Abstract
Erythema multiforme (EM) is usually caused by drug reactions or virus infection. We report a case of secondary syphilis presenting as EM in an HIV-infected patient, proved by immunohistochemical staining, which is rare in the literature. It is valuable to determine the etiology of EM to optimize treatment.
CASE REPORT
A 26-year-old homosexual man presented to our hospital with a 5-day history of fever episodes and pruritic skin rashes all over. The lesions had initially developed on his upper limbs and then extended to the entire body. He had been healthy except for one episode of chronic diarrhea due to Giardia infection that was completely treated 2 months previously and one episode of a perioral herpetic ulcer more than 6 months previously. He had no history of drug use before the occurrence of skin eruptions.
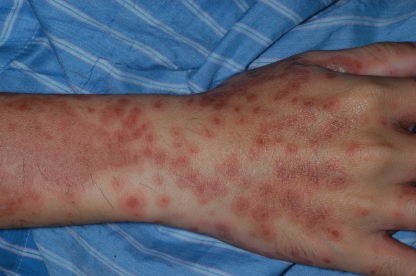
He was well oriented. His body temperature was 37.6°C, blood pressure was 128/66 mm Hg, pulse was 83 per minute, and respiratory rate was 18 per minute. On physical examination, there were numerous discrete, coin-sized annular erythemas with central dusky red areas (target lesions) over his four limbs, trunk, head, and neck (Fig. 1). The involved area was about 90% of the total body surface. Focal confluent patches and crusted erosions were also noted. The cutaneous lesions suggested erythema multiforme (EM). Other physical findings were unremarkable.
FIG. 1.
Numerous coin-sized target lesions with confluent patches presenting on the patient's entire body.
Laboratory tests disclosed a white blood cell count of 6,800/mm3 with 3% eosinophils, hemoglobin level of 11.6 g/dl, platelet count of 397,000/mm3, and C-reactive-protein level of 1.69 mg/dl. Serum creatinine and electrolyte levels and tests of liver function were normal. An enzyme-linked immunosorbent assay (ELISA) for human immunodeficiency virus (HIV) was positive. HIV infection was then confirmed by Western blotting. His CD4 count was 482/mm3, and the HIV viral load was 288,000 copies/ml (Roche Diagnostics, Basel, Switzerland). A venereal disease research laboratory (VDRL) test was positive with a titer of 1:128, and a Treponema pallidum hemagglutination assay (TPHA) (11, 13) was reactive with a titer of 1:2,560. A serological survey for recent infections by herpes simplex virus (HSV), cytomegalovirus (CMV), and toxoplasma was negative. Bacterial and fungal cultures from blood were also negative.
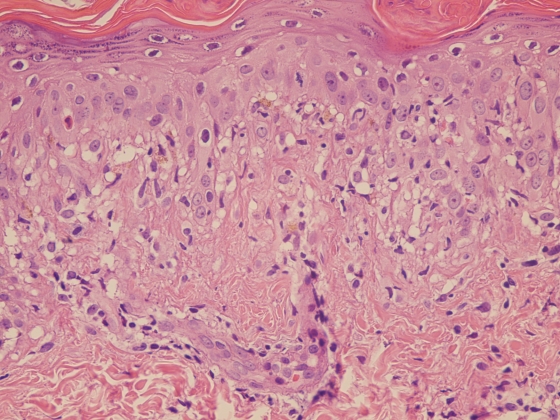
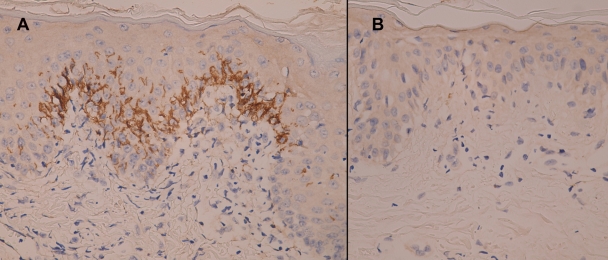
A skin specimen was taken from a lesion site. Microscopically, a hematoxylin-and-eosin-stained section showed mild hyperkeratosis, mild parakeratosis, lymphocytic exocytosis, and a few apoptotic keratinocytes in the epidermis (Fig. 2). Focal vacuolar degeneration of the basal cell layer and superficial perivascular lymphohistiocytic infiltration were also noted. The pathological features were compatible with EM. Further immunohistochemical study with an antispirochete antibody (Biocare Medical, California) (7) showed many intraepidermal spirochetes in the area of vacuolar degeneration (Fig. 3 A). However, no spirochetes were detected in the normal paralesional epidermis (Fig. 3B).
FIG. 2.
Vacuolar degeneration of the basal cell layer with a few apoptotic keratinocytes and perivascular lymphohistiocyte infiltration were noted in the lesion (hematoxylin and eosin; original magnification, ×400).
FIG. 3.
(A) Abundant intraepidermal spirochetes were identified in the area of vacuolar degeneration (immunohistochemical staining with a polyclonal antibody to spirochetes; magnification, ×400). (B) Spirochetes were not found in the paralesional site, which showed little inflammation in the hematoxylin-and-eosin section (immunohistochemical staining with a polyclonal antibody to spirochetes; magnification, ×400).
After clinicopathological correlation, this HIV-infected patient was diagnosed with T. pallidum-induced EM. He was treated with benzathine penicillin G (2.4 million units) by intramuscular injection once weekly for 3 consecutive weeks. His fever episodes and skin lesions gradually subsided, with some desquamation and postinflammatory hyperpigmentation of the skin.
To the best of our knowledge, cutaneous manifestations of secondary syphilis presenting as EM-type eruptions are very rare in HIV-infected patients in the literature. Only one case was reported in a Japanese article in 2005, in which a 28-year-old HIV-infected Australian man was found to have EM-like eruptions with spirochetes detected by immunostaining using an anti-Treponema pallidum antibody (12). Two other cases of secondary syphilis-related EM-like lesions were reported for immunocompetent adults (9, 10).
It is valuable for physicians to determine the etiology of EM in order to optimize treatment. An EM-type eruption, a kind of fixed circular erythematous patch with a central necrotic change or blistering, is considered a hypersensitivity reaction to various agents, such as foreign antigens, drugs, or infectious agents (8, 14). Drugs reported to give rise to EM include antibiotics, such as sulfonamides, aminopenicillins, cephalosporins, quinolones, tetracyclines, anticonvulsants, nonsteroidal anti-inflammatory drugs, antifungal agents, and others (1, 2). Infectious agents associated with EM include viruses, bacteria, fungi, parasites, and others (1, 6). HSV infection and adverse reactions to drugs account for the most common causes of EM in HIV-infected patients (4); however, all of these etiologies were excluded in our case.
The pathology of the EM lesion in our case showed typical vacuolar interface dermatitis with scattered necrotic keratinocytes and few perivascular plasma cells. There were no eosinophils in the inflammatory infiltrates. This picture was compatible with the diagnosis of EM, rather than the conventional papulosquamous lesion in secondary syphilis, which is characterized by psoriasiform hyperplasia and perivascular plasma cell infiltration (8, 10). Our immunohistochemical staining demonstrated abundant spirochetes in the lesional epidermis (i.e., T. pallidum, after correlations with the serological results of VDRL and TPHA), while no spirochetes were identified in the normal paralesional area. This finding might establish the causative role of spirochetes in inducing the change of interface dermatitis and keratinocyte necrosis, which clinically represented the EM eruptions. As a result, this patient responded well to standard penicillin treatment, and his symptoms resolved after treatment.
The traditional method for detecting spirochetes in tissue sections is the silver stain using either the Steiner modification of the Dieterle technique or the Warthin-Starry technique, which is hard to interpret due to marked background staining (7). Immunohistochemical staining with an antispirochete antibody, which consists of a rabbit purified IgG fraction, demonstrates better sensitivity and specificity than silver staining in localizing tissue spirochetes (7). Another useful ancillary tool in the diagnosis of syphilis is PCR. PCR can detect fewer organisms and can be complementary to immunohistocheminal staining in identifying T. pallidum from skin biopsy specimens (3, 9).
There is a high incidence of coinfection with HIV and T. pallidum among homosexual men (5). In HIV-infected patients with EM eruptions and elevated VDRL and TPHA titers, a diagnosis of EM-type syphilis cannot be made solely on the serology results because the eruptions may result from other causes, like viruses or drugs. Hence, we recommend a routine special stain or PCR method in order to identify spirochetes in the EM lesions of HIV-infected patients with elevated VDRL and TPHA titers.
In conclusion, we have reported a case of an HIV-infected patient with EM eruptions. Secondary syphilis should be included in the differential diagnosis when approaching such patients. Further, skin biopsy and special staining are essential for determining the causative relationship between spirochetes and EM lesions, especially when the patient's VDRL and TPHA titers are elevated. Such relationships would influence the treatment policy.
Acknowledgments
We thank Tseng-Tong Kuo (Department of Pathology, Chang Gung Memorial Hospital, Taipei, Taiwan) for his assistance with immunohistochemical staining for spirochetes.
Footnotes
Published ahead of print on 26 May 2010.
REFERENCES
- 1.Al-Johani, K. A., S. Fedele, and S. R. Porter. 2007. Erythema multiforme and related disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 103:642-654. [DOI] [PubMed] [Google Scholar]
- 2.Auquier-Dunant, A., M. Mockenhaupt, L. Naldi, O. Correia, W. Schroder, and J. C. Roujeau. 2002. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch. Dermatol. 138:1019-1024. [DOI] [PubMed] [Google Scholar]
- 3.Behrhof, W., E. Springer, W. Brauninger, C. J. Kirkpatrick, and A. Weber. 2008. PCR testing for Treponema pallidum in paraffin-embedded skin biopsy specimens: test design and impact on the diagnosis of syphilis. J. Clin. Pathol. 61:390-395. [DOI] [PubMed] [Google Scholar]
- 4.Coopman, S. A., R. A. Johnson, R. Platt, and R. S. Stern. 1993. Cutaneous disease and drug reactions in HIV infection. N. Engl. J. Med. 328:1670-1674. [DOI] [PubMed] [Google Scholar]
- 5.Dylewski, J., and M. Duong. 2007. The rash of secondary syphilis. CMAJ 176:33-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farthing, P., J. V. Bagan, and C. Scully. 2005. Mucosal disease series. Number IV. Erythema multiforme. Oral Dis. 11:261-267. [DOI] [PubMed] [Google Scholar]
- 7.Hoang, M. P., W. A. High, and K. H. Molberg. 2004. Secondary syphilis: a histologic and immunohistochemical evaluation. J. Cutan. Pathol. 31:595-599. [DOI] [PubMed] [Google Scholar]
- 8.Huff, J. C., W. L. Weston, and M. G. Tonnesen. 1983. Erythema multiforme: a critical review of characteristics, diagnostic criteria, and causes. J. Am. Acad. Dermatol. 8:763-775. [DOI] [PubMed] [Google Scholar]
- 9.Kim, Y. Y., J. H. Lee, S. Y. Yoon, J. D. Lee, and S. H. Cho. 2007. Erythema multiforme-like targetoid lesions in secondary syphilis. Acta Derm. Venereol. 87:381-382. [DOI] [PubMed] [Google Scholar]
- 10.Lee, J. Y., and E. S. Lee. 2003. Erythema multiforme-like lesions in syphilis. Br. J. Dermatol. 149:658-660. [DOI] [PubMed] [Google Scholar]
- 11.Lesinski, J., J. Krach, and E. Kadziewicz. 1974. Specificity, sensitivity, and diagnostic value of the TPHA test. Br. J. Vener. Dis. 50:334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okubo, R., S. Oyake, and R. Tsuboi. 2005. Secondary syphilis with HIV infection presenting erythema multiforme-like eruption. Rinsho Derma 47:134-135. [Google Scholar]
- 13.O'Neill, P. 1976. A new look at the serology of treponemal disease. Br. J. Vener. Dis. 52:296-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tonnesen, M. G., and N. A. Soter. 1979. Erythema multiforme. J. Am. Acad. Dermatol. 1:357-364. [DOI] [PubMed] [Google Scholar]