, Y., K. Endo, K. Suzuki, T. Mitsuoka, and S. Namioka. 1985. Use of nonprotein nitrogen in pigs: effects of dietary urea on the intestinal microflora. Am. J. Vet. Res. 46:959-962. [PubMed] [Google Scholar]
- 4.Chaban, B., K. M. Musil, C. G. Himsworth, and J. E. Hill. 2009. Development of cpn60-based real-time quantitative PCR assays for the detection of 14 Campylobacter species and application to screening canine fecal samples. Appl. Environ. Microbiol. 75:3055-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, M. D., P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, and J. A. Farrow. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812-826. [DOI] [PubMed] [Google Scholar]
- 6.Desai, A. R., K. M. Musil, A. P. Carr, and J. E. Hill. 2009. Characterization and quantification of feline fecal microbiota using cpn60 sequence-based methods and investigation of animal-to-animal variation in microbial population structure. Vet. Microbiol. 137:120-128. [DOI] [PubMed] [Google Scholar]
- 7.Dethlefsen, L., P. B. Eckburg, E. M. Bik, and D. A. Relman. 2006. Assembly of the human intestinal microbiota. Trends Ecol. Evol. 21:517-523. [DOI] [PubMed] [Google Scholar]
- 8.Dorsch, M., and E. Stackebrandt. 1992. Some modifications in the procedure of direct sequencing of PCR amplified 16S rDNA. J. Microbiol. Methods 16:271-279. [Google Scholar]
- 9.Dumonceaux, T. J., J. E. Hill, S. A. Briggs, K. K. Amoako, S. M. Hemmingsen, and A. G. Van Kessel. 2006. Enumeration of specific bacterial populations in complex intestinal communities using quantitative PCR based on the chaperonin-60 target. J. Microbiol. Methods 64:46-62. [DOI] [PubMed] [Google Scholar]
- 10.Dumonceaux, T. J., J. E. Hill, S. M. Hemmingsen, and A. G. Van Kessel. 2006. Characterization of intestinal microbiota and response to dietary virginiamycin supplementation in the broiler chicken. Appl. Environ. Microbiol. 72:2815-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumonceaux, T. J., J. E. Hill, C. Pelletier, M. G. Paice, A. G. Van Kessel, and S. M. Hemmingsen. 2006. Molecular characterization of microbial communities in Canadian pulp and paper activated sludge and quantification of a novel Thiothrix eikelboomii-like bulking filament. Can. J. Microbiol. 52:494-500. [DOI] [PubMed] [Google Scholar]
- 12.Duncan, S. H., G. L. Hold, H. J. Harmsen, C. S. Stewart, and H. J. Flint. 2002. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 52:2141-2146. [DOI] [PubMed] [Google Scholar]
- 13.Farris, M. H., and J. B. Olson. 2007. Detection of Actinobacteria cultivated from environmental samples reveals bias in universal primers. Lett. Appl. Microbiol. 45:376-381. [DOI] [PubMed] [Google Scholar]
- 14.Fernando, W. M. U., J. E. Hill, G. A. Zello, R. T. Tyler, W. J. Dahl, and A. G. Van Kessel. Diets supplemented with chickpea or its main oligosaccharide component raffinose modify fecal microbial composition in healthy adults. Beneficial Microbes, in press. [DOI] [PubMed]
- 15.Gueimonde, M., S. Tolkko, T. Korpimaki, and S. Salminen. 2004. New real-time quantitative PCR procedure for quantification of bifidobacteria in human fecal samples. Appl. Environ. Microbiol. 70:4165-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartemink, R., and F. M. Rombouts. 1999. Comparison of media for the detection of bifidobacteria, lactobacilli and total anaerobes from faecal samples. J. Microbiol. Methods 36:181-192. [DOI] [PubMed] [Google Scholar]
- 17.Hill, J. E., S. H. Goh, D. M. Money, M. Doyle, A. Li, W. L. Crosby, M. Links, A. Leung, D. Chan, and S. M. Hemmingsen. 2005. Characterization of vaginal microflora of healthy, nonpregnant women by chaperonin-60 sequence-based methods. Am. J. Obstet. Gynecol. 193:682-692. [DOI] [PubMed] [Google Scholar]
- 18.Hill, J. E., S. M. Hemmingsen, B. G. Goldade, T. J. Dumonceaux, J. Klassen, R. T. Zijlstra, S. H. Goh, and A. G. Van Kessel. 2005. Comparison of ileum microflora of pigs fed corn-, wheat-, or barley-based diets by chaperonin-60 sequencing and quantitative PCR. Appl. Environ. Microbiol. 71:867-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill, J. E., S. L. Penny, K. G. Crowell, S. H. Goh, and S. M. Hemmingsen. 2004. cpnDB: a chaperonin sequence database. Genome Res. 14:1669-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill, J. E., R. P. Seipp, M. Betts, L. Hawkins, A. G. Van Kessel, W. L. Crosby, and S. M. Hemmingsen. 2002. Extensive profiling of a complex microbial community by high-throughput sequencing. Appl. Environ. Microbiol. 68:3055-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill, J. E., J. R. Town, and S. M. Hemmingsen. 2006. Improved template representation in cpn60 PCR product libraries generated from complex templates by application of a specific mixture of PCR primers. Environ. Microbiol. 8:741-746. [DOI] [PubMed] [Google Scholar]
- 22.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley, R. E., M. Hamady, C. Lozupone, P. J. Turnbaugh, R. R. Ramey, J. S. Bircher, M. L. Schlegel, T. A. Tucker, M. D. Schrenzel, R. Knight, and J. I. Gordon. 2008. Evolution of mammals and their gut microbes. Science 320:1647-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ley, R. E., D. A. Peterson, and J. I. Gordon. 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837-848. [DOI] [PubMed] [Google Scholar]
- 25.Links, M. G., E. L. McCarthy, and W. L. Crosby. 2009. Another Portal for Examining DNA: APED. http://sourceforge.net/projects/aped.
- 26.Matsuki, T., K. Watanabe, J. Fujimoto, Y. Miyamoto, T. Takada, K. Matsumoto, H. Oyaizu, and R. Tanaka. 2002. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 68:5445-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuki, T., K. Watanabe, J. Fujimoto, T. Takada, and R. Tanaka. 2004. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 70:7220-7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliver, K. L., R. C. Hamelin, and W. E. Hintz. 2008. Effects of transgenic hybrid aspen overexpressing polyphenol oxidase on rhizosphere diversity. Appl. Environ. Microbiol. 74:5340-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. U. S. A. 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudi, K., M. Zimonja, I. M. Aasen, S. H. Knutsen, and S. Sahlstrom. 2009. Novel 16S rRNA gene analyses reveal new in vitro effects of insoluble barley fibres on the human faecal microbiota. Lett. Appl. Microbiol. 48:433-439. [DOI] [PubMed] [Google Scholar]
- 31.Schellenberg, J., M. G. Links, J. E. Hill, T. J. Dumonceaux, G. A. Peters, S. Tyler, B. Ball, A. Severini, and F. A. Plummer. 2009. Pyrosequencing of the chaperonin-60 universal target for phylogenetic analysis of microbial communities. Appl. Environ. Microbiol. 75:2889-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stackebrandt, E., I. Kramer, J. Swiderski, and H. Hippe. 1999. Phylogenetic basis for a taxonomic dissection of the genus Clostridium. FEMS Immunol. Med. Microbiol. 24:253-258. [DOI] [PubMed] [Google Scholar]
- 33.Turnbaugh, P. J., M. Hamady, T. Yatsunenko, B. L. Cantarel, A. Duncan, R. E. Ley, M. L. Sogin, W. J. Jones, B. A. Roe, J. P. Affourtit, M. Egholm, B. Henrissat, A. C. Heath, R. Knight, and J. I. Gordon. 2009. A core gut microbiome in obese and lean twins. Nature 457:480-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turroni, F., E. Foroni, P. Pizzetti, V. Giubellini, A. Ribbera, P. Merusi, P. Cagnasso, B. Bizzarri, G. L. de'Angelis, F. Shanahan, D. van Sinderen, and M. Ventura. 2009. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl. Environ. Microbiol. 75:1534-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turroni, F., J. R. Marchesi, E. Foroni, M. Gueimonde, F. Shanahan, A. Margolles, D. van Sinderen, and M. Ventura. 2009. Microbiomic analysis of the bifidobacterial population in the human distal gut. ISME J. 3:745-751. [DOI] [PubMed] [Google Scholar]
- 36.Walker, A. W., S. H. Duncan, H. J. Harmsen, G. Holtrop, G. W. Welling, and H. J. Flint. 2008. The species composition of the human intestinal microbiota differs between particle-associated and liquid phase communities. Environ. Microbiol. 10:3275-3283. [DOI] [PubMed] [Google Scholar]