Abstract
Recently, a new genus of Anaplasmataceae termed “Candidatus Neoehrlichia” was discovered in ticks and rodents. Here, we report on two patients who suffered from febrile bacteremia due to “Candidatus Neoehrlichia mikurensis” associated with thrombotic or hemorrhagic events. 16S rRNA and groEL gene sequencing provided evidence of three groups of sequence variants.
CASE REPORTS
Case 1. In June, July, and November 2007, a 69-year-old male patient from the district of Middle Frankonia, Germany, was repeatedly admitted to the Department of Rheumatology and Immunology at the University Hospital of Erlangen because of recurrent daily episodes of fever (up to 39°C, predominantly in the evening), night sweats, nonproductive cough, left thoracic pain, and intermittent bouts of diarrhea since April 2007. The patient had undergone immunosuppressive therapy with cyclophosphamide and prednisolone, as well as repeated courses of rituximab (anti-CD20 antibody; 0.7 g/dose, cumulative dose, 6.3 g) between 2005 and 2007 for suspected chronic inflammatory demyelinating polyneuropathy. The fever was sensitive to steroids above 20 mg/day. Blood counts and clinical chemistry revealed a hypochromic anemia (erythrocytes, 3.9 × 106 to 4.1 × 106/μl; hemoglobin, 10.8 to 11.7 g/dl; mean corpuscular hemoglobin, 26 to 27 pg; serum iron, 11 to 36 μg/dl), normal to slightly reduced numbers of leukocytes (3.1 to 5.6 × 103/μl) with a decreased percentage of lymphocytes (19%) and an increased proportion of monocytes (12 to 16%), and elevated levels of C-reactive protein (CRP; 77 to 114 mg/liter) but normal platelet counts (217,000/μl) and procalcitonin and hepatic transaminases. Due to the rituximab therapy, CD19+ B cells were <1% in the bone marrow and completely missing in the peripheral blood and the serum IgM level was 0.3 g/liter (normal, 0.4 to 2.3 g/liter). The history of the patient was remarkable for a clinically asymptomatic tick bite in 2005 and for two events of deep vein thrombosis of the upper left arm and lower right leg in October and November 2007, when he already had a fever of unknown origin (FUO).
Repeated microbiological, pathological, and immunological analyses (cultures of blood, bronchoalveolar lavage fluid, and bone marrow samples for bacteria, mycobacteria, and fungi; histologic examination of bone marrow, gastric, and colon biopsy specimens; autoantibody screening), as well as various imaging procedures (bronchoscopy, gastroscopy, colonoscopy, abdominal sonography, transesophageal echocardiography, whole-body positron emission tomography) failed to reveal an infectious, malignant, or autoimmune cause of the fever. There were no detectable antibodies against HIV-1 or -2, hepatitis B or C virus, cytomegalovirus, Anaplasma phagocytophilum, Borrelia burgdorferi, Bordetella pertussis, Brucella abortus/melitensis, Chlamydia psittaci, Coxiella burnetii, Ehrlichia chaffeensis, Francisella tularensis, Legionella pneumophila, Leptospira interrogans, Mycoplasma pneumoniae, Treponema pallidum, or Toxoplasma gondii, whereas antibodies against Herpes simplex virus, Varicella-zoster virus, Epstein-Barr virus, Parvovirus B19, Bartonella henselae, B. quintana, Chlamydia pneumoniae, and C. trachomatis were present at low levels with little or no IgM measurable. The serum levels of fungal antigens (Candida mannan, Aspergillus galactomannan) were within normal limits. All serological tests were performed with commercially available certified assays obtained from Abbott (Wiesbaden-Delkenheim, Germany), BD Diagnostics (Heidelberg, Germany), bioMérieux (Nürtingen, Germany), Bio-Rad (Munich, Germany), Dia-Sorin (Dietzenbach, Germany), Focus Diagnostics (Cypress, CA), Mast Diagnostica (Reinfeld, Germany), and Virion/Serion GmbH (Würzburg, Germany). Ultimately, a broad-range 16S rRNA gene-based PCR analysis which was performed with two independent aliquots of a bone marrow specimen (taken on 6 December 2007) and a peripheral blood sample (taken on 19 December) revealed an infection with “Candidatus Neoehrlichia mikurensis.” The patient was treated with doxycycline (1 × 200 mg/day) for 3 weeks, which led to rapid resolution of the fever and normalization of the acute-phase reactants and peripheral blood counts. One week later, prednisolone therapy could be stopped without any recurrence of fever. Peripheral blood B cells started to increase 2 months after initiation of doxycycline therapy and normalized 10 months thereafter. Follow-up broad-range PCR analyses of peripheral blood samples at 1, 14, and 21 months after doxycycline therapy were negative, and the patient has remained well ever since.
Case 2.
In November 2008, a 57-year-old previously healthy male patient from a rural forest area with an unknown tick bite history was found unconscious by his wife following a 3-day period of headaches. Upon emergency admission to the intensive care unit (ICU), an intracerebral and subarachnoid hemorrhage with an aneurysm of the left arteria carotis interna was diagnosed. The patient was afebrile at this stage. Despite external cerebrospinal fluid (CSF) drainage and neurosurgical clipping of the aneurysm (day 1), the intubated and mechanically ventilated patient developed a left-sided arteria cerebri media infarction and brain edema, which required decompressive hemicraniectomy on day 4. On day 3, the patient had a high body temperature (38.8°C), his CRP was 87.9 mg/liter, his total leukocyte count was 25,500/μl, and he required increasing dosages of norepinephrine and oxygen. His aspartate aminotransferase level was slightly elevated (72 to 122 U/ml), his platelet count was mildly decreased to normal (132,000 to 224,000/μl), and his alanine aminotransferase level was within normal limits. A chest X-ray revealed a pulmonary infiltration of the right lower lobe. Peripheral blood, CSF, and tracheal secretions were sampled for microbiological analysis, and antibiotic treatment with piperacillin-tazobactam (3 × 4.5 g intravenously [i.v.]) was started. As the patient did not respond (his CRP level on day 5 was 471 mg/liter) and a severe nosocomial infection was suspected, ciprofloxacin (3 × 400 mg i.v.) and fosfomycin (3 × 5 g i.v.) were added. The patient continued to have elevated body temperatures (>39°C) and infection parameters and remained dependent on vasopressors (>1 μg/kg/min norepinephrine). Except for the detection of oxacillin-sensitive Staphylococcus aureus in the tracheal secretion, all cultures remained sterile. The patient died on day 8 after admission to the ICU from septic multiorgan failure. A postmortem analysis of the blood sample taken on day 1 by universal 16S rRNA gene PCR demonstrated infection with “Candidatus N. mikurensis.” An autopsy was not performed.
Molecular methods.
DNA from Ixodes ricinus tick W330, which was collected from a dog in the district of Middle Frankonia (the region in which patient 1 also lives) as part of a different study (20), was prepared using the QIAamp DNA mini kit (Qiagen, Hilden, Germany). DNA was also isolated from EDTA-treated peripheral blood and a bone marrow aspirate of patient 1, which were processed separately using the High Pure PCR template preparation kit (Roche Diagnostics, Mannheim, Germany). The DNA (undiluted and diluted 1:10) was subjected to universal bacterial 16S rRNA gene amplification using primers 8f and 806r following previously published protocols, with minor modifications (10, 11). Furthermore, the 16S rRNA gene and the groEL gene of “Candidatus N. mikurensis” were amplified from DNA of patient 1, as well as from DNA from I. ricinus tick W330 (20), by nested PCR using the primers shown in Table 1. The nucleotide sequences of the primers are shown in Table 2. The PCR products were sequenced using an ABI Prism 310 or 3130 genetic analyzer (Applied Biosystems, Darmstadt, Germany) and the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems). Sequences were edited and assembled with the SeqMan program of the DNASTAR package (Lasergene, Madison, WI). For phylogenetic analysis of the 16S rRNA (1,426 bp) or groEL gene (1,233 bp) sequence, the program MEGA 4.1 (18) was used. Sequences were aligned by ClustalW applying the IUB matrix. Tree construction was achieved by the neighbor-joining method using the Jukes-Cantor matrix and the complete deletion option. Bootstrap analysis was conducted with 1,000 replicates. Condensed trees were calculated by applying a cutoff value of 95%.
TABLE 1.
Primers used for amplification and sequencing of the 16S rRNA and groEL genes of “Candidatus N. mikurensis” in patient 1
| Target | Primers used for: |
||
|---|---|---|---|
| First PCR | Nested PCR | Sequencing | |
| 16S rRNA gene | 8f + 1512r | 8f + Nehr16S re | 8f, Nehr16S re, Nehr16S seq1, Nehr16S seq2 |
| Nehr16S fo + 1512r | Nehr16S fo, 1512r, Nehr16S seq3, Nehr16S seq4 | ||
| groEL | Nehrgro fo + Nehrgro re | Nehrgro fo + Nehrgro seq1 | Nehrgro fo, Nehrgro seq1 |
| Nehrgro seq2 + Nehrgro seq4 | Nehrgro seq2, Nehrgro seq4 | ||
| Nehrgro seq3 + Nehrgro re | Nehrgro seq3, Nehrgro re | ||
TABLE 2.
Nucleotide sequences of primers used for amplification and sequencing of the 16S rRNA and groEL genes of “Candidatus N. mikurensis” in patient 1
| Primer | Nucleotide sequence |
|---|---|
| 8fa | 5′-AGA GTT TGA TCM TGG CTC AG-3′ |
| 806ra | 5′-GGACTACCAGGGTATCTAATCC-3′ |
| 1512ra | 5′-ACG GCT ACC TTG TTA CGA CTT C-3′ |
| Nehr16S fo | 5′-GCG ACT ATC TGG CTC AGT T-3′ |
| Nehr16S re | 5′-AGC CAA ACT GAC TCT TCC G-3′ |
| Nehr16S seq1 | 5′-TTG TTC GGA ATT ATT GGG CG-3′ |
| Nehr16S seq2 | 5′-CCA ATA ATT CCG AAC AAC GC-3′ |
| Nehr16S seq3 | 5′-CGT GTC GTG AGA TGT TGG GT-3′ |
| Nehr16S seq4 | 5′-CCC AAC ATC TCA CGA CAC GA-3′ |
| Nehrgro fo | 5′-GAA GCA TAG TCT AGT ATT TTT G-3′ |
| Nehrgro re | 5′-TCY TTA ACT TCT ACT TCA CTT GA-3′ |
| Nehrgro seq1 | 5′-ACA TCG CGC TTC ATA GAA AG-3′ |
| Nehrgro seq2 | 5′-AGG AAT TAG TAT TAG AAT CTT TG-3′ |
| Nehrgro seq3 | 5′-AAT ATA GCA AGA TCA GGT AGA C-3′ |
| Nehrgro seq4 | 5′-AAT CTT CCA TTT TAA CTG CTA AT-3′ |
With respect to patient 2, duplicate 1-ml aliquots of the EDTA-treated peripheral blood sample were individually subjected to combined pathogen DNA extraction and universal 16S rRNA gene-based PCR using the SepsiTest kit (Molzym, Bremen, Germany) (22). Four weeks later, the same procedure was repeated with two new 1-ml aliquots of the frozen blood sample. The 450-bp PCR amplicons were sequenced as described in the SepsiTest manual. The results obtained with all four aliquots were identical. Total DNA was also prepared from paraffin-embedded aneurysm tissue. Extensive precautionary measurements have been implemented in our molecular biology diagnostic laboratories to prevent false-positive results (e.g., preparation of reagents, isolation of DNA, and PCR amplification in three distinct rooms and use of barrier pipette tips and safety cabinets during all steps). The DNA from I. ricinus tick W330 (20) was prepared 10 years ago in a laboratory that was completely separated from the molecular diagnostic unit of the Microbiology Institute in Erlangen and has been stored at the Microbiology Department in Freiburg since 2003, thus excluding the remote possibility of cross-contamination.
16S rRNA and groEL gene sequencing.
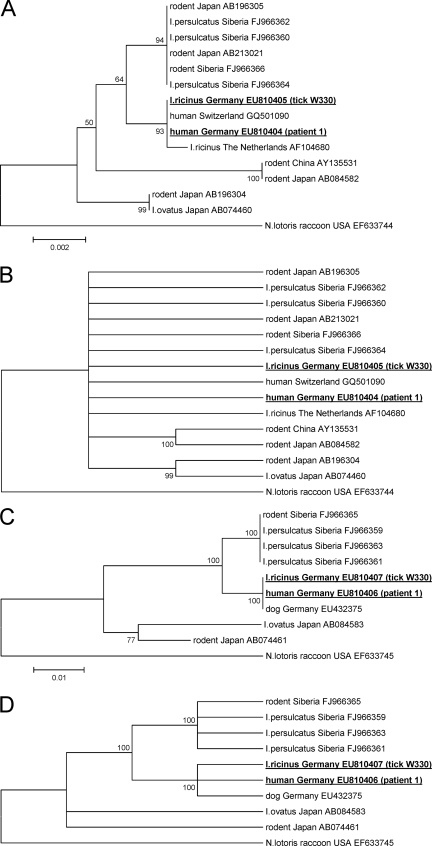
The 1,426 bp of the 16S rRNA gene sequence and the 1,233 bp of the groEL gene sequence obtained from the blood and bone marrow of patient 1 and from I. ricinus tick W330 were identical to each other. GenBank database analysis showed that the 16S rRNA gene sequence obtained from patient 1 was 100% identical (1,426/1,426 bp) to the 16S rRNA gene sequence from a Swiss patient with endocarditis (accession number GQ501090), 99.9% identical (1,413/1,414 bp) to the 16S rRNA gene sequence from a Dutch I. ricinus tick (accession number AF104680, initially reported as Ehrlichia-like sp. “Schotti variant” [14]), and 99.2% to 99.7% identical to the “Candidatus N. mikurensis” 16S rRNA gene sequences detected in rodents and Ixodes ovatus ticks in Japan (5, 8). However, the phylogenetic analysis revealed that 16S rRNA gene sequencing was not sufficient to differentiate between putative variants of the species “Candidatus N. mikurensis” (Fig. 1A and B).
FIG. 1.
Phylogenetic tree of the 16S rRNA (A, B) and groEL (C, D) gene sequences inferred using the neighbor-joining method. Bootstrap values are shown next to the nodes of the trees. The scale bar indicates the number of base substitutions per site. The final data sets contained 1,293 and 968 positions, respectively. Following the sequence names are the respective GenBank accession numbers. The accession numbers of sequences newly reported in this study are in bold and underlined (tick W330 and patient 1; the sequence data for patient 2 are not depicted because they did not fulfill the minimum length requirements for phylogenetic comparison to other published sequence data). The condensed trees (B, D) were calculated by applying a cutoff value of 95%.
The groEL sequence from patient 1 had an identity of 98.7% (1,217/1,233 bp) to groEL sequences from Siberian rodents and I. persulcatus ticks (accession numbers FJ966365 and FJ966363) and identities of 94.5% (1,165/1,233 bp) and 95.6% (1,179/1,233 bp) to the respective sequences from Japanese rodents and I. ovatus ticks (accession numbers AB084583 and AB074461). A shorter groEL sequence of 968 bp from a German dog (accession number EU432375) was 100% identical to our groEL sequences from patient 1 and Franconian I. ricinus tick W330. A phylogenetic analysis of all available and comparably long groEL sequences showed a highly significant clustering of the samples from Germany, Siberia, and Japan, respectively, which suggests the existence of three groups of sequence variants of “Candidatus N. mikurensis” (Fig. 1C and D).
Sequencing of the universal 16S rRNA gene PCR amplicon of the blood of patient 2 yielded a 390-bp sequence (accession number GU212868) that was 100% identical to a partial 16S rRNA gene sequence of “Candidatus N. mikurensis” (accession number FJ966366) and to the respective sequences of patient 1 and tick W330. DNA extracted from a paraffin-embedded sample of the aneurysm tissue of patient 2 was positive for human genomic DNA but not for bacterial DNA (data not shown), which might reflect the loss of sensitivity of PCR assays when using formalin-fixed tissue.
Serology.
Twofold serial dilutions (starting at 1:32) of the sera of patient 1 were analyzed for antibodies against A. phagocytophilum or E. chaffeensis by indirect immunofluorescence assays (Focus Diagnostics, Cypress, CA). All sera obtained from patient 1 prior to (5 December 2007) and after (28 January 2008 and 25 March and 28 October 2009) therapy were negative for IgM or IgG antibodies against A. phagocytophilum or E. chaffeensis. Sera from patient 2 were not available for retrospective analyses.
Discussion.
Anaplasmataceae is a family of Gram-negative, obligate intracellular alphaproteobacteria that has been suggested to consist of six genetically distinct genera (5): (i) the genera Ehrlichia, Anaplasma, and Neorickettsia, which are transmitted by ticks or fish parasites and cause disease in mammals, including humans (4); (ii) the genera Wolbachia and Aegyptianella, which only infect nematodes, arthropods, or birds (6, 12); and (iii) the newly proposed genus “Candidatus Neoehrlichia,” which was recently detected in ticks and wild animals but not in humans (5, 8, 17, 23). The reference strain of the new species “Candidatus N. mikurensis” was originally described in Japan (“Candidatus N. mikurensis” strain TK4456R from wild Rattus norvegicus rats on Mikura Island), but based on 16S rRNA and groEL gene analyses, homologous sequence variants were also found in ticks and wild mice in East Asia (Japan, China, Russia) (1, 8, 9, 15, 16) and Europe (Italy, Netherlands, Germany) (13, 14, 20). A closely related but distinct species, “Candidatus N. lotoris,” was found in raccoons in North America (23).
Until recently, “Candidatus N. mikurensis” was thought to be restricted to ticks and animals because the analysis of DNA samples from 62 patients with FUO in Japan failed to reveal infections with this bacterium (17). Very recently published data reported on the first case of human “Candidatus N. mikurensis” infection in an immunosuppressed 77-year-old patient with chronic B-cell lymphocytic leukemia who suffered from recurrent episodes of fever and erysipelas-like rashes, anemia, and an initial deep vein thrombosis (21). Together with this report, our cases suggest that “Candidatus N. mikurensis” is a novel human pathogen that can cause severe, blood culture-negative febrile illnesses and FUO.
Initial analyses described “Candidatus N. mikurensis” as an intracellular Gram-negative coccus that could be transferred from infected wild rats to laboratory rats, in which it replicated and was detectable in the blood, spleen, and liver. The infection did not elicit antibodies against A. phagocytophilum and Ehrlichia muris antigens (5, 8), which further supported the position of “Candidatus Neoehrlichia” as a separate genus within the family Anaplasmataceae. The 16S rRNA and groEL gene sequences of tick W330 and patient 1 and the respective phylogenetic analyses reported here strongly argue for the existence of a European sequence variant of “Candidatus N. mikurensis” that is able to infect humans (Fig. 1). The fact that patient 1 was seronegative for antibodies against A. phagocytophilum or E. chaffeensis is in accordance with the observations in infected rats but could also result from the severe immunosuppression of this patient.
The presence of “Candidatus N. mikurensis” in a putative rodent reservoir (5, 8, 17) and in ticks that feed on humans and animals (5, 8) (I. ricinus tick W330 [this study]), its repeated detection in independent blood specimens of patient 1 during his febrile illness, and its prompt disappearance following treatment with doxycycline and clinical cure of this patient strongly argue for the transmissibility and pathogenicity of this bacterium. In the case of patient 2, who died without having received treatment with doxycycline, we do not know whether the fatal outcome was causally related to the detection of “Candidatus N. mikurensis” in the blood. To date, the underlying disease-mediating mechanisms of “Candidatus N. mikurensis” are not known. In infected rats, “Candidatus N. mikurensis” was detected in membrane-bound inclusions within the cytosol of endothelial cells lining the splenic venous sinuses but not in blood cells (5). In accordance with these observations, no intracellular pathogens were detectable in Giemsa-stained blood smears of patient 1 (data not shown). It is possible that the severe thrombotic events in patient 1 and the aneurysm in patient 2 were somehow related to the putative endothelial cell tropism of “Candidatus N. mikurensis.” Intriguingly, a thromboembolic complication was also seen in the patient reported by Welinder-Olsson et al. (21) However, endotheliotropism as a pathogenic mechanism of “Candidatus N. mikurensis” currently remains speculative because nothing is known about potential endothelium-damaging, prothrombotic, or other virulence factors of “Candidatus N. mikurensis.” In addition, the histopathological and molecular analysis of the paraffin-embedded carotid aneurysm of patient 2 revealed vessel segments with thrombosis, intima fibrosis, or calcifications with intermittent lymphoplasmacellular infiltrates but no signs of acute infection. Pathogens were not detectable in the aneurysm tissue upon Giemsa staining or 16S rRNA gene PCR assay (data not shown). Nevertheless, the possibility of a low-level chronic infection that might have caused the bacteremia following the rupture of the aneurysm cannot be formally excluded.
With respect to factors that might predispose to infection with “Candidatus N. mikurensis,” it is noteworthy that patient 1 had been treated with several broadly immunosuppressive drugs and was lacking peripheral blood B lymphocytes. Interestingly, B-cell-dependent, as well as T-cell-dependent, mechanisms of protection have been described for infections with members of the family Anaplasmataceae (2, 3, 19). The B-cell deficiency of patient 1 offers an explanation for his very high bacterial burden of at least 100,000 bacterial cells/ml peripheral blood (data not shown), which was calculated based on the sensitivity of our broad-range 16S rRNA gene-based PCR assay and the assumption that the genome of “Candidatus N. mikurensis” harbors only one copy of the 16S rRNA gene, as has been shown for the genera Anaplasma and Ehrlichia.
In conclusion, this report identifies “Candidatus N. mikurensis” as a bacterium that is infectious for humans. It is possible that coagulopathies, which are rare in infections with A. phagocytophilum and E. chaffeensis, will turn out to be typical features of “Candidatus Neoehrlichia” infections. In the future, the in vitro culture of N. mikurensis” in host cells, which might be attempted using a recently established protocol for the closely related I. ovatus Ehrlichia bacteria (7), will be a prerequisite for the further characterization of its pathogenic potential.
Nucleotide sequence accession numbers.
The 16S rRNA and/or groEL gene sequences of the “Candidatus N. mikurensis” variants are available at GenBank under accession numbers EU810404 (16S rRNA gene, patient 1), EU810405 (16S rRNA gene, tick W330), EU810406 (groEL, patient 1), EU810407 (groEL, tick W330), and GU212868 (16S rRNA gene, patient 2).
Acknowledgments
We thank the medical technical assistants in the Diagnostic Department of the Microbiology Institute at the University Hospital of Erlangen for their help.
This study was supported in part by grants to C.B. from the German Research Foundation (DFG Bo 996/3-3) and from the Bavarian Ministry for Environment, Health, and Consumer Protection (VICCI, project 6).
F.D.V.L. and W.G. contributed equally to this article. C.B. and S.G.S. share senior authorship of this report.
Samir G. Sakka is a member of the Medical Advisory Board of Pulsion Medical Systems AG, Germany, and has received honoraria from this company and MSD Sharp & Dohme, Germany, for giving lectures. Claudia Disqué also holds a position at Molzym GmbH & Co. KG (Bremen, Germany), which produces the SepsiTest kit. We have no other potential conflict of interest to report.
Footnotes
Published ahead of print on 2 June 2010.
REFERENCES
- 1.Alekseev, A. N., H. V. Dubinina, I. Van De Pol, and L. M. Schouls. 2001. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J. Clin. Microbiol. 39:2237-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birkner, K., B. Steiner, C. Rinkler, Y. Kern, P. Aichele, C. Bogdan, and F. D. von Loewenich. 2008. The elimination of Anaplasma phagocytophilum requires CD4+ T cells, but is independent of Th1 cytokines and a wide spectrum of effector mechanisms. Eur. J. Immunol. 38:3395-3410. [DOI] [PubMed] [Google Scholar]
- 3.Bitsaktsis, C., B. Nandi, R. Racine, K. C. MacNamara, and G. Winslow. 2007. T-cell-independent humoral immunity is sufficient for protection against fatal intracellular Ehrlichia infection. Infect. Immun. 75:4933-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and HGE agent as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 5.Kawahara, M., Y. Rikihisa, E. Isogai, M. Takahashi, H. Misumi, C. Suto, S. Shibata, C. Zhang, and M. Tsuji. 2004. Ultrastructure and phylogenetic analysis of 'Candidatus Neoehrlichia mikurensis’ in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 54:1837-1843. [DOI] [PubMed] [Google Scholar]
- 6.Merçot, H., and D. Poinsot. 2009. Infection by Wolbachia: from passengers to residents. C. R. Biol. 332:284-297. [DOI] [PubMed] [Google Scholar]
- 7.Munderloh, U. G., D. J. Silverman, K. C. MacNamara, G. G. Ahlstrand, M. Chatterjee, and G. M. Winslow. 2009. Ixodes ovatus Ehrlichia exhibits unique ultrastructural characteristics in mammalian endothelial and tick-derived cells. Ann. N. Y. Acad. Sci. 1166:112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naitou, H., D. Kawaguchi, Y. Nishimura, M. Inayoshi, F. Kawamori, T. Masuzawa, M. Hiroi, H. Kurashige, H. Kawabata, H. Fujita, and N. Ohashi. 2006. Molecular identification of Ehrlichia species and 'Candidatus Neoehrlichia mikurensis’ from ticks and wild rodents in Shizuoka and Nagano Prefectures, Japan. Microbiol. Immunol. 50:45-51. [DOI] [PubMed] [Google Scholar]
- 9.Pan, H., S. Liu, Y. Ma, S. Tong, and Y. Sun. 2003. Ehrlichia-like organism gene found in small mammals in the suburban district of Guangzhou of China. Ann. N. Y. Acad. Sci. 990:107-111. [DOI] [PubMed] [Google Scholar]
- 10.Relman, D. A., P. W. Lepp, K. N. Sadler, and T. M. Schmidt. 1992. Phylogenetic relationships among the agent of bacillary angiomatosis, Bartonella bacilliformis, and other alphaproteobacteria. Mol. Microbiol. 6:1801-1807. [DOI] [PubMed] [Google Scholar]
- 11.Relman, D. A., T. M. Schmidt, R. P. MacDermott, and S. Falkow. 1992. Identification of the uncultured bacillus of Whipple's disease. N. Engl. J. Med. 327:293-301. [DOI] [PubMed] [Google Scholar]
- 12.Rikihisa, Y., C. Zhang, and B. M. Christensen. 2003. Molecular characterization of Aegyptianella pullorum (Rickettsiales, Anaplasmataceae). J. Clin. Microbiol. 41:5294-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanogo, Y. O., P. Parola, S. Shpynov, J. L. Camicas, P. Brouqui, G. Caruso, and D. Raoult. 2003. Genetic diversity of bacterial agents detected in ticks removed from asymptomatic patients in northeastern Italy. Ann. N. Y. Acad. Sci. 990:182-190. [DOI] [PubMed] [Google Scholar]
- 14.Schouls, L. M., I. Van De Pol, S. G. Rijpkema, and C. S. Schot. 1999. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J. Clin. Microbiol. 37:2215-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shpynov, S., P. E. Fournier, N. Rudakov, I. Tarasevich, and D. Raoult. 2006. Detection of members of the genera Rickettsia, Anaplasma, and Ehrlichia in ticks collected in the Asiatic part of Russia. Ann. N. Y. Acad. Sci. 1078:378-383. [DOI] [PubMed] [Google Scholar]
- 16.Spitalská, E., I. Literak, O. A. Sparagano, M. Golovchenko, and E. Kocianova. 2006. Ticks (Ixodidae) from passerine birds in the Carpathian region. Wien. Klin. Wochenschr. 118:759-764. [DOI] [PubMed] [Google Scholar]
- 17.Tabara, K., S. Arai, T. Kawabuchi, A. Itagaki, C. Ishihara, H. Satoh, N. Okabe, and M. Tsuji. 2007. Molecular survey of Babesia microti, Ehrlichia species and 'Candidatus Neoehrlichia mikurensis’ in wild rodents from Shimane Prefecture, Japan. Microbiol. Immunol. 51:359-367. [DOI] [PubMed] [Google Scholar]
- 18.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 19.Thirumalapura, N. R., E. C. Crossley, D. H. Walker, and N. Ismail. 2009. Persistent infection contributes to heterologous protective immunity against fatal ehrlichiosis. Infect. Immun. 77:5682-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Loewenich, F. D., B. U. Baumgarten, K. Schroppel, W. Geissdorfer, M. Rollinghoff, and C. Bogdan. 2003. High diversity of ankA sequences of Anaplasma phagocytophilum among Ixodes ricinus ticks in Germany. J. Clin. Microbiol. 41:5033-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welinder-Olsson, C., E. Kjellin, K. Vaht, S. Jacobsson, and C. Wenneras. 2010. First case of human 'Candidatus Neoehrlichia mikurensis’ infection in a febrile patient with chronic lymphocytic leukemia. J. Clin. Microbiol. 48:1956-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wellinghausen, N., A. J. Kochem, C. Disque, H. Muhl, S. Gebert, J. Winter, J. Matten, and S. G. Sakka. 2009. Diagnosis of bacteremia in whole-blood samples by use of a commercial universal 16S rRNA gene-based PCR and sequence analysis. J. Clin. Microbiol. 47:2759-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yabsley, M. J., S. M. Murphy, M. P. Luttrell, B. R. Wilcox, E. W. Howerth, and U. G. Munderloh. 2008. Characterization of 'Candidatus Neoehrlichia lotoris’ (family Anaplasmataceae) from raccoons (Procyon lotor). Int. J. Syst. Evol. Microbiol. 58:2794-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]