Abstract
Toxigenic Vibrio cholerae, the causative agent of the epidemic diarrheal disease cholera, interacts with diverse environmental bacteriophages. These interactions promote genetic diversity or cause selective enrichment of phage-resistant bacterial clones. To identify bacterial genes involved in mediating the phage-resistant phenotype, we screened a transposon insertion library of V. cholerae O1 El Tor biotype strain C6706 to identify mutants showing altered susceptibility to a panel of phages isolated from surface waters in Bangladesh. Mutants with insertion in cyaA or crp genes encoding adenylate cyclase or cyclic AMP (cAMP) receptor protein (CRP), respectively, were susceptible to a phage designated JSF9 to which the parent strain was completely resistant. Application of the cyaA mutant as an indicator strain in environmental phage monitoring enhanced phage detection, and we identified 3 additional phages to which the parent strain was resistant. Incorporation of the cyaA or crp mutations into other V. cholerae O1 strains caused similar alterations in their phage susceptibility patterns, and the susceptibility correlated with the ability of the bacteria to adsorb these phages. Our results suggest that cAMP-CRP-mediated downregulation of phage adsorption may contribute to a mechanism for the V. cholerae O1 strains to survive predation by multiple environmental phages. Furthermore, the cyaA or crp mutant strains may be used as suitable indicators in monitoring cholera phages in the water.
Bacteriophages contribute to the evolution of bacteria by mediating horizontal gene transfer and genomic rearrangements, as well as by bactericidal selection, in which bacterial strains that are able to resist phage predation thrive over competing phage-susceptible strains (5, 10, 11). Toxigenic Vibrio cholerae, the causative agent of the epidemic diarrheal disease cholera, interacts with diverse phages, both in the aquatic environment and in the host milieu, and these interactions may promote genetic diversity and/or cause selective enrichment of particular bacterial clones (10, 11, 26, 27).
Historically, cholera is an ancient disease with the occurrence of seven distinct pandemics since the first pandemic of cholera began in 1817, but the disease still affects millions of people (9, 16). The current seventh pandemic of cholera, which originated in Indonesia in 1961, is the most extensive in geographic spread and duration, and the causative agent is V. cholerae O1 of the El Tor biotype. The sixth pandemic and presumably the earlier pandemics were caused by the classical biotype, which now seems to be extinct.
Molecular epidemiological surveillance has revealed continually changing relative prevalences of different clones of pathogenic V. cholerae (9), and the emergence of new clones has been attributed to possible horizontal transfer of clusters of genes associated with virulence or environmental fitness as well as resistance to different antibiotics (9, 20). The recent recognition that phage predation may play a role in the natural control of cholera epidemics (10, 11, 14) reinforces predictions that changes in this pathogen and the prevalences of different clones may also be driven by environmental phages. The emergence of certain strains is likely to be enhanced by phages through the bactericidal mechanism in which phage-sensitive strains are killed while providing a selective advantage to phage-resistant strains. Therefore, the ability to evade phage predation constitutes an important factor in attaining increased evolutionary fitness.
In the present study we screened a transposon insertion library of V. cholerae O1 El Tor biotype strain C6706, to identify genes whose inactivation would enhance the susceptibility of the bacteria to environmental phages. Presumably, these genes contribute in mediating resistance to the relevant phages and thus allow the bacteria to survive phage predation. Bacteria with increased phage susceptibility due to mutations in the appropriate genes may also have application as improved indicator strains to monitor the prevalence of relevant phages in the environment.
MATERIALS AND METHODS
Bacterial strains and phages.
V. cholerae strains used as indicators in plaque assays or as hosts for phage preparations were from either clinical or environmental sources and were available in our collection. Clinical strains were originally obtained from cholera patients who attended the treatment center of the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B), located in Dhaka. Environmental isolates were from surface waters in Dhaka. The defined mutants were obtained from a previously described TnFGL3 insertion library of the El Tor biotype strain C6706 (6) or constructed in the present study. The different phages included in this study were isolated from environmental water samples collected in Dhaka. A description of different bacterial and phage strains included in the study is presented in Table 1.
TABLE 1.
Bacterial strains and phages used in this study
| Phage(s) or strain(s) | Description | Reference or source |
|---|---|---|
| JSF1, JSF3, JSF4, JSF141, JSF9, JSF10, JSF11, JSF12, JSF15 | Vibrio cholerae phages isolated from environmental waters in Bangladesh | 10, 24; this study |
| S224, O395, S262, L355, L396, L547, L362, B36921, C19385, D19316, E14850, E14983, AE4727, AE2883, AE7471 | Vibrio cholerae O1 classical biotype clinical isolates | Laboratory collection |
| C6706, 2924281, 2868921, 2868912, AF-1471, AL-30457, AM-33126, AN-32320, G-8747, G-3669, AP-13543, AM-33122, MG-116955, AN-24088, AO-29054, 2680370, 2706062, 2680335 | Vibrio cholerae O1 El Tor biotype clinical isolates | Laboratory collection |
| EC14235 (C6706; crp::TnFGL3) | Derivative of strain C6706 carrying TnFGL3 insertion in the crp gene encoding cAMP receptor protein (CRP) | 6 |
| EC13352 (C6706; cyaA::TnFGL3) | Derivative of strain C6706 carrying TnFGL3 insertion in the cyaA gene encoding adenylate cyclase | 6 |
| 2614, 3055, 112V214 | Environmental V. cholerae O1 El Tor isolates | Laboratory collection |
| 2614, crp::TnFGL3; 3055, crp::TnFGL3; 112V214, crp::TnFGL3 | Vibrio cholerae O1 El Tor strains with TnFGL3 insertion in crp gene | This study |
| 2614, cyaA::TnFGL3; 3055, cyaA::TnFGL3; 112V214, cyaA::TnFGL3 | V. cholerae O1 El Tor strains with TnFGL3 insertion in cyaA gene | This study |
| C-0273, C-1771, C-0270, AI-1852, Arg-03 | Vibrio cholerae O139 isolates | Laboratory collection |
| C-0273, crp::TnFGL3; C-771, crp::TnFGL3; C-0270, crp::TnFGL3 | Vibrio cholerae O139 carrying TnFGL3 insertion in crp gene | This study |
| C-0273, cyaA::TnFGL3; C-1771, cyaA::TnFGL3; C-0270, cyaA::TnFGL3 | Vibrio cholerae O139 carrying TnFGL3 insertion in cyaA gene | This study |
| V46, V51 | Vibrio cholerae O141 isolates | Laboratory collection |
| V46cr (V46; crp::TnFGL3), V51cr (V51; crp::TnFGL3) | Vibrio cholerae O141 carrying TnFGL3 insertion in crp gene | This study |
| V46c (V46; cyaA::TnFGL3), V51c (V51; cyaA::TnFGL3) | Vibrio cholerae O141 carrying TnFGL3 insertion in cyaA gene | This study |
Isolation and estimation of phage.
A panel of V. cholerae O1 and O139 strains were used as potential indicator strains to detect and isolate phages from environmental water using the soft agar plaque assay, as described previously (10). Plaques were counted to estimate the concentration of phage particles in the sample. Phages from representative plaques were further purified, and the specificity of each phage was tested using strains belonging to different species and serotypes.
Phage adsorption assay.
Adsorption of a phage on its host strain was studied according to a previously described method (23). To examine the adsorption of the phage to indicator bacterial cells, ∼2.5 × 108 cells of exponentially grown LB broth culture of each V. cholerae strain were mixed with 107 PFU of the phage. LB broth mixed with the phage without the bacteria was used as a control. Parallel samples were incubated for 20 min at 30°C, and then the concentrations of nonadsorbed phages were determined. To estimate the nonadsorbed phage in each sample, bacteria were removed by centrifugation for 10 min at 1,200 × g, and supernatants were filtered through 0.22-μm-pore-size filters (Millipore Corporation, Bedford, MA). Serial dilutions of the filtrate were plated on an indicator bacterial strain, and plaque counts were determined after 16 h of incubation at 37°C. In addition, phase-contrast microscopy was conducted to monitor possible agglutination of host bacteria in the presence of the phage.
Electron microcopy of phage particles.
High-titer phage preparations (∼1010 PFU/ml) were obtained using the plate lysis procedure as described previously (2). The phage particles were negatively stained with 2% uranyl acetate and were examined under a Philips transmission electron microscope (model 420T).
Screening of the transposon library.
A previously constructed TnFGL3 insertion library (6) of the El Tor strain C6706 was screened to identify mutants exhibiting altered susceptibilities to different phages. These phages either have been described previously (10) or were isolated in the present study (Table 1). The screening was done by cross streaking of the bacterial mutants and the phage on LB agar plates. The relevant mutants of C6706 identified by cross streaking were further confirmed by standard soft agar plaque assays as described above.
Chitin-induced transformation.
V. cholerae cells were transformed with genomic DNA fragments from appropriate donor strains (carrying TnFGL3 inserts) in the presence of chitin by using previously described methods (18, 25). Briefly, overnight cultures of the recipient V. cholerae strains were diluted 1:100-fold in LB medium and grown to an optical density at 600 nm (OD600) of ∼0.3. The bacteria were precipitated by centrifugation, washed, and resuspended in 1/10 volume of filter-sterilized environmental water or 0.5% sterile sea salt solution (SS). Aliquots of a 2-ml bacterial suspension were dispensed into the wells of a 12-well tissue culture plate containing sterile pieces of shrimp shell (25). After incubation at 30°C, with static growth for 24 h, the planktonic phase was removed and fresh water or SS was added. At the same time, 1 to 2 μg of the appropriate DNA was added to the wells. After 24 h, the shrimp shells were removed from the wells, washed in SS, and vortexed in SS to release the attached bacteria. The released bacteria were then plated onto appropriate antibiotic-containing LB agar plates. Suspected transformants were further analyzed using PCR and hybridization assays to confirm the presence of TnFGL3 insertion markers in the relevant genes.
RESULTS
Characteristics of relevant phages.
Initially the transposon insertion library was screened using a panel of 6 phages, and later 3 more phages were included (Table 1). A total of 4 phages to which the cyaA and crp mutants were susceptible, but the parent strain was completely resistant, were further characterized. These phages included JSF9, which was initially isolated from a sample of river water using classical biotype indicator strain S224, and 3 other phages, JSF10, JSF12, and JSF15, which were isolated using a cyaA mutant of El Tor biotype strain C6706 as the indicator (Table 2). The host specificity of these phages was examined using a panel of bacterial strains belonging to different species and serogroups (data not shown). Strains belonging to the classical biotype of V. cholerae O1 were susceptible to phages JSF9, JSF12, and JSF15, whereas the host for phage JSF10 was a specific nontoxigenic V. cholerae O139 strain, Arg-03. All wild-type V. cholerae O1 El Tor strains tested were resistant to these phages, but the cyaA or crp mutants of different El Tor strains were fully susceptible (Table 3). The wild-type strains of the classical biotype were susceptible to phages JSF9, JSF12, and JSF15 but were resistant to JSF10. However, cyA and crp mutants of a classical strain, S262, turned out to be susceptible to JSF10 phage as well (Table 3).
TABLE 2.
Characteristics of different bacteriophages analyzed in the study
| Phage designation | Indicator strain used for isolation | Morphologya | Family | Restriction profileb | DNA homologyc |
|---|---|---|---|---|---|
| JSF9 | V. cholerae O1 classical biotype strain S224 | Hexagonal head (50 nm), short tail (5 nm) | Podoviridae | 1 | JSF12, JSF15 |
| JSF10 | V. cholerae O139 strain Arg-03 | Hexagonal head (45 nm), long tail (100 nm) | Siphoviridae | 2 | None |
| JSF12 | V. cholerae O1 EC13352 (C6706; cyaA::TnFGL3) | Hexagonal head (50 nm), short tail (5 nm) | Podoviridae | 3 | JSF9, JSF15 |
| JSF15 | V. cholerae O1 EC13352 (C6706; cyaA::TnFGL3) | Hexagonal head (50 nm), short tail (5 nm) | Podoviridae | 4 | JSF9, JSF12 |
TABLE 3.
Susceptibilities of diverse V. cholerae strains to infection by different phages
| V. cholerae strain(s) | Description | Sensitivity to phagea: |
|||
|---|---|---|---|---|---|
| JSF9 | JSF10 | JSF12 | JSF15 | ||
| S224, O395, S262, S263, L355, L396, L547, L362, B36921, C19385, C19751, D19316, E14850, E14983, AE4727, AE2883, AE4731, AE7471, AE7485 | V. cholerae O1 classical biotype strains | S | R | S | S |
| C6706, 2924281, 2868921, 2868912, AF-1471, AL-30457, AM-33126, AN-32320, G-8747, G-3669, AP-13543, AM-33122, MG-116955, AN-24088, AO-29054, 2614, 3055, 112V214 | V. cholerae O1 El Tor biotype strains | R | R | R | R |
| C-0273, C-1771, C-0270 | V. cholerae O139 strains | R | R | R | R |
| Arg-03 | Nontoxigenic V. cholerae O139 | R | S | R | R |
| V46, V51 | V. cholerae O141 strains | R | R | R | R |
| C6706, crp::TnFGL3; 2614, crp::TnFGL3; 3055, crp::TnFGL3; 112V214, crp::TnFGL3 | Derivatives of El Tor strains, unable to produce cAMP receptor protein (CRP) | S | S | S | S |
| C6706, cyaA::TnFGL3; 2614, cyaA::TnFGL3; 3055, cyaA::TnFGL3; 112V214, cyaA::TnFGL3 | Derivatives of El Tor strains, carrying mutation in cyaA gene encoding adenylate cyclase; impaired in synthesis of cAMP | S | S | S | S |
| S262, crp::TnFGL3 | Derivative of classical strain, with mutation in crp gene | S | S | S | S |
| S262, cyaA::TnFGL3 | Derivative of classical strain, with mutation in cyaA gene | S | S | S | S |
| C-0273, crp::TnFGL3; C-771, crp::TnFGL3; C-0270, crp::TnFGL3 | V. cholerae O139 carrying mutations in crp gene encoding CRP | R | R | R | R |
| C-0273, cyaA::TnFGL3; C-1771, cyaA::TnFGL3; C-0270, cyaA::TnFGL3 | V. cholerae O139 carrying mutation in cyaA gene encoding adenylate cyclase | R | R | R | R |
| V46, crp::TnFGL3; V51, crp::TnFGL3 | V. cholerae O141 carrying mutation in crp gene | R | R | R | R |
| V46, cyaA::TnFGL3; V51, cyaA::TnFGL3 | V. cholerae O141 carrying mutation in cyaA gene | R | R | R | R |
Similar numbers of phage particles (∼8.1 × 108) were plated on different bacterial lawns. S, susceptible (plaque counts, 6.9 × 108 to 8.1 × 108); R, resistant (no plaques detected).
We further examined whether the resistance of the different wild-type V. cholerae strains to these phages was due to possible lysogeny of the phages in these strains, causing the bacteria to become immune to further infection by the same phage. All V. cholerae strains listed in Table 1 were probed with the phage DNA in colony blot hybridization. However, none of the strains hybridized with any of the 4 phage DNA probes, thus ruling out the possibility of the presence of these phage genomes in the bacteria in a lysogenic form.
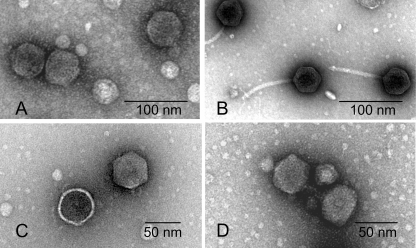
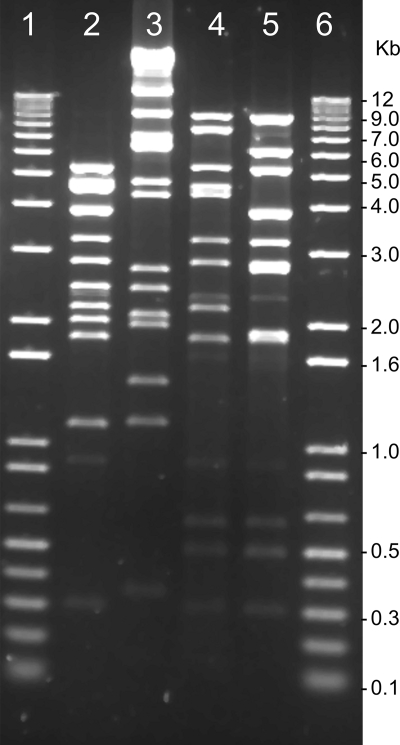
Electron microscopic examination of the morphology of the phages revealed that all these phages had hexagonal heads, had tails of various lengths (Fig. 1; Table 2), and belonged to defined phage families (1). Restriction endonuclease cleavage patterns and cross-hybridization analysis of the phage genomes suggested that although some of the phages shared DNA homology, all of them produced distinct restriction profiles (Fig. 2; Table 2) and were hence genetically distinguishable.
FIG. 1.
Electron micrographs of different phages, JSF9 (A), JSF10 (B), JSF12 (C), and JSF15 (D). The phages had hexagonal heads and tails of various lengths and belonged to defined phage families (Table 2).
FIG. 2.
Restriction endonuclease cleavage patterns of different phage genomes. Lanes 2 through 5 show BglI restriction patterns of DNA isolated from JSF9, JSF10, JSF12, and JSF15, respectively. Lanes 1 and 6 show 1-kb DNA Ladder Plus (Invitrogen) used as molecular size markers.
Mutations in cya or crp genes increase phage susceptibility of V. cholerae O1 strains.
We identified two defined transposon insertion mutants of strain C6706 which were susceptible to 4 different phages, while the parent strain was completely resistant to these phages. These two mutants, EC14235 and EC13352, respectively, carried insertions in the crp gene encoding cyclic AMP (cAMP) receptor protein (CRP) and in the cyaA gene encoding adenylate cyclase, the enzyme required for conversion of ATP to cAMP. Initial screening of the library comprising 3,156 mutants using 6 different phages led to the identification of these two mutants which were susceptible to phage JSF9, whereas the parent strain was resistant. Later these mutants were used as indicator strains to identify the 3 additional phages, JSF10, JSF12, and JSF15, from environmental water samples. All the 4 phages produced clear plaques on lawns of these two mutant strains, as well as high phage titers (∼108 PFU/ml) when grown with these strains in LB medium. None of the wild-type El Tor biotype strains and none of the other mutants in the library except the cyaA and crp mutants were susceptible.
To further verify the effect of cAMP and CRP mutations, we incorporated similar mutations in a number of other V. cholerae strains by chitin-induced transformation and recombination (16, 21) with genetically marked DNA carrying insertions in cyaA and crp genes (Table 1). The resulting mutants of the phage-resistant El Tor strains also became completely susceptible to infection by each of the 4 different phages (Table 3). Similarly, corresponding cyaA and crp mutants of a classical strain, S262, became susceptible to JSF10, while the parent strain was resistant. However, V. cholerae O139 and O141 strains subjected to the same mutations remained resistant to the phage despite sustaining the cAMP and CRP mutations (Table 3). Thus, the cAMP-CRP signaling system negatively regulates the phage susceptibility of V. cholerae O1 strains in particular, and the observed phage susceptibility of these strains was not due to possible growth defects arising from mutations in these ubiquitous regulatory genes.
The effect of cyaA and crp mutations occurs at the level of phage adsorption.
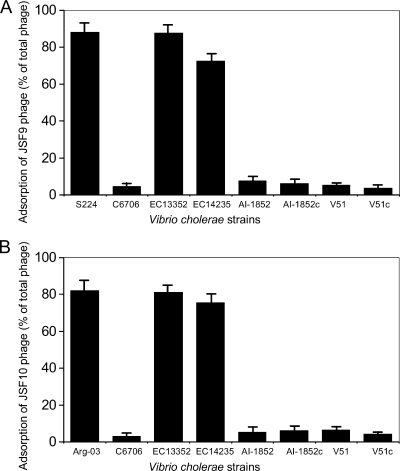
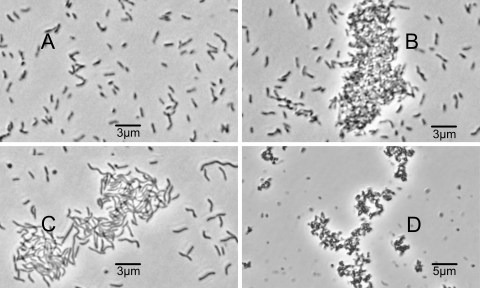
The adsorption of different phages to V. cholerae strains was studied both by a quantitative method and qualitatively by observing bacterial aggregation in the presence of phage by microscopy. The phage susceptibilities of different strains correlated with the abilities of the strains to adsorb these phage particles (Fig. 3; Table 3) and form bacterial aggregates (Fig. 4) in the presence of the phage. There was no apparent difference in the affinities of the 4 different phages for a particular bacterial strain. Whereas the phage-resistant El Tor strains did not agglutinate in the presence of any of these phages, the phage-susceptible mutants formed agglutinates (Fig. 4). This observation and the quantitative data on phage adsorption (Fig. 3) suggested that the effect of cyaA and crp mutations on the susceptibility of V. cholerae O1 strains to these phages occurred at the level of phage adsorption, possibly due to the availability of a functional phage receptor. Hence, in wild-type V. cholerae O1 strains which are resistant to the phages and fail to adsorb phage particles, the cAMP-CRP system may be involved in downregulation of the phage receptor on the target bacteria.
FIG. 3.
Adsorption of phages JSF9 (A) and JSF10 (B) to different V. cholerae strains. The descriptions of the strains are as follows. Strain S224, V. cholerae O1 classical biotype; C6706, El Tor; EC13352, derivative of strain C6706 defective in synthesis of adenylate cyclase; EC14235, derivative of strain C6706, defective in synthesis of cAMP receptor protein (CRP); AI-1852, O139; AI-1852c, derivative of strain AI-1852 defective in synthesis of adenylate cyclase; V51, O141; V51c, derivative of strain V51, defective in synthesis of adenylate cyclase; Arg-03, nontoxigenic V. cholerae O139 strain. Strains S224, EC13352, EC14235, and Arg-03 were susceptible to the different phages in the plaque assay. The phages were originally isolated from water using S224 and Arg-03 as indicator strains for JSF9 and JSF10, respectively.
FIG. 4.
The susceptibilities of different V. cholerae strains to diverse phages correlated with the formation of bacterial cell clumps in the presence of the phage. Clump formations in the presence of phage JSF9 by different strains are shown. The identities of strains and their susceptibilities were as follows: (A) strain C6706 (El Tor), phage resistant; (B) strain EC13352 (C6706; cyaA::TnFGL3), phage susceptible; (C) strain EC14253 (C6706; crp::TnFGL3), phage susceptible; and (D) strain S224 (classical), phage susceptible. The CRP-negative strain shows an elongated cell morphology.
Use of cyaA-mutated strain in environmental phage monitoring.
We tested the applicability of the cyaA-mutated derivative of El Tor strain C6706 as a convenient indicator strain and compared its applicability to that of the parent strain for monitoring phage prevalence in the environment. Aliquots of the same water samples were plated on different soft agar lawns prepared with the mutant and the parent strain. The detection of different phages and their apparent concentrations are presented in Table 4. It may be mentioned that the 3 additional phages, JSF10, JSF12, and JSF15, were isolated from this assay and were further characterized. The phages isolated using the cyaA mutant of C6706 as the indicator strain produced clear plaques on cyaA mutants as well as on crp mutants derived from other El Tor strains. Taken together, these results suggested that the cyaA and crp mutants were useful as indicator strains for enhanced estimation of V. cholerae O1-specific phages in environmental waters.
TABLE 4.
Detection of different phages in environmental water samples in Bangladesh using V. cholerae strain C6706 and its cyaA mutant as indicator strains during October 2008 and March 2009
| Date of sampling | No. of samples analyzeda | Estimated mean phage concn (PFU/ml) with different indicator strains (mean ± SD) |
P valueb | Phagesc identified | |
|---|---|---|---|---|---|
| C6706 | EC14235 | ||||
| October to December 2008 | 10 | 60.0 ± 31.6 | 155.0 ± 72.4 | 0.0005 | JSF2, JSF4, JSF10, JSF12 |
| January to March 2009 | 12 | 62.5 ± 22.6 | 116.6 ± 38.9 | 0.0011 | JSF9, JSF11, JSF15, JSF16 |
Samples were collected from 9 different environmental surveillance sites described previously (10) along Gulshan Lake, the Buriganga River, and the Turag River, in Dhaka, Bangladesh. Samples were pooled and tested for V. cholerae-specific phages.
The two-tailed P values from Mann-Whitney tests are shown.
Phages JSF2 and JSF4 have been described previously (10).
DISCUSSION
Molecular epidemiological surveillance of cholera has revealed temporal changes in the predominant clones of toxigenic V. cholerae associated with the disease and occasional replacement of one clone by another (9). However, the genetic, epidemiological, or ecological factors which lead to the occasional elimination of one clone of V. cholerae and promote the emergence of another clone in regions where the disease is endemic are not clear. Although pathogenic strains of V. cholerae cause severe infections in humans, V. cholerae as a species is a part of the normal aquatic flora, and presumably environmental factors may influence the predominance of diverse V. cholerae strains (7). Recent studies have shown that environmental bacteriophages acting on V. cholerae can significantly contribute to the distribution and abundance of different clones of V. cholerae (10, 11).
The ability to resist predation by one or more environmental phages that would kill a competing clone understandably provides increased fitness to the resistant clone. In this study we attempted to identify bacterial genes that presumably contribute to the resistance of certain groups of strains against particular environmental phages. Importantly, if the expression of these genes fluctuates under various environmental parameters, the bacteria may also be able to maintain a low concentration of the phage through conditional susceptibility and possibly use them against other competing bacteria. Interestingly, in this study, the mutant strains which became susceptible to infection by 4 different phages to which the parent strains were resistant carried insertions in the genes for production of cAMP or the cAMP receptor protein, either of which takes part in diverse metabolic regulations in different organisms.
The lysogeny of phage P22 in Salmonella enterica serovar Typhimurium is influenced by the cAMP-CRP system, in that wild-type strains are more readily lysogenized than are their cAMP or CRP mutants, and hence, these mutants are more susceptible to lysis (13). However, none of the wild-type strains in the present study was lysogenized by any of these 4 phages, and hence, the susceptibility of cAMP- or CRP-negative mutants in this study was not related to entering the lytic cycle instead of lysogeny.
Incorporation of the cyaA or crp mutations in a number of V. cholerae O1 strains showed similar alterations in their susceptibility to the phages studied. However, cAMP and CRP mutations in V. cholerae strains belonging to the O139 and O141 strain groups did not make these strains susceptible to these phages (Table 3). Therefore, the phage susceptibility of the cAMP- or CRP-negative V. cholerae O1 mutant strains was not an artifact due to a general growth defect caused by the absence of these regulators. Instead, the effect of cAMP-CRP is probably targeted toward certain surface components of V. cholerae O1 and, more particularly, of the El Tor biotype strains. The intracellular concentration of cAMP has been previously shown to affect membrane transport activity, fatty acid composition, and synthesis of certain membrane components in different bacteria (3, 8, 21). Thus, cyaA or crp may be associated with the expression of regulated phage receptors in certain groups of bacteria in contrast to other phage-susceptible strains in which the phage receptors are constitutively expressed.
The bacterial surface structures often used by phages as a receptor for adsorption to bacteria include lipopolysaccharide O antigen, outer membrane proteins (OMP), and pili. Previous studies have shown that cAMP and its receptor protein CRP can markedly influence the composition of these structures (12, 15, 17, 22). In Escherichia coli the downregulation of outer membrane proteins by noncoding RNAs has been shown to be controlled by a cAMP-CRP-dependent regulatory cascade (15). Insertion mutations in cya and crp genes in V. cholerae O1 have been shown to derepress the expression of cholera toxin and toxin-coregulated pilus (TCP), suggesting that the cAMP-CRP system negatively modulates the expression of these genes which are known to be coordinately regulated by ToxR, a transmembrane DNA-binding regulatory protein (22).
The involvement of the cAMP-CRP signaling system with the ToxR regulon is likely to have more profound effects in Vibrio cholerae, since ToxR regulates the expression of at least 20 genes, including those encoding important virulence factors and outer membrane proteins (17). For example, the outer membrane protein OmpT is known to be repressed by ToxR. Comparison of ompT transcription levels in the isogenic crp, toxR, and crp toxR mutants revealed that in the absence of ToxR, constitutive high-level ompT transcription is dependent on CRP; thus, both CRP and ToxR are required for the regulation of OmpT expression (17). Other changes in the bacterial surface structure that are influenced by the cAMP-CRP system include the levels of Vibrio exopolysaccharide (VPS) and matrix proteins required for bacterial biofilm formation. The ability of V. cholerae to form biofilms is crucial for its survival in aquatic habitats between epidemics and is advantageous for host-to-host transmission during epidemics. Biofilm formation is positively controlled by the transcriptional regulators VpsR and VpsT and is negatively regulated by the quorum-sensing transcriptional regulator HapR, as well as the cAMP-CRP regulatory complex (12). The interactions of phages with bacterial biofilms may depend on the susceptibility of the biofilm bacteria to the phage and the availability of receptors (24). If the phage possesses polysaccharide-degrading enzymes, or if considerable cell lysis is effected by the phage, the integrity of the biofilm may be destroyed. Alternatively, coexistence between phage and host bacteria within the biofilm may develop. Hence, the effect of cAMP-CRP on biofilm formation may also have a broader effect on the overall phage bacterial dynamics and their ecology.
Although this study did not precisely identify the receptor for the different phages studied, our assays confirmed that the differences in susceptibilities to the various phages occurred at the level of phage adsorption (Fig. 3). Further studies are under way to identify the relevant phage receptors and more precisely understand their expression in different V. cholerae strains. The ability of the El Tor strains to survive attack by three of the phages under conditions in which classical strains are susceptible might have involved inhibition of phage adhesion to El Tor strains through possible downregulation of the phage receptor. This difference in phage susceptibility between the classical and El Tor biotype strains remains to be explained. It seems likely that the receptor for these phages in most classical strains is constitutively expressed, whereas that in the El Tor biotype strains is negatively regulated by a cAMP-dependent mechanism, which may also involve other regulatory components. It may be mentioned that certain important differences between the regulation of gene expression in the classical biotype and that in the El Tor biotype of V. cholerae O1 are known to exist (4).
Recent studies of cholera phages are beginning to reveal that the prevalence of phages in the environment may influence the epidemiology of cholera and perhaps other waterborne bacterial infections (10, 11, 14, 19). Monitoring of environmental phage concentration involves the use of indicator bacterial strains on which the phages readily form plaques in a soft agar plaque assay. The findings that cyaA and crp mutants of El Tor strains are susceptible to certain phages to which the wild-type El Tor strains are resistant led us to examine the possibility that these mutants can be used instead of their parent strains as more sensitive indicator strains to estimate phage prevalence in the environment. Our results suggested that indeed these mutant strains have applicability in monitoring environmental phage prevalence, since significantly more phages were detected by these indicator strains than by their wild-type parent strains. Presumably, this difference arose from the fact that the wild-type El Tor strains resist predation by multiple environmental phages through a cAMP-CRP-dependent mechanism, and hence, plaque formation is not observed on a lawn of the bacteria. Besides, although the wild-type strain was not lysogenized by JSF9, JSF10, JSF12, or JSF15 phage used in this study, the existence of other environmental phages which might enter into a lysogenic form aided by the cAMP-CRP system (13) and hence confer on the bacteria immunity to infection by a similar phage cannot be ruled out.
In conclusion, the results of this study suggest that the ability to resist predation by multiple environmental phages might have contributed to the evolutionary fitness of V. cholerae O1. Furthermore, the phage resistance is conferred through a process involving the cAMP-CRP signaling system. Hence, this system may effectively alter a bacterial property that confers improved evolutionary fitness in an environment where phage predation modulates the prevalence of different bacterial clones, often associated with disease.
Acknowledgments
This research was funded in part by National Institutes of Health grants RO1-GM068851 and RO1-AI070963 under different subagreements between the Harvard Medical School and the ICDDR,B. The ICDDR,B is supported by countries and agencies which share its concern for the health problems of developing countries.
Footnotes
Published ahead of print on 14 May 2010.
REFERENCES
- 1.Ackermann, H.-W. 1987. Bacteriophage taxonomy. Microbiol. Sci. 4:214-218. [PubMed] [Google Scholar]
- 2.Albert, M. J., N. A. Bhuyan, A. Rahman, A. N. Ghosh, K. Hultenby, A. Weintraub, S. Nahar, A. K. M. G. Kibriya, M. Ansaruzzaman, and T. Shimada. 1996. Phage specific for Vibrio cholerae O139 Bengal. J. Clin. Microbiol. 34:1843-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alper, M. D., and B. N. Ames. 1978. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J. Bacteriol. 133:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyhan, S., A. D. Tischler, A. Camilli, and F. H. Yildiz. 2006. Differences in gene expression between the classical and El Tor biotypes of Vibrio cholerae O1. Infect. Immun. 74:3633-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brussow, H., C. Canchaya, and W. Hardt. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68:560-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, D. E., J. M. Urbach, and J. J. Mekalanos. 2008. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 105:8736-8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colwell, R. R., and W. M. Spira. 1992. The ecology of Vibrio cholerae, p. 107-127. In D. Barua and W. B. Greenough III (ed.), Cholera. Plenum Medical Book Co., New York, NY.
- 8.Dallas, W. S., Y. Tseng, and W. J. Dobrognsz. 1976. Regulation of membrane functions and fatty acid composition in Escherichia coli by cyclic AMP receptor protein. Arch. Biochem. Biophys. 175:295-302. [DOI] [PubMed] [Google Scholar]
- 9.Faruque, S. M., M. J. Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62:1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faruque, S. M., I. B. Naser, M. J. Islam, A. S. G. Faruque, A. N. Ghosh, G. B. Nair, D. A. Sack, and J. J. Mekalanos. 2005. Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc. Natl. Acad. Sci. U. S. A. 102:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faruque, S. M., M. J. Islam, Q. S. Ahmad, A. S. G. Faruque, D. A. Sack, G. B. Nair, and J. J. Mekalanos. 2005. Self-limiting nature of seasonal cholera epidemics: role of host-mediated amplification of phage. Proc. Natl. Acad. Sci. U. S. A. 102:6119-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fong, J. C., and F. H. Yildiz. 2008. Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J. Bacteriol. 190:6646-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong, J., G. R. Smith, and B. N. Ames. 1971. Adenosine 3′:5′-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc. Natl. Acad. Sci. U. S. A. 68:2258-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen, M. A., S. M. Faruque, J. J. Mekalanos, and B. R. Levin. 2006. Modeling the role of bacteriophage in the control of cholera outbreaks. Proc. Natl. Acad. Sci. U. S. A. 103:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansen, J., M. Eriksson, B. Kallipolitis, and P. Valentin-Hansen. 2008. Down-regulation of outer membrane proteins by noncoding RNAs: unraveling the cAMP-CRP and sigmaE-dependent CyaR-ompX regulatory case. J. Mol. Biol. 383:1-9. [DOI] [PubMed] [Google Scholar]
- 16.Kaper, J. B., J. G. Morris, and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, C. C., D. S. Merrell, A. Camilli, and J. B. Kaper. 2002. ToxR interferes with CRP-dependent transcriptional activation of ompT in Vibrio cholerae. Mol. Microbiol. 43:1577-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meibom, K. L., M. Blokesch, N. A. Dolganov, C. Wu, and G. K. Schoolnik. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824-1827. [DOI] [PubMed] [Google Scholar]
- 19.Nelson, E. J., J. B. Harris, J. G. Morris, Jr., S. B. Calderwood, and A. Camilli. 2009. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 7:693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman, M. H., K. Biwas, M. A. Hossain, R. B. Sack, J. J. Mekalanos, and S. M. Faruque. 2008. Distribution of genes for virulence and ecological fitness among diverse Vibrio cholerae population in a cholera endemic area: tracking the evolution of pathogenic strains. DNA Cell Biol. 27:347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saier, M. H., M. R. Schmidt, and M. Leibowitz. 1978. Cyclic AMP-dependent synthesis of fimbriae in Salmonella typhimurium: effects of cya and pts mutations. J. Bacteriol. 134:356-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skorupski, K., and R. K. Taylor. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 94:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stent, G. S. 1963. Molecular biology of bacterial viruses, p. 94-96. W. H. Freeman & Co., San Francisco, CA.
- 24.Suthereland, I. W., K. A. Hughes, L. C. Skillman, and K. Tait. 2004. The interaction of phage and biofilms. FEMS Microbiol. Lett. 232:1-6. [DOI] [PubMed] [Google Scholar]
- 25.Udden, S. M. N., M. S. H. Zahid, K. Biswas, Q. S. Ahmad, A. Cravioto, G. B. Nair, J. J. Mekalanos, and S. M. Faruque. 2008. Acquisition of classical CTX prophage from Vibrio cholerae O141 by El Tor strains aided by lytic phages and chitin-induced competence. Proc. Natl. Acad. Sci. U. S. A. 23:11951-11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous bacteriophage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 27.Zahid, M. S. H., S. M. N. Udden, A. S. G. Faruque, S. B. Calderwood, J. J. Mekalanos, and S. M. Faruque. 2008. Effect of phage on the infectivity of Vibrio cholerae and emergence of genetic variants. Infect. Immun. 76:5266-5273. [DOI] [PMC free article] [PubMed] [Google Scholar]