Abstract
We investigated the impact of probiotics on the intestinal carriage of vancomycin-resistant enterococci (VRE). Administration of Lactobacillus rhamnosus Lcr35 but not Escherichia coli Nissle reduced, although not significantly, the density of VRE colonization in a murine model. No effect of Lcr35 was observed in a double-blind placebo randomized study, involving nine patients.
Vancomycin-resistant enterococci (VRE) are widespread nosocomial pathogens, whose main site of colonization is the intestinal tract, leading to asymptomatic digestive carriage and hence to the formation of a reservoir of VRE (4). Once colonized, patients are at greater risk of developing subsequent infections or of transmitting VRE to other patients. While reducing VRE intestinal carriage is a major step in limiting dissemination and infection, there still exists no effective VRE decolonization treatment. Because VRE persistence in the gut may be due both to intestinal flora disruption and to inhibition of the intestinal immune function, we hypothesize that probiotics are good candidates for achieving VRE clearance.
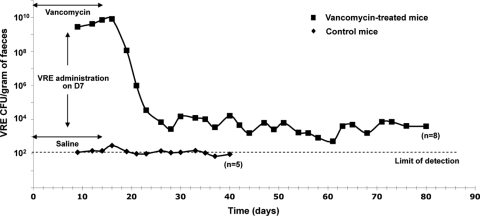
We developed a murine model of digestive VRE colonization and investigated the effects of two probiotic bacterial strains, one Gram-positive, Lactobacillus rhamnosus Lcr35 (Lcr35), and the other Gram-negative, Escherichia coli Nissle 1917 (EcN), on established VRE intestinal colonization. We used a resistant VanA-type Enterococcus faecium strain isolated from a bacteremic patient (2). Female IOPS OF1A mice, none of which was initially colonized, were individually caged and screened for VRE prior to any treatment. They were assigned to receive either sterile drinking water (control) or water containing vancomycin (250 μg/ml) for 14 days (D0 to D14). Gastric gavage of VRE (106 CFU in 0.2 ml) was performed on D7, and 7 days after VRE inoculation (D14) the mice received either Lcr35 (108 CFU) once a day for three or eight consecutive days or EcN (109 CFU) for eight consecutive days. The density (CFU/g) of VRE in the animal feces was determined three times a week over a 40-day period. Eight out of eight mice who had received prior oral vancomycin became colonized with VRE, with counts ranging from 3.2 × 106 to 8.3 × 109 CFU/g of feces on D9 (48 h after VRE inoculation) (Fig. 1), similar to the observations of Donskey et al. (1). These levels of VRE fecal concentration persisted for 9 days, including 2 days after vancomycin was stopped, and then rapidly declined to reach a level of 103 to 104 CFU/g of feces on D26, which was maintained in five mice up to D80 (Fig. 1). Only one mouse out of five in the control group (receiving neither Lcr35 nor EcN) had detectable VRE over a 21-day period.
FIG. 1.
Persistence and density of vancomycin-resistant Enterococcus faecium (VRE) intestinal colonization in mice. Mice received either vancomycin (▪) or saline (⧫) 7 days before and after VRE gastric inoculation (from D0 to D14). Stool VRE levels (CFU/g of feces) were quantified every 2 or 3 days from D8 until D80. In the control group, the number of VRE CFU was under the limit of detection (1.4 × 102 CFU/g) from D41.
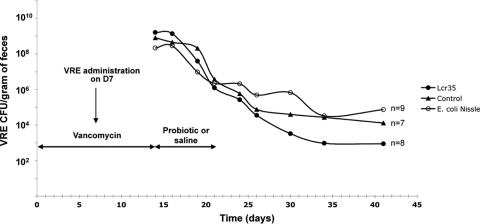
Mean fecal counts of VRE were similar in 3-day Lcr35-treated mice and in the control group (data not shown). In the group with 8-day treatment (n = 8), the density of VRE was lower, although not statistically different, than that in the control group (n = 7) from D30 (i.e., 9 days after probiotic discontinuation) until D41 (Fig. 2). With EcN, there was no difference in VRE density between the test (n = 9) and the control groups throughout the 26-day period (Fig. 2).
FIG. 2.
Effect of an 8-day course of administration of either Lactobacillus rhamnosus Lcr35 or Escherichia coli Nissle on intestinal carriage of vancomycin-resistant Enterococcus faecium (VRE). All mice received vancomycin (250 μg/ml) in drinking water for 14 days, and VRE gastric inoculation was performed on D7. On D14, vancomycin was stopped and mice received once daily either probiotic (•, Lcr35, 108 CFU; ○, E. coli Nissle, 109 CFU) or saline (▴), for 8 days. Stool VRE levels were quantified over a period of 4 weeks.
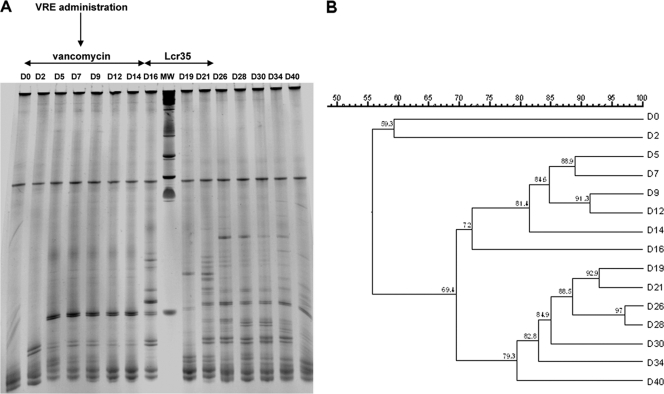
The dominant fecal microbiota was analyzed by temporal temperature gradient gel electrophoresis (TTGE), as previously described (5, 7). Feces samples were collected before treatment and three times a week from D0 to D40 from 8-day Lcr35-treated (n = 5) and control mice (n = 5). Total DNA was extracted from 150 to 200 mg fecal samples. Primers U968-GC and L1401 (7) were used to amplify the V6 to V8 regions of bacterial 16S rRNA genes, and PCR-TTGE profiles were compared using GelCompar software (Quantity One; Bio-Rad). The TTGE profiles obtained were composed of 11 to 32 bands representing the dominant flora. Figure 3 shows an example of a TTGE profile and its relevant dendrogram. Dice's coefficient was used to calculate distances between the samples. As from 48 h of vancomycin administration (D2), a few major bands appeared, with TTGE patterns exhibiting a similarity varying from 44.3 to 72.9% on D2 compared to D0. These new bands were detected throughout the antibiotic treatment (Fig. 3). VRE administration on D7 did not induce any major modification in the TTGE profiles, whereas major changes occurred after discontinuation of vancomycin. Comparison of the TTGE profiles of Lcr35-treated mice with those of control mice showed no major difference, indicating that administration of this probiotic did not drastically modify the evolution of fecal microbiota in the animals.
FIG. 3.
TTGE analysis of 16S rRNA (V6 to V8) amplification products from feces samples collected over time (40 days) of mice receiving vancomycin (D0 to D14) followed (test group) or not (control group) by daily administration of Lcr35 (D15 to D21). Representative example of TTGE community profile (A) and its phylogenetic tree constructed using Dice's coefficient (B) of an animal from the test group. The periods of vancomycin and probiotic administration are indicated by horizontal double arrows, and VRE gastric inoculation is indicated by a vertical arrow. MW, molecular weight markers.
Lcr35 was also tested in VRE cross-transmission among mice. Twenty-one VRE-free mice were placed in communal cages (three mice per cage), and 12 were supplied with 108 CFU of Lcr35 via daily gastric gavage from D0 to D9. At the same time, all mice were supplied with vancomycin in drinking water (250 μg/ml). On D7, one VRE-colonized mouse was placed in each communal cage. Four hours after cohousing, 3 of the 12 separately housed naïve mice were colonized (mean VRE density, 5.2 × 108 CFU/g) versus 5 of the 9 control mice (mean VRE density, 1.6 × 108 CFU/g). No statistical difference was observed either in the number of colonized mice or in the mean VRE density. At 8 and 12 h of cohousing, all 21 mice were colonized.
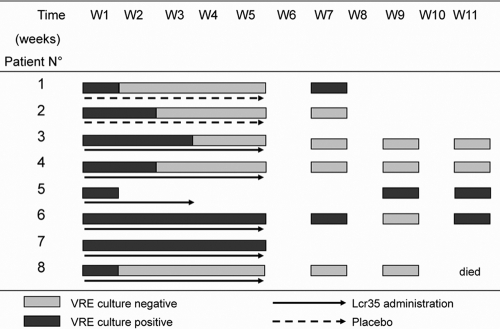
In addition, we looked at the effectiveness of Lcr35 in clearing VRE colonization in a limited number of VRE-carrying patients. After written informed consent, patients were included in a double-blind randomized pilot study (ClinicalTrials.gov identifier, NCT00437580), conducted over a 2-year period (2007-2008). Inclusion criteria were age of ≥18 years and positive stool screening for VRE. Exclusion criteria were inability to take pills, neutropenia of <1,000/mm3, and immunosuppressive therapy. Patients were randomly assigned to orally receive a 5-week course of Lcr35 (109 active cells daily) or a placebo. The patients were asked to provide stool samples every week for 5 weeks and then every 2 weeks until three consecutive negative VRE cultures were obtained. Results are summarized in Table 1 and Fig. 4. No significant effect of Lcr35 was observed on VRE intestinal colonization.
TABLE 1.
Characteristics of VRE-positive patients included in the study to compare the effects of a 5-week course of oral administration of Lactobacillus rhamnosus Lcr35 with those of a placebo on VRE intestinal carriage clearance
| Patient no. | Arm | Age (yr) | Other VRE-positive colonization site | Previous antibiotic therapy | Antibiotic therapy during treatment (no. of days) |
|---|---|---|---|---|---|
| 1 | Placebo | 82 | None | Amoxicillin-clavulanic acid | None |
| 2 | Placebo | 84 | Urine | Ofloxacin | Ofloxacin (9) |
| 3 | Lcr35 | 75 | Urine | Ciprofloxacin | None |
| Vancomycin | |||||
| Metronidazole | |||||
| 4 | Lcr35 | 52 | Urine | Cefotaxime | Norfloxacin (77) |
| Norfloxacin | |||||
| 5 | Lcr35 | 75 | None | Imipenem | None |
| Ceftriaxone | |||||
| 6 | Lcr35 | 92 | Urine | Vancomycin | None |
| Ceftriaxone | |||||
| Amoxicillin | |||||
| Metronidazole | |||||
| 7 | Lcr35 | 66 | Urine | Linezolid | None |
| Teicoplanin | |||||
| Ofloxacin | |||||
| Ticarcillin | |||||
| 8 | Lcr35 | 89 | None | Amoxicillin-clavulanic acid | None |
| Ofloxacin | |||||
| Amoxicillin |
FIG. 4.
Outcome of VRE colonization in patients receiving Lcr35 (solid arrow, n = 6) versus placebo (dashed arrow, n = 2) for a 5-week period (W1 to W5). VRE was detected in patient stools from W1 to W11; black bars, positive VRE cultures; gray bars, negative VRE cultures. Nine VRE-positive patients were recruited. One patient died 2 days after inclusion and was excluded from the analysis. Of the eight remaining patients, six received Lcr35 and two received placebos. Patients 1 and 2 completed the treatment with placebo but stopped the study after W7. Patient 5 stopped the treatment with Lcr35 after 3 weeks. The Charlson index of comorbidity was 3 to 4 for two patients and higher than 5 for six of them.
Our study validated a mouse model of VRE colonization remarkably similar to that seen in humans, with VRE carriage sustained for 66 days after discontinuation of vancomycin and spontaneous clearance in some animals. Vancomycin treatment prior to VRE inoculation seems to play a key role in VRE colonization of the gastrointestinal tract since it was associated with high levels of VRE intestinal colonization (Fig. 1). When vancomycin was discontinued, the VRE was quickly cleared from the gut and sustained colonization was not achieved (data not shown). We hypothesize that administration of vancomycin promotes VRE colonization by disrupting the barrier formed by the intestinal microbiota and hence provides a selective advantage for resistant bacteria such as VRE to the detriment of the normal flora. TTGE analysis showed that vancomycin administration induces changes in the intestinal microbiota that could corroborate this hypothesis. Whether the influence of antibiotic-linked microbiota changes on VRE colonization is direct or not remains to be determined. It has been shown elsewhere that Gram-negative probiotic bacteria are potentially able to regulate intestinal homeostasis (6). However, in our study, the administration of E. coli Nissle to VRE-colonized mice failed to decrease VRE density in the gut, whereas administration of Lcr35 lowered VRE density, albeit not to a level of significance.
Several studies performed either in murine models or in humans have suggested that the administration of probiotics improves intestinal microbial balance and the modulation of immune functions and that probiotics may be interesting agents to eradicate or prevent VRE intestinal colonization (3). Our study showed that the two probiotics tested had little or no effect. Since the action of probiotics in VRE-colonized humans is likely to be influenced by host-, VRE-, and probiotic-related factors, further investigations are required to determine if probiotics have any effect on VRE clearance.
Acknowledgments
We thank Michaël Picard, Gwenael Le Moal, Céline Fageon, Lemlih Duchchone, and Sophie Marquet for technical assistance.
Footnotes
Published ahead of print on 26 April 2010.
REFERENCES
- 1.Donskey, C. J., J. A. Hanrahan, R. A. Hutton, and L. B. Rice. 1999. Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J. Infect. Dis. 180:384-390. [DOI] [PubMed] [Google Scholar]
- 2.Lesens, O., L. Mihaila, F. Robin, O. Baud, J. P. Romaszko, O. Tourniac, J. M. Constantin, B. Souweine, R. Bonnet, A. Bouvet, J. Beytout, O. Traore, and H. Laurichesse. 2006. Outbreak of colonization and infection with vancomycin-resistant Enterococcus faecium in a French university hospital. Infect. Control Hosp. Epidemiol. 27:984-986. [DOI] [PubMed] [Google Scholar]
- 3.Manley, K. J., M. B. Fraenkel, B. C. Mayall, and D. A. Power. 2007. Probiotic treatment of vancomycin-resistant enterococci: a randomised controlled trial. Med. J. Aust. 186:454-457. [DOI] [PubMed] [Google Scholar]
- 4.Muto, C. A., J. A. Jernigan, B. E. Ostrowsky, H. M. Richet, W. R. Jarvis, J. M. Boyce, and B. M. Farr. 2003. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect. Control Hosp. Epidemiol. 24:362-386. [DOI] [PubMed] [Google Scholar]
- 5.Seksik, P., L. Rigottier-Gois, G. Gramet, M. Sutren, P. Pochart, P. Marteau, R. Jian, and J. Dore. 2003. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut 52:237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonnenburg, J. L., C. T. Chen, and J. I. Gordon. 2006. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 4:e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoetendal, E. G., A. D. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]