Abstract
Rothia aeria is a recently described Gram-positive rod from the family Micrococcaceae. An elderly woman with rheumatoid arthritis and dental abscesses who was undergoing immunosuppression had R. aeria isolated from synovial fluid. This report characterizes this rare organism and contributes to the literature on its pathogenicity and likely oral source.
CASE REPORT
An 88-year-old New Zealand European woman with seropositive rheumatoid arthritis presented to hospital for multidisciplinary assessment and physiotherapy. Her main symptom was painful swollen shoulders. Her rheumatoid arthritis had been diagnosed 5 years earlier. Immunosuppressive medications were methotrexate at 12.5 mg weekly, prednisone at 2 mg daily, and hydrocortisone at 10 mg daily in addition to medications for cardiac disease and osteoporosis. She had taken low-dose oral prednisone and methotrexate for over 4 years, in addition to alternative therapies with unknown doses of corticosteroid-containing medication. She had been referred to a dentist for her dental caries but had been advised against dental clearance because of her advanced age and frail condition. Physical examination showed extensive dental decay with loss of all of the native crowns and large bilateral shoulder joint effusions. No other joints were swollen, but she did have significant widespread joint deformity and pain associated with secondary degenerative arthritis.
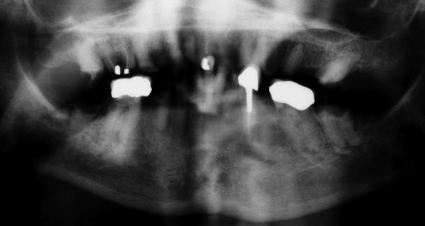
Blood tests showed no significant abnormality on routine hematological and biochemical analyses. The C-reactive protein level was not raised. Review of her most recent dental radiograph confirmed extensive dental caries and multiple apical abscesses (Fig. 1).
FIG. 1.
Recent dental X-ray with arrows marking multiple lucent areas at the apices that denote abscesses. Adjacent bone is sclerotic secondary to chronic inflammation.
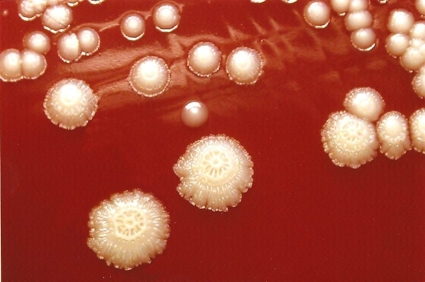
Therapeutic aspiration of synovial fluid from both shoulders obtained viscous yellow fluid. The right shoulder aspirate had a white cell count of 2,300 × 106/liter. The left aspirate had a white cell count of 800 × 106/liter with 85% polymorphonuclear leukocytes, 12% lymphocytes, and 3% monocytes. Cholesterol and calcium pyrophosphate crystals were present. Some of the fluid from both shoulders was inoculated into pediatric blood culture bottles, and then the remainder was centrifuged and the deposit was cultured directly on blood and chocolate agar incubated in 5% CO2. Growth was observed only in the enrichment broth from the left shoulder, and a Gram-positive bacillus was seen on Gram staining after 4 days. A pure growth of white raised colonies 1.5 to 2.2 mm in diameter with a spoke-and-wheel appearance was obtained at 48 h (Fig. 2). The API Coryne V3.0 identification strip (bioMérieux, Marcy l'Etoile, France) profile obtained (7054125) identified the isolate as Rothia dentocariosa with 99.9% probability (Table 1).
FIG. 2.
Appearance of R. aeria colonies on blood agar.
TABLE 1.
Biochemical characteristics of the isolate
| Test | Resulta |
|---|---|
| Nitrate reduction | + |
| Pyrazinamidase | + |
| Alanine-phenylalanine-proline arylamidase | + |
| Alkaline phosphatase | − |
| β-Glucuronidase | − |
| β-Galactosidase | − |
| α-Glucosidase | + |
| N-Acetyl-d-glucosaminidase | − |
| Esculin hydrolysis | + |
| Urease activity | − |
| Gelatin hydrolysis | − |
| Catalase | + |
| Fermentation of: | |
| Glucose | + |
| d-Ribose | − |
| d-Xylose | − |
| d-Mannitol | − |
| Maltose | + |
| Lactose | − |
| Saccharose | + |
| Glycogen | − |
Symbols: +, positive; −, negative.
Initial antibiotic susceptibilities were determined by Etest (MIC Evaluator; Oxoid, Cambridge, United Kingdom) as follows: penicillin, 0.002 mg/liter; gentamicin, 4.0 mg/liter. Further susceptibilities were determined by disc diffusion on chocolate agar (Table 2) using Clinical and Laboratory Standards Institute staphylococcal standards (15).
TABLE 2.
Antibiotic susceptibilities of the isolatea
| Time (h) on chocolate agar | MIC (mg/liter) |
Inhibition zone (mm) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillin | Erythromycin | Vancomycin | Cefotaxime | Ceftriaxone | Amoxicillin | Gentamicin | Doxycycline | Cotrimoxazole | Imipenem | Clindamycin | Augmentinb | Piperacillin | |
| 24 | 0.015 (S) | 0.03 (S) | 2.0 (S) | 0.03 (S) | 0.03 (S) | 0.016 (S) | 4.0 (S) | 34 (S) | 24 (S) | 47 (S) | 32 (S) | 52 (S) | 47 |
| 48 | 0.015 | 0.03 | 4.0 (I) | 0.03 | 0.03 | 0.016 | 4.0 | 28 (S) | 16 (S) | 47 | 20 (I) | 52 | 47 |
Antibiotic susceptibilities are shown in parentheses as follows: S, susceptible; I, intermediate.
Augmentin is a trade name for amoxicillin-clavulanic acid.
The 16S rRNA gene was amplified and sequenced (1, 16) (accession number GU44512; National Center for Biotechnology Information [NCBI], National Library of Medicine, Bethesda, MD). The query sequence of 1,335 bp was found to be 100% homologous to the 16S rRNA gene sequences for R. aeria (NCBI accession number EU293888). The next closest species match, R. dentocariosa, had 98.6% homology. We identified the isolate as R. aeria according to the guidelines recommended by Janda and Abbott (6). The 16S rRNA gene sequence we obtained is 99.8% (1,300/1,302 sequence coverage) homologous to the 2004 strain described by Li et al. (9) (NCBI accession number AB071952).
On day 9 after admission, with culture results, biochemical characteristics, and antibiotic susceptibilities known, a 14-day course of treatment for septic arthritis was commenced by continuous intravenous infusion of penicillin at 2.4 g/day. Blood cultures taken immediately prior to the start of antibiotic therapy recorded no growth. Transesophageal echocardiography excluded cardiac vegetations, and subsequent blood cultures remained negative. A second aspirate of left shoulder synovial fluid, obtained 2 weeks after the first, was sterile. In the 6 months since this episode, the patient's shoulder symptoms have improved and she has had no further problems with infection.
The presence of a rare organism in pure culture and a plausible source support the idea that this is a significant isolate even though the clinical findings are less pronounced than typically seen with septic arthritis. Moreover, septic arthritis can occur in immunocompetent patients when the synovial fluid leukocyte count is less than 50,000 × 106 (8). The combination of corticosteroids and methotrexate significantly increases the likelihood of infection in persons with rheumatoid arthritis, and it is likely that immunosuppression attenuated the clinical and laboratory findings of septic arthritis.
Monju et al. reported the only other published instance of R. aeria causing invasive disease (13). That report described a bloodstream infection in a neonate born to a mother who had undergone extraction of a decaying tooth 4 days prior to delivery. Similarly, we postulate the oral cavity to be the source of R. aeria and hematogenous spread to be the mechanism of septic arthritis. A series characterizing normal oral flora with 16S rRNA gene sequencing has identified R. aeria as a rare oral cavity-colonizing microorganism (5).
Other species of Rothia colonize the oral cavity and have caused septic arthritis. R. mucilaginosa has been reported as a cause of a late prosthetic joint infection that arose following a dental extraction (12). As in the present case, the R. mucilaginosa infection was not associated with a prominent inflammatory response. R. dentocariosa has caused invasive infections, particularly endocarditis (2), dialysis-associated peritonitis (3), and intrauterine fetal death (7).
This isolate has the same biochemical characteristics and 16S rRNA gene sequence as that reported by Li et al., who described the first isolate of R. aeria from air filters on the Mir space station. Both differ from the isolate reported by Monju et al. in their ability to reduce nitrates and produce catalase. R. dentocariosa is known to be catalase variable, and this may also explain this difference in our findings (14). The colonial morphology appears indistinguishable from that of R. dentocariosa (3).
This report shows that routine biochemical methods may misidentify R. aeria as R. dentocariosa. We took the opportunity to 16S rRNA sequence two isolates previously identified as R. dentocariosa from our laboratory culture collection and the New Zealand Reference Culture Collection of the Institute of Environmental Science and Research. Sequencing confirmed that they were R. dentocariosa. In our experience, Rothia spp. are very rare isolates. Our practice of subjecting unusual but clinically significant organisms to 16S RNA gene sequencing allows us to distinguish R. aeria from the better-described species R. dentocariosa.
Finally, this case illustrates the consequences of dental infection in the context of immunosuppression. Preventive dental care is established as a means of preventing infection in the setting of immunocompromise due to advanced HIV and transplantation or cancer chemotherapy (4, 17). However, recommendations are scarce for less intensive immunosuppressive therapy (10), despite the high rates of periodontal disease that complicate rheumatoid arthritis (11). Recommendations concerning this relatively common clinical problem could benefit many, though sadly not this patient, who is too frail for dental clearance. Moreover, newer immunosuppressive regimens may have more profound effects on dental and periodontal disease, which makes further consideration of preventive dentistry all the more important.
Footnotes
Published ahead of print on 26 May 2010.
REFERENCES
- 1.Borriello, S. P., P. R. Murray, and G. Funke (ed.). 2005. Topley & Wilson's microbiology & microbial infections, 10th ed., p. 242-243. Hodder Arnold, London, United Kingdom.
- 2.Braden, D. S., S. Feldman, and A. L. Palmer. 1999. Rothia endocarditis in a child. South. Med. J. 92:815-816. [DOI] [PubMed] [Google Scholar]
- 3.Ergin, C., M. T. Sezer, C. Agalar, S. Katirci, T. Demirdal, and G. Yayli. 2000. A case of peritonitis due to Rothia dentocariosa in a CAPD patient. Perit. Dial Int. 20:242-243. [PubMed] [Google Scholar]
- 4.Guggenheimer, J., D. Mayher, and B. Eghtesad. 2005. A survey of dental care protocols among US organ transplant centers. Clin. Transplant. 19:15-18. [DOI] [PubMed] [Google Scholar]
- 5.Haraszthy, V. I., J. J. Zambon, P. K. Sreenivasan, M. M. Zambon, D. Gerber, R. Rego, and C. Parker. 2007. Identification of oral bacterial species associated with halitosis. J. Am. Dent. Assoc. 138:1113-1120. [DOI] [PubMed] [Google Scholar]
- 6.Janda, J. M., and S. L. Abbott. 2007. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J. Clin. Microbiol. 45:2761-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson, M. D., and B. Jacobsson. 2007. Intrauterine fetal death associated with Rothia dentocariosa: a case report. Am. J. Obstet. Gynecol. 197:e6-e7. [DOI] [PubMed] [Google Scholar]
- 8.Li, S. F., J. Henderson, E. Dickman, and R. Darzynkiewicz. 2004. Laboratory tests in adults with monoarticular arthritis: can they rule out a septic joint? Acad. Emerg. Med. 11:276-280. [DOI] [PubMed] [Google Scholar]
- 9.Li, Y., Y. Kawamura, N. Fujiwara, T. Naka, H. Liu, X. Huang, K. Kobayashi, and T. Kzaki. 2004. Rothia aeria sp. nov., Rhodococcus baikonurensis sp. nov. and Arthrobacter russicus sp. nov., isolated from air in the Russian space laboratory Mir. Int. J. Syst. Evol. Microbiol. 54:827-835. [DOI] [PubMed] [Google Scholar]
- 10.McCullough, M. 2009. Dental notes. Immunosuppressive drugs. Aust. Prescr. 32:75. http://www.australianprescriber.com/magazine/32/3/artid/1027. [Google Scholar]
- 11.Mercado, F., R. I. Marshall, A. C. Klestov, and P. M. Bartold. 2000. Is there a relationship between rheumatoid arthritis and periodontal disease? J. Clin. Periodontol. 27:267-272. [DOI] [PubMed] [Google Scholar]
- 12.Michels, F., J. Colaert, F. Gheysen, and T. Scheerlinck. 2007. Late prosthetic joint infection due to Rothia mucilaginosa. Acta Orthop. Belg. 73:263-267. [PubMed] [Google Scholar]
- 13.Monju, A., N. Shimizu, M. Yamamoto, K. Oda, Y. Kawamoto, and K. Ohkusu. 2009. First case report of sepsis due to Rothia aeria in a neonate. J. Clin. Microbiol. 47:1605-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.). 2007. Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 15.National Committee for Clinical Laboratory Standards. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—eighth edition. Clinical Laboratory Standards Institute, Wayne, PA.
- 16.Stackebrandt, E., and M. Goodfellow. 1991. Nucleic acid techniques in bacterial systematics. Wiley, New York, NY.
- 17.Weinert, M., R. M. Grimes, and D. P. Lynch. 1996. Oral manifestations of HIV infection. Ann. Intern. Med. 125:485-496. [DOI] [PubMed] [Google Scholar]