Abstract
Methylocella silvestris BL2, a facultative methane utilizer, can grow on monomethylamine (MMA) as a sole carbon and nitrogen source. No activity of MMA dehydrogenase was detectable. Instead, this bacterium utilizes a methylated amino acid pathway (γ-glutamylmethylamide [GMA] and N-methylglutamate [NMG]) for MMA metabolism. The activities of the two key enzymes in this pathway, GMA synthetase and NMG dehydrogenase, were found when the bacterium was grown on MMA. GMA was detected by high-performance liquid chromatography-mass spectrometry only when the bacterium was grown on MMA but not when it was grown on methanol. Proteomic analysis of soluble and membrane fractions of the proteome from MMA- and methanol-grown cultures revealed that an eight-gene cluster (Msil2632 to Msil2639) was induced by MMA and cotranscribed as an operon, as shown by reverse transcription-PCR. GMA-dissimilating enzyme activity was also detected when it was grown on MMA. Formaldehyde and ammonium production from GMA was dependent on glutamate but not on α-ketoglutarate. Marker exchange mutagenesis of a putative GMAS gene homologue (gmas, Msil2635) within this eight-gene cluster, with a kanamycin gene cassette, abolished growth of M. silvestris on MMA as either a sole carbon or a sole nitrogen source. Overall, our results suggest that gmas is essential in MMA metabolism by M. silvestris.
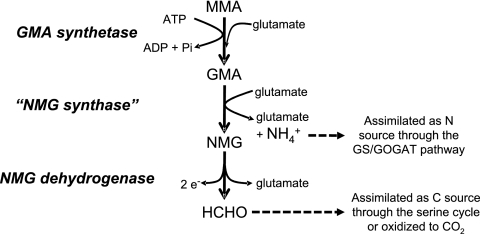
Monomethylamine (MMA) is ubiquitous in the environment. For example, putrefaction of proteins (14a, 17) and degradation of many nitrogen-containing pesticides and herbicides can release MMA (5, 16b, 18). In the marine environment, MMA is released from the degradation of quaternary amines, such as betaine, carnitine, choline, and trimethylamine N-oxide, which are used as osmolytes by many marine organisms (3, 6). Once released, MMA can be used by some microorganisms as a sole carbon and nitrogen source through different pathways. Methanogenic archaea, such as Methanosarcina and Methanomicrobium, can use MMA anaerobically as a substrate to produce methane via a methyltransferase system (28). Gram-positive bacteria, such as Arthrobacter, metabolize MMA aerobically via an oxidase, which breaks down MMA into formaldehyde and ammonium (39). Gram-negative bacteria such as Methylobacterium extorquens and Paracoccus denitrificans utilize MMA dehydrogenase, a multisubunit enzyme that generates formaldehyde and ammonium from MMA aerobically (9, 16). Many other Gram-negative bacteria, such as Aminobacter aminovorans (previously known as strain MA and strain MS), can use MMA as a sole carbon and nitrogen source aerobically; however, they lack MMA dehydrogenase. It has been shown that in these microorganisms, two unusual amino acids, γ-glutamylmethylamide (GMA) and N-methylglutamate (NMG), are involved in MMA metabolism (1, 23, 33). In strain MA, an enzyme proposed as “NMG synthase” (“NMGS”) converted MMA to NMG, which was subsequently oxidized to formaldehyde, regenerating glutamate (Glu) by a membrane-bounded particulate, NMG dehydrogenase (NMGDH) (33). The reactions carried out by these enzymes are summarized below. In strain MS, GMA was found to be a key metabolite in MMA metabolism (23). The synthesis of GMA was proposed to be carried out by a glutamine synthetase-like protein, and the reaction was dependent on ATP and Mg2+. However, the fate of GMA in such a GMA-dependent MMA pathway is not clear.
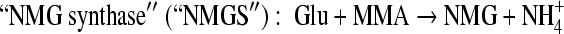 |
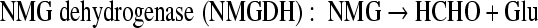 |
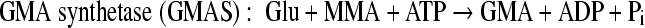 |
Although enzymes in these methylated amine-mediated pathways have been purified earlier, such as “NMGS” (31), NMGDH (2), and GMAS (21, 25, 37), the genes involved in these pathways have been studied only recently (24, 38).
Methylocella silvestris BL2 is a facultative methane-oxidizing bacterium belonging to the Alphaproteobacteria (11, 13, 35). It can grow on methylamine as a sole carbon and nitrogen source. Here we report the characterization of a gene cluster in M. silvestris BL2 involved in MMA metabolism and demonstrate that gmas is essential in MMA metabolism by this bacterium.
MATERIALS AND METHODS
Cultivation of Methylocella silvestris.
Cells were grown at 25°C in a fed-batch mode in a 5-liter fermentor using diluted nitrate mineral (DNMS) medium, as described by Theisen and colleagues (35). A total of 10 mM methylamine hydrochloride or 10 mM methanol plus 2 mM NH4Cl was used as a carbon and nitrogen source. Cells were harvested at late exponential phase, resuspended in 10 mM 1,4-piperazinediethanesulfonic acid (PIPES) buffer (pH 7.6), and then stored at −80°C. To test whether sarcosine could be used as a substrate for M. silvestris, 10 mM sarcosine was used with or without 10 mM methanol.
To test if the gmas mutant could grow on MMA, a nitrogen-free DNMS medium was used. MMA was added at 5, 10, or 20 mM as the sole carbon and nitrogen source. A control, wild-type M. silvestris, was grown on either DNMS medium with methanol or nitrogen-free DNMS medium with 10 mM MMA. All growth experiments were set up in triplicate using 120-ml serum vials containing 20 ml medium, with an inoculum size of 5%. The serum vials were incubated at 25°C in a shaker (150 rpm).
HPLC-MS analyses of GMA.
GMA was extracted from 100 μl frozen cells by vigorously vortexing them in 1 ml methanol for 1 min. Cells were then removed by centrifugation. The supernatant was heated at 60°C to remove methanol by evaporation. The residual powder was dissolved in 20 μl of water, and the solution was kept at −20°C prior to mass spectrometry (MS) analyses, which were carried out using an Agilent 1100 high-performance liquid chromatography (HPLC) system, with a diode array detector (DAD) coupled to a Bruker HCTplus (high-capacity ion trap) mass spectrometer. The HPLC column was obtained from Agilent (Zorbax reverse-phase HPLC column Rx-C18, 150 by 4.6 mm and particle size of 5 μm with C18 guard cartridge). Samples were filtered through a 0.2-μm microspin filter before injection. The mobile phases used were water with 0.1% trifluoroacetic acid (TFA) (A) and methanol with 0.1% TFA (B). Gradient settings used were 0 to 2 min at 100% A, 2 to 25 min from 100% A to 100% B, 25 to 30 min at 100% B, 30 to 35 min of equilibration to 100% A, and 35 to 45 min at 100% A. The flow rate was 1 ml min−1, and 10% of the flow was diverted to MS. The DAD setting was 210 nm, 254 nm, and 280 nm. The MS setting was full scan, with electrospray ionization in positive mode, nebulizer gas at 10 liters min−1, dry gas at 300°C and 40 lb/in2, and capillary exit at 65 V. GMA was identified based on the elution time, and molecular mass was compared to synthesized standards, which were prepared and purified according to a previously published method (27).
Protein analyses.
Cells were broken for protein analyses and enzyme assays by passing them three times through a French pressure cell (American Instrument Co.) at 110 MPa. Cell debris was removed by centrifugation at 6,000 × g for 15 min. Where necessary, soluble and membrane protein fractions were further separated by ultracentrifugation at 150,000 × g for 2 h, and the membrane fraction was washed once with buffer (10 mM PIPES, pH 7.6), followed by ultracentrifugation for 1 h under the same conditions. Protein concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad). One-dimensional protein analyses were carried out using a precast NuPage Bis-Tris gel (10%), according to the manufacturer's protocol (Invitrogen). Gels were stained with Coomassie brilliant blue R-250, and bands were excised, digested with trypsin, and analyzed using matrix-assisted laser desorption ionization-mass spectrometry (MALDI-MS) and tandem mass spectrometry at the Biological Mass Spectrometry and Proteomics Facility, Department of Biological Sciences, University of Warwick, as described previously (32).
RNA extraction and reverse transcription (RT)-PCR.
RNA was extracted from frozen cells using either an RNeasy mini kit (Qiagen) or the method described by Gilbert and colleagues (14). Trace DNA contaminants in the RNA were further removed by DNase I (Qiagen) digestion, and RNA was then recovered using the RNeasy minikit (Qiagen). Reverse transcription was performed using the SuperScript II system (Invitrogen). Reverse transcription and PCR were carried out using a Bio-Rad thermocycler. Primers used are listed in Table 1.
TABLE 1.
List of oligonucleotides used in this study
| Target | Sequence (5′-3′) |
Product size (bp) | Annealing temp (°C) | |
|---|---|---|---|---|
| Forward primer | Reverse primer | |||
| Msil2632 | GCGGCATAGTTGGATTGTTT | CATTCCAGCAAGATCGAACC | 412 | 55 |
| Msil2633 | CTGCGAAAATCTTCGACCTC | GATCGAGCCTTTGACGAGAA | 400 | 55 |
| Msil2634 | CTGAAAATTCCCGTCACCAT | TTCTCCCAATCGGTGATTTC | 421 | 55 |
| Msil2635 | CAAAGAGTGTGGCGTCAAGA | TCTCGGTGATGACGTCGTAG | 460 | 55 |
| Msil2636 | CGTGCTCAATCTCTGCCATA | GAATCCCTTGGTGGTTTCAA | 327 | 55 |
| Msil2638 | GGCCGCAATATTTCCCTATT | TCGCAGATTGTGATCTCAGC | 510 | 60 |
| Msil2639 | GACCGTGTTTGACGTCTCG | TCCGTCAACGCATGATAGAG | 250 | 60 |
| Msil2632/Msil2633 | CCGATCAATATGTCGCCTTC | TGACGTCGCCTTTCACATAG | 423 | 55 |
| Msil2633/Msil2634 | GCGCCTTGTATCATTTCCAC | GCGCTTGGAGATTTTCTGAC | 598 | 55 |
| Msil2634/Msil2635 | CGCCTTGCAAACTACCTTTC | TATCAAGCCATGTCGCAAAG | 411 | 55 |
| Msil2635/Msil2636 | CATCAGGAATGGCAGGATTT | CGAATTCCTTGGCGAGATAA | 333 | 55 |
| Msil2636/Msil2638 | AATCGAAGGGCTGTTCTTCA | CGTGTCGCCGAAATATCC | 591 | 60 |
| Msil2638/Msil2639 | CGCTGAACGATCGCAAAC | GAGCGACGCCAACAGCTC | 547 | 60 |
| Construction of gmas mutant | CCTGAGAATTCATGATCATGTCGGCATCC | CTGGAAGGATCCTCAGCAGTCGAGCGTCTGCTC | 1,308 | 52 |
| Amplification of kan gene cassette | GGTAGGTCGACGCATGCGAGCTCGGAAAGCCACGTTGTGTCTC | GGTAGGTCGACGCATGCGAGCTCAAGGTGTTGCTGACTCATAC | 1,184 | 55 |
| Confirmation of gmas::kan mutant | GCCTTGCAAACTACCTTTCG | CGAATTCCTTGGCGAGATAA | 2,591 (for mutant), 1,665 (for wild type) | 55 |
Enzyme assays.
All enzyme assays were carried out in triplicate using cell-free crude extract in 10 mM PIPES buffer (pH 7.6) at room temperature (22°C), unless otherwise stated. GMA synthetase (MMA + glutamate + ATP → ADP + Pi + GMA) activity was measured by quantifying the production of γ-glutamylhydroxamate from glutamate and hydroxylamine in the presence of ATP and Mg2+ by monitoring the change in absorbance at 540 nm. The assay was originally developed to quantify the ability of glutamine synthetase to form glutamine (4) but later adapted to quantify GMAS activity (24, 37). The following concentrations of substrates were used: glutamate (50 mM), hydroxylamine hydrochloride (15 mM), MgCl2 (20 mM), and ATP (5 mM).
NMGDH activity was measured using 2,6-dichlorophenolindophenol (DCPIP) as an artificial electron acceptor with or without KCN (1 mM), as described by Bamforth and Large (2). The initial rate of decrease in A600 was recorded continuously for 5 min (within the linear phase). MMA dehydrogenase was assayed in the same way, except that methylamine hydrochloride (final concentration, 10 mM) was used as the substrate to initiate the reaction. In addition, NMGDH activity was also measured by quantifying NMG-dependent formaldehyde production using the method described below.
The activity of GMA dissimilation enzymes was measured using the following two methods: (i) GMA-dependent formaldehyde production and (ii) GMA-dependent ammonium production. Formaldehyde concentration was determined colorimetrically using 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Purpald reagent; Sigma). The reaction was started by adding GMA, and absorbance at 550 nm was recorded before and after incubation for 10 min to measure the initial rate of conversion within the linear phase (12). Prior to assaying ammonium, the cell-free enzyme extract was dialyzed to remove ammonium and MMA from the culture using a Slide-A-Lyzer 3.5-kDa-molecular-mass-cutoff dialysis cassette (Thermo Scientific), dialyzing for 2 h against 50 mM PIPES buffer (1 liter, pH 7.6) at 4°C. GMA-dependent ammonium released before and after 30 min of incubation was determined colorimetrically using Nessler's reagent (Sigma), measuring the change of absorbance at 440 nm (7). A decrease in absorbance was observed when α-ketoglutarate was present in the assay. This was due to the glutamine synthetase/glutamate synthase activity in the presence of residual ammonium after dialysis (data not shown).
“NMGS” activity was measured indirectly by quantifying glutamate and MMA-dependent formaldehyde production as described previously (24). The assay was initiated by adding either glutamate (final concentration, 10 mM) or MMA (final concentration, 10 mM).
Bioinformatics.
Sequence similarity searches were performed with GenBank using the BLASTP program against the nonredundant protein sequence database and the Swiss-Prot protein sequence database. Protein sequences of type I, type II, and type III glutamine synthetases were downloaded from GenBank and aligned using the ClustalX program (36). Phylogenetic analysis was performed using the MEGA4 program (34).
Marker exchange mutagenesis of gmas.
To construct a gmas mutant of M. silvestris, the gmas gene was amplified by PCR and inserted into pUC19 under the EcoRI and BamHI sites. The 258-bp SacI fragment from the gmas gene was then removed and replaced with a kanamycin gene cassette, which was amplified from the plasmid pCM184 by PCR using the primers listed in Table 1 (30). The plasmid was cut with EcoRI and BamHI, and the 2.2-kb fragment containing the kanamycin gene cassette was then gel purified and electroporated into M. silvestris using the method of Kim and Wood (19), with minor modifications. A total of 500 ng of DNA was added to 100 μl of cells. The electroporation settings used were resistance at 400 Ω, voltage at 2.2 kv, and a cuvette of 1 mm. Cells were recovered at 25°C overnight with DNMS medium containing methanol (10 mM) before plating. Potential mutants were selected on DNMS (20 μg ml−1 Kan) agar plates, with methanol (10 mM) as the sole carbon source. Mutation of the gmas gene was confirmed by diagnostic PCR and subsequent DNA sequencing.
RESULTS
MMA metabolism by Methylocella silvestris involves GMA.
M. silvestris can utilize MMA as the sole carbon and nitrogen source (13). Its genome is available in GenBank (accession number CP001280), and the genome does not contain any candidate coding sequences for MMA oxidase or MMA dehydrogenase. In addition, dye-linked MMA dehydrogenase assays were performed, and no activity was found using cell extract from cells grown on MMA as a sole carbon and nitrogen source.
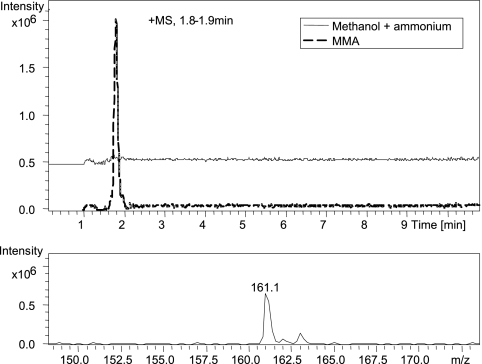
Experiments were then carried out to investigate if M. silvestris used the methylated amino acid pathway for MMA metabolism. HPLC-MS analysis showed that when M. silvestris was grown on MMA, GMA could be detected, whereas no GMA was found when M. silvestris was grown on methanol (Fig. 1). No NMG was detected in either MMA-grown cultures or methanol-grown cultures by HPLC-MS analysis. Assays were then carried out to determine activities of key enzymes involved in the GMA- and NMG-mediated metabolic pathways (Table 2). The γ-glutamylhydroxamate assay showed that GMAS/glutamine synthetase activities were approximately 3-fold higher in MMA-grown cultures than in methanol-grown cultures. The GMAS/glutamine synthetase activity detected by the γ-glutamylhydroxamate assay in methanol-grown cultures was due to glutamine synthetase rather than GMAS, since GMAS is not expressed under these conditions (see below). Furthermore, assays for NMGDH were carried out using two methods. Dye-linked NMGDH activity was found only when the bacterium was grown on MMA and not when it was grown on methanol plus ammonium. This activity was relatively low (4.4 ± 0.6 nmol min−1 mg protein−1). Dye-linked NMGDH activity was approximately 4-fold lower without KCN (final concentration, 1 mM), added in order to inhibit cytochrome oxidases, as suggested by Bamforth and Large (2). A more robust assay for NMGDH was used to quantify the production of formaldehyde from NMG. This activity was, again, detectable only in MMA-grown cultures but was higher (16.6 ± 0.7 nmol min−1 mg protein−1) than that obtained using the dye-linked NMGDH assay, indicating that DCPIP was a poor artificial electron acceptor for this enzyme. Assays using cytochrome c from horse heart did not yield any activity for NMGDH from M. silvestris. In addition, no activity of “NMGS” was detected in either MMA-grown or methanol-grown cultures using the method described by Latypova and colleagues (24).
FIG. 1.
(Top) Comparison of extracted ion chromatograms (EIC; m/z 161) of methanol extract of Methylocella silvestris cells grown on methylamine (dashed line) and methanol plus ammonium (solid line). (Bottom) The peak identified with a mass-to-charge ratio of 161.1 is γ-glutamylmethylamide.
TABLE 2.
Key enzyme activities in cells grown on MMA or methanol
| Assay | Activity (nmol min−1 mg protein−1) in cells grown ona: |
|
|---|---|---|
| MMA | Methanol plus ammonium | |
| GMAS | 31.1 ± 0.4 | 11.9 ± 0.9 |
| NMGDH | ||
| DCPIP | 4.4 ± 0.6 | ND |
| Formaldehyde | 16.0 ± 0.7 | ND |
| GMA dissimilation activity | ||
| Formaldehyde | 4.74 ± 0.12 | ND |
| Ammonium | 7.98 ± 0.49 | ND |
Values are means ± standard deviations from three assays. ND, not detectable.
Identification and characterization of a gene cluster involved in MMA metabolism.
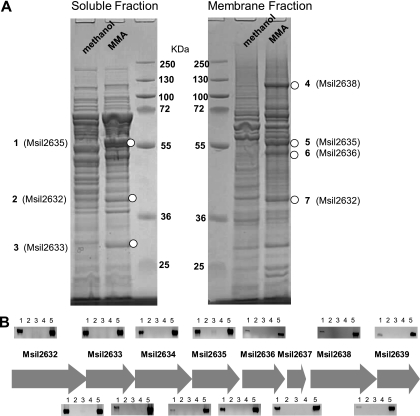
A proteomic analysis was carried out to investigate the proteins involved in MMA metabolism by M. silvestris. By comparing polypeptide profiles of cells grown on methanol or MMA, it was obvious that several proteins were highly induced by MMA in both the soluble fraction and membrane fraction of MMA-grown cells (Fig. 2 A). These polypeptides were cut from the gel and sequenced by tandem mass spectrometry. The results indicated that proteins encoded by a gene cluster were highly induced in MMA-grown cells and were, therefore, likely to be involved in MMA metabolism by M. silvestris (Table 3; Fig. 2A).
FIG. 2.
(A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses of soluble and membrane proteins from cells grown on MMA alone and methanol plus ammonium. Bands highlighted (1 to 7) were cut from the gel and analyzed by matrix-assisted laser desorption ionization-mass spectrometry and tandem mass spectrometry. (B) Agarose gel photos of RT-PCR products showing the eight-gene cluster, which was induced by MMA. Agarose gel photos above each gene show the induction of each gene by MMA only, and gel photos above intergenic regions show that this gene cluster is cotranscribed. RT-PCR products using mRNA extracted from MMA-grown cells and methanol-grown cells are shown in lanes 1 and 3, respectively. Corresponding controls without reverse transcriptase are shown in lanes 2 and 4, respectively. Lane 5 is the positive control for PCR using genomic DNA from Methylocella silvestris.
TABLE 3.
Identification of polypeptides induced by MMA
| Band | ORF identification | Calculated molecular mass from protein sequence (kDa) | No. of polypeptides detected | Sequence coverage (%) |
|---|---|---|---|---|
| 1 | Msil2635 | 48.4 | 5 | 21 |
| 2 | Msil2632 | 32.0 | 5 | 21 |
| 3 | Msil2633 | 24.7 | 4 | 24 |
| 4 | Msil2638 | 104.3 | 7 | 10 |
| 5 | Msil2635 | 48.4 | 8 | 17 |
| 6 | Msil2636 | 44.8 | 6 | 18 |
| 7 | Msil2632 | 32.0 | 4 | 20 |
The results from BLAST searches for each gene in this cluster are shown in Table 4. The first three open reading frames (ORFs; Msil2632 to Msil2634) encode polypeptides with high similarities to individual domains of the glutamate synthase large subunit, containing a glutamine amidotransferase domain, a GXGXG motif, and a highly conserved flavin mononucleotide (FMN)-binding domain, respectively. All the BLASTP hits matched with significant identities are putative genes with no known experimentally validated functions. ORF Msil2635 encodes a glutamine synthetase-like protein; however, sequence analysis indicated that this gene is lacking a conserved ammonia-binding site (D50, S53, and Y179), which is commonly found in glutamine synthetases (26). This gene also shows 41% identity to a newly characterized GMAS from Methylovorus mays (38) (Fig. 3). In both Msil2635 and GMAS from Methylovorus mays, the three key ammonium-binding residues are nonpolar rather than polar (D50 → G50, S53 → A53, and Y179 → C179). The last four ORFs in this gene cluster showed 35 to 75% sequence identities to putative heterotetrameric sarcosine oxidase subunits. Msil2636 and Msil2638 had 36% and 49% identity, respectively, to characterized sarcosine oxidase subunits of Corynebacterium sp. P-1 (10). However, when tested, M. silvestris failed to grow on sarcosine supplied as either a sole carbon source or a sole nitrogen source.
TABLE 4.
Gene description and BLAST search results
| ORF | Length (aa) | Top BLASTP match (identity [%]) | Gene assignmenta |
Note | |
|---|---|---|---|---|---|
| a | b | ||||
| Msil2632 | 299 | Glutamine amidotransferase, class II (Azorhizobium caulinodans ORS 571 [67]) | gltB1 | mgsA | Contains a GlxB-like glutamine amidotransferase domain |
| Glutamine amidotransferase, class II (Xanthobacter autotrophicus Py2 [65]) | |||||
| Msil2633 | 235 | Glutamate synthase domain 3-like protein (Methylobacterium populi BJ001 [72]) | gltB3 | mgsB | Contains a GXGXG motif commonly found in glutamate synthase |
| Glutamate synthase family protein (Xanthobacter autotrophicus Py2 [66]) | |||||
| Msil2634 | 444 | Putative glutamate synthase GltB2 subunit (Methylobacillus flagellatus KT [87]) | gltB2 | mgsC | Contains a highly conserved FMN-binding domain in GltS |
| Glutamate synthase domain 2-like protein (Azorhizobium caulinodans ORS 571 [86]) | |||||
| Msil2635 | 435 | Putative type III glutamine synthetase (Rhodopseudomonas palustris HaA2 [69]) | gltIII | gmas | Does not contain key residues for ammonium binding |
| γ-Glutamylmethylamide synthetase (Methylovorus mays [41]) | |||||
| Msil2636 | 416 | Putative sarcosine oxidase β subunit (Azorhizobium caulinodans ORS 571 [75]) | soxB | mgdA | |
| Sarcosine oxidase β subunit (Corynebacterium sp. P-1 [49]) | |||||
| Msil2637 | 95 | Putative sarcosine oxidase, δ subunit (Bradyrhizobium sp. BTAi1 [55]) | soxD | mgdB | |
| Sarcosine oxidase δ subunit (Corynebacterium sp. P-1 [37]) | |||||
| Msil2638 | 984 | Putative sarcosine oxidase α subunit (Methylobacterium populi BJ001 [55]) | soxA | mgdC | Contains two domains of the glycine cleavage T-protein |
| Sarcosine oxidase α subunit (Corynebacterium sp. P-1 [36]) | |||||
| Msil2639 | 209 | Putative sarcosine oxidase γ subunit (Rhodopseudomonas palustris HaA2 [37]) | soxG | mgdD | |
| Sarcosine oxidase γ subunit (Corynebacterium sp. P-1 [31]) | |||||
a, assigned based on BLASTP matches; b, assigned based on the similarity between Msil2632 to Msil2639 and the gene cluster of Methyloversatilis universalis (24).
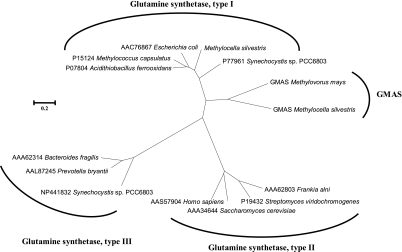
FIG. 3.
An unrooted phylogenetic tree showing the relationship between γ-glutamylmethylamide synthetases (GMAS) and three types of glutamine synthetases. Amino acid sequences were aligned using the ClustalX program, and the tree was constructed using MEGA4 (∼350 amino acids [aa] for γ-glutamylmethylamide synthetases, ∼370 aa for type I glutamine synthetases, ∼335 aa for type II glutamine synthetases, and ∼440 aa for type III glutamine synthetases). The scale bar represents 2 substitutions per 10 amino acids.
To further investigate whether this gene cluster is induced by MMA, transcriptional analyses were performed with RNA extracted from MMA- and methanol-grown cultures. The results (Fig. 2B) indicated that the genes in this cluster were induced by MMA, whereas no transcripts could be detected in methanol-grown cells. Furthermore, RT-PCR targeting the intergenic region yielded products of the expected sizes, indicating that the genes were cotranscribed as a single operon.
The activity of the GMA-dissimilating enzyme is dependent on glutamate.
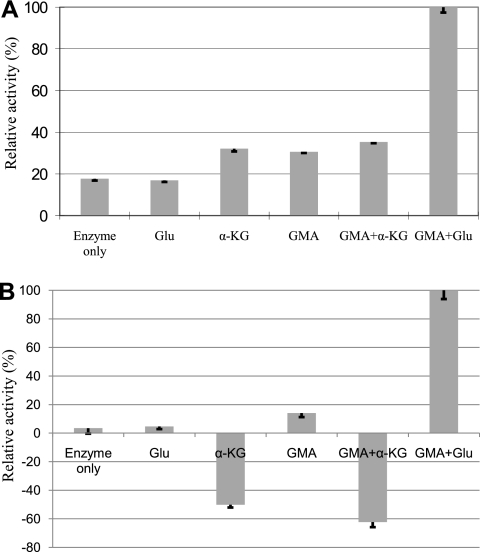
The fate of GMA in microorganisms which use the methylated amine pathway for MMA metabolism is controversial. We therefore decided to further explore whether GMA could be dissimilated by M. silvestris. The results are shown in Fig. 4. Formaldehyde production was seen when GMA was incubated with cell-free crude extract, and this was stimulated by glutamate. Ammonium production from GMA was also observed. This was also dependent on glutamate. These experiments indicated that GMA, via the presence of glutamate and release of ammonium, may be transformed into NMG, which is further oxidized by NMG dehydrogenase to yield formaldehyde.
FIG. 4.
GMA-dissimilating enzyme activities determined by quantifying GMA-dependent formaldehyde production (A) or ammonium production (B). The activities with GMA and Glu were chosen as 100%, which were 4.74 ± 0.12 nmol min−1 mg protein−1 for the formaldehyde assay and 7.98 ± 0.49 nmol min−1 mg protein−1 for the ammonium assay. Glu, glutamate; α-KG, α-ketoglutarate; GMA, γ-glutamylmethylamide. Means and standard deviations of the results from three replicates are shown.
A gmas mutant cannot grow on MMA as either a sole carbon source or a sole nitrogen source.
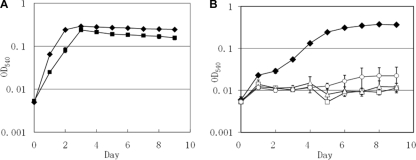
To investigate if the putative gmas gene, which was induced by MMA, is essential in MMA metabolism by M. silvestris, we constructed a mutant by marker exchange. This mutant can grow on methanol in the presence of ammonium but failed to grow on MMA as a sole carbon and nitrogen source at 5, 10, or 20 mM (Fig. 5). In addition, unlike wild-type M. silvestris, this mutant cannot grow on MMA as a sole nitrogen source in the presence of methanol using nitrogen-free DNMS medium (data not shown).
FIG. 5.
(A) Growth curves of the wild type (filled diamonds) and the gmas::kan mutant (filled squares) of Methylocella silvestris grown on methanol (10 mM) with ammonium (2 mM). (B) Growth curves of the wild type (filled diamonds, 10 mM) and the gmas::kan mutant (open triangles, 5 mM; open squares, 10 mM; open circles, 20 mM) of Methylocella silvestris grown on MMA alone at different concentrations. Means and standard deviations of the results from three replicates are shown. OD540, optical density at 540 nm.
DISCUSSION
Through comparative proteomic analyses, we revealed that a cotranscribed cluster of eight genes is involved in MMA metabolism by M. silvestris. Recently, a similar gene cluster was identified in Methyloversatilis universalis and was designated NMGS (mgsABC), GMAS, and NMGDH (mgdABCD), respectively (24). No attempt was made to elucidate the functions of putative mgsABC (Msil2632 to Msil2634) and mgdABCD (Msil2636 to Msil2639) identified in M. silvestris in this study; however, it is likely that they are NMGS and NMGDH, respectively.
GMA, which is induced by MMA, was found as an early product in the metabolism of MMA by Aminobacter aminovorans strain MS (23), suggesting a key role for GMA in MMA metabolism by this bacterium. It was also found that the enzyme, which was responsible for GMA synthesis, was similar to, but distinct from, glutamine synthetase. GMAS and glutamine synthetase from Aminobacter aminovorans strain MS were subsequently purified, and it was demonstrated that GMAS was more specific for GMA (25). In M. silvestris, a separate bona fide type I glutamine synthetase gene is present in the genome, and this enzyme is expressed in both methanol- and methylamine-grown cells (data not shown). The gmas homolog (Msil2635), which is induced by MMA (Fig. 2B) in M. silvestris, is likely to be GMAS. The translated amino acid of this protein showed high sequence identity to a characterized GMAS from Methylovorus mays (38), and phylogenetic analyses showed that these two proteins, which are related to type I glutamine synthetases, form a separate branch (Fig. 3). Moreover, similar to GMAS from Methylovorus mays, GMAS from M. silvestris lacks key residues that are crucial in the binding of ammonium, suggesting that these enzymes do not prefer ammonium and thus are likely to be GMAS rather than glutamine synthetase.
The fate of GMA in bacterial MMA metabolism is controversial. Latypova and colleagues found that GMA was a key intermediate in Methyloversatilis universalis when it was grown on MMA, but no GMA-dissimilating enzyme activity could be detected (24). Different observations were made in other studies. For example, using 14C-labeled GMA, Loginova and colleagues demonstrated that GMA was converted to NMG in Hyphomicrobium vulgare and that the reaction was dependent on glutamate (29). GMA dissimilation activity was also found in a Methylophaga strain, and the reaction was dependent on α-ketoglutarate and ammonium (20, 22). In M. silvestris, we were unable to show a direct conversion of GMA to NMG (data not shown); however, we demonstrated that there is glutamate-dependent, but not α-ketoglutarate-dependent, GMA-dissimilating enzyme activity which releases formaldehyde and ammonium from GMA (Fig. 4). This is similar to what has been found in Hyphomicrobium vulgare, where the conversion of GMA to NMG was also dependent on the presence of glutamate. The demonstration of α-ketoglutarate- plus ammonium-dependent, but not glutamate-dependent, GMA dissimilation enzyme activity in Methylophaga spp. is probably misleading, since the assay was carried out in a buffer containing 100 mM ammonium (20), which might have inhibited the glutamate-dependent GMA dissimilation activity. In fact, high glutamine synthetase/glutamate synthase activity was found in this bacterium when it was grown on MMA (20), and α-ketoglutarate and ammonium may have been converted to glutamate, which might have served as a true substrate for the GMA-dissimilating enzyme in this Methylophaga strain. GMAS in M. silvestris seems to be essential, and mutation of the corresponding gene (gmas homologue, Msil2635) resulted in a mutant which failed to grow on MMA as either a sole carbon source or a sole nitrogen source (Fig. 4B).
We therefore propose the pathway of MMA metabolism by M. silvestris shown in Fig. 6, in which MMA is metabolized, via GMA and NMG, to formaldehyde and ammonium. Formaldehyde produced is either further oxidized to CO2 to generate energy and reducing equivalents or assimilated into cell biomass, probably through the serine cycle. Ammonium produced in this pathway is used as a nitrogen source which is probably assimilated through bona fide glutamine synthetase and glutamate synthase (genes encoding glutamate dehydrogenase or alanine dehydrogenase are not present in the genome). However, we were unable to detect NMG from MMA-grown cells of M. silvestris. This is probably due to the fact that accumulation of GMA or NMG is related to growth state, as demonstrated previously (16a). Although it was suggested that GMA-dependent and NMG-dependent MMA metabolism by bacteria involved two different pathways (1, 24), our data and those of others suggest that it is more likely that these two may indeed be the same pathway (8, 29). It has been shown that glutamate-dependent ammonium and formaldehyde production from GMA also occurs in Aminobacter aminovorans strain MA when it is grown on MMA (8). This strain has been studied extensively, and it uses the NMG-mediated pathway for MMA metabolism (15, 31, 33). The NMG-mediated pathway for MMA metabolism was proposed, based mainly on studies using this strain. The key lies in whether GMA or MMA is the true substrate for this so-called “NMG synthase” (“NMGS”). The purified “NMGS” from strain MA is specific for glutamate but not for MMA, and indeed, a number of amines can substitute for MMA (31). There is, therefore, an urgent need to purify “NGMS” and to reexamine the specificity of this enzyme. Kinetic studies are also required to finally resolve the controversial roles of the GMA- and NMG-dependent pathways for MMA metabolism by bacteria.
FIG. 6.
Proposed pathway of MMA metabolism by Methylocella silvestris. Formaldehyde produced from this pathway is either assimilated as a carbon source through the serine cycle or further oxidized to CO2, and the ammonium produced is assimilated as a nitrogen source through the glutamine synthetase (GS)/glutamate synthase (GOGAT) pathway. e−, electron.
Acknowledgments
We thank M. Kalyuzhnaya, S. Yamamoto, S. Dedysh, P. Dunfield, D. P. Kelly, P. J. Large, and C. Anthony for advice on enzyme assays and helpful discussions and R. Boden for his advice on GMA synthesis. S. Slade is acknowledged for helping with the MALDI-MS analyses.
This work was supported by NERC research grant NE/E016855/1 to J. C. Murrell. We are grateful to the Joint Genome Institute for providing the genome sequence of M. silvestris BL2 (community sequencing program 2007; ID 4023905).
Footnotes
Published ahead of print on 14 May 2010.
REFERENCES
- 1.Anthony, C. 1982. The biochemistry of methylotrophs. Academic Press, London, United Kingdom.
- 2.Bamforth, C. W., and P. J. Large. 1977. Solubilization, partial purification and properties of N-methylglutamate dehydrogenase from Pseudomonas aminovorans. Biochem. J. 161:357-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, E. L., and H. S. Kwan. 1985. Bacterial reduction of trimethylamine oxide. Annu. Rev. Microbiol. 39:131-149. [DOI] [PubMed] [Google Scholar]
- 4.Bender, R. A., K. A. Janssen, A. D. Resnick, M. Blumenberg, F. Foor, and B. Magasanik. 1977. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J. Bacteriol. 129:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhadbhade, B. J., S. S. Sarnaik, and P. P. Kanekar. 2002. Biomineralization of an organophosphorous pesticide, monocrotophos, by soil bacteria. J. Appl. Microbiol. 93:224-234. [DOI] [PubMed] [Google Scholar]
- 6.Burg, M. B., and J. D. Ferraris. 2008. Intracellular organic osmolytes: function and regulation. J. Biol. Chem. 283:7309-7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, E. A., C. A. Streuli, and P. R. Averell (ed.). 1970. The analytical chemistry of nitrogen and its compounds. Wiley Interscience, New York, NY.
- 8.Chandler, S. R. 1983. The utilization of methylamine-nitrogen by the methazotrophic bacterium Pseudomonad P. Ph.D. thesis. University of Reading, Reading, United Kingdom.
- 9.Chistoserdov, A. Y., L. V. Chistoserdova, W. S. Mclntire, and M. E. Lidstrom. 1994. Genetic organization of mau gene cluster in Methylobacterium extorquens AM1: complete nucleotide sequence and generation and characteristics of mau mutants. J. Bacteriol. 176:4052-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chlumsky, L. J., L. Zhang, and M. S. Jorns. 1995. Sequence analysis of sarcosine oxidase and nearby genes reveals homologies with key enzymes of folate one-carbon metabolism. J. Biol. Chem. 270:18252-18259. [DOI] [PubMed] [Google Scholar]
- 11.Dedysh, S. N., C. Knief, and P. F. Dunfield. 2005. Methylocella species are facultatively methanotrophic. J. Bacteriol. 187:4665-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson, R. G., and N. W. Jacobsen. 1970. A new sensitive and specific test for the detection of aldehydes: formation of 6-mercapto-3-substituted-s-triazolo [4,3-b]-s-tetrazines. Chem. Commun. (Camb.) 24:1719-1721. [Google Scholar]
- 13.Dunfield, P. F., V. N. Khmelenina, N. E. Suzina, Y. A. Trotsenko, and S. N. Dedysh. 2003. Methylocella silvestris sp. nov., a novel methanotrophic bacterium isolated from an acidic forest cambisol. Int. J. Syst. Evol. Microbiol. 53:1231-1239. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert, B., I. R. McDonald, R. Finch, G. P. Stafford, A. K. Nielsen, and J. C. Murrell. 2000. Molecular analysis of the pmo (particulate methane monooxygenase) operons from two type II methanotrophs. Appl. Environ. Microbiol. 66:966-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Hepworth, H. 1924. Some simple natural organic bases, p. 170-186. In Chemical synthesis: studies in the investigation of natural organic products. Read Books, London, England.
- 15.Hersh, L. B., J. A. Peterson, and A. A. Thompson. 1971. An N-methyl glutamate dehydrogenase from Pseudomonas M.A. Arch. Biochem. Biophys. 145:115-120. [DOI] [PubMed] [Google Scholar]
- 16.Husain, M., and V. L. Davidson. 1987. Purification and properties of methylamine dehydrogenase from Paracoccus denitrificans. J. Bacteriol. 169:1712-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Jones, J. G., and E. Bellion. 1991. In vivo 13C and 15N NMR studies of methylamine metabolism in Pseudomonas species MA. J. Biol. Chem. 266:11705-11713. [PubMed] [Google Scholar]
- 16b.Kamanavalli, C. M., and H. Z. Ninnekar. 2000. Biodegradation of propoxur by Pseudomonas species. World J. Microbiol. Biotechnol. 16:329-331. [Google Scholar]
- 17.Kamiya, A., and O. Youki. 1984. Study of odorous compounds produced by putrefaction of foods. J. Chromatogr. A 292:383-391. [Google Scholar]
- 18.Kaplan, D. L., and A. M. Kaplan. 1985. Biodegradation of N-nitrosodimethylamine in aqueous and soil systems. Appl. Environ. Microbiol. 50:1077-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, C., and T. K. Wood. 1998. Electroporation of pink-pigmented methylotrophic bacteria. Appl. Biochem. Biotechnol. 73:81-88. [Google Scholar]
- 20.Kimura, T., I. Sugahara, K. Hayashi, M. Kobayashi, and M. Ozeki. 1990. Primary metabolic pathway of methylamine in Methylophaga sp. AA-30. Agric. Biol. Chem. 54:2819-2826. [Google Scholar]
- 21.Kimura, T., I. Sugahara, K. Hanai, and Y. Tonomura. 1992. Purification and characterization of γ-glutamylmethylamide synthetase from Methylophaga sp. AA-30. Biosci. Biotechnol. Biochem. 56:708-711. [DOI] [PubMed] [Google Scholar]
- 22.Kimura, T., I. Sugahara, K. Hanai, and Y. Tonomura. 1995. Purification and characterization of a new γ-glutamylmethylamide-dissimilating enzyme system from Methylophaga sp. AA-30. Biosci. Biotechnol. Biochem. 59:648-655. [DOI] [PubMed] [Google Scholar]
- 23.Kung, H.-F., and C. Wagner. 1969. γ-Glutamylmethylamide: a new intermediate in the metabolism of methylamine. J. Biol. Chem. 244:4136-4140. [PubMed] [Google Scholar]
- 24.Latypova, E., S. Yang, Y.-S. Wang, T. Wang, T. A. Chavkin, M. Hackett, H. Schäfer, and M. G. Kalyuzhnaya. 2010. Genetics of the glutamate-mediated methylamine utilization pathway in the facultative methylotrophic beta-proteobacterium Methyloversatilis universalis FAM5. Mol. Microbiol. 75:426-439. [DOI] [PubMed] [Google Scholar]
- 25.Levitch, M. E. 1976. The demonstration of two discrete enzymes catalyzing the synthesis of glutamine and γ-glutamylmethylamide in Pseudomonas MS. Biochem. Biophys. Res. Commun. 76:609-614. [DOI] [PubMed] [Google Scholar]
- 26.Liaw, S.-H., I. Kuo, and D. Eisenberg. 1995. Discovery of the ammonium substrate site on glutamine synthetase, a third cation binding site. Protein Sci. 4:2358-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenstein, N. 1942. Preparation of γ-alkylamides of glutamic acid. J. Am. Chem. Soc. 65:1021-1022. [Google Scholar]
- 28.Liu, Y., and W. B. Whitman. 2008. Metabolic, phylogenetic and ecological diversity of the methanogenic Archaea. Ann. N. Y. Acad. Sci. 1125:171-189. [DOI] [PubMed] [Google Scholar]
- 29.Loginova, N. V., V. N. Shishkina, and Y. A. Trotsenko. 1976. Primary metabolic pathways of methylated amines in Hyphomicrobium vulgare. Mikrobiologiia 45:41-47. (In Russian.) [PubMed] [Google Scholar]
- 30.Marx, C. J., and M. E. Lidstrom. 2002. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria. Biotechniques 33:1062-1067. [DOI] [PubMed] [Google Scholar]
- 31.Pollock, R. J., and L. B. Hersh. 1971. N-methylglutamate synthetase: purification and properties of the enzyme. J. Biol. Chem. 246:4737-4743. [PubMed] [Google Scholar]
- 32.Schäfer, H., I. R. McDonald, P. D. Nightingale, and J. C. Murrell. 2005. Evidence for the presence of a CmuA methyltransferase pathway in novel marine methyl halide-oxidising bacteria. Environ. Microbiol. 7:839-852. [DOI] [PubMed] [Google Scholar]
- 33.Shaw, W. V., L. Tsai, and E. R. Stadtman. 1966. The enzymatic synthesis of N-methylglutamic acid. J. Biol. Chem. 241:935-945. [PubMed] [Google Scholar]
- 34.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 35.Theisen, A. R., M. H. Ali, S. Radajewski, M. G. Dumont, P. F. Dunfield, I. R. McDonald, S. N. Dedysh, C. B. Miguez, and J. C. Murrell. 2005. Regulation of methane oxidation in the facultative methanotroph Methylocella silvestris BL2. Mol. Microbiol. 58:682-692. [DOI] [PubMed] [Google Scholar]
- 36.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto, S., M. Wakayama, and T. Tachiki. 2007. Characterization of theanine-forming enzyme from Methylovorus mays no. 9 in respect to utilization of theanine production. Biosci. Biotechnol. Biochem. 71:545-552. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto, S., M. Wakayama, and T. Tachiki. 2008. Cloning and expression of Methylovorus mays no. 9 gene encoding gamma-glutamylmethylamide synthetase: an enzyme usable in theanine formation by coupling with the alcoholic fermentation system of baker's yeast. Biosci. Biotechnol. Biochem. 72:101-109. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, X., J. H. Fuller, and W. S. McIntire. 1993. Cloning, sequencing, expression and regulation of structural gene for the copper/topa quinine-containing methylamine oxidase from Arthrobacter strain P1, a gram-positive facultative methylotroph. J. Bacteriol. 175:5617-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]