Abstract
Microsatellite-based genotyping for Candida albicans can give discrepant results between laboratories when expressed in fragment sizes, because their determination depends on electrophoretic conditions. The interlaboratory reproducibility was assessed in six laboratories provided with an allelic ladder. Despite variations in size determinations, alleles were correctly assigned, making data transportable between laboratories.
Candida albicans is a diploid yeast responsible for a wide range of fungal infections in both immunocompetent and immunocompromised individuals. It is the most frequent fungal species involved in recurrent infections, outbreaks, and nosocomial infections (18). Genotyping of C. albicans can be performed using fluorescence-based microsatellite length polymorphism (MLP) analysis of PCR-amplified DNA fragments (2, 4, 5, 9, 11, 20, 21). Microsatellite sequences are defined as tandemly repeated stretches of 1 to 6 nucleotides and are excellent candidates for genetic analysis as they are polymorphic. Microsatellite alleles are often expressed as DNA fragments of different sizes obtained after PCR amplification with primers flanking the microsatellite region (22). Based on the variations in repeat numbers, genetic relatedness between different isolates can be assessed.
MLP analysis is a method with a high discriminatory power and reproducibility, and it allows high throughput. It has proved its efficacy in genotyping large collections of samples in epidemiological studies of not only C. albicans but also other yeasts, such as Candida glabrata (10, 13), Candida tropicalis (6), and filamentous fungi, such as Aspergillus fumigatus or Penicillium marneffei (1, 7, 17). However, interlaboratory exchange of microsatellite data can be difficult, because the use of different equipment and diverse capillary electrophoresis conditions can lead to altered assessment of PCR fragment lengths. In the past, calibration by means of allelic ladders was applied for the analysis of population data (12, 14, 19, 23). More recently, de Valk et al. (8) produced locus-specific allelic ladders for the microsatellite-based analysis of Aspergillus fumigatus and tested its utility for assigning allele sizes in an interlaboratory study. In order to normalize C. albicans microsatellite results, we developed an allelic ladder that includes the most frequently occurring alleles of the CDC3 polymorphic microsatellite marker (2). Our objective was to test interlaboratory genotyping results by using the allelic ladder as an internal reference for a given marker.
First, the allelic ladder for the tetranucleotide repeat (AGTA) CDC3 marker was constructed. Seven alleles in C. albicans were identified in our previous studies (2, 11). Genomic DNA of isolates known to contain the different alleles was extracted using the High Pure PCR template preparation kit as described by the manufacturer (Roche Applied Biosciences, Germany). Alleles were amplified in single-plex PCRs in a 20-μl reaction volume, consisting of 2 μl DNA, 1× PCR buffer (Roche Diagnostics GmbH, Mannheim, Germany), 0.2 mM each deoxynucleoside triphosphate, 5 mM MgCl2, and 5 pmol of each CDC3 primer, with the sense primer labeled with hexachlorocarboxyfluorescein (HEX) (2) and 1.25 U of Taq Gold polymerase (Applied Biosystems, les Ulis, France). PCR was performed in an iCycler thermocycler (Bio-Rad, Hercules, CA) and consisted of an initial denaturation step at 95°C for 10 min, followed by 30 cycles of 30 s at 95°C, 30 s at 55°C, and 1 min at 72°C, with a final extension step of 30 min at 72°C. PCR products were then pooled in equivalent volumes, and 2 μl of this allelic pool was added to 13.5 μl of Hi-Di formamide (Applied Biosystems) and 0.5 μl 6-carboxy-X-rhodamine (ROX)-labeled Geneflo 625 size standard (Eurx, Gdansk, Poland). Capillary electrophoresis of the allelic ladder was performed using the ABI Prism 3730XL sequencer (Fig. 1). Sizes of the seven alleles were calculated with GeneMapper (version 4; Applied Biosystems). Reproducibility was tested in 10 separate experiments that resulted in allele size variation of ≤0.08 nucleotides for the seven alleles.
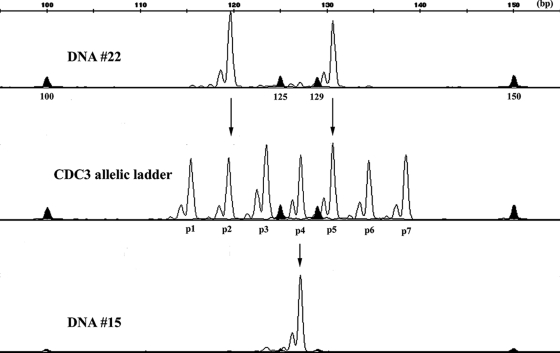
FIG. 1.
Example of allele assignment using the CDC3 allelic ladder for two isolates, numbers 22 and 15. Allele peaks in the ladder (black lines) are marked as p1 to p7. GeneFlo 625 internal size standards (black filled peaks) with sizes in bp are shown beneath each peak. Isolate 15 is p2-p5 heterozygous, and isolate 15 is p4 homozygous.
The CDC3 ladder (10 μl) was sent to six participating laboratories with previous experience in MLP analysis. The National Reference Center for Mycoses and Antifungals (NRCMA) also provided five C. albicans DNA samples of known CDC3 genotypes (DNA samples 1 to 5) and the CDC3 primers (2). To test the influence of the DNA extraction method, each participant laboratory selected five isolates from their own strain collection, extracted the genomic DNA, and performed MLP genotyping on these samples (samples 6 to 35). The 35 DNA samples were sent to the NRCMA for blind MLP analysis.
Participants were made aware of the number of allele peaks identified in the ladder (one to seven) and were asked to perform MLP using the NRCMA's PCR amplification/cycling conditions and their own equipment and reagents (Table 1). For each DNA sample, genotyping results were expressed as peak numbers (1 to 7), and fragment sizes were expressed in bp. As shown in Table 2, reported fragment sizes varied between the laboratories, with size differences of up to 4.3 bp. However, upon comparing the results to the allelic ladder, all laboratories correctly assigned peak numbers to the five DNA samples.
TABLE 1.
Conditions and equipment used by NRCMA and the six participant teams
| Laboratory | DNA extraction method (source or reference) | DNA polymerase (source) | Thermocycler (manufacturer) | Size standard (source) | Software (manufacturer) | Capillary electrophoresis instrument (manufacturer) |
|---|---|---|---|---|---|---|
| NRCMA | DNA EasyPlant (Qiagen) | Taq (Roche) | iCycler (Bio-Rad) | Geneflo625 (EurX) | GeneMapper version 4 (Applied Biosystems) | ABI 3730 XL (Applied Biosystems) |
| Lab 1 | Ultraclean microbial DNA isolation kit (MoBio) | Taq (Roche) | ABI 9700 (Applied Biosystems) | ABI GS500 (Applied Biosystems) | GeneMapper version 2.1 (Applied Biosystems) | ABI 3130 (Applied Biosystems) |
| Lab 2 | MagnaLyser/MagNA Pure (Roche) | FastStart (Roche) | T1 (Biometra) | ET400-R (GE Healthcare) | Fragment profiler (GE Healthcare) | MegaBACE 500 (GE Healthcare) |
| Lab 3 | Manual (15) | Go Taq (Promega) | TC 412 (Techne) | ROX 500 (Applied Biosystems) | Peak Scanner (Applied Biosystems) | CEQ 8800 (Beckman Coulter) |
| Lab 4 | Manual (16) | Taq (Fermentas) | iCycler (Bio-Rad) | TAMRA 500 (Applied Biosystems) | GeneScan version 3.1 (Applied Biosystems) | ABI 310 (Applied Biosystems) |
| Lab 5 | Boiling with chelating resin (Chelex) | Eurobiotaq (Eurobio) | PTC 100 (MJ Research) | GeneScan 500 LIZ (Applied Biosystems) | GeneMapper version 4.0 (Applied Biosystems) | ABI 3130 (Applied Biosystems) |
| Lab 6 | Manual (3) | AmpliTaq (Applied Biosystems) | GeneAmp PCR system 9700 (Applied Biosystems) | ROX 500 (Applied Biosystems) | GeneMapper version 3.0 (Applied Biosystems) | ABI 3730 XL (Applied Biosystems) |
TABLE 2.
Genotyping results for the five DNA samples based on CDC3 ladder peak number and fragment size
| Laboratory | Fragment size (bp) for indicated allele and peak no.a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 |
Sample 2 |
Sample 3 |
Sample 4 |
Sample 5 |
||||||
| Allele 1 (peak 5) | Allele 2 (peak 6) | Allele 1 (peak 1) | Allele 2 (peak 4) | Allele 1 (peak 1) | Allele 2 (peak 3) | Allele 1 (peak 2) | Allele 2 (peak 6) | Allele 1 (peak 3) | Allele 2 (peak 6) | |
| NRCMA | 130.6 | 134.5 | 115.4 | 127.1 | 115.4 | 123.5 | 119.5 | 134.5 | 123.5 | 134.5 |
| Lab 1 | 128.0 | 132.0 | 112.0 | 124.0 | 112.0 | 120.0 | 116.0 | 132.0 | 120.0 | 132.0 |
| Lab 2 | 132.2 | 136.1 | 115.9 | 128.3 | 115.9 | 124.2 | 120.1 | 136.1 | 124.2 | 136.1 |
| Lab 3 | 129.2 | 133.2 | 112.9 | 124.8 | 113.5 | 120.8 | 117.5 | 133.2 | 120.8 | 133.2 |
| Lab 4 | 129.0 | 133.0 | 114.0 | 125.0 | 114.0 | 121.0 | 118.0 | 133.0 | 121.0 | 133.0 |
| Lab 5 | 130.0 | 134.0 | 114.0 | 126.0 | 114.0 | 122.0 | 118.0 | 134.0 | 122.0 | 134.0 |
| Lab 6 | 129.0 | 133.0 | 113.0 | 125.0 | 113.0 | 121.0 | 117.0 | 133.0 | 121.0 | 133.0 |
The peak numbers represent the allele peaks in the CDC3 allelic ladder. Genotypes were identical for all the participant laboratories and the NCRMA. Peak 7 was not present in the DNA isolates tested.
The influence of DNA extraction method on allele assignment was assessed using DNA samples provided by the participating laboratories. For every DNA sample supplied (samples 6 to 35), the allele assignations by the participating laboratory and the NCRMA were identical based on the peak number (data not shown). Therefore, the DNA extraction method does not seem to interfere with allele assignment.
Although reproducibility for a given capillary electrophoresis platform is high, according to previous reports (2, 8, 20), we demonstrated here that DNA fragment size determinations differed between laboratories due to differences in equipment, separation matrices, size standards, and software. However, the application of the C. albicans CDC3 ladder as an internal standard allowed the correct allele assignment for the samples provided, and there was excellent agreement between the NRCMA and the participating laboratory for unknown DNA samples. The demonstration here is for one C. albicans marker only. The question of how many loci should be tested depends on the objectives. For some issues, a single locus may be sufficient. If a high discriminatory power is needed, several markers can be analyzed (2, 20). Following the same principle, allelic ladders could be designed for these additional microsatellite markers.
In conclusion, once primers and fluorescent labels have been designed for microsatellite-based typing systems, the use of an allelic ladder is an important asset that allows standardization of results and transfer and comparison of data between laboratories.
Acknowledgments
We gratefully acknowledge the Institut Pasteur sequencing facility (PF8) and Anne Whitney in the CDC Genomics Unit for technical help.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 28 April 2010.
REFERENCES
- 1.Bart-Delabesse, E., J. F. Humbert, E. Delabesse, and S. Bretagne. 1998. Microsatellite markers for typing Aspergillus fumigatus isolates. J. Clin. Microbiol. 36:2413-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botterel, F., C. Desterke, C. Costa, and S. Bretagne. 2001. Analysis of microsatellite markers of Candida albicans used for rapid typing. J. Clin. Microbiol. 39:4076-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman, B. 1993. A model PCR/probe system for the identification of fungal pathogens, p. 423-430. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, DC.
- 4.Costa, J. M., O. Eloy, F. Botterel, G. Janbon, and S. Bretagne. 2005. Use of microsatellite markers and gene dosage to quantify gene copy numbers in Candida albicans. J. Clin. Microbiol. 43:1387-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalle, F., N. Franco, J. Lopez, O. Vagner, D. Caillot, P. Chavanet, B. Cuisenier, S. Aho, S. Lizard, and A. Bonnin. 2000. Comparative genotyping of Candida albicans bloodstream and nonbloodstream isolates at a polymorphic microsatellite locus. J. Clin. Microbiol. 38:4554-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desnos-Ollivier, M., S. Bretagne, C. Bernede, V. Robert, D. Raoux, E. Chachaty, E. Forget, C. Lacroix, and F. Dromer. 2008. Clonal population of flucytosine-resistant Candida tropicalis from blood cultures, Paris, France. Emerg. Infect. Dis. 14:557-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Valk, H. A., J. F. Meis, I. M. Curfs, K. Muehlethaler, J. W. Mouton, and C. H. Klaassen. 2005. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J. Clin. Microbiol. 43:4112-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Valk, H. A., J. F. Meis, S. Bretagne, J. M. Costa, B. A. Lasker, S. A. Balajee, A. C. Pasqualotto, M. J. Anderson, L. Alcazar-Fuoli, E. Mellado, and C. H. Klaassen. 2009. Interlaboratory reproducibility of a microsatellite-based typing assay for Aspergillus fumigatus through the use of allelic ladders: proof of concept. Clin. Microbiol. Infect. 15:180-187. [DOI] [PubMed] [Google Scholar]
- 9.Eloy, O., S. Marque, F. Botterel, F. Stephan, J. M. Costa, V. Lasserre, and S. Bretagne. 2006. Uniform distribution of three Candida albicans microsatellite markers in two French ICU populations supports a lack of nosocomial cross-contamination. BMC Infect. Dis. 6:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foulet, F., N. Nicolas, O. Eloy, F. Botterel, J. C. Gantier, J. M. Costa, and S. Bretagne. 2005. Microsatellite marker analysis as a typing system for Candida glabrata. J. Clin. Microbiol. 43:4574-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Hermoso, D., O. Cabaret, G. Lecellier, M. Desnos-Ollivier, D. Hoinard, D. Raoux, J. M. Costa, F. Dromer, and S. Bretagne. 2007. Comparison of microsatellite length polymorphism and multilocus sequence typing for DNA-based typing of Candida albicans. J. Clin. Microbiol. 45:3958-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glock, B., D. W. Schwartz, E. M. Schwartz-Jungl, and W. R. Mayr. 1996. Allelic ladder characterization of the short tandem repeat polymorphism in intron 6 of the lipoprotein lipase gene and its application in an Austrian Caucasian population study. J. Forensic Sci. 41:579-581. [PubMed] [Google Scholar]
- 13.Grenouillet, F., L. Millon, J. M. Bart, S. Roussel, I. Biot, E. Didier, A. S. Ong, and R. Piarroux. 2007. Multiple-locus variable-number tandem-repeat analysis for rapid typing of Candida glabrata. J. Clin. Microbiol. 45:3781-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths, R. A., M. D. Barber, P. E. Johnson, S. M. Gillbard, M. D. Haywood, C. D. Smith, J. Arnold, T. Burke, A. J. Urquhart, and P. Gill. 1998. New reference allelic ladders to improve allelic designation in a multiplex STR system. Int. J. Legal Med. 111:267-272. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman, C. S., and F. Winston. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 17.Lasker, B. A., and Y. Ran. 2004. Analysis of polymorphic microsatellite markers for typing Penicillium marneffei isolates. J. Clin. Microbiol. 42:1483-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puers, C., H. A. Hammond, L. Jin, C. T. Caskey, and J. W. Schumm. 1993. Identification of repeat sequence heterogeneity at the polymorphic short tandem repeat locus HUMTH01[AATG]n and reassignment of alleles in population analysis by using a locus-specific allelic ladder. Am. J. Hum. Genet. 53:953-958. [PMC free article] [PubMed] [Google Scholar]
- 20.Sampaio, P., L. Gusmao, A. Correia, C. Alves, A. G. Rodrigues, C. Pina-Vaz, A. Amorim, and C. Pais. 2005. New microsatellite multiplex PCR for Candida albicans strain typing reveals microevolutionary changes. J. Clin. Microbiol. 43:3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephan, F., M. S. Bah, C. Desterke, S. Rezaiguia-Delclaux, F. Foulet, P. Duvaldestin, and S. Bretagne. 2002. Molecular diversity and routes of colonization of Candida albicans in a surgical intensive care unit, as studied using microsatellite markers. Clin. Infect. Dis. 35:1477-1483. [DOI] [PubMed] [Google Scholar]
- 22.Wan, Q. H., H. Wu, T. Fujihara, and S. G. Fang. 2004. Which genetic marker for which conservation genetics issue? Electrophoresis 25:2165-2176. [DOI] [PubMed] [Google Scholar]
- 23.Watson, S., R. Allsop, L. Foreman, Z. Kelsey, and P. Gill. 2001. Sequenced allelic ladders and population genetics of a new STR multiplex system. Forensic Sci. Int. 115:207-217. [DOI] [PubMed] [Google Scholar]