Abstract
We report eight cases of airway colonization by Geosmithia argillacea in patients with cystic fibrosis. This filamentous fungus, resembling members of the genera Penicillium and Paecilomyces, was identified by molecular analysis. All patients carried a mutation on each CFTR (cystic fibrosis transmembrane conductance regulator) allele, with at least one copy of the F508del mutation. The first isolation of this fungus occurred from F508del-homozygous patients at a younger age than in F508del-heterozygous patients. Before recovery of G. argillacea, all patients were treated with itraconazole; two of them had also received voriconazole for an Aspergillus fumigatus infection. However, antifungal susceptibility patterns showed high MICs of voriconazole for all isolates, and high MICs of amphotericin B and itraconazole for the majority of them, but mostly low minimum effective concentrations (MECs) of caspofungin. The appearance and persistence of G. argillacea in the airways were not associated with exacerbation of the disease. However, the clinical implications of G. argillacea, particularly in immunocompromised patients, remain a concern, particularly given recent observations suggesting that this fungus may also cause disseminated infections.
With a frequency of about 1/2,500 births in France, cystic fibrosis (CF) is the most common genetic inherited disease in the European Caucasian population (3). The disease is caused by mutations in the CFTR (cystic fibrosis transmembrane conductance regulator) gene, which encodes a chloride channel in the plasma membranes of various epithelial cell types. Several organs are affected, but the prognosis of CF essentially depends on the severity of lesions in the lungs (4). The defect in the chloride channel leads to thickening of the bronchial mucus, facilitating the entrapment of the inhaled bacteria and fungal conidia and providing a suitable environment for the growth of microorganisms. Nevertheless, airway colonization by filamentous fungi in CF is rarely found in young children and usually follows episodes of bacterial infection (mainly due to Staphylococcus aureus or Pseudomonas aeruginosa). Thus, previous bronchoalveolar epithelial lesions related to these infections may be required for the establishment of fungi in the respiratory tract. The clinical significance of isolating filamentous fungi from respiratory secretions remains a matter of debate. Recent studies, however, clearly show that they cause increased morbidity and an increased number of hospital admissions (1).
Aspergillus fumigatus, Scedosporium apiospermum, and Exophiala dermatitidis are the most common clinically relevant fungi and are usually responsible for chronic airway colonization (13). Other filamentous fungi that are frequently, but only transiently, present in respiratory secretions include Paecilomyces variotii and some species belonging to the genus Alternaria, Cladosporium, or Penicillium. Some thermophilic filamentous fungi have also been described in humans, almost exclusively in CF patients. Indeed, we have previously described cases of chronic colonization by Penicillium emersonii (the anamorph state of Talaromyces emersonii) (6) and Acrophialophora fusispora (7) in CF patients.
Here we report colonization by Geosmithia argillacea in eight CF patients attending three different French hospitals (Angers, Giens, and Rouen) between 1999 and 2009. All the isolates were identified by sequencing the ribosomal DNA (rDNA) genes. Additionally, the in vitro antifungal susceptibilities of the isolates were compared to the evolution of airway colonization during antifungal treatment.
MATERIALS AND METHODS
Isolates and culture conditions.
Thirty-six clinical isolates were detected from respiratory secretions of eight patients who were followed up in hospitals at Rouen (two patients), Giens (four patients), or Angers (two patients) between 1999 and 2009. Some isolates could not be subcultured due to the concomitant growth of other microorganisms. All available isolates were freeze-dried and deposited at the IHEM Culture Collection in the Scientific Institute of Public Health (Brussels, Belgium) to be publically available. Two isolates from an additional patient (patient I), whom we had described in a previous report as being colonized by P. emersonii (6), were also studied.
Isolates were maintained by regular passage on yeast extract-peptone-dextrose agar (YPDA) plates (containing the following, in g/liter: yeast extract, 5; peptone, 10; glucose, 20; and agar, 20) supplemented with 0.5% chloramphenicol. Cultures were incubated at 37°C (or 25°C for determination of preferred growth temperature). Morphology was studied on YPDA plates both macroscopically and microscopically after mounting in lactic blue stain. For DNA extraction, isolates were grown in yeast extract-peptone-dextrose (YPD) broth.
Analysis of the internal transcribed spacer (ITS) regions.
Mycelium was harvested from 7-day-old cultures in YPD broth (10 ml) and ground in liquid nitrogen with a mortar and pestle. DNA was recovered using the DNeasy plant mini kit (Qiagen Inc., Valencia, CA) protocol with minor modifications, including additional steps for fungal tissue disruption. Samples (0.2 g) were resuspended in 400 μl of AP1 buffer with 4 μl of RNase A solution. Glass beads (0.25 g [0.1 mm in diameter] and 0.25 g [0.5 mm in diameter]) were added, and the mixture was homogenized for 1 min in a Disruptor Genie (Scientific Industries Inc., Bohemia, NY) before incubation at 65°C and twice during the incubation step. DNA was then extracted according to the manufacturer's protocol but using a 50-μl elution volume. The DNA concentration was determined using a NanoDrop spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE) at 260 nm.
The ITS regions of the nuclear rRNA gene were amplified by nested PCR with the outer primer pair V9D and LS266 (8) and the inner primer pair ITS5 (18) and ITSseq rev (5′CCTACCTGATCCGAGGTCAA3′). The first-step PCR mixture (25 μl) contained 75 ng of fungal DNA template, 2 mM MgCl2 (Eurogentec France SASU, Angers, France), 0.2 μM each primer, 0.2 μM each deoxynucleoside triphosphate (dNTP) (Eurogentec), and 1 U of HotGoldStar DNA polymerase (Eurogentec). PCR was performed in a 96-well MyCycler thermal cycler (Bio-Rad Laboratories Inc., Marnes-la-Coquette, France) that was programmed as follows: initial denaturation at 95°C for 10 min; 25 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 45 s; and final extension at 72°C for 5 min. The amplified products were diluted 10-fold, and 5 μl was used for the second amplification step. This second-step PCR was performed as described above but in a final volume of 50 μl and with an increased number (30) of cycles. Final products were subjected to agarose gel electrophoresis and purified using a QIAquick gel extraction kit (Qiagen Inc.). Purified samples were stored at −20°C until they were sent to Eurofins/MWG for sequencing.
Antifungal drug susceptibility testing.
Antifungal susceptibility testing was performed by the microdilution broth reference method of the Antifungal Susceptibility Testing Subcommittee of EUCAST (15). Amphotericin B (Sigma-Aldrich, Saint Quentin Fallavier, France), itraconazole (Janssen-Cilag, Issy-les-Moulineaux, France), voriconazole (Pfizer Central Research, Sandwich, United Kingdom), posaconazole (Schering-Plough Research Institute, Kenilworth, NJ), and caspofungin (Merck & Co., Inc., Rahway, NJ) were obtained as powders of known potency. Briefly, testing was performed for azole drugs and caspofungin in RPMI 1640 medium with l-glutamine, but without sodium bicarbonate, buffered to pH 7.0 with 0.165 M MOPS (morpholinepropanesulfonic acid), with a final inoculum of 105 conidia/ml. Amphotericin B was tested in antibiotic medium 3 (AM3) (Difco, Le Pont de Claix, France) supplemented with 2% glucose. After inoculation, microplates were incubated at 35°C for 48 h and MICs determined visually. For caspofungin, the minimum effective concentration (MEC) was determined by reading microplates with an inverted microscope as recommended by EUCAST (15). The reference strains Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were included to ensure quality control.
Nucleotide sequence accession numbers.
All sequences obtained were deposited in the GenBank database under the accession numbers indicated in Table 1.
TABLE 1.
Geosmithia argillacea isolates recovered from CF patients and GenBank accession numbers of the rRNA gene ITS sequences
| Patient | Isolation date | IHEM no. | GenBank accession no. |
|---|---|---|---|
| A | 18 September 2007 | 22636 | GU165722 |
| 29 November 2007 | 22637 | GU165723 | |
| 5 December 2007 | 22638 | ||
| 26 February 2008 | 22929 | GU165724 | |
| 15 April 2008 | 22931 | ||
| 22 September 2008 | 22894 | GU165725 | |
| 3 November 2008 | 22911 | ||
| B | 14 December 2006 | 22640 | GU165726 |
| 16 February 2007 | NPa | ||
| 14 March 2007 | NP | ||
| C | 10 September 1999 | 16291 | GU165727 |
| 7 December 1999 | 16292 | ||
| D | 31 August 2005 | 22641 | GU165728 |
| 7 November 2007 | 22642 | GU165729 | |
| E | 28 August 2007 | 22643 | GU165730 |
| 9 October 2007 | 22644 | GU165731 | |
| 30 October 2007 | 22645 | GU165732 | |
| 7 January 2008 | 22682 | ||
| 17 January 2008 | 22683 | ||
| 5 February 2008 | NP | ||
| 20 February 2008 | 23433 | GU165733 | |
| 4 June 2008 | NP | ||
| F | 15 February 2008 | 23425 | |
| 2 October 2008 | 22928 | GU165734 | |
| 25 June 2009 | 23426 | ||
| 3 August 2009 | 23427 | ||
| 22 September 2009 | 23428 | ||
| G | 20 April 2007 | 22647 | GU165736 |
| 25 June 2007 | 22899 | GU165737 | |
| 26 June 2007 | 22685 | GU165735 | |
| 12 November 2007 | 22648 | GU165738 | |
| 1 October 2008 | 23431 | GU165739 | |
| 16 March 2009 | 23432 | ||
| H | September 2004 | NP | |
| January 2005 | NP | ||
| 1 February 2009 | 22033 | GU165740 | |
| Ib | 25 September 1997 | 14262 | DQ317583/DQ317584 |
| 27 October 1997 | 22896 | ||
| 5 December 1997 | 23429 | GU165741 | |
| 11 December 1997 | 22898 | ||
| 21 January 1998 | 23430 |
NP, not preserved.
This patient was reported previously (6) as having chronic airway colonization by Penicillium emersonii.
RESULTS
Morphology.
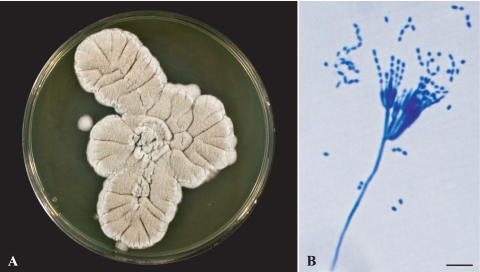
Isolates included in the study were collected from respiratory secretions of eight CF patients who were followed up in Rouen (two patients), Giens (four patients), or Angers (two patients) hospitals. All isolates, which were initially identified as Penicillium sp. or Paecilomyces sp., were morphologically identical, both macroscopically and microscopically. Macroscopic analysis on YPDA plates (Fig. 1 A) showed cream- to beige-colored colonies with no color at the reverse. Growth was slow and restricted at room temperature but was enhanced at 37°C, with colonies reaching 2 to 3 cm in diameter after 7 days at this temperature. Microscopic analysis showed the presence of hyaline, rough-walled, septate, and often branched conidiophores arising from the vegetative hyphae. These conidiophores bore biverticillate or triverticillate-asymmetrical penicilli, which consisted of metulae in verticils of three to five, each bearing 5 to 10 parallel-ranged phialides with a tapering tip (Fig. 1B). Conidia were smooth walled, hyaline to pale brown, and cylindrical to ellipsoidal and measured 3.5 to 5 by 1.5 to 2.7 μm.
FIG. 1.
Morphological features of Geosmithia argillacea. (A) Whitish to olive powdery colonies grown on yeast extract-peptone-dextrose agar. (B) Microscopic analysis with lactic blue stain, showing biverticillate and asymmetrical penicilli consisting of metulae in verticils of three to five, each bearing 5 to 10 phialides with a cylindrical base and a tapering tip which produce one-celled smooth-walled, ellipsoidal to cylindrical conidia. Bar, 10 μm.
Molecular analysis.
The ITS sequences were used in individual BLASTn (Basic Local Alignment Search Tool) searches using NCBI (National Center for Biotechnology Information) BLAST and UNITE databases (11). The two programs produce similar results. The highest identities (between 96 and 100%) for the ITS regions were for Geosmithia argillacea strains with accession numbers (i) AF033389 (strain NRRL 5177; ARS Culture Collection), (ii) EU862337, and (iii) EU862335. The GenBank alignments revealed that the mismatches occurred in both the ITS1 and ITS2 regions. No mismatches occurred in the 5.8S rDNA region.
On the basis of their morphological features, these isolates were initially considered to be P. emersonii. However, sequencing of the ITS regions led to their reclassification as G. argillacea. In light of these results, we retested the fungus isolated in 1997 from respiratory secretions of another CF patient, which had been previously described as P. emersonii. Molecular analysis of two of the available isolates revealed that the initial analysis suffered from misidentification (Table 1).
Patients.
We did not find any correlation between G. argillacea colonization and patient sex (Table 2). All of our patients carried a mutation on each allele, with at least one copy of the F508del. Five of the eight patients, as well as patient I, who was initially described as being colonized by P. emersonii, were homozygous for the F508del mutation. The frequency of homozygosity for the F508del mutation (66%) was higher than that in the general CF population (48.9%, according to the 2006 data from the French Registry of Cystic Fibrosis).
TABLE 2.
Clinical characteristics of CF patients colonized by Geosmithia argillacea
| Patient | Hospital location | Sexa | Age (yr) at CF diagnosis | Relevant CFTR mutation | Age (yr) at first isolation of Geosmithia argillacea |
|---|---|---|---|---|---|
| A | Angers | F | 14 | F508del/C.622-248-4del20pb | 23 |
| B | Angers | M | Birth | F508del/F508del | 8 |
| C | Giens | F | 2 | F508del/F508del | 7 |
| D | Giens | M | Birth | F508del/F508del | 6 |
| E | Giens | M | 20 | F508del/E92K | 36 |
| F | Giens | M | 4 | F508del/F508del | 17 |
| G | Rouen | F | Birth | F508del/F508del | 13 |
| H | Rouen | F | 14 | F508del/I336K | 48 |
| Ib | Giens | M | Birth | F508del/F508del | 12 |
F, female; M, male.
This patient was reported previously (6) as having chronic airway colonization by Penicillium emersonii.
The patients could be classified into two groups on the basis of their CFTR genotype. The first isolation of G. argillacea occurred from F508del-homozygous patients (B, C, D, F, G, and I) at a mean age of 10 years (range, 6 to 17) and from heterozygous patients (A, E, and H) at a mean age of 36 years (range, 23 to 48) (Table 2).
Four of the patients were chronically colonized with G. argillacea (Table 1), with at least two positive samples over a period of more than 6 months. Chronic colonization was suspected for the four other patients. Over the same time period, all but one of the patients were also found to be chronically colonized with S. aureus (Table 3). Chronic colonization by other bacterial or fungal CF pathogens, including P. aeruginosa, A. fumigatus, Aspergillus flavus, and Aspergillus terreus, was less frequent. Exophiala dermatitidis and S. apiospermum were each found in a single patient. Almost all patients also had chronic colonization of the airways caused by A. fumigatus that had been eradicated by the time of isolation of G. argillacea (Table 3).
TABLE 3.
Other microorganisms recovered from sputum samples from patients colonized by Geosmithia argillacea
| Patient | No. of sputum samples positive for G. argillacea (duration [mo] of chronic colonization) | Microorganisms colonizing the airways (beginning or period of colonization)a |
Antifungal drug(s) (beginning or period of treatment)b | |
|---|---|---|---|---|
| Bacteria | Fungi | |||
| Ac | 7 (14) | Staphylococcus aureus (September 2007 →) | Aspergillus fumigatus (2001-October 2006, September 2007 →), Candida albicans (2005 →) Aspergillus flavus (September 2007 →) | ITZ (February 2006-July 2007) |
| B | 3 | Staphylococcus aureus (January 2006 →) Pseudomonas aeruginosad (March 2007 →) | Candida albicans (2001 →) Candida parapsilosis (2002-October 2006) Aspergillus fumigatus (2005-November 2006, April 2007 →) Aspergillus flavus (2005-November 2006) Scedosporium apiospermum (October 2006 →) | ITZ (February 2007 →) |
| C | 2 | Pseudomonas aeruginosad (1996-1998) Staphylococcus aureus (1997 →) | Aspergillus fumigatus (1997-August 1999, October 1999 →) Aspergillus terreus (1998-March 1999, November 1999 →) | ITZ (September 1999-February 2000) |
| D | 2 | Staphylococcus aureus (2002 →) | Aspergillus fumigatus (2003 →) Aspergillus terreus (2006 →) Exophiala dermatitidis (2006 →) Aspergillus flavus (2007 →) | ITZ (July 2003-February 2004, September 2004 →) |
| Ee | 8 (10) | Staphylococcus aureus (2004 →) Pseudomonas aeruginosa (2002 →) | Aspergillus fumigatus (2001-October 2007, September 2008 →) Candida albicans (2007 →) | VCZ (June 2004-July 2006) ITZ (July 2006-January 2008) PCZ (February 2008-June 2008, September 2008-May 2009) |
| F | 5 (19) | Staphylococcus aureus (1996 →) Pseudomonas aeruginosa (2003 →) | Aspergillus fumigatus (2003-February 2008) Aspergillus flavus (2004-2005) | ITZ (August 2007-February 2008, June 2008 →) |
| G | 6 (23) | Staphylococcus aureus (2004 →) Pseudomonas aeruginosad (2004 →) | Candida albicans (July 2005 →) Aspergillus fumigatus (2004-November 2005, April 2006-December 2006) | VCZ (February 2007-March 2007) PCZ (March 2007-November 2007) ITZ (January 2008-February 2008) PCZ (February 2008-April 2008, November 2008-October 2009, November 2009 →) |
| H | 3 | Pseudomonas aeruginosa (2000 →) | Aspergillus fumigatus (2000-2004, February 2009 →) | ITZ (December 2004) CS (March 2009) ITZ (March 2009-December 2009) |
Arrows indicate the continuation of the airway colonization at the date of last isolation of G. argillacea. Unless otherwise indicated, all microorganisms recovered from sputum samples were responsible for chronic colonization of the patient (defined as at least two isolations over a period of 6 months).
Arrows indicate the continuation of the antifungal treatment at the date of last isolation of G. argillacea. CS, caspofungin; ITZ, itraconazole; PCZ, posaconazole; VCZ, voriconazole.
Patients A was infected by a Mycobacterium sp. in September 2007.
Not responsible for chronic colonization.
Patient E was infected by a Mycobacterium sp. in 1994.
Antifungal drug susceptibility testing.
Table 4 summarizes the results from in vitro antifungal susceptibility testing performed using the EUCAST reference method for at least one isolate for each patient (two isolates for patients A, C, D, E, and G, with the first one being collected at the beginning of the colonization and the second being one of the final isolates collected). All isolates tested showed a high MIC of 16 μg/ml to voriconazle, and the majority also showed high MICs to itraconazole (MIC of ≥2 μg/ml for 7 of the 13 isolates tested). The susceptibility to amphotericin B and posaconazole was variable. Amphotericin B and posaconazole MICs were ≥2 μg/ml for 10 isolates and 5 isolates, respectively. The majority of isolates (9 of 13) exhibited low MECs of ≤0.5 μg/ml to caspofungin.
TABLE 4.
Antifungal drug susceptibilities of Geosmithia argillacea isolates
| Patient | Isolate (IHEM no.) | MIC (μg/ml) |
||||
|---|---|---|---|---|---|---|
| Amphotericin B | Itraconazole | Voriconazole | Posaconazole | Caspofungina | ||
| A | 22636 | 2 | 4 | 16 | 2 | 0.25 |
| 22894 | 1 | 2 | 16 | 2 | 0.5 | |
| B | 22640 | 2 | 16 | 16 | 16 | 0.5 |
| C | 16291 | 1 | 0.5 | 16 | 0.25 | 1 |
| 16292 | 1 | 0.5 | 16 | 0.25 | 1 | |
| D | 22641 | 2 | 2 | 16 | 1 | 0.5 |
| 22642 | 8 | 1 | 16 | 1 | 0.25 | |
| E | 22643 | 2 | 0.5 | 16 | 0.25 | 2 |
| 23433 | 2 | 1 | 16 | 0.25 | 0.5 | |
| F | 22928 | 2 | 1 | 16 | 0.5 | 0.25 |
| G | 22685 | 8 | 4 | 16 | 2 | 2 |
| 23431 | 4 | 2 | 16 | 2 | 0.5 | |
| H | 22033 | 2 | 2 | 16 | 1 | 0.5 |
For caspofungin, the minimum effective concentration (MEC) in μg/ml is shown.
All patients had received itraconazole orally, and two of them (patients E and G) also received voriconazole, for treatment of an A. fumigatus infection before the recovery of G. argillacea. This treatment led to the eradication of A. fumigatus, thus allowing the detection of G. argillacea in the following samples. In most cases, G. argillacea was not detected in culture several months later, when new colonizations by A. fumigatus occurred.
Patient E also received posaconazole from February to May 2009. Geosmithia argillacea was no longer detected in later samples from this patient, whereas the same drug administered to patient G during 6 months from March 2007 was ineffective, consistent with the susceptibility patterns seen for the corresponding isolates. Of note, patient H, who was colonized once again by A. fumigatus in February 2009, was the only patient treated with an echinocandin (caspofungin for 10 days), and no filamentous fungi (including G. argillacea) were recovered from later samples. Nevertheless, the forced expiratory volume in 1 s (FEV1) was not determined before and after caspofungin treatment, and we could not specify whether eradication of the fungus improved this patient's condition.
DISCUSSION
Geosmithia is a polyphyletic genus created to accommodate Penicillium species forming conidia that are borne as cylinders from cylindrical, rough-walled phialides lacking narrow necks (as in Penicillium and Paecilomyces) and which do not produce green colonies (14). Geosmithia argillacea was initially described by Stolk et al. in 1969 (16) as a new thermotolerant Penicillium species. Recent molecular analysis revealed that Talaromyces eberneus, isolated for the first time in 1994 in Taiwan (20), is the teleomorph (sexual form) of G. argillacea (12, 19). Here, we report the colonization of the airways by this unusual fungal pathogen in eight CF patients. This fungus had previously been isolated from a CF patient in our laboratory, although at the time it was misidentified as a Penicillium emersonii (6) on the basis of its morphological features. Sequencing the ITS regions of the rRNA genes from these isolates allowed us to correct this misidentification. It is likely that similar misidentifications are present in other published reports, attributing infections caused by G. argillacea to Penicillium species.
More than 850 mutations of the CFTR gene have been reported to cause disease (CFGAC website, http://www.genet.sickkids.on.ca/cftr/). The F508del mutation accounts for approximately two-thirds (66%) of all CF alleles worldwide; the remaining third display considerable mutational heterogeneity. All of our patients carried at least one copy of the mutated allele F508del, giving a higher frequency of homozygosity than in the general CF population (66% versus 48.9%). We also found that colonization by G. argillacea was first detected at a younger age in patients homozygous for the F508del mutation than in heterozygous patients. It is well known that CF patients homozygous for the F508del mutation generally have more severe pulmonary disease than heterozygous CF patients (21). A large-scale multicenter study will be needed to confirm the association between a particular CFTR genotype and colonization by G. argillacea or the potential differences in age of acquisition.
All but one of the patients colonized by G. argillacea were also chronically colonized by S. aureus. Complementary studies should be carried out to characterize the interactions in this microbial community. In our case, colonization of the airways by G. argillacea always succeeded infections by various bacteria. Thus, bronchial epithelial lesions due to chronic bacterial infections or to the resulting inflammatory response may be required for the establishment of this filamentous fungus (5).
Repeated isolation of G. argillacea from these patients suggested that the fungus was continuously present in their airways, thus representing a chronic colonization. This was particularly true for four of the patients, since several successive samples were culture positive for this fungus. Chronic colonization could not be confirmed for patient D due to new colonizations by some aspergilli between culture-positive samples (with the rapid and extensive growth of the aspergilli possibly masking the presence of G. argillacea). Geosmithia argillacea is highly thermophilic, with optimal growth at between 37°C and 40°C. This partly explains its ability to colonize the bronchial tubes of CF patients. Nevertheless, little is known about the natural habitat of this fungus. Studies on the environment of CF patients will thus help to define the origin of the contamination and to establish prophylactic measures.
Almost all cases reported here were diagnosed after eradication of the other filamentous fungi colonizing the airways of the patients, in particular the rapidly growing fungus A. fumigatus. All patients were treated with itraconazole for an A. fumigatus infection, including allergic bronchopulmonary aspergillosis for two of them, and two were treated with voriconazole. Accordingly, in vitro antifungal drug susceptibility testing performed on a large number of isolates revealed that they all showed high voriconazole MICs and that the majority also showed high itraconazole MICs. Posaconazole was also administered to patients E and G and caspofungin to patient H, leading to the eradication of G. argillacea in patients E and H. This was consistent with the antifungal susceptibility patterns of the corresponding isolates, showing variable results with posaconazole and mostly low caspofungin MECs.
Since 1999, 17 cases of colonization by G. argillacea in CF patient have been reported: eight in this study, one in 1999 (6), and eight presented by Barton et al. (2) at the 31st European Cystic Fibrosis conference. Improved methods of detection and identification of fungi in CF patients and a growing number of sequences available from the databases may at least partly explain this increased incidence. However, these reports suggest that G. argillacea is an emerging pathogen in CF lung disease. Six of the eight cases reported here were diagnosed after 2005. Even though the appearance and persistence of the fungus in the airways do not seem to be associated with exacerbation of the disease, the potential clinical implications of its recovery from respiratory secretions are still a matter of concern. Grant et al. (9) reported a systemic mycosis in a German Shepherd dog with suspected predisposing immunodeficiency. Moreover, colonization by bacteria and A. fumigatus may contribute to the chronic inflammatory response, which seems to play a more important role than the microorganisms themselves in the development of slowly progressing pulmonary disease in CF (10). Finally, given the thermophilic properties and the persistence of G. argillacea, this fungus may be a likely candidate for causing invasive disease in cases of immunosuppression related to lung transplantation, as has been previously reported for S. apiospermum (17).
Acknowledgments
We gratefully acknowledge Françoise Symoens from the IHEM Culture Collection for her help in preserving the strains.
S.G., M.P., B.R., J.C., L.F., J.P.B., and A.C. are members of the ISHAM (International Society for Human and Animal Mycology) working group on fungal respiratory infections in cystic fibrosis.
Footnotes
Published ahead of print on 12 May 2010.
REFERENCES
- 1.Amin, R., A. Dupuis, S. D. Aaron, and F. Ratjen. 2010. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in cystic fibrosis patients. Chest 137:171-176. [DOI] [PubMed] [Google Scholar]
- 2.Barton, F., A. Borman, E. Johnson, R. Hobson, S. Conway, T. Lee, D. Peckham, and K. Brownlee. 2008. Geosmithia argillacea—a potential new pathogen in cystic fibrosis lung disease? Abstr. 31st Eur. Cystic Fibrosis Conf. J. Cyst. Fibros. 7:S50. [Google Scholar]
- 3.Bellis, G., M. H. Cazes, A. Parant, M. Gaimard, C. Travers, E. Le Roux, S. Ravilly, and G. Rault. 2007. Cystic fibrosis mortality trends in France. J. Cyst. Fibros. 6:179-186. [DOI] [PubMed] [Google Scholar]
- 4.Bobadilla, J. L., M. Macek, Jr., J. P. Fine, and P. M. Farrell. 2002. Cystic fibrosis: a worldwide analysis of CFTR mutations—correlation with incidence data and application to screening. Hum. Mutat. 19:575-606. [DOI] [PubMed] [Google Scholar]
- 5.Cimon, B., J. Carrère, J. Chazalette, J. Giniès, P. Six, J. Vinatier, D. Chabasse, and J. Bouchara. 1995. Fungal colonization and immune response to fungi in cystic fibrosis. J. Mycol. Med. 5:211-216. [Google Scholar]
- 6.Cimon, B., J. Carrere, J. P. Chazalette, J. F. Vinatier, D. Chabasse, and J. P. Bouchara. 1999. Chronic airway colonization by Penicillium emersonii in a patient with cystic fibrosis. Med. Mycol. 37:291-293. [PubMed] [Google Scholar]
- 7.Cimon, B., S. Challier, H. Beguin, J. Carrere, D. Chabasse, and J. P. Bouchara. 2005. Airway colonization by Acrophialophora fusispora in patients with cystic fibrosis. J. Clin. Microbiol. 43:1484-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerrits Van Den Ende, A. H. G., and G. S. De Hoog. 1999. Variability and molecular diagnostics of the neutropic species Cladophialophora bantiana. Studies Mycol. 1999:151-162. [Google Scholar]
- 9.Grant, D. C., D. A. Sutton, C. A. Sandberg, R. D. Tyler, Jr., E. H. Thompson, A. M. Romanelli, and B. L. Wickes. 2009. Disseminated Geosmithia argillacea infection in a German shepherd dog. Med. Mycol. 47:221-226. [DOI] [PubMed] [Google Scholar]
- 10.Koch, C., and N. Hoiby. 1993. Pathogenesis of cystic fibrosis. Lancet 341:1065-1069. [DOI] [PubMed] [Google Scholar]
- 11.Koljalg, U., K. H. Larsson, K. Abarenkov, R. H. Nilsson, I. J. Alexander, U. Eberhardt, S. Erland, K. Hoiland, R. Kjoller, E. Larsson, T. Pennanen, R. Sen, A. F. Taylor, L. Tedersoo, T. Vralstad, and B. M. Ursing. 2005. UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol. 166:1063-1068. [DOI] [PubMed] [Google Scholar]
- 12.Mouchacca, J. 2007. Heat tolerant fungi and applied research: addition to the previously treated group of strictly thermotolerant species. World J. Microbiol. Biotechnol. 23:1755-1770. [DOI] [PubMed] [Google Scholar]
- 13.Pihet, M., J. Carrere, B. Cimon, D. Chabasse, L. Delhaes, F. Symoens, and J. P. Bouchara. 2009. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis—a review. Med. Mycol. 47:387-397. [DOI] [PubMed] [Google Scholar]
- 14.Pitt, J. 1979. Geosmithia gen nov for Penicillium lavendulum and related species. Can. J. Bot. 57:2021-2030. [Google Scholar]
- 15.Rodriguez-Tudela, J. L., M. C. Arendrup, S. Arikan, F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, E. Dannaoui, D. W. Denning, J. P. Donnelly, W. Fegeler, C. Lass-Flörl, C. Moore, M. Richardson, P. Gaustad, A. Schmalreck, A. Velegraki, and P. Verweij. 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982-984. [DOI] [PubMed] [Google Scholar]
- 16.Stolk, A., H. Evans, and T. Nilsson. 1969. Penicillium argillaceum sp nov a thermotolerant Penicillium. Trans. Br. Mycol. Soc. 53:307-311. [Google Scholar]
- 17.Symoens, F., C. Knoop, M. Schrooyen, O. Denis, M. Estenne, N. Nolard, and F. Jacobs. 2006. Disseminated Scedosporium apiospermum infection in a cystic fibrosis patient after double-lung transplantation. J. Heart Lung Transplant. 25:603-607. [DOI] [PubMed] [Google Scholar]
- 18.White, T., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. Innis and D. Gelfand (ed.), PCR protocols: a guide to methods and applications. Academic Press, New York, NY.
- 19.Yaguchi, T., S. Edagawa, and K. Nishimura. 2005. Geosmithia argillacea is the anamorh of Talaromyces eberneus as a heat resistant fungus. Cryptogamie Mycol. 26:131-141. [Google Scholar]
- 20.Yaguchi, T., A. Someya, and K. Nihimura. 1994. Two new species of Talaromyces from Taiwan and Japan. Mycoscience 35:249-255. [Google Scholar]
- 21.Zielenski, J. 2000. Genotype and phenotype in cystic fibrosis. Respiration 67:117-133. [DOI] [PubMed] [Google Scholar]