Abstract
Sex differences occur in most species and involve a variety of biological characteristics. The nematode Caenorhabditis elegans consists of two sexes, self-fertile hermaphrodites (XX) and males (XO). Males differ from hermaphrodites in morphology, behavior, and life span. Here, we find that male C. elegans worms are much more sensitive than hermaphrodites to oxidative stress and show that the DM domain transcription factor MAB-3 plays a pivotal role in determining this male hypersensitivity. The hypersensitivity to oxidative stress does not depend on the dosage of X chromosomes but is determined by the somatic sex determination pathway. Our analyses show that the male hypersensitivity is controlled by MAB-3, one of the downstream effectors of the master terminal switch TRA-1 in the sex determination pathway. Moreover, we find that MAB-3 suppresses expression of several transcriptional target genes of the ELT-2 GATA factor, which is a global regulator of transcription in the C. elegans intestine, and show that RNA interference (RNAi) against elt-2 increases sensitivity to oxidative stress. These results strongly suggest that the DM domain protein MAB-3 regulates oxidative stress sensitivity by repressing transcription of ELT-2 target genes in the intestine.
Sex differences occur in most species and not only create male or female gametes but also affect a variety of somatic traits, including morphology, behavior, and life history. In addition, there are often differences between the sexes in terms of disease susceptibility (23), many of which cannot be explained in terms of behavioral or anatomical differences. Most of these sexual dimorphisms are thought to be controlled by differences in gene regulation in each sex.
The nematode Caenorhabditis elegans is widely used as an experimental multicellular organism. C. elegans occurs as a population comprising self-fertilizing hermaphrodites that are diploid for the X chromosome and a low percentage of males that possess a single X chromosome. Males are produced either as a result of nondisjunction of the X chromosomes during gametogenesis in the hermaphrodite or from a male-hermaphrodite mating. All somatic differences between the sexes result from the differential activity of a “global” sex determination regulatory pathway that is controlled by the number of X chromosomes. A series of inhibitory interactions in this signal transduction pathway ultimately sets the activity of the terminal transcription factor tra-1. The hermaphrodite fate is specified when TRA-1 is active, and the male fate is specified when TRA-1 is inactive. It has been suggested that TRA-1 regulates transcription of sex differentiation genes and determines somatic sexual fate cell autonomously (37).
C. elegans males are morphologically distinct from hermaphrodites: the male body is thinner, and the tail is specialized to deliver sperm to the hermaphrodite during mating. There are also behavioral differences between the sexes. Males are more active and engage in mate searching and frequent “backing” behavior, which are not seen in hermaphrodites. There are also physiological sex differences in C. elegans. The male life span is intrinsically longer than that of hermaphrodite animals, although under normal conditions, this increased longevity is dramatically reduced by male-specific behaviors, like mating (10). In addition, males are more resistant than hermaphrodites to a fungal pathogen, Cryptococcus neoformans. This resistance can be induced in hermaphrodites by inappropriate activation of the male sex determination pathway. These differences are molecularly determined and may not result from behavioral changes or reproductive differences (33).
As seen above, physiological sex differences are common. In this study, we identify a new physiological sex difference in C. elegans involving the male hypersensitivity to oxidative stress. Essentially, all organisms must defend themselves against reactive oxygen species (ROS), which are derived from both mitochondrial respiration and exogenous sources. Moreover, oxidative stress contributes to the etiology of various degenerative diseases, such as atherosclerosis, hypertension, diabetes, and cancer, in humans (7). In addition, we demonstrate that the sensitivity depends on one of the male determinants, MAB-3, a DM domain transcription factor that is a downstream target of TRA-1, the terminal regulator of sex determination in C. elegans. The DM domain is a zinc finger motif first identified in a gene affecting sexual regulation in Drosophila (8, 25). Finally, our data suggest that ELT-2, a GATA transcription factor, is involved in male oxidative stress sensitivity. Thus, our results suggest that MAB-3 regulates sensitivity to oxidative stress by repressing transcription of ELT-2 target genes.
MATERIALS AND METHODS
C. elegans strains and culture.
All strains were maintained at 20°C or 16°C (temperature-sensitive strains) on nematode growth medium (NGM) as described previously (3). The strains of C. elegans used in this study were N2 Bristol (wild type [WT]) and the tra-1(e1099), tra-2(e1094) (12), fem-1(hc17) (6), fem-2(b245) (16), mab-3(e1240) (28), and him-8(e1489) (13) strains. The him-8(e1489) mutation causes a high incidence (6,537,440%) of males due to X chromosome nondisjunction. The strains of other Caenorhabditis species used in this study were AF16 (Caenorhabditis briggsae) and SB146 (Caenorhabditis remanei).
Assays for stress sensitivity.
To assay arsenite sensitivity, well-fed young males and hermaphrodites (or females) were picked and transferred to NGM agar plates containing 1.5, 3, 5, 6, 7.5, or 9 mM sodium arsenite. Worms were incubated at 20°C, and the numbers of surviving worms were counted at the indicated times. To assay paraquat or hydrogen peroxide sensitivity, worms were transferred to M9 buffer containing 100 mM paraquat or 3 mM hydrogen peroxide and incubated at 20°C. To assay UV stress sensitivity, worms were transferred to NGM agar plates without bacteria, and UV irradiation (1,500 J/m2) was performed with a UV cross-linker (FS-800; Funakoshi). Then worms were transferred to NGM agar plates with bacteria and incubated at 20°C. To assay heat stress sensitivity, worms were transferred to NGM agar plates with bacteria and incubated at 35°C for the indicated times.
RNAi.
RNA interference (RNAi) by feeding was performed as described previously (15). Fragments designated for RNAi were obtained by PCR from cDNA and were cloned into the L4440 feeding vector (pPD129.36). The RNAi clones were transformed into HT115 strains, and cultures were incubated with IPTG (isopropyl-β-d-thiogalactopyranoside) to induce expression.
Plasmid construction.
The ges-1 promoter, a fragment containing 2.5 kb upstream of the initiation ATG, was constructed by PCR and subcloned into pPD95.75 at the SphI and SalI sites (pPD95.75::Pges-1). For constructing Pges-1::mab-3, a PCR fragment of the mab-3 gene with the SalI and KpnI sites was subcloned into pPD95.75::Pges-1.
Microinjection.
The plasmid containing Pges-1::mab-3 was injected, in combination with the rol-6 morphological marker plasmid pRF4, into the distal gonad of young adult WT hermaphrodites at concentrations of 25 ng/μl for Pges-1::mab-3 and 50 ng/μl for rol-6. Transgenic animals were identified by their characteristic movement (a roller phenotype).
Quantitative RT-PCR.
Total RNA was isolated from young adult worms by use of Sepasol-RNA I Super (Nacalai Tesque, Inc.). Reverse transcription (RT) was performed with SuperScript III reverse transcriptase (Invitrogen), followed by quantitative real-time PCR using FastStart universal SYBR green master mix (ROX) (Roche Applied Science).
Oligonucleotides.
The full sequences of the double-stranded oligonucleotides used for this work are available at http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html.
RESULTS AND DISCUSSION
C. elegans males are hypersensitive to oxidative stress.
When C. elegans wild-type (N2 Bristol) males and hermaphrodites were treated with sodium arsenite at 3 mM, males died rapidly but hermaphrodites survived at least 24 h (Fig. 1 A) (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). This dimorphism held true for a wide range of arsenite concentrations (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). As inorganic arsenic is known to cause oxidative stress by inducing a rapid burst of ROS (18), we tested whether the male hypersensitivity to inorganic arsenite was due to oxidative stress. To this end, animals were treated with a canonical oxidative stressor (hydrogen peroxide or paraquat). Following either treatment, males died rapidly compared with hermaphrodites (Fig. 1B and C), indicating that males are much more sensitive to oxidative stress than hermaphrodites. In contrast to the large difference in sensitivity to oxidative stress, there was only a small difference in sensitivity to heat or UV stress between males and hermaphrodites (Fig. 1D and E). Heat stress causes detrimental effects for proteins, such as misfolding and aggregation (21), and UV irradiation causes the formation of pyrimidine dimers, which leads to mutations (22). However, both heat shock and UV irradiation cause some oxidative stress through formation of free radicals (9, 22). Therefore, it is likely that the slightly higher sensitivity of males to heat or UV stress results from their hypersensitivity to oxidative stress.
FIG. 1.
C. elegans males show hypersensitivity to oxidative stress. (A to E) WT (N2 Bristol) males and hermaphrodites were scored for survival following exposure to 3 mM sodium arsenite on NGM agar plates (A), 3 mM hydrogen peroxide (B) and 100 mM paraquat (C) in M9 buffer, or 35°C heat (D) and 1,500 J/m2 UV irradiation (E) on NGM agar plates for the indicated times. (F) Effect of male mating on oxidative stress sensitivity. Solitary or grouped males and hermaphrodites were scored for survival following exposure to NGM agar plates containing 3 mM sodium arsenite. The mean ± standard deviation (SD) for the fraction of live animals on each of four plates (50 animals per plate) is shown.
Although the life span of C. elegans males is intrinsically longer than that of hermaphrodites, this male longevity is markedly shortened by mating with hermaphrodites or by male clumping (10, 34). Thus, we tested whether the male hypersensitivity to oxidative stress resulted from mating or male clumping. To this end, we isolated males before they have started mating behavior. These solitary males were also hypersensitive to oxidative stress (Fig. 1F). This result indicates that male sensitivity to oxidative stress is not caused by mating behavior or male clumping. Furthermore, the long male life span does not correlate with male sensitivity to oxidative stress.
To see if the sex difference in oxidative stress sensitivity occurs in other Caenorhabditis species, we used C. briggsae and C. remanei, which are closely related to C. elegans. Males were much more sensitive to oxidative stress than hermaphrodites in C. briggsae and females in C. remanei (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). These results suggest that the male hypersensitivity to oxidative stress is common to Caenorhabditis species.
Male fate signaling in the global sex determination pathway provides hypersensitivity to oxidative stress.
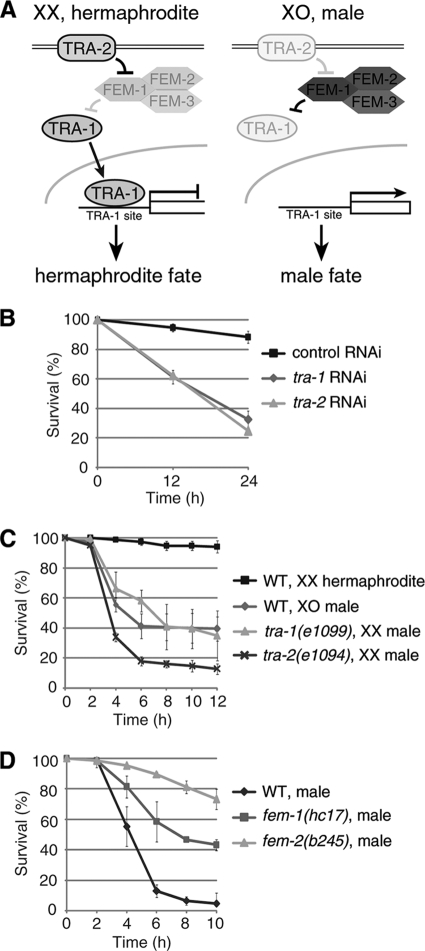
The global pathway that regulates sex determination in C. elegans (Fig. 2 A) is largely conserved in other Caenorhabditis species (32). Recently, it has been reported that the pathway is involved in physiological sex differences in C. elegans. Chromosome dosage affects the life span of C. elegans (11), and a male soma confers resistance to C. neoformans-mediated killing (33). Thus, we determined if the male hypersensitivity to oxidative stress depended on the differences in chromosome dosage or in somatic development between the sexes. To this end, we measured the oxidative stress sensitivity of sexually reversed animals.
FIG. 2.
Male hypersensitivity to oxidative stress is determined by the somatic sex determination pathway. (A) Schematic models of the C. elegans somatic sex determination signaling pathways. (B) X chromosome dosage is not involved in the male hypersensitivity. Both tra-1(e1099) XX males and tra-2(e1094) XX males show hypersensitivity to oxidative stress, similar to WT XO males. (C) tra-1 or tra-2 RNAi-treated hermaphrodites also show hypersensitivity to oxidative stress. (D) Survival of temperature-sensitive mutants of fem-1(hc17) and fem-2(b245) or WT males. Animals were scored for survival following exposure to NGM agar plates containing 3 mM sodium arsenite (B, C, and D). The mean ± SD for the fraction of live animals on each of four plates (50 animals per plate) is shown.
The sexual fates of all somatic cells are controlled by a regulatory pathway whose activities differ between the sexes. The terminal regulator is TRA-1 (tra for transformer), which is homologous to Drosophila Ci and vertebrate GLI (36). Active TRA-1 promotes hermaphrodite development and prevents male development (12, 36). Thus, we measured the sensitivity to oxidative stress of tra-1(e1099) animals, which are fertile males in spite of their XX chromosomal composition (12). The tra-1(e1099) XX males showed hypersensitivity to oxidative stress, like wild-type males (Fig. 2B). In the global sex determination pathway, TRA-2 inhibits the three FEM (feminization) proteins which negatively regulate TRA-1 (Fig. 2A) (1, 24, 29, 31). Thus, we examined the sensitivity of tra-2(e1094) XX animals, which are imperfect males. They also showed hypersensitivity to oxidative stress (Fig. 2B). Furthermore, RNAi against tra-1 or tra-2 in hermaphrodites, which were developed as imperfect males, also resulted in a marked increase in the sensitivity to oxidative stress (Fig. 2C). These results strongly suggest that the male hypersensitivity depends on the somatic sex determination pathway but not on the dosage of X chromosomes.
To confirm this idea, we examined XO animals of temperature-sensitive fem alleles for their sensitivity to oxidative stress. Thus, fem-1(hc17) and fem-2(b245) XO animals, which are temperature sensitive, were kept at restrictive temperatures to repress male fates during larval development. These fem XO animals appeared morphologically male, although their tails were partially defective and their bodies were bigger than those of WT males, and they had an oily substance that seemed to be yolk protein. They showed significantly less sensitivity to oxidative stress than wild-type males (Fig. 2D). These results suggest that genes in the global sex determination pathway that promote male development also cause hypersensitivity to oxidative stress.
MAB-3, one of the TRA-1 targets, is involved in the male hypersensitivity to oxidative stress.
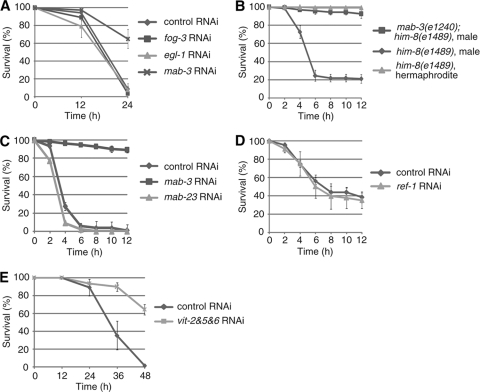
We next focused on downstream targets of TRA-1, the terminal switch in the sex determination pathway. To date, three major targets of TRA-1 have been identified (egl-1, fog-3, and mab-3). EGL-1 is a global regulator of programmed cell death (5). FOG-3 blocks oogenesis and induces spermatogenesis in germ cells (4). MAB-3 is a DM domain transcription factor, which directs differentiation of sensory ray neuroblasts into male sense organs (V rays) in the peripheral nervous system (28) and represses expression of vitellogenin (yolk protein) genes in the male intestine (35). Thus, we examined the effect of RNAi suppression of these genes on male hypersensitivity to oxidative stress. RNAi against egl-1 or fog-3 did not affect the male hypersensitivity (Fig. 3 A), suggesting that male-specific apoptosis and oogenesis/spermatogenesis have little or no effect on the sensitivity to oxidative stress. In contrast, mab-3 RNAi significantly reduced the male hypersensitivity (Fig. 3A). To confirm this result, we studied mab-3(e1240) null-mutant males. Whereas control him-8(e1489) males showed hypersensitivity, like wild-type males, mab-3(e1240); him-8(e1489) males showed a reduced sensitivity, which was nearly identical to that of WT or him-8(e1489) hermaphrodites (Fig. 3B) (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). In addition, mab-3(e1240); him-8(e1489) males showed a reduced sensitivity to other oxidative stressors, such as hydrogen peroxide and paraquat (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). These results indicate that the C. elegans male hypersensitivity to oxidative stress depends on the function of the TRA-1 target gene mab-3. One other DM domain transcription factor, mab-23, is known to regulate male development (17). However, unlike mab-3 RNAi, mab-23 RNAi did not affect the male hypersensitivity to oxidative stress (Fig. 3C), suggesting that mab-3 is specifically involved in the male hypersensitivity.
FIG. 3.
The MAB-3 gene, one of the TRA-1 target genes, plays a pivotal role in determining the male hypersensitivity to oxidative stress. (A) Effect of RNAi against TRA-1 target genes (fog-3, egl-1, or mab-3) on the male hypersensitivity to oxidative stress. (B) mab-3(e1240) males show strong resistance to oxidative stress, similar to WT hermaphrodites. (C) mab-23 is not involved in the male hypersensitivity. mab-23 RNAi or mab-3 RNAi was performed. (D) ref-1, one of the major MAB-3 targets, is not involved in the male hypersensitivity. ref-1 RNAi was performed with mab-3(e1240); him-8(e1489) males. (E) Effect of knockdown of all vit genes on the oxidative stress sensitivity of WT hermaphrodites. Animals were scored for survival following exposure to NGM agar plates containing 3 mM (A, B, and C) or 5 mM (D and E) sodium arsenite. The mean ± SD for the fraction of live animals on each of four plates (50 animals per plate) is shown.
MAB-3 represses several ELT-2 target genes in the intestine.
When a plasmid containing the Pges-1::mab-3 transgene was introduced as an extrachromosomal array into mab-3(e1240); him-8(e1489) males, the resistance to arsenite was decreased (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). To identify mechanisms by which MAB-3 regulates the male hypersensitivity, we began by studying the known MAB-3 targets, ref-1 and the vitellogenin genes, which are repressed by MAB-3. First, ref-1 is known to be the primary target of MAB-3 in the V ray lineage in males (26). We found that ref-1 RNAi-treated mab-3(e1240); him-8(e1489) males showed nearly the same sensitivity as control RNAi-treated ones (Fig. 3D), suggesting that ref-1 is not involved in the male hypersensitivity. Second, vitellogenin is a yolk protein precursor, and its level of expression is high in hermaphrodites but very low in males. In C. elegans, five vit genes (vit-2 to vit-6; vit-1 is a pseudogene) have been identified (30), and vit-2 expression was known to be repressed by MAB-3 (35). Moreover, we found that the expressions of vit-5 and vit-6 are also repressed by MAB-3 (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). To reduce yolk protein, we constructed a vit-2, vit-5, and vit-6 tandem RNAi vector and performed feeding RNAi with this vector in WT hermaphrodites. As vit-3 and vit-4 genes are more than 95% identical to vit-5 at the nucleotide sequence level, this tandem RNAi construct should effectively knock down all vit genes. Indeed, the expression levels of all vit mRNAs and yolk proteins were markedly decreased in the RNAi-treated hermaphrodites (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). However, the RNAi-treated hermaphrodites did not show an increased sensitivity to oxidative stress; in fact, their sensitivity was somewhat decreased (Fig. 3E). The vit RNAi treated mab-3(e1240); him-8(e1489) males did not show an increased sensitivity either (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). These results suggest that the male hypersensitivity to oxidative stress does not result from the absence or low-level expression of yolk protein, which was surprising, since an increase in vitellogenin protein was shown to provide stress resistance in queen honey bees (27). The reason for this difference between C. elegans worms and honey bees is not known. However, our results suggest that hitherto unknown target genes of MAB-3 are involved in the male hypersensitivity to oxidative stress.
To identify MAB-3 target genes involved in oxidative stress sensitivity, we considered the following things. First, it is likely that the intestine is a major site of response to toxins and chemical stressors in C. elegans (2, 14). Second, the ELT-2 GATA factor is a global regulator of transcription in the C. elegans intestine (19, 20). Third, ELT-2 is a major activator of transcription of the vit genes, which are known MAB-3 targets (35). On the basis of these considerations, we hypothesized that MAB-3, which is also expressed in the intestine, might repress the transcription of other ELT-2 target genes. Thus, we performed quantitative RT-PCR analysis of vit genes and several intestinal ELT-2 target genes, which belong to the detoxification and stress response family (19). Our analysis showed that vit-2, vit-5, and vit-6 are strongly repressed in wild-type males but that the repression is canceled in mab-3(e1240) males (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). Furthermore, expression of 4 detoxification and stress responsive ELT-2 target genes was also repressed in wild-type males but not in mab-3(e1240) males (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). To examine whether MAB-3 is directly involved in the repression of expression of ELT-2 target genes in the intestine, we expressed mab-3 under the control of the intestine-specific ges-1 promoter. When a plasmid containing the Pges-1::mab-3 transgene was introduced as an extrachromosomal array into him-8(e1489) hermaphrodites, the expression levels of not only vit genes but also 2 detoxification and stress response ELT-2 target genes, thn-2 and alh-12, were significantly reduced (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). These results suggest that MAB-3 represses several ELT-2 target genes in the intestine. However, RNAi against these detoxification and stress response genes did not significantly enhance the stress sensitivity of WT hermaphrodites (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html), suggesting that these detoxification and stress response genes may constitute only part of the MAB-3 and ELT-2 target and would not be sufficient to determine the male hypersensitivity to oxidative stress.
MAB-3 increases the oxidative stress sensitivity, at least partly, through repression of ELT-2 target genes.
We then hypothesized that transcriptional repression of ELT-2 target genes by MAB-3 should be, at least in part, responsible for the male hypersensitivity to oxidative stress. To test this idea, we examined whether inhibition of ELT-2 by RNAi increases oxidative stress sensitivity. To avoid potential developmental defects, we performed elt-2 RNAi on young adult hermaphrodites and males. RNAi against elt-2 in hermaphrodites resulted in a significant increase in the arsenite stress sensitivity (Fig. 4 A). Moreover, elt-2 RNAi in mab-3(e1240); him-8(e1489) males, but not in him-8(e1489) males, resulted in a significant increase in the sensitivity (Fig. 4B). elt-2 RNAi-treated mab-3(e1240); him-8(e1489) males also led to a slight increase in the sensitivity to other oxidative stressors (hydrogen peroxide and paraquat) (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). These results suggest that MAB-3 increases oxidative stress sensitivity, at least partly, by repressing some ELT-2 target genes. Interestingly, our quantitative RT-PCR measurements have shown that the expression level of elt-2 itself is significantly lower in males than in hermaphrodites and that elt-2 expression is increased in mab-3(e1240) animals (Fig. 4C). Thus, it is likely that not only ELT-2 function but also elt-2 expression is downregulated by MAB-3.
FIG. 4.
Suppression of elt-2 increases oxidative stress sensitivity. (A) elt-2 RNAi-treated WT [him-8(e1489)] hermaphrodites show higher oxidative stress sensitivity. (B) elt-2 RNAi affects oxidative stress sensitivity in mab-3(e1240); him-8(e1489) males but not in WT males. Animals were scored for survival following exposure to NGM agar plates containing 7.5 mM (A) or 3 mM [him-8(e1489)] or 5 mM [mab-3(e1240); him-8(e1489)] (B) sodium arsenite. The mean ± SD for the fraction of live animals on each of three plates (50 animals per plate) is shown. (C) MAB-3 represses elt-2 expression. Bars show relative mRNA levels of elt-2 in WT [him-8(e1489)] and mab-3(e1240) [mab-3(e1240); him-8(e1489)] worms. The level in WT [him-8(e1489)] hermaphrodites is set to 1.0. The averages of results from 5 independent experiments are shown. Error bars represent standard errors of the means (SEM). (D) Hypothetical model for the action of MAB-3 in determining the male hypersensitivity to oxidative stress. See the text for details.
We have found that C. elegans males are much more sensitive than hermaphrodites to oxidative stress and that this male hypersensitivity to oxidative stress is determined by the DM domain transcription factor MAB-3, which is one of the major downstream targets of the master terminal switch TRA-1 in the sex determination pathway. Our results also showed that MAB-3-induced repression of transcriptional target genes of the ELT-2 GATA factor, which is a global regulator of transcription in the intestine, partly mediates the male hypersensitivity to oxidative stress (Fig. 4D). The promoters of vit genes contain potential MAB-3-binding sites (35), and four detoxification and stress responsive ELT-2 target genes also possess putative MAB-3-binding sites in their promoter region (see http://www.ritsumei.ac.jp/lifescience/bm/hori/sup.html). These results may support our model, but we do not rule out another possibility, namely, that MAB-3 directly suppresses ELT-2 transcriptional activity. The molecular mechanism by which MAB-3 represses ELT-2 targets should be elucidated in future studies. The DM domain transcription factors are evolutionally conserved from nematodes to mammals and are also shown also to regulate sexual development in mammals. We can speculate that mammalian DM domain transcription factors would also function to repress GATA factor-induced transcription. Thus, this study has identified a novel sex difference, oxidative stress sensitivity, in C. elegans and defined the underlying mechanism by which the interaction of the DM domain transcription factor MAB-3 with the GATA factor ELT-2 has been revealed.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to E.N.). H.I. is a fellow of the Japan Society for the Promotion of Science (JSPS).
C. elegans strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). We thank the members of the Nishida laboratory for technical comments and helpful discussion.
Footnotes
Published ahead of print on 24 May 2010.
REFERENCES
- 1.Ahringer, J., T. A. Rosenquist, D. N. Lawson, and J. Kimble. 1992. The Caenorhabditis elegans sex determining gene fem-3 is regulated post-transcriptionally. EMBO J. 11:2303-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, J. H., K. Vranas, M. Lucke, H. Inoue, N. Hisamoto, K. Matsumoto, and T. K. Blackwell. 2005. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc. Natl. Acad. Sci. U. S. A. 102:16275-16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, P., and R. E. Ellis. 2000. TRA-1A regulates transcription of fog-3, which controls germ cell fate in C. elegans. Development 127:3119-3129. [DOI] [PubMed] [Google Scholar]
- 5.Conradt, B., and H. R. Horvitz. 1999. The TRA-1A sex determination protein of C. elegans regulates sexually dimorphic cell deaths by repressing the egl-1 cell death activator gene. Cell 98:317-327. [DOI] [PubMed] [Google Scholar]
- 6.Doniach, T., and J. A. Hodgkin. 1984. A sex-determining gene, fem-1, required for both male and hermaphrodite development in C. elegans. Dev. Biol. 106:223-235. [DOI] [PubMed] [Google Scholar]
- 7.Droge, W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82:47-95. [DOI] [PubMed] [Google Scholar]
- 8.Erdman, S. E., and C. B. Kenneth. 1993. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J. 12:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanagan, S. W., P. L. Moseley, and G. R. Buettner. 1998. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett. 431:285-286. [DOI] [PubMed] [Google Scholar]
- 10.Gems, D., and D. L. Riddle. 2000. Genetic, behavioral and environmental determinants of male longevity in Caenorhabditis elegans. Genetics 154:1597-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartman, P. S., and N. Ishii. 2007. Chromosome dosage as a lifespan determinant in Caenorhabditis elegans. Mech. Ageing Dev. 128:437-443. [DOI] [PubMed] [Google Scholar]
- 12.Hodgkin, J. A., and S. Brenner. 1977. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics 86:275-287. [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgkin, J., H. R. Horvitz, and S. Brenner. 1979. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics 91:67-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue, H., N. Hisamoto, J. H. An, R. P. Oliveira, E. Nishida, T. K. Blackwell, and K. Matsumoto. 2005. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 19:2278-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath, R. S., M. Martinez-Campos, P. Zipperlen, A. G. Fraser, and J. Ahringer. 2002. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2:research0002.1-research0002.10. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed]
- 16.Kimble, J., L. Edgar, and D. Hirsh. 1984. Specification of male development in Caenorhabditis elegans: the fem genes. Dev. Biol. 105:234-239. [DOI] [PubMed] [Google Scholar]
- 17.Lints, R., and S. W. Emmons. 2002. Regulation of sex-specific differentiation and mating behavior in C. elegans by a new member of the DM domain transcription factor family. Genes Dev. 16:2390-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu, S. X., M. Athar, I. Lippai, C. Waldren, and T. K. Hei. 2001. Induction of oxyradicals by arsenic: Implication for mechanism of genotoxicity. Proc. Natl. Acad. Sci. U. S. A. 98:1643-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGhee, J. D., M. C. Sleumer, M. Bilenky, K. Wong, S. J. McKay, B. Goszczynski, H. Tian, N. D. Krich, J. Khattra, R. A. Holt, D. L. Baillie, Y. Kohara, M. A. Marra, S. J. Jones, D. G. Moerman, and A. G. Robertson. 2007. The ELT-2 GATA-factor and the global regulation of transcription in the C. elegans intestine. Dev. Biol. 302:627-645. [DOI] [PubMed] [Google Scholar]
- 20.McGhee, J. D., T. Fukushige, M. W. Krause, S. E. Minnema, B. Goszczynski, J. Gaudet, Y. Kohara, O. Bossinger, Z. Yongjun, J. Khattra, M. Hirst, S. J. M. Jones, M. M. Marra, P. Ruzanov, A. Warner, R. Zapf, D. G. Moerman, and J. M. Kalb. 2009. ELT-2 is the predominant transcription factor controlling differentiation and function of the C. elegans intestine, from embryo to adult. Dev. Biol. 327:551-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto, R. I. 1998. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12:3788-3796. [DOI] [PubMed] [Google Scholar]
- 22.Murakami, S., and T. E. Johnson. 1996. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics 143:1207-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ober, C., D. A. Loisel, and Y. Gilad. 2008. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 9:911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilgrim, D., A. McGregor, P. Jackle, T. Johnson, and D. Hansen. 1995. The C. elegans sex-determining gene fem-2 encodes a putative protein phosphatase. Mol. Biol. Cell 6:1159-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raymond, C. S., C. E. Shamu, M. M. Shen, K. J. Seifert, B. Hirsch, J. Hodgkin, and D. Zarkower. 1998. Evidence for evolutionary conservation of sex-determining genes. Nature 391:691-695. [DOI] [PubMed] [Google Scholar]
- 26.Ross, J. M., A. K. Kallis, M. W. Murphy, and D. Zarkower. 2005. The DM domain protein MAB-3 promotes sex-specific neurogenesis in C. elegans by regulating bHLH proteins. Dev. Cell 8:881-892. [DOI] [PubMed] [Google Scholar]
- 27.Seehuus, S. C., K. Norberg, U. Gimsa, T. Krekling, and G. V. Amdam. 2006. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 103:962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen, M. M., and J. Hodgkin. 1988. mab-3, a gene required for sex-specific yolk protein expression and a male-specific lineage in C. elegans. Cell 54:1019-1031. [DOI] [PubMed] [Google Scholar]
- 29.Spence, A. M., A. Coulson, and J. Hodgkin. 1990. The product of fem-1, a nematode sex-determining gene, contains a motif found in cell cycle control proteins and receptors for cell-cell interactions. Cell 60:981-990. [DOI] [PubMed] [Google Scholar]
- 30.Spieth, J., and T. Blumenthal. 1985. The Caenorhabditis elegans vitellogenin gene family includes a gene encoding a distantly related protein. Mol. Cell. Biol. 5:2495-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starostina, N. G., J. Lim, M. Schvarzstein, L. Wells, A. M. Spence, and E. T. Kipreos. 2007. A CUL-2 ubiquitin ligase containing three FEM proteins degrades TRA-1 to regulate C. elegans sex determination. Dev. Cell 13:127-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stothard, P., and D. Pilgrim. 2003. Sex-determination gene and pathway evolution in nematodes. Bioessays 25:221-231. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg, M. C. W., J. Z. Woerlee, H. Ma, and R. C. May. 2006. Sex-dependent resistance to the pathogenic fungus Cryptococcus neoformans. Genetics 173:677-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Voorhies, W. A. 1992. Production of sperm reduces nematode lifespan. Nature 360:456-458. [DOI] [PubMed] [Google Scholar]
- 35.Yi, W., and D. Zarkower. 1999. Similarity of DNA binding and transcriptional regulation by Caenorhabditis elegans MAB-3 and Drosophila melanogaster DSX suggests conservation of sex determining mechanisms. Development 126:873-881. [DOI] [PubMed] [Google Scholar]
- 36.Zarkower, D., and J. Hodgkin. 1992. Molecular analysis of the C. elegans sex-determining gene tra-1: a gene encoding two zinc finger proteins. Cell 70:237-249. [DOI] [PubMed] [Google Scholar]
- 37.Zarkower, D. 2006. Somatic sex determination. In WormBook. doi: 10.1895/wormbook.1.84.1. http://www.wormbook.org/chapters/www_somaticsexdeterm/somaticsexdeterm.pdf. [DOI] [PMC free article] [PubMed]