Abstract
In this study, we demonstrate that differences within the P2 domain of norovirus genogroup I (GI) strains can be used to segregate outbreaks which are unrelated, whereas complete conservation within this region allows tracking of strains that are part of a single outbreak and likely to have a common source.
Noroviruses (NoVs) are members of the Caliciviridae family (7) and the leading cause of outbreaks of acute gastroenteritis worldwide (14). NoV outbreaks are frequently associated with semiclosed or closed institutions such as hospitals and homes for the elderly (11, 22), but outbreaks also occur in other settings, including eating establishments, cruise ships, concert halls (2, 10, 20), and schools (16). Transmission of NoVs is usually person to person (15), although food and water (1, 3, 5, 9, 17, 18, 21) and environmental or airborne contamination (6, 19) have all been implicated in transmission.
Human NoVs are genetically diverse, and three distinct genogroups (GI, GII, and GIV) and many genotypes/genetic clusters exist (8, 13, 26). Diversity among NoVs is generated through the accumulation of point mutations associated with the error-prone nature of RNA replication and genetic recombination involving the exchange of sequences between related RNA viruses.
The NoV capsid is divided into the S domain, which constitutes the 5′ end (amino acids [aa] 1 to 225), and the P (protruding) domain (aa 226 to 530) (23). The P domain can be further subdivided into two subdomains, P1 and P2. The P2 domain is the hypervariable region of the capsid and corresponds to the most exposed area likely to be involved in immune recognition and attachment. Due to the high diversity within this region, it is not possible to design a single cross-reactive primer pair capable of amplifying all genotypes within a genogroup; therefore, genotype-specific primers are required in order to amplify this region, as previously seen for NoV genogroup II (25).
Fecal samples were collected from genogroup I outbreaks of gastroenteritis as part of the ongoing National Surveillance Programme of the molecular epidemiology of norovirus genotypes.
Outbreaks were defined as including two or more cases of gastroenteritis linked in place and time. A new outbreak was arbitrarily defined as occurring at least 7 days after the last case in a previous outbreak or as occurring in a different patient care unit such as a ward or hospital.
Outbreak 714124 occurred in a nursing home in Blackburn, Lancashire, United Kingdom, in August 2007; outbreak 414003 occurred in a restaurant in Chorley, Lancashire, in January 2004 (4); outbreak 512057 occurred in a bar/restaurant in Liverpool, United Kingdom, in November 2005; outbreak 612057 occurred in a nursing home and outbreak 612058 occurred in a hotel in Liverpool in November 2006; outbreak E/2005/UK occurred in a cruise ship in December 2007; outbreak Q1/2007/US occurred in a cruise ship in the United States in February 2007; and outbreak Newquay/2008/UK occurred in Newquay, Cornwall, United Kingdom, in August 2008.
Fecal specimens were prepared as previously described, and nucleic acid extraction, Norovirus detection through amplification of a small region spanning the open reading frame 1/2 (Orf1/2) junction, and genotyping through sequence analysis of the S domain were all performed as previously described (4).
Oligonucleotide primers for the amplification of a region encompassing the P2 domain of NoV GI genotypes 1 to 7 were designed from alignments of complete Orf2 nucleotide sequence data. See Table 1 for primer sequences and positions and amplification conditions. Separate monoplex reactions were carried out for each of the genotypes, and amplicons were separated by agarose gel electrophoresis and sequenced directly after purification using the same P domain genotype-specific primers.
TABLE 1.
Norovirus genogroup I genotype-specific primers for amplification of a region encompassing the P2 domaina
| Genotype | Primer nameb | Primer sequencec | Annealing temp (°C) | Amplicon size (bp) | P2 domain size (nt) |
|---|---|---|---|---|---|
| GI-1 | P2 GI-1 F | 5′ TCNAAYTCACGTGCTCCTCTT 3′ | 47 | 682 | 407 |
| P2 GI-1 R | 5′ TCCGNCCNGTATCAGGGTCAA 3′ | ||||
| GI-2 | P2 GI-2 F | 5′ TCCAATTCTAGGTTTCCTTCCCT 3′ | 47 | 670 | 437 |
| P2 GI-2 R | 5′ GGGCTTGTTCACTGACAAAGTG 3′ | ||||
| GI-3 | P2 GI-3 F | 5′ TCWAAYTCAAGRGTCCCTTCT 3′ | 50 | 684 | 437 |
| P2 GI-3 R | 5′ GCTTCMCCTCTAGTGGGGGCCT 3′ | ||||
| GI-4 | P2 GI-4 F | 5′ TCTAATTCYAGGATCCCAAAT 3′ | 45 | 665 | 434 |
| P2 GI-4 R | 5′ GCCTGCTCACTAATAAAGTGTG 3′ | ||||
| GI-5 | P2 GI-5 F | 5′ TCCAATTCCCGTGTTCCCAAT 3′ | 47 | 678 | 437 |
| P2 GI-5 R | 5′ CATNGAKGGGGCTTGTTCACT 3′ | ||||
| GI-6 | P2 GI-6 F | 5′ TCAAATTCTCGTGTCCCTGTGT 3′ | 45 | 646 | 425 |
| P2 GI-6 R | 5′ GTTCATTRCAGAAGTGGGTAAT 3′ | ||||
| GI-7 | P2 GI-7 F | 5′ GCTAACTCCAGAGTGCCCGCAA 3′ | 50 | 674 | 428 |
| P2 GI-7 R | 5′ GCGGCTTCACCTCGGATTGGTG 3′ |
The PCR cycling conditions were 94°C for 2 min, followed by 40 cycles at 94°C for 30s, 45°C for 1 min, and 72°C for 1 min and finally 72°C for 5 min.
F, forward; R, reverse. The forward primers correspond to nucleotide positions 6081 to 6101 and the reverse primers to nucleotide positions 6742 to 6761 on GI-1 strain Norwalk/1968/US (M87661). The P2 domain region of GI strains corresponds to nucleotide positions 6165 to 6572 on the GI-1 strain Norwalk/1968/US (M87661).
Y = C or T, R = A or G, and N = C or G or T or A.
Sequence analysis of the region encoding the P2 domain (nucleotides [nt] 6165 to 6572) on the GI-1 strain Norwalk/1968/US (M87661) was performed using Bionumerics version 3.5 (Applied Maths, Kortrijk, Belgium). Sequence alignments were performed using the Clustal algorithm. Of the P domain sequences, only the region corresponding to the P2 domain was used in comparisons.
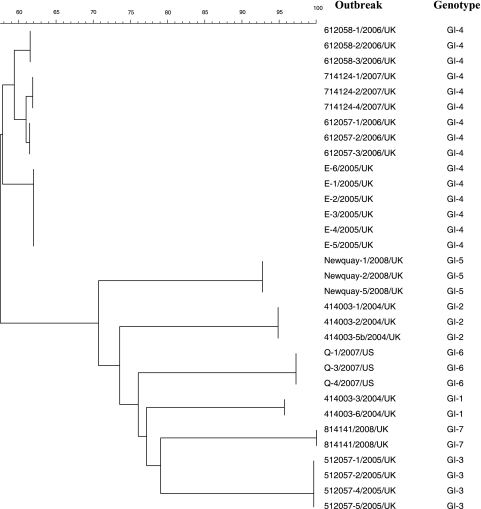
NoV genogroup I genotypes 1 to 7 were all successfully amplified using the genotype-specific primers designed to provide amplicons encompassing the P2 domain (Table 1). Phylogenetic analysis of the P2 domain sequences differentiated between each genotype (Fig. 1).
FIG. 1.
Dendrogram of P2 domain sequences derived from strains of 10 outbreaks, constructed using the neighbor-joining algorithm. Strain denomination indicates the outbreak number, year of isolation, and country. Genogroup and genotype are indicated in the second column.
Genotyping and subsequent analysis of the P2 domain showed that outbreak 414003, which was linked to the ingestion of fecally contaminated oysters in 2004 (3) contained a mixture of GI-1 and GI-2 genotypes. Conservation within the P2 domain of GI-1 or GI-2 indicated that the customers were infected by a common source (Fig. 1).
Among four GI-4 outbreaks detected between 2005 and 2007, strains within outbreaks showed 100% identity within the P2 domain but were different among the 4 outbreaks (Fig. 1). Two of these outbreaks occurred in the same geographical region: one in November and the second in December 2006. Diversity of the P2 domain between strains in these two outbreaks clearly distinguished them as separate events, whereas the strains within each of the outbreaks clearly linked them as having a common source.
Similarly three unrelated outbreaks caused by GI-3, GI-5, or GI-6 demonstrated that strains within an outbreak were identical (Fig. 1).
The analysis of sequence from the S domain region of GI NoVs is valid for genotyping and benefits from the use of a single set of consensus primers (4, 12). Previously we reported that sequence identity within the P2 domain among GII strains was a useful tool for outbreak tracking and monitoring transmission events between outbreaks which, using then common epidemiological definition, were identified as separate events (24, 25). Similarly, data from this study show the validity of using P2 domain sequences to link GI strains within an outbreak and segregate outbreaks which are unrelated.
Nucleotide sequence accession numbers.
The GenBank nucleotide sequence accession numbers are as follows; 414003-3/2004/UK, GQ487570; 414003-1/2004/UK, GQ487571; 512057-1/2005/UK, GQ487572; 714124-1/2007/UK, GQ487573; 612057/2006, GQ487574; 612058/2006/UK, GQ487575; E-1/2005/UK, GQ487576; Newquay-1/2008/UK, GQ487577; and Q-1/2007/US, GQ487578.
Acknowledgments
We thank Richard Cooke (Aintree Hospital), Barry Vipond (Bristol HPA), John Cheesbrough (Preston HPA), and Hilary Cotterill (Manchester HPA) for sending us fecal samples and information from outbreaks.
Footnotes
Published ahead of print on 5 May 2010.
REFERENCES
- 1.Anonymous. 2007. Norovirus outbreak associated with ill food-service workers—Michigan, January-February 2006. MMWR Morb. Mortal. Wkly. Rep. 56:1212-1216. [PubMed] [Google Scholar]
- 2.Evans, M. R., R. Meldrum, W. Lane, D. Gardner, C. D. Ribeiro, C. I. Gallimore, and D. Westmoreland. 2002. An outbreak of viral gastroenteritis following environmental contamination at a concert hall. Epidemiol. Infect. 129:355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallimore, C. I., J. S. Cheesbrough, K. Lamden, C. Bingham, and J. J. Gray. 2005. Multiple norovirus genotypes characterised from an oyster-associated outbreak of gastroenteritis. Int. J. Food Microbiol. 103:323-330. [DOI] [PubMed] [Google Scholar]
- 4.Gallimore, C. I., M. Iturriza-Gomara, J. Xerry, J. Adigwe, and J. Gray. 2007. Inter-seasonal diversity of Norovirus genotypes: emergence and selection of virus variants. Arch. Virol. 152:1295-1303. [DOI] [PubMed] [Google Scholar]
- 5.Gallimore, C. I., C. Pipkin, H. Shrimpton, A. D. Green, Y. Pickford, C. McCartney, G. Sutherland, D. W. G. Brown, and J. J. Gray. 2005. Detection of multiple enteric viruses within a foodborne outbreak of gastroenteritis: an indication of the source of contamination. Epidemiol. Infect. 133:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green, J., P. A. Wright, C. I. Gallimore, O. Mitchell, P. Morgan-Capner, and D. W. G. Brown. 1998. The role of environmental contamination with small round structured viruses in a hospital outbreak investigated by reverse-transcriptase polymerase chain reaction assay. J. Hosp. Infect. 39:39-45. [DOI] [PubMed] [Google Scholar]
- 7.Green, K. Y., T. Ando, M. S. Balayan, T. Berke, I. N. Clarke, M. K. Estes, D. O. Matson, S. Nakata, J. D. Neill, M. J. Studdert, and H. J. Thiel. 2000. Taxonomy of the Caliciviruses. J. Infect. Dis. 181:S322-S330. [DOI] [PubMed] [Google Scholar]
- 8.Hansman, G. S., K. Natori, H. Shirato-Horikoshi, S. Ogawa, T. Oka, K. Katayama, T. Tanaka, T. Miyoshi, K. Sakae, S. Kobayashi, M. Shinohara, K. Uchida, N. Sakurai, K. Shinozaki, M. Okada, Y. Seto, K. Kamata, N. Nagata, K. Tanaka, T. Miyamura, and N. Takeda. 2006. Genetic and antigenic diversity among noroviruses. J. Gen. Virol. 87:909-919. [DOI] [PubMed] [Google Scholar]
- 9.Hewitt, J., D. Bell, G. C. Simmons, M. Rivera-Aban, S. Wolf, and G. E. Greening. 2007. Gastroenteritis outbreak caused by waterborne norovirus at a New Zealand ski resort. Appl. Environ. Microbiol. 73:7853-7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho, M., S. S. Monroe, S. Stine, D. Cubitt, R. I. Glass, H. P. Madore, P. F. Pinsky, C. Ashley, and E. O. Caul. 1989. Viral gastroenteritis aboard a cruise ship. Lancet ii:961-965. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, X., E. Turf, E. Hu, E. Barrett, X. M. Dai, S. Monroe, C. Humphrey, L. K. Pickering, and D. O. Matson. 1996. Outbreaks of gastroenteritis in elderly nursing homes and retirement facilities associated with human caliciviruses. J. Med. Virol. 50:335-341. [DOI] [PubMed] [Google Scholar]
- 12.Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kageyama, T., M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, S. Kojima, R. Takai, T. Oka, N. Takeda, and K. Katayama. 2004. Coexistence of multiple genotypes, including newly identified genotypes, in outbreaks of gastroenteritis due to norovirus in Japan. J. Clin. Microbiol. 42:2988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan, J. E., G. W. Gary, R. C. Baron, N. Singh, L. B. Schonberger, R. Feldman, and H. B. Greenberg. 1982. Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann. Intern. Med. 96:756-761. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan, J. E., L. B. Schonberger, G. Varano, N. Jackman, J. Bied, and G. W. Gary. 1982. An outbreak of acute nonbacterial gastroenteritis in a nursing home. Demonstration of person-to-person transmission by temporal clustering of cases. Am. J. Epidemiol. 116:940-948. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, S., T. Morishita, T. Yamashita, K. Sakae, O. Nishio, T. Miyake, Y. Ishihara, and S. Isomura. 1991. A large outbreak of gastroenteritis associated with a small round structured virus among schoolchildren and teachers in Japan. Epidemiol. Infect. 107:81-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuritsky, J. N., M. T. Osterholm, H. B. Greenberg, J. A. Korlath, J. R. Godes, C. W. Hedberg, J. C. Forfang, A. Z. Kapikian, J. C. McCullough, and K. E. White. 1984. Norwalk gastroenteritis: a community outbreak associated with bakery product consumption. Ann. Intern. Med. 100:519-521. [DOI] [PubMed] [Google Scholar]
- 18.Lysen, M., M. Thorhagen, M. Brytting, M. Hjertqvist, Y. Andersson, and K. O. Hedlund. 2009. Genetic diversity among foodborne and waterborne norovirus outbreaks in Sweden. J. Clin. Microbiol. 47:2411-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marks, P. J., I. B. Vipond, D. Carlisle, D. Deakin, R. E. Fey, and E. O. Caul. 2000. Evidence for airborne transmission of Norwalk-like virus (NLV) in a hotel restaurant. Epidemiol. Infect. 124:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parashar, U. D., L. Dow, R. L. Fankhauser, C. D. Humphrey, J. Miller, T. Ando, K. S. Williams, C. R. Eddy, J. S. Noel, T. Ingram, J. S. Bresee, S. S. Monroe, and R. I. Glass. 1998. An outbreak of viral gastroenteritis associated with consumption of sandwiches: implications for the control of transmission by food handlers. Epidemiol. Infect. 121:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sala, M. R., N. Cardenosa, C. Arias, T. Llovet, A. Recasens, A. Dominguez, J. Buesa, and L. Salleras. 2005. An outbreak of food poisoning due to a genogroup I norovirus. Epidemiol. Infect. 133:187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid, D., I. Lederer, A. M. Pichler, C. Berghold, E. Schreier, and F. Allerberger. 2005. An outbreak of Norovirus infection affecting an Austrian nursing home and a hospital. Wien. Klin. Wochenschr. 117:802-808. [DOI] [PubMed] [Google Scholar]
- 23.Tan, M., and X. Jiang. 2007. Norovirus-host interaction: implications for disease control and prevention. Exp. Rev. Mol. Med. 9:1-22. [DOI] [PubMed] [Google Scholar]
- 24.Xerry, J., C. I. Gallimore, M. Iturriza-Gomara, and J. J. Gray. 2009. Tracking the transmission routes of genogroup II noroviruses in suspected food-borne or environmental outbreaks of gastroenteritis through sequence analysis of the P2 domain. J. Med. Virol. 81:1298-1304. [DOI] [PubMed] [Google Scholar]
- 25.Xerry, J., C. I. Gallimore, M. Iturriza Gomara, D. J. Allen, and J. J. Gray. 2008. Transmission events within outbreaks of gastroenteritis determined through the analysis of nucleotide sequences of the P2 domain of genogroup II noroviruses J. Clin. Microbiol. 46:947-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng, D. P., T. Ando, R. L. Fankhauser, R. S. Beard, R. I. Glass, and S. S. Monroe. 2006. Norovirus classification and proposed strain nomenclature. Virology 346:312-323. [DOI] [PubMed] [Google Scholar]