Abstract
We report the repeated isolation of the fungus Geosmithia argillacea from sputum samples of people with cystic fibrosis. Identification was based on morphology and DNA sequence analysis. Isolation of G. argillacea did not appear to be associated with clinical deterioration. The pathogenic potential of G. argillacea is discussed.
People with cystic fibrosis (CF) are at risk of colonization by and, in some cases, subsequent allergic reaction to or infection with a number of fungi. Most notable among these are Aspergillus species, Scedosporium apiospermum, and Exophiala dermatitidis (7). Recently, the fungus Geosmithia argillacea has been repeatedly isolated from sputum of several people with CF attending clinics at the Leeds Regional Cystic Fibrosis Centre. This report describes these findings and associated clinical features and discusses their possible clinical significance.
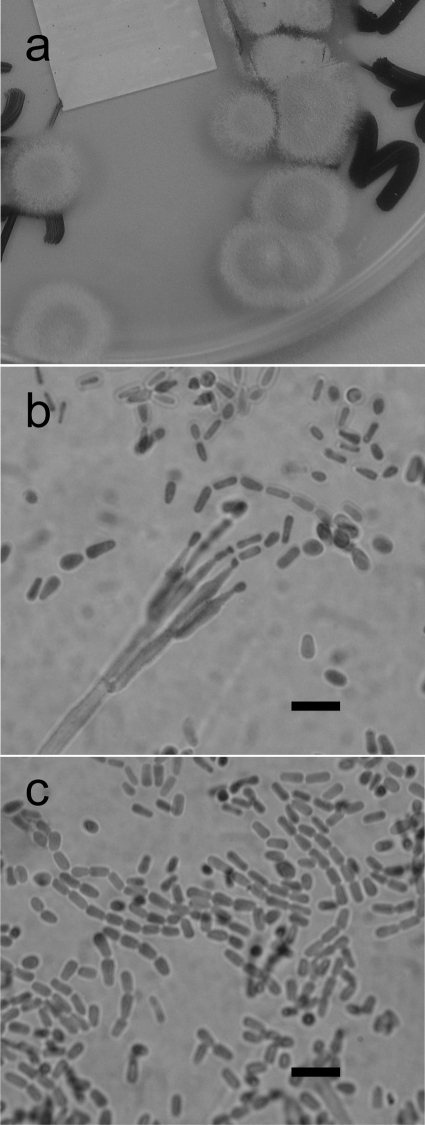
Approximately 500 subjects (150 pediatric and 330 adult) with a confirmed diagnosis of CF attending clinics at the Leeds Teaching Hospitals Trust were examined between 1 January 2005 and 30 June 2007. Sputum culture was carried out following treatment with 0.1% (wt/vol) solution of dithiothreitol inoculated onto Sabouraud's agar plates with 20 mg/liter colistin and gentamicin (E&O Laboratories) and incubated at 29, 36, and 45°C for 7 days. A relatively slow-growing, pale-brown, powdery mold was isolated from sputum samples of eight CF subjects (Fig. 1 a), in many cases from several sputum samples from the same person (Table 1). Growth was seen on plates incubated at all temperatures, in some cases with other fungi (Table 1). Microscopic examination of cultures revealed an extensively sporing, Penicillium-like fungus with phialides produced in a terverticillate mode of branching (Fig. 1b). Phialides were cylindrical with tapering apices and produced chains of columnar or “box-shaped” conidia that later became ovoid to globose (Fig. 1c). This fungus also grew on Columbia blood agar at a slightly lower rate than it did on Sabouraud's agar. Molecular identification was carried out as described previously (1, 2). Briefly, DNA was extracted from fungal cultures and subjected to PCR using primers designed to amplify large subunit (LSU) or internal transcribed spacer 1 (ITS1) regions of the nuclear rRNA gene cassette (1). For certain isolates, regions of the β-tubulin gene were also analyzed. Sequences of the PCR amplicons were used to search the GenEMBL database and a database generated by the HPA Mycology Reference Laboratory, Bristol, United Kingdom. The sequences from all three strains shared very high homologies with Geosmithia argillacea sequences in the public databases. A representative isolate of G. argillacea from the present study has been stored in the National Collection of Pathogenic Fungi (NCPF) as NCPF 7710. Antifungal susceptibility testing of representative G. argillacea isolates was performed according to the CLSI M38-A methodologies (4). G. argillacea exhibited low MICs to amphotericin B, itraconazole, posaconazole, and caspofungin but high MICs to voriconazole (16 mg/liter) (Table 2).
FIG. 1.
Morphology of G. argillacea. (a) Photograph of plate of G. argillacea growing on Sabouraud dextrose agar after 7 days at 37°C. (b) Microscopic appearance of the conidiophore and conidia of G. argillacea. Bar = 5 μm. (c) Microscopic appearance of conidia of G. argillacea.
TABLE 1.
Number of sputum samples and sputum samples positive for G. argillacea, clinical details, and other culture results
| Characteristic | Value/description for subject: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| No. of sputum samples | 79 | 73 | 68 | 13 | 11 | 26 | 78 | 91 |
| No. of positive samples (%) | 29 (36.7) | 1 (1.4) | 11 (16.8) | 9 (69) | 15 (73.3) | 2 (7.7) | 1 (1.3) | 29 (31.9) |
| Age at first isolation (yr) | 26 | 24 | 27 | 21 | 6 | 19 | 8 | 14 |
| CF genotype | DF508 DF508 | DF508 DF508 | DF508 2184delA | DF508 Q493X | DF508 DF508 | DF508 DF508 | DF508 1717-1(G>T) | DF508 DF508 |
| FEV1 at first isolation (% predicted)c | 15 | 82 | 17 | 55 | 73 | 58 | 58 | 27 |
| Other organismsa | Af, As, Mv | Af, Sa, MRSA, Pa, Mc | Bccm | Af, Sa, Pa | Af, Sm | Af, Pa, Mc | Bccc, Pa, Af, Ma | Af, Psp, Pa, Ps, Ax, As, Mv |
| Any associated decline? | No | No | No | No | No | No | No | No |
| Comment(s)b | Listed for lung transplantation prior to isolation | Two episodes of hemoptysis within 2 months of isolation, not; to be related | Subsequently listed for lung transplantation | CT chest shows plugged dilated bronchus and nodularity in right upper lobe | Listed for lung transplantation prior to isolation thought has subsequently undergone transplantation | |||
Af, Aspergillus fumigatus; As, Alcaligenes sp.; Ma, Mycobacterium abscessus; Sa, Staphylococcus aureus; MRSA, methicillin-resistant S. aureus; Pa, Pseudomonas aeruginosa; Mc, Mycobacterium chelonae; Bccm, Burkholderia cepacia complex sp. B. multivorans; Sm, Streptococcus milleri; Bccc, Burkholderia cepacia complex sp. B. cenocepacia; Psp, Paecilomyces sp.; Ps, Pseudoxanthomonas sp.; Ax, Achromobacter xylosoxidans; Mv, Mycobacterium avium; Hf, Haemophilus influenzae.
CT, computed tomography.
FEVI, forced expiratory volume in 1 s.
TABLE 2.
Antifungal susceptibility of G. argillacea isolates to amphotericin B, itraconazole, voriconazole, posaconazole, and caspofungin obtained using the NCCLS/CLSI broth microdilution methoda
| Isolate | Source | MIC (mg/liter) for: |
||||
|---|---|---|---|---|---|---|
| Amphotericin | Itraconazole | Voriconazole | Caspofungin | Posaconazole | ||
| NCPF 2801 | Sputum, CF patient, UK, 1991 | 1 | 0.5 | 16 | 2 | ND |
| NCPF 7594 | Isolated from blood culture, patient with peritonitis, UK, 2002 | 2 | 0.5 | 16 | 0.5 | ND |
| NCPF 7596 | Isolated from peritoneal dialysis fluid of same patient as NCPF 7594 | 2 | 0.5 | 16 | 0.25 | ND |
| CF isolate 1 | Leeds Teaching Hospitals Trust, CF subject 4 | 0.5 | 0.5 | >16 | 0.25 | 0.5 |
| CF isolate 2 | Leeds Teaching Hospitals Trust, CF subject 3 | 2 | 0.5 | >16 | 0.5 | 0.5 |
UK, United Kingdom; ND, not determined.
The clinical characteristics of CF subjects from whom G. argillacea had been isolated were examined for any apparent associated decline in clinical status and to establish any other relevant clinical associations (Table 1). The first recognized isolation of G. argillacea in CF sputum culture at this center coincided with results determined by a different unit within the Department of Microbiology carrying out identification of filamentous fungi. It is likely that G. argillacea was present prior to the study period but not recognized or reported as such. No association between isolation of G. argillacea and either age or lung function was apparent. There was no subjective evidence of clinical decline in these subjects that could be attributed to G. argillacea upon detailed review of the case notes. No obvious epidemiological links could be identified between people with CF with positive sputum culture for G. argillacea. The subjects were distributed widely throughout the catchment area for the CF clinics involved; they included both pediatric and adult patients with CF, and as such, they would have been seen at different clinics and by different clinicians; and there was no evidence that acquisition of G. argillacea correlated with bronchoscopy. A full analysis of risk factors will be required in order to determine why we were able to grow G. argillacea from samples from some people with CF and not from those of others.
Previously, G. argillacea, which is related to Penicillium, has been described as a causal agent of food spoilage, where its heat resistance was also noted (10). We assume that the subjects in the current report acquired G. argillacea through inhalation, though the precise environmental source is unknown. However, we cannot formally rule out that isolation resulted from contamination from food via the oropharynx as the quality of sputa was not routinely assessed. Recently Giraud et al. (5) have determined that a fungus initially identified as Penicillium emersonii in a patient with CF (3) was in fact G. argillacea and that this organism is isolated increasingly from sputa in people with CF in France. The only report of G. argillacea causing a disseminated fungal infection to date has been one of infection in a dog (6).
It is possible that G. argillacea may be isolated more commonly than has been realized up to now and overlooked given its morphological similarities to Penicillium and Paecilomyces spp. A molecular characterization of fungal isolates stored in the NCPF that might represent misidentified G. argillacea isolates supports this contention. Isolate NCPF 2801, from sputum of a CF patient from the United Kingdom in 1991, was indistinguishable genetically from G. argillacea (data not shown), and G. argillacea was recently isolated from a bronchoalveolar lavage specimen from a 51-year-old patient in Leeds without CF being treated for a persistent cough following smoke inhalation. Furthermore, two more isolates, NCPF 7594 and 7596, isolated from blood culture and peritoneal dialysis fluid from a patient from the United Kingdom with peritonitis in 2001 were also revealed to be G. argillacea by β-tubulin gene sequencing (data not shown).
While it appears that colonization of people with CF by G. argillacea does not present a significant clinical problem, a concern remains where people with CF become immunocompromised, for example, if they undergo lung transplantation. Aspergillus fumigatus and Scedosporium apiospermum have been documented to cause invasive disease that is frequently fatal in this clinical setting (8, 9). It remains possible that G. argillacea might cause systemic disease when colonized patients become immunocompromised following lung transplantation. It is pleasing to note, however, that subsequent to the study period, two of the CF patients have undergone heart lung transplantation at another center, and to date, there was no evidence of systemic fungal infection by G. argillacea posttransplantation in either patient.
Nucleotide sequence accession numbers.
Sequences from the G. argillacea isolates described here have been deposited into GenBank under accession numbers AM744972 to AM744975.
Footnotes
Published ahead of print on 26 April 2010.
REFERENCES
- 1.Borman, A. M., C. K. Campbell, C. J. Linton, P. D. Bridge, and E. M. Johnson. 2006. Polycytella hominis is a mutated form of Scedosporium apiospermum. Med. Mycol. 44:33-39. [DOI] [PubMed] [Google Scholar]
- 2.Borman, A. M., C. J. Linton, S.-J. Miles, C. K. Campbell, and E. M. Johnson. 2006. Ultra-rapid preparation of total genomic DNA from isolates of yeast and mould using Whatman FTA filter paper technology—a re-usable DNA archiving system. Med. Mycol. 44:389-398. [DOI] [PubMed] [Google Scholar]
- 3.Cimon, B., J. Carrere, J. P. Chazalette, J. F. Vinatier, D. Chabasse, and J. P. Bouchara. 1999. Chronic airway colonization by Penicillium emersonii in a patient with cystic fibrosis. Med. Mycol. 37:291-293. [PubMed] [Google Scholar]
- 4.CLSI. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard M38-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Giraud, S., M. Pihet, B. Razafimandimby, J. Carrère, N. Nicolas Degand, L. Mely, L. Favennec, J. P. Bouchara, and A. Calenda. 2009. Geosmithia argillacea: an emerging pathogen in cystic fibrosis patients? Abstr. 1st Meet. ISHAM Working Group Fungal Respiratory Infect. Cystic Fibrosis. [DOI] [PMC free article] [PubMed]
- 6.Grant, D. C., D. A. Sutton, C. A. Sandberg, R. D. Tyler, Jr., E. H. Thompson, A. M. Romanelli, and B. L. Wickes. 2009. Disseminated Geosmithia argillacea infection in a German Shepherd dog. Med. Mycol. 47:221-226. [DOI] [PubMed] [Google Scholar]
- 7.Nagano, Y., B. C. Millar, E. Johnson, C. E. Goldsmith, J. S. Elborn, J. Rendall, and J. E. Moore. 2007. Fungal infections in patients with cystic fibrosis. Rev. Med. Microbiol. 18:11-17. [Google Scholar]
- 8.Nunley, D. R., P. Ohori, W. F. Grgurich, A. T. Iacono, P. A. Williams, R. J. Keenan, and J. H. Dauber. 1998. Pulmonary aspergillosis in cystic fibrosis lung transplant recipients. Chest 114:1321-1329. [DOI] [PubMed] [Google Scholar]
- 9.Symoens, F., C. Knoop, M. Schrooyen, O. Denis, M. Estenne, N. Nolard, and F. Jacobs. 2006. Disseminated Scedosporium apiospermum infection in a cystic fibrosis patient after double-lung transplantation. J. Heart Lung Transplant. 25:603-607. [DOI] [PubMed] [Google Scholar]
- 10.Yaguchi, T., S. Udagawa, and K. Nishimura. 2005. Geosmithia argillacea is the anamorph of Talaromyces eburneus as a heat resistant fungus. Crypt. Mycol. 26:133-141. [Google Scholar]