Abstract
Microorganisms can use complex photosystems or light-dependent proton pumps to generate membrane potential and/or reduce electron carriers to support growth. The discovery that proteorhodopsin is a light-dependent proton pump that can be expressed readily in recombinant bacteria enables development of new strategies to probe microbial physiology and to engineer microbes with new light-driven properties. Here, we describe functional expression of proteorhodopsin and light-induced changes in membrane potential in the bacterium Shewanella oneidensis strain MR-1. We report that there were significant increases in electrical current generation during illumination of electrochemical chambers containing S. oneidensis expressing proteorhodopsin. We present evidence that an engineered strain is able to consume lactate at an increased rate when it is illuminated, which is consistent with the hypothesis that proteorhodopsin activity enhances lactate uptake by increasing the proton motive force. Our results demonstrate that there is coupling of a light-driven process to electricity generation in a nonphotosynthetic engineered bacterium. Expression of proteorhodopsin also preserved the viability of the bacterium under nutrient-limited conditions, providing evidence that fulfillment of basic energy needs of organisms may explain the widespread distribution of proteorhodopsin in marine environments.
Classic experiments in microbial bioenergetics used light-driven reactions from halobacterial bacteriorhodopsin or the photosynthetic reaction center to provide a temporary driving force for understanding transport and chemiosmotic coupling (6, 7, 19, 35). However, light-driven reactions have not been used in metabolic engineering to alter microbial physiology and production of chemicals. The recent discovery of proteorhodopsin (PR) in ocean microorganisms and the ease with which this membrane protein can be functionally expressed by recombinant bacteria have made possible many engineering strategies previously not available (1, 16). In this paper, we describe progress toward the goal of integrating light-driven reactions with biocatalysis.
In contrast to the situation for established industrial microorganisms, such as Escherichia coli, our current understanding of less-studied algal and phototrophic bacteria may limit metabolic engineering strategies which require genetic manipulation. Metabolic engineering strategies using photosynthetic bacteria have focused largely on methods to increase hydrogen production, and improvements rely mainly on engineering of nitrogenase and hydrogenase to produce H2. Algae appear to be suited to large-scale cultivation for lipid production, but so far little has been done to engineer these organisms (36). In principle, platform microbial hosts capable of producing a diverse range of products could be boosted by addition of light-driven processes from phototrophic metabolism.
To demonstrate the feasibility of transferring a light-driven process into a nonphotosynthetic bacterium, we chose to study proteorhodopsin (PR) first because it is one of the simplest mechanisms for harnessing the energy from light. The proteorhodopsins are a group of transmembrane proteins that use the light-induced isomerization of retinal, the oxidative cleavage product of the carotenoid β-carotene, either to initiate signaling pathways or to catalyze the transfer of ions across cell membranes (8). PR was discovered by metagenomic analysis of marine samples (1) and is related to the well-studied bacteriorhodopsin of archaea (33) and rhodopsin (34), a eukaryotic light-sensing protein. The membrane potential generated by light-driven proton pumping by PR has been confirmed to drive ATP synthesis in a heterologous system (25). However, bacteria expressing heterologous PR were shown not to benefit from this pumping activity, as no significant increases in growth rates were observed (9). This led to the suggestion that PR may benefit the organism only under starvation conditions. In agreement with this hypothesis, Gomez-Consarnau et al. (10) have reported that the light-dependent growth rates of a marine flavobacterium that has a native PR are increased only when the organism is cultured under energy-limited conditions.
Studies of both native and recombinant systems in which rhodopsins are expressed have generated light-dependent membrane potentials. In membrane vesicles isolated from a native host, the light-dependent membrane potential generated by bacteriorhodopsin provides the driving force for ATP synthesis (35) and uptake of leucine and glutamate (20, 22). More recently, studies of recombinant systems have coupled the membrane potential to other transport processes. In one example, the membrane potential-dependent export of specific toxic molecules increased when E. coli cells expressing both an archaeal rhodopsin and a specific efflux pump were exposed to light (17). In another experiment, starved E. coli cells expressing PR increased the swimming motion of their flagella when they were illuminated (44). Based upon measurements of flagellar motion as a function of light intensity and azide concentration, the proton motive force generated by PR was estimated to be −0.2 V, a value similar to the value for aerobic respiration in E. coli (42).
As a nonphotosynthetic host for recombinant PR expression, we chose the dissimilatory metal-reducing bacterium Shewanella oneidensis strain MR-1, which is genetically tractable for engineering and is able to use a variety of terminal electron acceptors, including insoluble metal oxides (11, 30). Key to the ability of this bacterium to reduce metal oxides is a multicomponent extracellular respiratory pathway that transports electrons from menaquinol to cytochromes in the outer membrane. This pathway is composed of a cytoplasmic membrane tetraheme protein (CymA), a periplasmic decaheme protein (MtrA), an integral outer membrane protein (MtrB), and a decaheme lipoprotein (MtrC) that is associated with MtrB (14, 37, 40). The ability of S. oneidensis to reduce extracellular metal oxides has made it possible to harvest electrons from this organism by coupling it to an electrode which serves as the electron acceptor (21). The electron flow to the outer surface allows respiration rates to be measured directly by electrochemistry.
In the current work, we introduced PR into an electricity-generating bacterium, S. oneidensis strain MR-1, and demonstrated that there was integration of a light-driven process into the metabolism of a previously nonphotosynthetic organism that resulted in a useful output. We show here that PR allows cells to survive for extended periods in stationary phase and that the presence of light results in an increase in electricity generation. A possible physiological model to explain these effects is discussed.
MATERIALS AND METHODS
Microbiological methods.
E. coli JM109 was used for all molecular biology procedures, and plasmids were transferred to S. oneidensis strain MR-1 by conjugation with the mating strain E. coli WM3064. S. oneidensis was cultivated at 30°C either in Luria-Bertani (LB) medium or in minimal medium containing 20 mM lactate (15, 23). Anaerobic cultures of S. oneidensis contained 40 mM fumarate as an electron acceptor when they were not grown in electrochemical chambers with an electrode as the electron acceptor. When required, 50 μg ml−1 kanamycin and 10 μM retinal were included for plasmid selection and PR reconstitution, respectively. Optical densities at 600 nm (OD600) of cell cultures were determined with a Bausch and Lomb Spectronic 20. Estimates of the number of cells ml−1 in a culture were obtained by counting the number of colonies after a dilution series of the culture was plated on LB agar.
Gene cloning.
The PR gene of a marine bacterium in the SAR86 phylogenetic group (original contig GenBank accession no. AF279106; proteorhodopsin gene GenBank accession no. AAG10475) was amplified by PCR from a plasmid containing the PR gene (1) using gene-specific oligonucleotides (5′-GGTCTAGAAGGAGGAGATCTACATATGAAATTATTACTGATATTAG-3′ and 5′- ACTAGCGGCCGCTTAAGCATTAGAAGATTCTTTAAC-3′) with added XbaI and NotI restriction sites and a Shine-Dalgarno sequence. The PCR product was cloned into the constitutive expression vector pUCmod (the lac repressor binding sequence was deleted) (39) and sequenced to ensure that the PR gene was not changed. The PR gene and the upstream constitutive lac promoter then were subcloned into the BamHI site of the broad-host-range vector pBBR1MCS-2 (18) for expression in S. oneidensis (3). Subsequent digestion of the pBBR1MCS-2 plasmid containing the fragment cloned into the BamHI restriction site revealed that the modified promoter and PR gene were oriented opposite the endogenous pBBR1MCS-2 promoter sequence.
PR absorption spectroscopy.
Cultures of S. oneidensis harboring pBBR1MCS-2 containing the PR gene or an empty control plasmid were incubated anaerobically in minimal medium containing 10 μM retinal at 30°C for approximately 16 h. The cells were centrifuged and resuspended in 50 mM Tris-HCl (pH 8), 0.1 M NaCl. It was difficult to measure the difference spectrum because of the background caused by scattering and absorption by native cytochromes expressed by S. oneidensis. To reduce the scattering of a sample, resuspended cells were sonicated using 10-s pulses at 30% power for a total of 90 s (Branson Sonifier). Absorption spectra were measured (Varian CARY 50 spectrophotometer), and the effects of scattering were removed by subtracting a cubic polynomial fit to the data at 310 nm and 700 to 750 nm. To remove absorption by native cytochromes, the difference spectrum for cultures of S. oneidensis containing pBBR1MCS-2 with the PR gene and cultures of S. oneidensis containing an empty control plasmid was calculated. To account for minor differences in cytochrome absorption at 400 nm and the numbers of cells in each culture, the two baseline-corrected spectra were normalized at the cytochrome absorption peak (λmax, 410 nm) by scaling the spectrum for the culture of S. oneidensis containing the empty plasmid. To estimate the number of PR molecules cell−1, the concentration of PR in the sample was calculated by assuming that the molar extinction coefficient at 520 nm was 50,000 M−1 cm−1 (2), and the number of viable cells was determined by plating serial dilutions on agar plates. The estimate was based on three replicate experiments
Illumination methods.
Light for both the growth rate measurement experiments and the electrochemical experiments was generated by circuit boards containing arrays of green (λmax, 530 nm) high-power light-emitting diodes (LEDs) (Luxeon III Emitter; LXHL-PM09; Future Electronics) powered by a direct current power supply (Mastech model HY3030E). In the growth experiments, the samples were illuminated by LEDs spaced approximately 3 cm apart. In the electrochemical experiments, both faces of the electrode were illuminated by separate arrays of LEDs (see Fig. 4A). To provide sufficient light intensity, the circuit boards were coated with thin layers of clear epoxy (EPO-TEK 302-3 M; Epoxy Technology) and were submerged in the temperature-controlled water bath <1 cm from the electrochemical chamber. Light intensities were measured with a light meter (AEMC Instruments model CA813), and photometric units (lux) were converted to radiometric units (mW cm−2) using the standard photopic response and power spectrum of the LEDs.
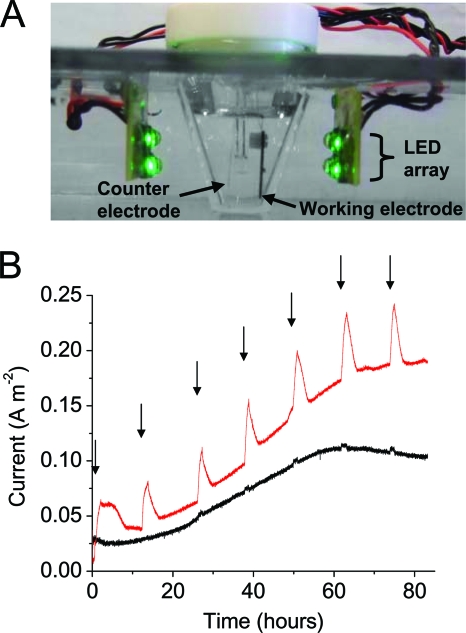
FIG. 4.
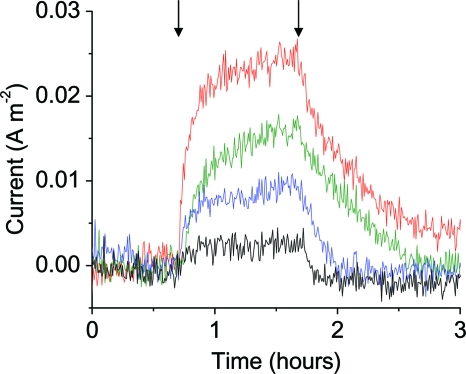
Light-dependent current increases in electrochemical chambers containing S. oneidensis expressing PR. (A) LED arrays used for illumination and glass cone of the electrochemical chamber containing both the working electrode and the counter electrode. So that details of the interior of the chamber could be seen, it was necessary to reduce the light intensity compared to the intensity used during the experiments and to remove reflections of the LEDs on the glass surfaces. (B) Oxidation current of an electrochemical chamber inoculated with S. oneidensis expressing PR (red trace) or containing a control plasmid (black trace). The arrows indicate the beginning of each 1-h illumination period. The light intensity was 10 mW cm−2, and the traces are representative of three independent experiments.
Membrane potential measurement.
The membrane potential for S. oneidensis was measured using a membrane potential-sensitive dye and flow cytometry (31). Cells grown aerobically in minimal medium with 20 mM lactate were centrifuged and then washed and resuspended in an equal volume of minimal medium without lactate as the electron donor. After incubation at 30°C for 30 to 40 h in the dark, the cells were diluted to obtain a concentration of 106 cells ml−1 by addition of minimal medium containing either 0 or 10 mM lactate. Following incubation for 1 h at 30°C either in the dark or in the presence of approximately 3 mW cm−2 of light, the cells were exposed to the cyanine dye DioC2(3) at a concentration of 10 μM for 5 min in the dark. Fluorescence intensities at wavelengths greater than 640 nm were measured for 10,000 cells using a FACSCalibur flow cytometer (Becton Dickinson). An EDTA treatment that is often required to disrupt the cell membrane and allow the dye to enter the cells of Gram-negative bacteria was not required for S. oneidensis. For depolarization of the membrane potential, the protonophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP) was added at a concentration of 5 μM.
Electrochemical studies.
Electrochemical measurements for S. oneidensis were determined using 20-ml electrochemical chambers as described previously (23, 24). Ten milliliters of an anaerobic culture of S. oneidensis grown to an OD600 of 0.4 to 0.5 at 30°C in minimal medium containing 30 mM lactate, 40 mM fumarate, 50 μg ml−1 kanamycin, and 10 μM retinal was transferred to a sterile, oxygen-sparged, stirred electrochemical chamber. During medium exchanges, the medium surrounding the electrode was replaced with fresh medium containing 30 mM lactate, 50 μg ml−1 kanamycin, 10 μM retinal, and 0.5 μM riboflavin (23). During periods of light, both faces of a graphite working electrode (AXF-5Q; Poco Graphite, Inc.) were illuminated. For experiments in which periodic illumination was used, the electrochemical chambers (with 10 ml of medium) contained electrodes that were 2 cm by 0.5 cm by 0.1 cm; for metabolite experiments in which continuous illumination was used, the electrochemical chambers (with 15 ml of medium) contained electrodes that were 2 cm by 2 cm by 0.1 cm and the chambers were flushed with nitrogen gas. Heat from the LED circuit was dissipated using a temperature-controlled circulating water bath (NESLAB, RTE-5B), and the measured temperature did not vary by more than 0.3°C. Radiant heating due to light was negligible because the LED light sources do not emit infrared light. The potential at the working electrode was maintained at 0.245 V versus standard hydrogen electrode, and the current production at 30°C was measured at 30-s intervals.
To measure metabolite concentrations in the medium from electrochemcial chambers, samples were removed from the chambers, centrifuged to remove cells, and loaded (2 μl) onto an Agilent Zorbax SB-Aq C18 column (5 μm; 4.6 by 250 mm), and chromatography was performed using an Agilent 1100 high-performance liquid chromatography (HPLC) system equipped with a photodiode array detector. Metabolites were separated with an isocratic mobile phase containing 20 mM sodium phosphate (pH 2) and 0.5% acetonitrile at a rate of 1 ml min−1, and the absorbance at 210 nm was monitored. Standard curves were generated using known concentrations of each chemical, and peak areas were integrated using the Agilent ChemStation software.
RESULTS
Functional expression of PR by S. oneidensis.
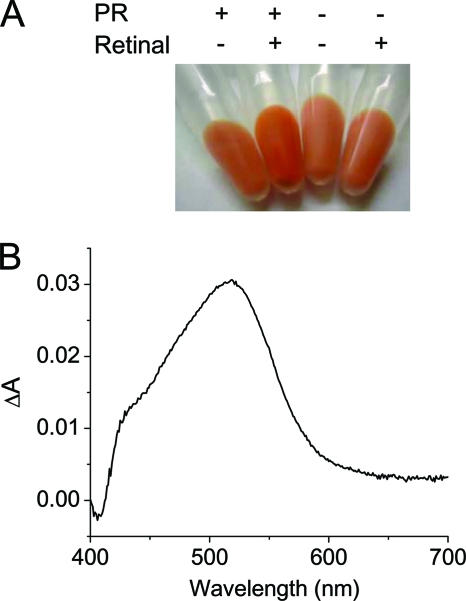
We first established that expression and reconstitution of PR bound to retinal in S. oneidensis lead to accumulation of red pigment in the cell membrane (Fig. 1 A). While wild-type cultures are naturally pink due to expression of c-type cytochromes under O2-limiting conditions, quantitative changes in absorption due to functional reconstitution of PR were measured by calculating the difference spectrum for retinal-expressing PR cultures and cultures containing an empty control plasmid (Fig. 1B). A comparison of the difference spectrum obtained to previous PR spectra suggested that there was functional reconstitution of recombinant PR in S. oneidensis (1). Using an extinction coefficient for rhodopsins absorbing in the visible region and the number of cells ml−1 culture, we estimated that there were 40,000 molecules of PR per S. oneidensis cell grown anaerobically in minimal medium. Our estimate is similar to previous results reported for native PR expression in bacteria belonging to the SAR86 phylogenetic group and in Pelagibacter cells, which contain 24,000 and 10,000 molecules of PR per cell, respectively (2, 9).
FIG. 1.
Expression of functional PR by S. oneidensis. (A) Cell pellets of S. oneidensis expressing PR (+) or containing an empty control plasmid (−) grown overnight at 30°C in LB medium supplemented (+) or not supplemented (−) with retinal. Cells expressing PR in the presence of retinal have additional red pigment. (B) Difference in the absorption spectra of cultures of S. oneidensis expressing PR and cultures of S. oneidensis containing an empty control plasmid grown anaerobically in minimal medium containing 10 μM retinal.
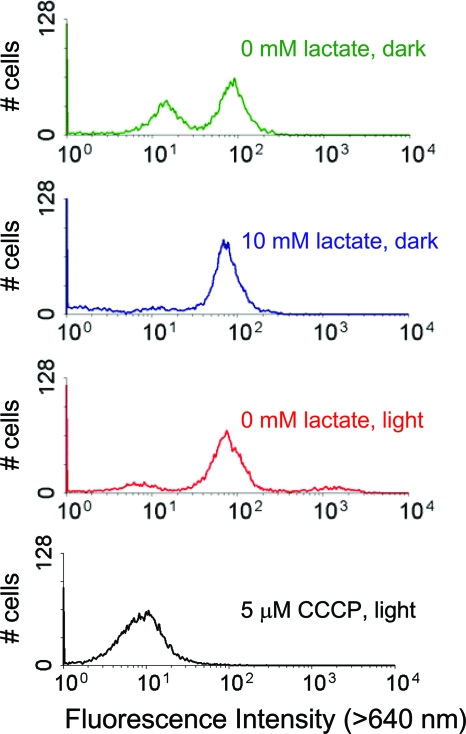
Having established that there was expression in S. oneidenis, we next examined generation of light-dependent membrane potentials by monitoring the fluorescence of cells labeled with the lipophilic cyanine dye DioC2(3), which aggregates at high concentrations, causing its fluorescence to shift to longer wavelengths (31). Because the interior of energized cells has a negative potential relative to the exterior, the positively charged dye accumulates in the cytoplasm and the fluorescence intensity at longer wavelengths increases. Membrane potentials of cells deenergized through starvation or addition of the protonophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP) become depolarized and no longer drive accumulation of the dye in the cytoplasm.
Cultures of S. oneidensis expressing PR were incubated in the dark without an electron donor to starve the cells and to deenergize the membrane potential. Cells then were incubated in the light or in the dark with and without lactate as an electron donor and exposed to DioC2(3), and the fluorescence intensities of individual cells were measured by flow cytometry (Fig. 2). While cultures incubated in the dark without lactate contained both energized cells (high fluorescence intensity) and deenergized cells (low fluorescence intensity), cultures incubated either in the dark with lactate or in the light without lactate contained only energized cells. Addition of the protonophore CCCP depolarized the membrane potential, demonstrating that PR pumps protons out of the cytoplasm to increase the membrane potential. Incubation of starved cells expressing the empty control plasmid in the light did not generate a population of energized cells (see Fig. S1 in the supplemental material).
FIG. 2.
Illumination of S. oneidensis expressing PR increases the membrane potential: histograms of red fluorescence intensities measured using flow cytometry for aerobic cultures of S. oneidensis expressing PR stained with the cyanine dye DioC2(3). Cultures grown to an OD600 of 1.0 in minimal medium with lactate were washed and then were resuspended in minimal medium without lactate. After 40 h, the cells starved of an electron donor were diluted into medium containing 0 or 10 mM lactate, were incubated for 1 h either in the dark or in the light, and then were stained and analyzed. Starved cultures incubated in the dark without lactate (green trace) contained both energized and deenergized cells; cultures incubated in the dark with lactate (blue trace) and cultures incubated in the light without lactate (red trace) contained only energized cells; addition of CCCP to the cells incubated in the light depolarized the membrane potential (black trace).
PR enhances survival during stationary phase.
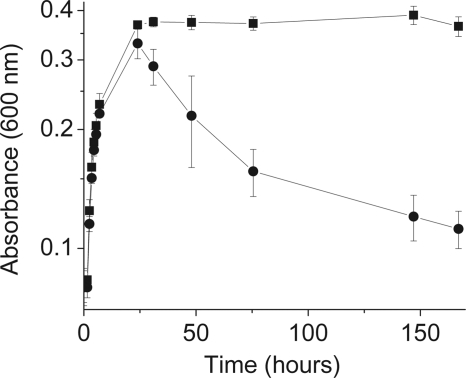
Although the results described above showed that recombinant PR operates as a light-driven proton pump, it is not clear how PR influences the physiology of S. oneidensis. In bacteria that naturally contain PR, light-driven proton pumping is hypothesized to provide a selective advantage during stationary phase (9, 10, 25). To explore whether this benefit can be observed in an engineered bacterium that does not express PR naturally, we measured the anaerobic growth of cultures of S. oneidensis expressing PR in minimal medium supplemented with retinal both in the light and in the dark (Fig. 3). While the optical densities at 600 nm of both cultures grown in the light and cultures grown in the dark peaked at 25 h, only cultures grown in the light remained in stationary phase for >6 days with a density of 109 viable cells ml−1. For cultures grown in the dark, the optical density decreased immediately after the maximum value was reached, and after 6 days the density was 106 viable cells ml−1. Regardless of illumination, the viability of control cultures of S. oneidensis harboring an empty control plasmid or cultures of S. oneidensis expressing PR without retinal steadily decreased after the maximum cell density was reached at 25 h (see Fig. S2 in the supplemental material).
FIG. 3.
PR extends the viability of S. oneidensis during stationary growth. Cell growth was monitored by measuring the optical density at 600 nm for anaerobic cultures of S. oneidensis expressing PR in the presence of 3 mW cm−2 of light (squares) or in the dark (circles). Cultures of S. oneidensis were incubated anaerobically at 30°C in minimal medium containing lactate, fumarate, and retinal. The values are means ± standard deviations for three independent cultures.
PR enhances electricity generation.
With the demonstration that recombinant PR is functional and integrated into the physiology of S. oneidensis, we investigated whether light-driven proton pumping by PR can influence extracellular respiration and generation of electricity. In a series of reactions in S. oneidensis that have not been fully elucidated, oxidation of lactate to acetate leads to reduction of menaquinone and formation of biosynthetic precursors. In the presence of an electrode at an appropriate potential, the redox loop is completed by oxidation of menaquinol and subsequent transfer of electrons via several cytochromes to the outside of the cell, where small-molecule mediators assist in transfer to the electrode (21, 23). Electron transfer to the electrode can be observed directly by measuring the oxidation current (24). After inoculation, cells attach to the electrode, which is a requirement for respiration and growth because mediators do not efficiently carry electrons from planktonic cells to an electrode (23).
Illumination of recombinant S. oneidensis cultures expressing PR growing anaerobically on electrodes (Fig. 4 A) resulted in light-dependent increases in the oxidation current (Fig. 4B). Light-dependent changes were not observed in cultures containing an empty control plasmid. The oxidation current measured in electrochemical chambers inoculated with S. oneidensis either expressing PR or containing the empty control plasmid increased for approximately 70 h as bacteria colonized the electrode. After reaching asymptotic values that did not depend on expression of PR and were sensitive to conditions during inoculation, the current remained steady until lactate was depleted from the medium.
The effect of light-dependent proton pumping by PR on the respiration rate of anaerobically growing cells was measured by illuminating the cultures periodically, and the sawtooth response to changes in light intensity shown in Fig. 4B was representative of all experiments. Typically, after 10 min of illumination the current increased to a value that was 0.04 ± 0.01 A m−2 greater than the value before illumination; when the light was removed, the current decreased within 1 h to a value expected for an identical experiment in which there was no illumination. The magnitude of the light-dependent increase in current represented a 100% increase during early stages of growth. As the absolute light-dependent current was relatively constant as more cells attached to the electrode, illumination at later stages resulted in a 30% increase in the respiration current, indicating that there may have been changes in light penetration for thicker biofilms.
The light-dependent increase in current was proportional to the intensity of the light used to illuminate fully colonized electrodes (Fig. 5). Saturation of the light-dependent increase did not occur with intensities of 10 mW cm−2. Previous studies have shown that saturation of the light-dependent increases for planktonic E. coli expressing PR (44) and halobacteria expressing bacteriorhodopsin (13) occurs at approximately 20 mW cm−2. Addition of exogenous riboflavin, which was previously identified as an extracellular electron mediator produced by S. oneidensis (23, 43), did not alter the magnitude of the light-dependent change in current.
FIG. 5.
Magnitude of the oxidation current depends on the light intensity. The change in oxidation current was measured for an electrochemical chamber containing an electrode fully colonized (>70 h) by cells of S. oneidensis expressing PR during illumination using the following light intensities: 0.7 mW cm−2 (black trace), 1.9 mW cm−2 (blue trace), 3.6 mW cm−2 (green trace), and 9.0 mW cm−2 (red trace). Each illumination period was 1 h long, and the current was allowed to stabilize before the next experiment. The arrows indicate the beginning and end of the illumination period.
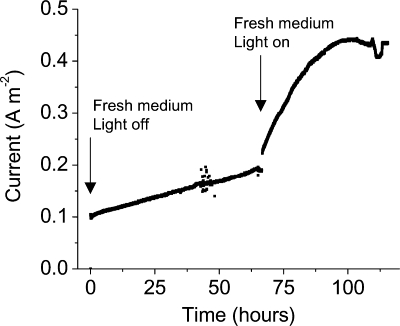
The effects of continuous illumination on the current and metabolite concentrations for an electrochemical chamber containing S. oneidensis expressing PR were measured (Fig. 6 and Table 1). After inoculation and attachment of cells to the electrode, planktonic cells and the medium surrounding the electrode were removed and replaced with fresh medium (zero time). In the dark, the current increased as cell growth continued on the electrode. After a second medium exchange and illumination of the chamber, the average rate of current production increased, and lactate was consumed more rapidly.
FIG. 6.
Current for an electrochemical chamber containing S. oneidensis expressing PR during periods of constant darkness or constant light. After inoculation of the electrochemical chamber and attachment to the electrode, planktonic cells and the medium surrounding the electrode were removed and replaced with fresh medium (zero time). After a second medium exchange (at 67 h), the chamber was illuminated continuously using a light intensity of 10 mW cm−2.
TABLE 1.
Carbon balance for electrochemical chamber in dark and light conditionsa
| Conditions | Time (h) | Concn (mM) of: |
Lactate consumption rate (μmol h−1) | Avg current (A m−2) | ||
|---|---|---|---|---|---|---|
| Lactate | Acetate | Pyruvate | ||||
| Dark | 0 | 32.0 | 0.0 | 0.4 | ||
| 67 | 13.7 | 15.3 | 2.5 | 4.1 | 0.15 | |
| Light | 68 | 32.4 | 4.6 | 0.2 | ||
| 117 | 6.7 | 25.2 | 0.4 | 7.8 | 0.39 | |
For experimental details, see Materials and Methods and the legend to Fig. 6.
For both light and dark periods, decreases in lactate concentration were matched by increases in acetate and pyruvate concentrations. To obtain precise carbon recovery values and to prevent complete oxidation of lactate and acetate to CO2 by aerobic respiration or consumption of H2 produced at the counter electrode, it was necessary to sparge the chambers with N2. Under these conditions and regardless of illumination, the number of electrons recovered at the electrode was less than the expected value. As observed previously (4), it is likely that high N2 flushing rates decrease the H2 partial pressure and increase the rate of hydrogenase-catalyzed oxidation of menaquinol and reduction of protons to form H2 (26). Regardless of the mode of electron disposal and variations in cell growth and attachment to the electrode, illumination of PR-containing chambers was always correlated with an increased rate of oxidation of lactate to acetate.
DISCUSSION
As evidenced by the occurrence of proteorhodopsins in environments and clades of bacteria, a PR is expected to provide a selective advantage. Given the role of this molecule as a light-dependent proton pump, it was hypothesized that PR could provide an advantage under nutrient-limited conditions (9, 10). Despite attempts to measure this advantage, most growth rates of bacteria expressing PR are not higher in the light (10). We provide evidence here that PR allows cells to remain viable for extended periods. Our results not only suggest that PR provides a selective advantage by maintaining a membrane potential during stationary phase but also show that this benefit can be transferred easily into a bacterium that does not contain a PR naturally, which may explain the widespread occurrence of this protein in many different groups of marine bacteria that live under long-term nutrient limitation conditions in the open ocean (38).
The results of our electrochemical studies demonstrate that light energy can be used by cultures of S. oneidensis expressing a light-dependent proton pump to enhance generation of electric current. Compared to the time required for S. oneidensis to divide during anaerobic growth or to increase its biomass, light-induced changes in current were rapid, suggesting that rather than altering protein expression, illumination increased the rates of extracellular respiration by individual cells. Because all electrons deposited on an electrode must originate from lactate, any increase in current is proportional to an increase in lactate consumption. Our measurements demonstrate that electrochemical chambers containing S. oneidensis expressing PR generate more current and consume more lactate in the light than in the dark (Table 1).
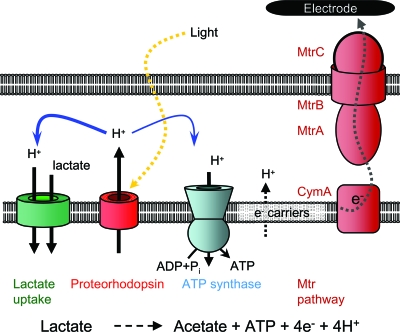
Well-established observations have demonstrated that small changes in membrane potential lead to large increases in transport rates and accumulation ratios for small molecules (6, 7, 19). Small increases in the intracellular pH also result in increased accumulation of weak acids, such as lactate. For example, for symport of a molecule such as lactate compensated by one charge (32), the Nernst equation (12) predicts that a 7- to 18-mV increase should cause a 30 to 100% increase in the intracellular concentration. Similarly, 0.1- to 0.3-unit ΔpH changes can result in a similar accumulation ratio. Such modest changes in the membrane potential or ΔpH are consistent with the proton-pumping capacity of PR (27, 29), suggesting that illumination leads to increased lactate uptake, increased reduction of electron carriers such as NAD+/NADH and menaquinone/menaquinol, and a need for faster regeneration of oxidized electron carriers (Fig. 7). A similar mechanism may be involved in the light-induced increases in current in a photosynthetic Rhodopseudomonas species isolated using amorphous iron(III) as an electron acceptor (45).
FIG. 7.
Schematic diagram of current generation by S. oneidensis expressing PR under anaerobic conditions with lactate as a carbon source. Excitation of PR by light drives the transport of protons (H+) across the membrane to increase the proton membrane potential, which is coupled to the import of lactate and synthesis of ATP. Oxidation of lactate leads to production of acetate, ATP, and reduced electron carriers, such as NADH and menaquinol. The enzymes that likely are involved in reducing the electron carriers include lactate dehydrogenase, NADH dehydrogenase, and formate dehydrogenase; the reactions may also transport protons. Electrons are passed through the Mtr extracellular respiratory pathway to the electrode (37).
While the increase in lactate uptake due to PR could be predicted, the increase in the S. oneidensis respiration rate was unexpected. Previous bioenergetic experiments with respiring E. coli and mitochondria have shown that the membrane potential and the rate of respiration are inversely related (5, 28). In a regulatory loop known as respiratory control, increases in the membrane potential slow processes that transport protons out of the cell by increasing the energy required to pump protons across the membrane. As electron transport is linked to pumping of many protons (10 H+ pumped for 2 e− transferred from NADH to oxygen) (41), a high membrane potential feeds back to limit respiration. However, if the net respiratory reaction is coupled to less proton pumping, respiratory control would not be expected to have such a powerful influence. For anaerobic growth of S. oneidensis, the 4-electron oxidation of lactate should not result in translocation of more than 2 net protons (2 H+ pumped for 2 e− transferred from formate to menaquinone) (41), and the translocation could be less if protons are also used in import and export of solutes. The observation that S. oneidensis does not exhibit respiratory control makes this bacterium an attractive platform for strategies combining electron transport and proton motive force generation and suggests further experiments to examine the role of the membrane potential of S. oneidensis during anaerobic growth.
While the amount of electrical energy generated is smaller than the amount of light energy used to illuminate the electrode under laboratory conditions, our work shows that it is possible to integrate light-driven reactions into a nonphotosynthetic bacterium to alter survival and metabolism, with effects such as acceleration of electricity generation. In addition to increasing the rates of oxidation of carbon sources, light-induced proton gradients incorporated into engineered bacteria may be used to drive energy-demanding synthetic reactions for production of fine chemicals. More sophisticated light-converting systems, such as photosynthetic reaction centers, may further improve the efficiency and yield of light energy capture and conversion.
Supplementary Material
Acknowledgments
We thank Ed DeLong (Massachusetts Institute of Technology) for his gift of the plasmid containing the proteorhodopsin gene, Tony Dean (University of Minnesota) for use of the flow cytometer, and Erin Marasco (Cargill, Inc.) for performing preliminary experiments.
This research was supported by the Institute on the Environment (University of Minnesota) and by National Science Foundation grant CBET-0756296.
Footnotes
Published ahead of print on 7 May 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Beja, O., L. Aravind, E. V. Koonin, M. T. Suzuki, A. Hadd, L. P. Nguyen, S. Jovanovich, C. M. Gates, R. A. Feldman, J. L. Spudich, E. N. Spudich, and E. F. DeLong. 2000. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science 289:1902-1906. [DOI] [PubMed] [Google Scholar]
- 2.Beja, O., E. N. Spudich, J. L. Spudich, M. Leclerc, and E. F. DeLong. 2001. Proteorhodopsin phototrophy in the ocean. Nature 411:786-789. [DOI] [PubMed] [Google Scholar]
- 3.Beliaev, A. S., D. A. Saffarini, J. L. McLaughlin, and D. Hunnicutt. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. [DOI] [PubMed] [Google Scholar]
- 4.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein, C., L. Tiankova, and A. Kepes. 1979. Respiratory control in Escherichia coli K 12. Eur. J. Biochem. 94:387-392. [DOI] [PubMed] [Google Scholar]
- 6.Crielaard, W., A. J. Driessen, D. Molenaar, K. J. Hellingwerf, and W. N. Konings. 1988. Light-driven amino acid uptake in Streptococcus cremoris or Clostridium acetobutylicum membrane vesicles fused with liposomes containing bacterial reaction centers. J. Bacteriol. 170:1820-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elferink, M. G., I. Friedberg, K. J. Hellingwerf, and W. N. Konings. 1983. The role of the proton-motive force and electron flow in light-driven solute transport in Rhodopseudomonas sphaeroides. Eur. J. Biochem. 129:583-587. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman, J. A., M. S. Schwalbach, and U. Stingl. 2008. Proteorhodopsins: an array of physiological roles? Nat. Rev. Microbiol. 6:488-494. [DOI] [PubMed] [Google Scholar]
- 9.Giovannoni, S. J., L. Bibbs, J. C. Cho, M. D. Stapels, R. Desiderio, K. L. Vergin, M. S. Rappe, S. Laney, L. J. Wilhelm, H. J. Tripp, E. J. Mathur, and D. F. Barofsky. 2005. Proteorhodopsin in the ubiquitous marine bacterium SAR11. Nature 438:82-85. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Consarnau, L., J. M. Gonzalez, M. Coll-Llado, P. Gourdon, T. Pascher, R. Neutze, C. Pedros-Alio, and J. Pinhassi. 2007. Light stimulates growth of proteorhodopsin-containing marine flavobacteria. Nature 445:210-213. [DOI] [PubMed] [Google Scholar]
- 11.Gralnick, J. A., and D. K. Newman. 2007. Extracellular respiration. Mol. Microbiol. 65:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harold, F. M. 1986. The vital force: a study of bioenergetics. W. H. Freeman and Company, New York NY.
- 13.Hartmann, R., and D. Oesterhelt. 1977. Bacteriorhodopsin-mediated photophosphorylation in Halobacterium halobium. Eur. J. Biochem. 77:325-335. [DOI] [PubMed] [Google Scholar]
- 14.Hartshorne, R. S., C. L. Reardon, D. Ross, J. Nuester, T. A. Clarke, A. J. Gates, P. C. Mills, J. K. Fredrickson, J. M. Zachara, L. Shi, A. S. Beliaev, M. J. Marshall, M. Tien, S. Brantley, J. N. Butt, and D. J. Richardson. 2009. Characterization of an electron conduit between bacteria and the extracellular environment. Proc. Natl. Acad. Sci. U. S. A. 106:22169-22174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hau, H. H., A. Gilbert, D. Coursolle, and J. A. Gralnick. 2008. Mechanism and consequences of anaerobic respiration of cobalt by Shewanella oneidensis strain MR-1. Appl. Environ. Microbiol. 74:6880-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, E. T., and C. Schmidt-Dannert. 2008. Light-energy conversion in engineered microorganisms. Trends Biotechnol. 26:682-689. [DOI] [PubMed] [Google Scholar]
- 17.Kamo, N., T. Hashiba, T. Kikukawa, T. Araiso, K. Ihara, and T. Nara. 2006. A light-driven proton pump from Haloterrigena turkmenica: functional expression in Escherichia coli membrane and coupling with a H+ co-transporter. Biochem. Biophys. Res. Commun. 341:285-290. [DOI] [PubMed] [Google Scholar]
- 18.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 19.Lanyi, J. K., R. Renthal, and R. E. MacDonald. 1976. Light-induced glutamate transport in Halobacterium halobium envelope vesicles. II. Evidence that the driving force is a light-dependent sodium gradient. Biochemistry 15:1603-1610. [DOI] [PubMed] [Google Scholar]
- 20.Lanyi, J. K., V. Yearwooddrayton, and R. E. Macdonald. 1976. Light-induced glutamate transport in Halobacterium halobium envelope vesicles. 1. Kinetics of light-dependent and sodium-gradient-dependent uptake. Biochemistry 15:1595-1603. [DOI] [PubMed] [Google Scholar]
- 21.Lovley, D. R. 2008. The microbe electric: conversion of organic matter to electricity. Curr. Opin. Biotechnol. 19:564-571. [DOI] [PubMed] [Google Scholar]
- 22.Macdonald, R. E., and J. K. Lanyi. 1975. Light-induced leucine transport in Halobacterium halobium envelope vesicles—chemiosmotic system. Biochemistry 14:2882-2889. [DOI] [PubMed] [Google Scholar]
- 23.Marsili, E., D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick, and D. R. Bond. 2008. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. U. S. A. 105:3968-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsili, E., J. B. Rollefson, D. B. Baron, R. M. Hozalski, and D. R. Bond. 2008. Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Appl. Environ. Microbiol. 74:7329-7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez, A., A. S. Bradley, J. R. Waldbauer, R. E. Summons, and E. F. DeLong. 2007. Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host. Proc. Natl. Acad. Sci. U. S. A. 104:5590-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meshulam-Simon, G., S. Behrens, A. D. Choo, and A. M. Spormann. 2007. Hydrogen metabolism in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 73:1153-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel, H., and D. Oesterhelt. 1976. Light-induced changes of the pH gradient and the membrane potential in H. halobium. FEBS Lett. 65:175-178. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell, P., and J. Moyle. 1969. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur. J. Biochem. 7:471-484. [DOI] [PubMed] [Google Scholar]
- 29.Nagel, G., B. Mockel, G. Buldt, and E. Bamberg. 1995. Functional expression of bacteriorhodopsin in oocytes allows direct measurement of voltage dependence of light induced H+ pumping. FEBS Lett. 377:263-266. [DOI] [PubMed] [Google Scholar]
- 30.Nealson, K. H., and J. Scott. 2006. Ecophysiology of the genus Shewanella, p. 1133-1151. In M. Dworkin, S. Falkow, E. Rosenbergy, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, vol. 6. Springer, New York NY. [Google Scholar]
- 31.Novo, D., N. G. Perlmutter, R. H. Hunt, and H. M. Shapiro. 1999. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 35:55-63. [DOI] [PubMed] [Google Scholar]
- 32.Nunez, M. F., O. Kwon, T. H. Wilson, J. Aguilar, L. Baldoma, and E. C. C. Lin. 2002. Transport of l-lactate, d-lactate, and glycolate by the LldP and GlcA membrane carriers of Escherichia coli. Biochem. Biophys. Res. Commun. 290:824-829. [DOI] [PubMed] [Google Scholar]
- 33.Oesterhelt, D., and W. Stoeckenius. 1971. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat. New Biol. 233:149-152. [DOI] [PubMed] [Google Scholar]
- 34.Palczewski, K., T. Kumasaka, T. Hori, C. A. Behnke, H. Motoshima, B. A. Fox, I. Le Trong, D. C. Teller, T. Okada, R. E. Stenkamp, M. Yamamoto, and M. Miyano. 2000. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739-745. [DOI] [PubMed] [Google Scholar]
- 35.Racker, E., and W. Stoeckenius. 1974. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J. Biol. Chem. 249:662-663. [PubMed] [Google Scholar]
- 36.Rosenberg, J. N., G. A. Oyler, L. Wilkinson, and M. J. Betenbaugh. 2008. A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 19:430-436. [DOI] [PubMed] [Google Scholar]
- 37.Ross, D. E., S. S. Ruebush, S. L. Brantley, R. S. Hartshorne, T. A. Clarke, D. J. Richardson, and M. Tien. 2007. Characterization of protein-protein interactions involved in iron reduction by Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 73:5797-5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rusch, D. B., A. L. Halpern, G. Sutton, K. B. Heidelberg, S. Williamson, S. Yooseph, D. Y. Wu, J. A. Eisen, J. M. Hoffman, K. Remington, K. Beeson, B. Tran, H. Smith, H. Baden-Tillson, C. Stewart, J. Thorpe, J. Freeman, C. Andrews-Pfannkoch, J. E. Venter, K. Li, S. Kravitz, J. F. Heidelberg, T. Utterback, Y. H. Rogers, L. I. Falcon, V. Souza, G. Bonilla-Rosso, L. E. Eguiarte, D. M. Karl, S. Sathyendranath, T. Platt, E. Bermingham, V. Gallardo, G. Tamayo-Castillo, M. R. Ferrari, R. L. Strausberg, K. Nealson, R. Friedman, M. Frazier, and J. C. Venter. 2007. The Sorcerer II global ocean sampling expedition: Northwest Atlantic through Eastern Tropical Pacific. PLoS Biol. 5:398-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt-Dannert, C., D. Umeno, and F. H. Arnold. 2000. Molecular breeding of carotenoid biosynthetic pathways. Nat. Biotechnol. 18:750-753. [DOI] [PubMed] [Google Scholar]
- 40.Shi, L., T. C. Squier, J. M. Zachara, and J. K. Fredrickson. 2007. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol. Microbiol. 65:12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon, J., R. J. M. van Spanning, and D. J. Richardson. 2008. The organisation of proton motive and non-proton motive redox loops in prokaryotic respiratory systems. Biochim. Biophys. Acta 1777:1480-1490. [DOI] [PubMed] [Google Scholar]
- 42.Tran, Q. H., and G. Unden. 1998. Changes in the proton potential and the cellular energetics of Escherichia coli during growth by aerobic and anaerobic respiration or by fermentation. Eur. J. Biochem. 251:538-543. [DOI] [PubMed] [Google Scholar]
- 43.von Canstein, H., J. Ogawa, S. Shimizu, and J. R. Lloyd. 2008. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 74:615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walter, J. M., D. Greenfield, C. Bustamante, and J. Liphardt. 2007. Light-powering Escherichia coli with proteorhodopsin. Proc. Natl. Acad. Sci. U. S. A. 104:2408-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing, D. F., Y. Zuo, S. A. Cheng, J. M. Regan, and B. E. Logan. 2008. Electricity generation by Rhodopseudomonas palustris DX-1. Environ. Sci. Technol. 42:4146-4151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.