Abstract
Disseminated microsporidiosis is a life-threatening opportunistic infection. Here, we report about a previously undescribed genovar of Encephalitozoon cuniculi causing disseminated infection in a non-HIV-infected renal transplant recipient. Disseminated microsporidiosis must be considered in the differential diagnosis of chronic fever in renal allograft recipients, even those without urinary symptoms.
CASE REPORT
A 38-year-old woman with end-stage renal disease due to IgA nephropathy received a renal transplant. Her immunosuppressive therapy consisted of thymoglobulin, mycophenolate mofetil (MMF), and cyclosporine A (cyA).
Four weeks posttransplantation, the patient presented with intermittent fever. The clinical examination was unremarkable. No travel history was known. Laboratory examination showed a white blood cell count of 16.4 × 109 liter−1, with a C-reactive protein level of 6 mg/liter (reference values < 6). Graft biopsy and magnetic resonance imaging (MRI) provided no evidence for rejection and no vascular or urologic complication. Urine, blood, stool and sputum cultures showed no fungal or bacterial growth. A PCR-based assay on blood for cytomegalovirus (CMV) was positive (5.15 log). The patient was successfully treated with ganciclovir: the fever resolved, and she was discharged to home after a 4-day hospitalization.
Two weeks later, she developed a fever (38°C), a cough, nonspecific abdominal pain, and anorexia without transit troubles and was readmitted to our hospital. Initial investigations included full blood examination, demonstrating nonregenerative anemia and leucopenia without inflammatory syndrome. Renal function revealed a serum creatinine level of 86 μmol/liter (reference range, 44 to 80). CMV DNA was undetectable in blood. Urine contained numerous cells, but cultures showed no fungal or bacterial growth. Thoracic and sinus computed tomography scans did not reveal any lesions, and the brain MRI was unremarkable. No tubercle bacillus was detected on 3 successive sputa.
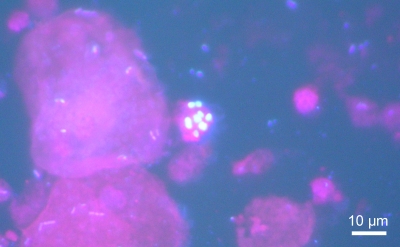
Stools were repeatedly negative for microsporidia, Cryptosporidium spp., and other parasites. After a 1-month hospitalization, all drugs (excepting MMF and cyA) were stopped to eliminate toxic etiology. At that time, many spores of microsporidia were detected in urine samples (Fig. 1), the kidney biopsy specimen, and sputum smears with the use of Uvitex 2B staining (15). No spore of microsporidia was found in stool, duodenal biopsy, or cerebrospinal fluid (CSF) specimens. No culture of the organism was performed. There was no evidence of microsporidia in the feces of the patient's dog; unfortunately, its urine and serum could not be sampled. The patient was given albendazole at 400 mg twice daily for 4 weeks and 400 mg daily until her CD4 cell count increased to 100/mm3 (9 months). MMF was switched to azathioprine. This treatment led to clinical improvement, including resolution of fever after 5 days of treatment and reduction of abdominal pain after 2 weeks of treatment. The serum creatinine level decreased to 63 μmol/liter. However, rare microsporidian spores continued to be shed in urine. Complete clearance of spores was observed only 5 and a half months after treatment initiation.
FIG. 1.
Patient's urine specimen showing reniform Encephalitozoon spores, as visualized by Uvitex 2B staining (×1,000 magnification).
Molecular specific identification.
DNA was extracted from the patient's specimens (urine, kidney biopsy, sputum, CSF, stool, and duodenal biopsy) by using a QIAamp DNA minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions after an initial 30-min incubation step with 10 U lyticase at 37°C (Sigma Aldrich, Saint Quentin Fallavier, France). Species detection was performed by amplifying a 938-bp fragment of the Encephalitozoon cuniculi small subunit rRNA gene by using the 5′-GTGGTCTGCCCCTGTGGGGT-3′ and 5′-CCCTCACAGCAGGCAGAAGC-3′ primers (13). Amplification was performed with a model 9700 PCR system (Applied Biosystems, Foster City, CA) in a 50-μl volume containing 2 mM MgCl2, 1× Applied Biosystems Gold buffer, 200 μM each deoxynucleoside triphosphate (dNTP), 0.4 μM each primer, 2 U of Applied Biosystems AmpliTaq Gold, and 10 μl of extracted DNA. After 9 min at 95°C, amplification consisted of 38 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 90 s, followed by a 5-min terminal extension step at 72°C. The presence of E. cuniculi DNA was evidenced by this specific PCR on urine, sputum, and renal biopsy specimens. E. cuniculi DNA was undetectable in duodenal biopsy, stool, CSF, and blood specimens, in accordance with the absence of microsporidian spores at microscopic observation in these specimens. E. cuniculi DNA became undetectable in urine only 5 and a half months after the initiation of treatment with albendazole.
Molecular subspecific typing.
Subspecific typing was made by PCR and sequence analysis of a 403-bp fragment containing the internal transcribed spacer (ITS) of the rRNA genes, as previously described (1). PCR products were purified with a QIAquick PCR purification kit (Qiagen) and sequenced on both strands with the PCR primers and a BigDye Terminator kit (Applied Biosystems) on an Applied Biosystems 3730 automated sequencer. Sequence analysis showed the presence of five repeats of 5′-GTTT-3′ in the ITS region of all tested specimens collected from our patient (1 sputum sample, 1 renal biopsy sample, and 4 urine samples), indicating that she was infected with a previously undescribed strain, which we propose to name the type IV strain (Fig. 2).
FIG. 2.
Alignment of the ITS sequences of the 3 known E. cuniculi strain types with our patient's strain, which we propose to name the type IV strain (GenBank accession no. HM045511).
Immunological methods.
By use of an indirect immunofluorescence technique (IFAT) (16), a serum sample taken early after infection showed a moderately strong IgG antibody response against the spore wall of E. cuniculi, but no reaction against the polar tube was observed. After 3 months, the titer of IgG against the spore wall increased 2-fold, with an IgG-positive response against the parasite polar tube.
Several species of microsporidia can cause disease in humans. Most cases have been described to occur in HIV-infected patients, but microsporidia are being considered emerging pathogens in transplant recipients (8). The most frequently recognized species in humans is Enterocytozoon bieneusi. It is mainly found in the upper gastrointestinal tract and associated with diarrheal illness. Infections with Encephalitozoon spp. are less frequently identified and are characterized by their potential to disseminate (4, 7). Disseminated microsporidiosis due to Encephalitozoon spp. have been described most commonly for patients with AIDS and only rarely for those with other forms of immunosuppression. To our knowledge, only 5 cases of E. cuniculi infections in non-HIV-infected immunocompromised patients have been reported in the literature, in addition to our case. Among the 6 cases, 5 occurred in transplant recipients. A sixth patient who presented with iris tumor caused by E. cuniculi infection had idiopathic CD4+ T lymphocytopenia (6). The clinical characteristics of our patient and of the other non-HIV-infected patients are reported in Table 1. All patients had severe immunosuppression that could facilitate E. cuniculi infection. Disseminated infection was described to occur in 4 patients (4/6 patients). One patient had only respiratory distress, and another one had ocular manifestation. The most commonly reported clinical manifestations of disseminated infection were keratoconjunctivitis, fever, abdominal pain, and respiratory symptoms (cough and thoracic pain).
TABLE 1.
Characteristics of non-HIV-infected immunocompromised patients with E. cuniculi infectiona
| Source or reference(s) | Location (yr) | Graft type | Immunosuppressive treatment or immunodepressive condition | Clinical symptom(s) | Specimen(s) positive for microsporidial spores | Method(s) for species identification | Treatment(s) | Outcome | Clearance of spores | Strain type |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | Canada (2002) | Kidney | High-dose methylprednisolone, anti-CD3 antibodies | Keratoconjunctivitis, fever, allograft tenderness | Urine, sputum, stool, conjonctival scraping, brain, kidney | TEM, IFA, PCR | Albendazole (800 mg/day for 4 wk), fumagillin eye drops | Death | Relapse after clearance of spores | III |
| 5 | Mexico (2003) | Kidney | High-dose prednisolone, rapamycin, cyclosporine A | Fever, diarrhea, thoracic pain, ocular discomfort, abdominal pain | Urine, grafted kidney | TEM, IFA | Albendazole (400 mg/day for 2 wk), fumagillin eye drops | Clinical improvement | Relapse after clearance of spores | NA |
| 9 | U.S. (2003) | Kidney | High-dose steroid therapy | Bilateral keratoconjunctivitis, fever, graft tenderness | Urine, sputum, stool, conjonctival scraping, brain, kidney | TEM, PCR | None | Death | No | NA |
| 12, 14 | U.S. (2004) | Bone marrow | Thiotepa, cyclophosphamide, total body irradiation, antilymphocyte globulin, cyclosporine A | Respiratory distress | Lung biopsy | TEM, PCR, DNA sequencing | None | Death | No | III |
| 6 | Switzerland (2005) | Idiopathic CD4 lymphopenia | Iris tumor | Tumor biopsy, urine | TEM, PCR | Albendazole (800 mg/day for 4 wk), fumagillin eye drops | Clinical improvement | After 5 mo | I | |
| This case | France (2008) | Kidney | Cyclosporine A, mycophenolate mofetil (replaced by aziathioprine) | Fever, cough, abdominal pain | Urine, sputum, kidney biopsy | PCR, DNA sequencing, IFA | Albendazole (800 mg/day for 2 wk, 400 mg/day for 9 mo) | Clinical improvement | After 5.5 mo | IV |
TEM, transmission electron microscopy; IFA, indirect immunofluorescence assay; NA, not available.
In all these cases, microsporidia were isolated in various body fluids or tissues, including urine, sputum, stool, conjunctival scraping, brain, and kidney biopsy specimens. Urine specimens seem to be the most contributive samples. Indeed, five patients had positive urine specimens. The last patient data were not provided, because diagnosis was made postmortem on the lung biopsy specimen.
In our patient, microsporidian spores were isolated from urine, sputum, and renal biopsy specimens and visualized microscopically. Species identification was confirmed by specific PCR. Sequence analysis of the ITS region was used to establish the E. cuniculi strain type on the basis of the number of 5′-GTTT-3′ repeats. Three types of strains had previously been identified by ITS sequence analysis (types I, II, and III, also named “rabbit strain,” “mouse strain,” and “dog strain,” respectively) (3). E. cuniculi genotype III had been isolated in 2 non-HIV-infected immunocompromised patients (10, 12, 14). E. cuniculi type I had been detected in the iris tumor biopsy specimen of one patient (6). Our patient was infected with a newly discovered genotype.
Identification of the infecting species of microsporidia is determinant for treatment choice. Albendazole has demonstrated activity against E. cuniculi in vitro (2) and E. intestinalis in vivo in patients with AIDS (11). Four out of six non-HIV-infected immunocompromised patients with E. cuniculi infection have been treated with albendazole, together with fumagillin eye drops for 3 patients with ocular infection (Table 1). Clinical improvement was seen in all treated patients, but relapses occurred after treatment interruption in two patients: one patient died from cerebral E. cuniculi infection 4 months after treatment (10), and the other one experienced a relapse 1 year after (5). In our patient, parasite shedding in urine decreased but did not cease completely until 5 and a half months after the beginning of treatment.
Disseminated microsporidiosis must be considered in the differential diagnosis of chronic fever in renal allograft recipients, even those without urinary symptoms. Because of the broad range of infected sites and symptoms that microsporidia can cause in severely immunocompromised patients, the search for microsporidian spores in not only stool specimens but also urine specimens should be performed in cases of unexplained fever and abdominal pain, particularly if urines contain numerous cells and bacteriologic cultures remain sterile.
Nucleotide sequence accession number.
The nucleotide sequence for the type IV strain was deposited in GenBank under accession no. HM045511.
Acknowledgments
We declare that we have no conflicting interests in relation to this work.
Footnotes
Published ahead of print on 12 May 2010.
REFERENCES
- 1.Asakura, T., S. Nakamura, M. Ohta, Y. Une, and K. Furuya. 2006. Genetically unique microsporidian Encephalitozoon cuniculi strain type III isolated from squirrel monkeys. Parasitol. Int. 55:159-162. [DOI] [PubMed] [Google Scholar]
- 2.Beauvais, B., C. Sarfati, S. Challier, and F. Derouin. 1994. In vitro model to assess effect of antimicrobial agents on Encephalitozoon cuniculi. Antimicrob. Agents Chemother. 38:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Didier, E. S., C. R. Vossbrinck, M. D. Baker, L. B. Rogers, D. C. Bertucci, and J. A. Shadduck. 1995. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology 111(4):411-421. [DOI] [PubMed] [Google Scholar]
- 4.Didier, E. S., and L. M. Weiss. 2006. Microsporidiosis: current status. Curr. Opin. Infect. Dis. 19:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gamboa-Dominguez, A., J. De Anda, J. Donis, F. Ruiz-Maza, G. S. Visvesvara, and H. Diliz. 2003. Disseminated Encephalitozoon cuniculi infection in a Mexican kidney transplant recipient. Transplantation 75:1898-1900. [DOI] [PubMed] [Google Scholar]
- 6.Kodjikian, L., J. G. Garweg, M. Nguyen, T. Schaffner, P. Deplazes, and S. Zimmerli. 2005. Intraocular microsporidiosis due to Encephalitozoon cuniculi in a patient with idiopathic CD4+ T-lymphocytopenia. Int. J. Med. Microbiol. 294:529-533. [DOI] [PubMed] [Google Scholar]
- 7.Kotler, D. P., and J. M. Orenstein. 1998. Clinical syndromes associated with microsporidiosis. Adv. Parasitol. 40:321-349. [DOI] [PubMed] [Google Scholar]
- 8.Lanternier, F., D. Boutboul, J. Menotti, M. O. Chandesris, C. Sarfati, M. F. Mamzer Bruneel, Y. Calmus, F. Mechai, J. P. Viard, M. Lecuit, M. E. Bougnoux, and O. Lortholary. 2009. Microsporidiosis in solid organ transplant recipients: two Enterocytozoon bieneusi cases and review. Transpl. Infect. Dis. 11:83-88. [DOI] [PubMed] [Google Scholar]
- 9.Mahmood, M. N., M. E. Keohane, and E. M. Burd. 2003. Pathologic quiz case: a 45-year-old renal transplant recipient with persistent fever. Arch. Pathol. Lab. Med. 127:e224-e226. [DOI] [PubMed] [Google Scholar]
- 10.Mohindra, A. R., M. W. Lee, G. Visvesvara, H. Moura, R. Parasuraman, G. J. Leitch, L. Xiao, J. Yee, and R. del Busto. 2002. Disseminated microsporidiosis in a renal transplant recipient. Transpl. Infect. Dis. 4:102-107. [DOI] [PubMed] [Google Scholar]
- 11.Molina, J. M., C. Chastang, J. Goguel, J. F. Michiels, C. Sarfati, I. Desportes-Livage, J. Horton, F. Derouin, and J. Modai. 1998. Albendazole for treatment and prophylaxis of microsporidiosis due to Encephalitozoon intestinalis in patients with AIDS: a randomized double-blind controlled trial. J. Infect. Dis. 177:1373-1377. [DOI] [PubMed] [Google Scholar]
- 12.Orenstein, J. M., P. Russo, E. S. Didier, C. Bowers, N. Bunin, and D. T. Teachey. 2005. Fatal pulmonary microsporidiosis due to Encephalitozoon cuniculi following allogeneic bone marrow transplantation for acute myelogenous leukemia. Ultrastruct. Pathol. 29:269-276. [DOI] [PubMed] [Google Scholar]
- 13.Schuitema, A., R. Hartskeerl, T. van Gool, R. Laxminarayan, and W. Terpstra. 1993. Application of the polymerase chain reaction for the diagnosis of microsporidiosis. AIDS 7:S57-S61. [Google Scholar]
- 14.Teachey, D. T., P. Russo, J. M. Orenstein, E. S. Didier, C. Bowers, and N. Bunin. 2004. Pulmonary infection with microsporidia after allogeneic bone marrow transplantation. Bone Marrow Transplant. 33:299-302. [DOI] [PubMed] [Google Scholar]
- 15.van Gool, T., F. Snijders, P. Reiss, J. K. Eeftinck Schattenkerk, M. A. van den Bergh Weerman, J. F. Bartelsman, J. J. Bruins, E. U. Canning, and J. Dankert. 1993. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J. Clin. Pathol. 46:694-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Gool, T., J. C. Vetter, B. Weinmayr, A. Van Dam, F. Derouin, and J. Dankert. 1997. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J. Infect. Dis. 175:1020-1024. [DOI] [PubMed] [Google Scholar]