Abstract
We describe a case and summarize six additional cases of cervical lymphadenitis in otherwise healthy adults caused by Mycobacterium haemophilum. The organism causes cervicofacial lymphadenitis in healthy children and severe disease in immunocompromised patients but has not been previously reported to cause cervical lymphadenitis in nonimmunocompromised, healthy adults.
CASE REPORT
A 27-year-old woman, with a medical history of asthma and not on systemic corticosteroid therapy, presented with persistent swelling in the right neck and cheek. Her symptoms began several weeks after she bit herself on the right buccal mucosa while eating. She denied any drainage from the buccal or submandibular lesions, nor did she complain of any fevers, chills, or night sweats. Her medical history was otherwise unremarkable except for a root canal 6 months prior. She was initially evaluated by her primary care physician and prescribed a 10-day course of augmentin without relief. The swelling persisted, and she was subsequently treated with a 10-day course of clindamycin in combination with a 7-day course of methylprednisolone, again without response. She was referred to an otolaryngologist because of concern about a possible abscess. An exam at that time revealed a 2-cm hard mass in the submucosal region on the right buccal area along with tender palpable lymphadenopathy in the right submandibular region. She was placed on levofloxacin for 14 days with mild improvement; her symptoms relapsed, however, after discontinuation. A computed tomography (CT) scan of the head and neck at 3 months into her course revealed a loculated abscess in the right buccal region as well as a cluster of enlarged necrotic lymph nodes in the right submandibular region measuring 3.0 by 2.0 by 1.5 cm. She underwent intraoral incision and drainage of the right buccal cheek abscess containing mucopurulent fluid, as well as excisional lymph node biopsy of the deep submandibular cervical nodes. Lymph node histopathology revealed necrotizing granulomatous lymphadenitis (Fig. 1 ) with negative acid-fast and fungal stains. Routine bacterial and fungal cultures of pus from the abscess cavity remained negative throughout their incubation. Mycobacterial cultures, however, finally revealed Mycobacterium haemophilum growing only on chocolate agar slants incubated at 30°C. The isolate was presumptively identified by its characteristic growth only on iron-supplemented media incubated at lower temperatures (30°C), and the identity was confirmed by high-pressure liquid chromatographic (HPLC) analysis showing typical mycolic acids (14). In vitro susceptibility studies were not performed. She recovered without antimicrobial therapy after surgical drainage of the buccal abscess and lymph node excision.
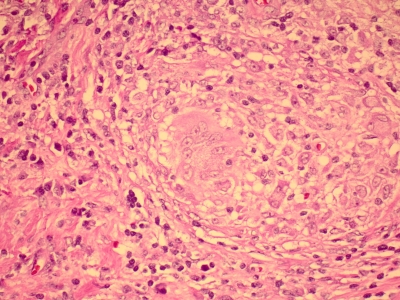
FIG. 1.
Histopathology of an excisional lymph biopsy specimen from a 27-year-old, otherwise healthy female with cervicofacial lymphadenitis showing granulomas with giant cells in center (magnification, ×1,000; hematoxylin and eosin [H&E] stain).
To our knowledge, our case is the first to describe cervical lymphadenitis caused by M. haemophilum in a healthy adult patient, although a number of nontuberculous mycobacteria (NTM), including M. haemophilum, have been reported to cause cervical lymphadenitis, especially in children (8). We additionally retrospectively reviewed the available chart information of six other cases of cervicofacial lymphadenitis caused by M. haemophilum in otherwise healthy adults seen over a 15-year period in the greater Phoenix metropolitan area of Arizona (Table 1). The study was approved by the Institutional Review Board of Banner Good Samaritan Medical Center. Together with the described patient, the ages of the seven adult patients ranged from 19 to 79 years, with a median age of 53 years. None were reported to have any debilitating or highly immunocompromising condition, and all were otherwise seemingly healthy. Interestingly, four of the seven cases (57%) had histories of either recent buccal trauma (the patient described here) or tooth extraction (three patients) prior to the onset of lymphadenitis; histories of buccal trauma or tooth extraction were not noted in the charts for the remaining three cases.
TABLE 1.
Descriptions of six additional cases of Mycobacterium haemophilum causing cervicofacial lymphadenitis in otherwise healthy adults over a 15-year period
| Case no. | Patient description | Presentation | Histopathology | Culture results | Follow-up | Additional history |
|---|---|---|---|---|---|---|
| 1 | 60-yr-old female | Left neck mass, nontender lymphadenopathy; lymphadenitis continued despite antibacterial therapy and incision and drainage; patient presented some mos later with same clinical picture | Fine needle aspirate (FNA) showed necrotic granulomatous inflammation at both presentations | All bacterial cultures negative; acid-fast hacillas (AFB) studies performed at second presentation had negative direct fluorochrome smears, but culture grew M. haemophilum | Patient given clarithromycin and nodes excised; no recurrence | |
| 2 | 65-yr-old female | Painless right cheek and submandibular neck mass; inflammation continued despite 3-mo trial of augmentin; patient presented for additional studies | FNA showed granulomatous lymphadenitis | All bacterial cultures negative; AFB studies performed at second presentation had negative direct fluorochrome smears, but culture grew M. haemophilum | Patient given clarithromycin; no recurrence | Patient had recent extraction of right submandibular molar tooth prior to presentation |
| 3 | 53-yr-old female | 10-day history of worsening left neck and facial swelling; she did not respond to a 10-day course of cephalexin; she was seen again and placed on clindamycin for 10 days without improvement; excisional lymph node biopsy | No malignancy; no organisms; necrotizing lymphadenitis | All bacterial cultures negative; AFB studies performed at final presentation had negative direct fluorochrome smears, but culture grew M. haemophilum | Not available | 6 wks prior to onset she had undergone tooth extraction |
| 4 | 22-yr-old female | Firm, nontender right neck mass on right anterior cleidomastoid muscle in zone II of neck, no erythema | FNA showed necrotizing granulomatous lymphadenitis | All bacterial cultures negative; AFB studies performed at second presentation had negative direct fluorochrome smears, but culture grew M. haemophilum | Not available | Patient was from East India and had undergone tooth extraction in India 4 wks prior to presentation |
| 5 | 19-yr-old male | 6-wk history of left cervical lymphadenitis which had failed a course of antibacterial therapy; mass measured 2 by 2 cm by CT scan and was described as consisting of matted lymph nodes located high in anterior cervical chain triangle; excisional lymph node biopsy performed | Necrotizing lymphadenitis | All bacterial cultures negative; AFB studies performed at second presentation had negative direct fluorochrome smears, but culture grew M. haemophilum | Not available | Tuberculin skin test negative |
| 6 | 79-yr-old female | Patient afebrile, with solitary mass on right side of neck; excisional lymph biopsy performed | Necrotizing granulomatous lymphadenitis | All bacterial cultures negative; AFB studies performed at second presentation had negative direct fluorochrome smears, but culture grew M. haemophilum | Not available |
Mycobacterium haemophilum, a slow-growing nontuberculous mycobacterium, is more commonly associated with infections involving the lymph nodes, skin and soft tissues, bone and joints, and lungs in patients with profound immunocompromise, including those with hematological malignancies, those with AIDS, transplant recipients, and those receiving immunosuppressive medications for autoimmune conditions (1, 2, 5, 7, 9, 10, 12, 14, 15, 18). In immunocompetent patients the organism has been described as a cause of a pulmonary nodule in an adult and of localized lymphadenitis, typically cervicofacial, in healthy pediatric patients, the latter being hypothesized to be due to lymphatic drainage from the oropharynx (3, 11, 16, 17).
Failure to recognize M. haemophilum as an etiology of cervicofacial lymphadenitis in otherwise healthy adults may be due to its stringent growth requirements and clinicians' low level of suspicion of its presence in the differential diagnosis. Routine cultures for bacteria, fungi, and mycobacteria will not normally recover M. haemophilum. For its isolation, culture media have to be supplemented with 0.4% hemoglobin, 60 μM hemin (factor X), or 15 mg/ml ferric ammonium citrate. M. haemophilum is unique among mycobacteria in its requirement for hemin or ferric ammonium citrate for growth (7, 13, 14, 15, 17, 18). Its growth is very slow or nonexistent on Löwenstein-Jensen medium or Middlebrook 7H11 agar at 35°C. Furthermore, the organism grows far better at 28 to 30°C than at 35°C and growth is absent at 37°C. These characteristics can be used to presumptively identify M. haemophilum in the laboratory; alternatively, laboratories with access to high-pressure liquid chromatography can rapidly identify an isolate by its characteristic chromatogram (14). As seen with our patients, the lack of suspicion for M. haemophilum often results in a delay of diagnosis, inadequate therapy with usual antibacterial antimicrobics, and repeated biopsies and microbiologic studies.
Mycobacterium haemophilum is known to have a worldwide distribution, however rare its incidence may be; its ecological niche is unknown, and its means of transmission and acquisition remain elusive (1, 4, 6, 7, 14, 17, 18). A large majority of the cases presented in this report were associated with recent dental manipulation and/or oral trauma; this finding raises the question of whether the organism normally or transiently resides among dental and gum tissue and seeds the lymphatics from that route, as is hypothesized for pediatric cervicofacial lymphadenitis.
The organism is most susceptible in vitro to clarithromycin, ciprofloxacin, amikacin, rifabutin, and rifampin; for most patients, antimicrobial choice and length of therapy are guided by the underlying condition and clinical response (1, 4, 6, 14, 15, 17, 18). Complete surgical excision as opposed to incision and drainage seems paramount to treatment of NTM lymphadenitis in children (6, 8). In vitro susceptibility testing is not currently standardized for M. haemophilum and is not recommended (6).
Among our seven cases, five patients underwent surgical excision of the infected lymph nodes, whereas the remaining two patients were diagnosed by fine-needle aspirate (FNA). Follow-up information after the diagnosis of M. haemophilum infection was available for three of the seven cases: our case report patient, who was treated with surgical intervention alone, one patient who received clarithromycin after surgical excision (case 1), and the third patient, who received clarithromycin without surgical excision (case 2); all resulted in complete recovery. It is difficult to speculate on the role of the various treatment modalities on patient recovery based on this limited information. Notably, our case did demonstrate documented improvement with levofloxacin but subsequently relapsed, suggesting the inadequacy of treatment involving short-duration antimicrobial therapy or a lack of surgical intervention.
These cases are reported to alert clinicians and medical microbiologists that M. haemophilum should be considered in the differential diagnosis of chronic cervicofacial lymphadenitis in otherwise healthy adults, especially after oral manipulation or trauma. When considering a mycobacterial etiology in adult and pediatric immunocompetent patients presenting with chronic lymphadenitis, lymph node specimens should be additionally cultured in a manner enabling recovery of M. haemophilum (addition of chocolate agar plates, slants, or other hemin- or ferric ammonium citrate-containing media incubated at a temperature ranging from 28 to 30°C). Alternatively, recovery of M. haemophilum may be sought in immunocompetent patients with lymphadenitis of any duration, including acute disease, if biopsy specimens reveal granulomatous inflammation on histopathology.
Footnotes
Published ahead of print on 19 May 2010.
REFERENCES
- 1.Armstrong, K. L., R. W. James, D. J. Dawson, P. W. Francis, and B. Masters. 1992. Mycobacterium haemophilum causing perihilar or cervical lymphadenitis in healthy children. J. Pediatr. 121:202-205. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. 1991. Mycobacterium haemophilum infections—New York City metropolitan area, 1990-1991. MMWR Morb. Mortal. Weekly Rep. 40:636-637, 643. [PubMed] [Google Scholar]
- 3.Davis, J. P., P. R. Prinsley, and P. Robinson. 1993. Cervical lymphadenopathy due to mycobacterial infection: a diagnostic protocol. J. Laryngol. Otol. 107:614-617. [DOI] [PubMed] [Google Scholar]
- 4.Dawson, D. J., Z. M. Blacklock, and D. W. Kane. 1981. Mycobacterium haemophilum causing lymphadenitis in an otherwise healthy child. Med. J. Aust. 2:289-290. [DOI] [PubMed] [Google Scholar]
- 5.Dever, L. L., J. W. Martin, B. Seaworth, and J. H. Jorgensen. 1992. Varied presentations and responses to treatment of infections caused by Mycobacterium haemophilum in patients with AIDS. Clin. Infect. Dis. 14:1195-1200. [DOI] [PubMed] [Google Scholar]
- 6.Griffith, D. E., T. Aksamit, B. A. Brown-Elliott, A. Catanzaro, C. Daley, F. Gordin, S. M. Holland, R. Horsburgh, G. Huitt, M. F. Iademarco, M. Iseman, K. Olivier, S. Ruoss, C. F. von Reyn, R. J. Wallace, Jr., and K. Winthrop. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367-416. [DOI] [PubMed] [Google Scholar]
- 7.Harrison, A. C., and T. Jayasundera. 1999. Mycobacterial cervical lymphadenitis in Auckland, diagnosis by fine needle aspirate. N. Z. Med. J. 112:6-9. [PubMed] [Google Scholar]
- 8.Jarzembowski, J. A., and M. B. Young. 2008. Nontuberculous mycobacterial infections. Arch. Pathol. Lab. Med. 132:1333-1341. [DOI] [PubMed] [Google Scholar]
- 9.Kiehn, T. E., M. White, K. J. Pursell, N. Bonne, M. Tsivitis, A. E. Brown, B. Polsky, and D. Armstrong. 1993. A cluster of four cases of Mycobacterium haemophilum infection. Eur. J. Clin. Microbiol. Infect. Dis. 12: 14-118. [DOI] [PubMed] [Google Scholar]
- 10.McBride, M. E., A. H. Rudolph, J. A. Tschen, P. Cernoch, J. Davis, B. A. Brown, and R. J. Wallace. 1991. Diagnostic and therapeutic considerations for cutaneous Mycobacterium haemophilum infections. Arch. Dermatol. 127:276-277. [PubMed] [Google Scholar]
- 11.Piersimoni, C., and C. Scarparo. 2009. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg. Infect. Dis. 15:1351-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers, P. L., R. E. Walker, H. C. Lane, F. G. Witebsky, J. A. Kovacs, J. E. Parillo, and H. Masur. 1988. Disseminated Mycobacterium haemophilum infection in two patients with the acquired immunodeficiency syndrome. Am. J. Med. 84:640-642. [DOI] [PubMed] [Google Scholar]
- 13.Samra, Z., L. A. Kaufmann, S. Zeharia, J. Ashkenazi, J. Amir, J. Bahar, U. Reischl, and L. Naumann. 1999. Optimal detection and identification of Mycobacterium haemophilum in specimens from pediatric patients with cervical lymphadenopathy. J. Clin. Microbiol. 37:832-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saubolle, M. A., T. E. Kiehn, M. H. White, M. F. Rudinsky, and D. Armstrong. 1996. Mycobacterium haemophilum: microbiology and expanding clinical and geographic spectra of disease in humans. Clin. Microbiol. Rev. 9:435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thibert, L., F. Lebel, and B. Martineau. 1990. Two cases of Mycobacterium haemophilum infection in Canada. J. Clin. Microbiol. 28:621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van De Griendt, E. J., P. J. Rietra, and R. N. Van Andel. 2003. Mycobacterium haemophilum as cause of lymphadenitis in the neck of otherwise healthy boy. Ned. Tijdschr. Geneeskd. 147:1367-1369. (In Dutch.) [PubMed] [Google Scholar]
- 17.White, D. A., T. E. Kiehn, A. Y. Bondoc, and S. A. Massarella. 1999. Pulmonary nodule due to Mycobacterium haemophilum in an immunocompetent host. Am. J. Respir. Crit. Care Med. 160:1366-1368. [DOI] [PubMed] [Google Scholar]
- 18.White, M. H., E. B. Papdopoulos, T. N. Small, T. E. Kiehn, and D. Armstrong. 1995. Mycobacterium haemophilum infections in bone marrow transplant recipients. Transplantation 60:957-960. [PubMed] [Google Scholar]