Abstract
The receptor tyrosine kinase Ror2 acts as a receptor or coreceptor for Wnt5a to mediate Wnt5a-induced activation of the Wnt/JNK pathway and inhibition of the β-catenin-dependent canonical Wnt pathway. However, little is known about how Ror2 cooperates with another receptor component(s) to mediate Wnt5a signaling. We show here that Ror2 regulates Wnt5a-induced polymerization of Dishevelled (Dvl) and that this Ror2-mediated regulation of Dvl is independent of the cytoplasmic region of Ror2. Ror2 can associate with Frizzled7 (Fz7) via its extracellular cysteine-rich domain to form a receptor complex that is required for the regulation of Dvl and activation of the AP-1 promoter after Wnt5a stimulation. Suppressed expression of Fz7 indeed results in the inhibition of Wnt5a-induced polymerization of Dvl and AP-1 activation. Interestingly, both the DIX and the DEP domains of Dvl are indispensable for Dvl polymerization and subsequent AP-1 activation after Wnt5a stimulation. We further show that polymerized Dvl is colocalized with Rac1 and that suppressed expression of Rac1 inhibits Wnt5a-induced AP-1 activation. Collectively, our results indicate that Ror2/Fz receptor complex plays an important role in the Wnt5a/Rac1/AP-1 pathway by regulating the polymerization of Dvl.
Wnt proteins can elicit β-catenin-dependent and -independent signaling pathways (2, 20, 46). Ror2 is a member of the Ror family of receptor tyrosine kinases and plays essential roles in developmental morphogenesis (21, 26, 31, 32, 44). Ror2 has been shown to act as a receptor or coreceptor for Wnt5a to activate the β-catenin-independent signaling pathway, involving JNK/c-Jun (AP-1), Src and Ca2+, which are essential for cell polarity, migration, and cancer cell invasion (8, 14, 28-31, 37). Wnt5a/Ror2 signaling also plays a crucial role in inhibiting the β-catenin-dependent signaling pathway (25). Structure-function analyses of Ror2 revealed that Ror2 mediates Wnt5a signaling through distinct mechanisms dependent on and independent of its kinase activity, i.e., Wnt5a-induced migration of fibroblast cells requires the cytoplasmic C-terminal portion of Ror2 but not its intrinsic kinase activity (28), whereas the intrinsic kinase activity of Ror2 is indispensable for extracellular matrix (ECM) degradation of osteosarcoma cells (8). In addition, inhibition of the β-catenin-dependent signaling pathway by Wnt5a also requires the intrinsic kinase activity of Ror2 (24). Importantly, the Caenorhabditis elegans ortholog of Ror2, CAM-1, also has the kinase activity-dependent and -independent functions (9, 12, 13). Furthermore, CAM-1 exhibits the cytoplasmic region-independent functions, including cell migration (17), synaptic transmission at the neuromuscular junction (10), and inhibition of the β-catenin-dependent signaling pathway (11), although their underlying molecular mechanisms remain to be determined. However, it is unknown whether or not Ror2 also exhibits the cytoplasmic region-independent functions in other organisms.
Dishevelled (Dvl) is an essential mediator of both the β-catenin-dependent and -independent signaling pathways. We have previously reported that both Ror2 and Dvl are required for Wnt5a-induced cell migration (28). However, the relationship between Ror2 and Dvl in Wnt5a signaling remains unclear. It has been reported that Dvl has an ability to form dynamic polymers, which are crucial for activating the β-catenin-dependent signaling pathway probably by serving as a scaffold for Axin recruitment (39, 41). However, there is no direct evidence showing that Wnt stimulation indeed induces dynamic formation of Dvl polymers. In addition, it remains unclear whether or not the polymerization of Dvl is involved in the β-catenin-independent signaling pathway.
In the present study we show that Wnt5a induces dynamic polymerization of Dvl2 via a receptor complex containing both Ror2 and Frizzled (Fz)7, even in the absence of the cytoplasmic region of Ror2. We further provide evidence indicating that Ror2/Fz7 receptor complex plays an important role in Wnt5a/Rac1/AP-1 pathway by regulating polymerization of Dvl2.
MATERIALS AND METHODS
Materials.
The following antibodies were used: mouse monoclonal antibodies against Flag (F3165; Sigma, St. Louis, MO; a 1:2,500 dilution for immunoblotting [IB] and immunoprecipitation [IP]), hemagglutinin (HA; MMS-101R; Covance Research Products, Berkeley, CA; 1:2,000 for IB and 1:1,000 for IP), α-tubulin (CP06; Calbiochem, La Jolla, CA; 1:1,000 for IB), AP2 (CP46; Calbiochem; 1:200 for immunofluorescence [IF]), green fluorescent protein (GFP; which also recognize cyan and yellow fluorescent protein [CFP and YFP, respectively]) (632381; Clontech, Mountain View, CA; 1:1,000 for IB), Dvl3 (sc-8027; Santa Cruz Biotechnology, Santa Cruz, CA; 1:500 for IB), Rac1 (610650 [BD Biosciences, San Jose, CA], 1:500 for IB, and 16-637 [Millipore, Temecula, CA], 1:250 for IF and 1:1,000 for IB), β-catenin (610154; BD Biosciences; 1:1,000 for IB), caveolin (610406; BD Biosciences; 1:200 for IF), clathrin (610499; BD Biosciences; 1:200 for IF), and EEA1 (610456; BD Biosciences; 1:500 for IF); rabbit polyclonal antibodies against Dvl2 (3216; Cell Signaling Technology, Danvers, MA; 1:500 for IB), P-LRP6 (Ser1490, 2568; Cell Signaling Technology; 1:1,000 for IB), and GFP (which also recognizes CFP and YFP) (ab290; Abcam, Cambridge, MA; 1:1,000 for IP); rabbit monoclonal antibodies against LRP6 (2560; Cell Signaling Technology; 1:1,000 for IB). The following small interfering RNAs (siRNAs) were purchased from Sigma Genosys: Fz7 #1 (5′-UGAGCUACGCUUCUUCUUAUU-3′); Fz7 #2 (5′-GCGAGAAAGGCAUCUCGGUTT-3′); Fz2 #1 (5′-GCUAUAAGUUUCUGGGUGATT-3′); Fz2 #2 (5′-GGGUAAGCCAGCACUGCAATT-3′); Rac1 #1 (5′-GUUCUUAAUUUGCUUUUCCTT-3′); Rac1 #2 (5′-GAGGAAGAGAAAAUGCCUGTT-3′); and control (5′-GUACCGCACGUCAUUCGUAUC-3′). Mouse purified Wnt5a and Wnt3a were purchased from R&D Systems (Minneapolis, MN). FM4-64 and MitoTracker Red were purchased from Invitrogen (Carlsbad, CA) and Lonza (Walkersville, MD), respectively. Wnt5a conditioned medium (CM) and control CM were prepared as described previously (29).
Plasmid construction.
Plasmids encoding mouse Ror2 tagged with Flag, HA, GFP, or CFP at the C termini and the derivative kinase-dead and deletion mutants were constructed as described previously (15, 22, 28, 31). Full-length cDNAs for mouse Dvl2 and human Fz7 were subcloned into pEYFP-C1 and pECFP-N3 vectors (Clontech), respectively. Plasmids for the YFP-Dvl2 mutants, M2 (V67A,K68A) and KM (K446M), were constructed by site-directed mutagenesis. To construct expression plasmids for the sr-Dvl2 (WT, M2, or KM), three bases in the targeting sequence in the corresponding YFP-Dvl2 cDNA were altered by PCR-based mutagenesis (CATGGAGAAATATAATTT). A plasmid encoding mouse Wnt5a-HA was constructed by introducing an HA epitope sequence in pPGKWnt5a (43). Plasmids for Dvl2 siRNA and control siRNA in pSUPER vector (OligoEngine, Seattle, WA) were constructed as described previously (28). To construct the AP-1-driven luciferase reporter construct (pAP-1-Luc), oligonucleotides containing seven tandem AP-1 binding sites (TGACTAA) were annealed and subcloned into the pGL4.27 (luc2P/minP/Hygro) firefly luciferase vector (Promega, Madison, WI). The SuperTopFlash was kindly provided by Randall T. Moon (University of Washington, Seattle).
Cells, transfection, and cell treatments.
L cells were cultured in Dulbecco modified Eagle medium (DMEM) containing 5% fetal calf serum. Ror2/L cells were established and maintained as described previously (28). Cells were transfected with expression plasmids or siRNA duplexes using Lipofectamine 2000 or Lipofectamine RNAiMax (Invitrogen), respectively. The pSUPER siRNA plasmids were transfected by an electroporation as described previously (28). Cells were treated with 400 ng of Wnt5a/ml or 1,000 ng of Wnt3a/ml unless specified otherwise.
Time-lapse imaging and IF microscopy.
Transfected cells grown on fibronectin-coated glass-bottom dishes were serum starved with DMEM containing 0.1% bovine serum albumin (BSA) and 20 mM HEPES (pH 7.4) for 2 h. Fluorescence images were collected from living cells every 15 s at 37°C in DMEM containing 0.1% BSA and 20 mM HEPES (pH 7.4) using an inverted microscope (Axiovert 200M; Carl Zeiss MicroImaging, Inc., Thornwood, NY) equipped with a laser scanning confocal imaging system (LSM510; Carl Zeiss) and a Plane Apochromat 1.4-numerical-aperture ×63 oil immersion objective lens (Carl Zeiss). Images were processed with ImageJ (National Institutes of Health, Bethesda, MD). For IF analysis, cells were fixed and stained with the respective antibodies as previously described (22). Fluorescent images were obtained using the same system and processed by using Photoshop CS (Adobe, San Jose, CA).
Analysis of Dvl2 puncta.
Cells transiently transfected with YFP-Dvl2 were imaged as described above, and any spots greater than 0.2 μm in diameter and with a >2-fold-higher mean fluorescence intensity compared to the adjacent region were defined as puncta. Cells without any puncta before Wnt stimulation were visually selected and used for the analysis. The number of puncta per cell was counted from confocal images.
Immunoprecipitation, immunoblotting, and reporter assay.
Whole-cell lysates were prepared and subjected to analyses by immunoprecipitation and immunoblotting as described previously (28). Coculture IP assay was performed as described previously (49). For the reporter assay, the cells were transfected with the pAP-1-Luc or the SuperTopFlash, together with the internal control plasmid pGL4.74[hRluc/TK] (Promega) at a ratio of 49:1. Cells were serum starved with DMEM containing 0.1% BSA for 12 h and treated with Wnt5a for 24 h. The luciferase activities were measured by using a dual-luciferase reporter assay system (Promega) and a GloMax 96 microplate luminometer (Promega).
RT-PCR.
Total RNA was extracted by using Isogen (Nippon Gene, Toyama, Japan), and 0.5 μg of total RNA was analyzed by reverse transcription-PCR (RT-PCR) using the SuperScript One-Step RT-PCR with Platinum Taq system (Invitrogen) with the following primers: Fz7, 5′-GAAGCTGGAGAAGCTGATGG-3′ (forward) and 5′-ATCTCTCGCCCCAAATCTCT-3′ (reverse); Fz2, 5′-TCGCCTACAACCAGACCATC-3′ (forward) and 5′-CATTGGAAGCCGAACTTGT-3′ (reverse); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-AGGTCGGTGTGAACGGATTTG-3′ (forward) and 5′-AAGTGGGCCCCGGCCTTCTCCAT-3′ (reverse).
RESULTS AND DISCUSSION
We used mouse fibroblast L cells, which do not express both Ror1 and Ror2 proteins at detectable levels and exhibit migratory responses to Wnt5a only when Ror2 is expressed exogenously (28). Since Wnt5a is able to activate both β-catenin-dependent and -independent signaling pathways, depending on the cellular or receptor context (25), we first determined which signaling pathways are activated in Wnt5a-treated L cells in the presence or absence of exogenous Ror2. Although Wnt5a failed to induce accumulation of β-catenin (Fig. 1A) and phosphorylation of LRP6 (Fig. 1B) even in the presence of exogenous Ror2, it induced phosphorylation of Dvl, as detected by electrophoretic mobility shift, in a Ror2-dependent manner (Fig. 1A). On the other hand, Wnt3a induced all of these events irrespective of the presence or absence of Ror2 (Fig. 1A and B), indicating that Wnt5a activates the β-catenin-independent, but not the β-catenin-dependent, signaling pathway in a Ror2-dependent manner in L cells. Consistent with these findings, it has been reported that Wnt5a fails to induce transcriptional activation of a T-cell factor/lymphoid enhancer factor-driven luciferase reporter gene (SuperTopFlash), a readout for the β-catenin-dependent signaling (16), either in the presence or in the absence of Ror2 in L cells (25). On the other hand, Wnt5a could induce the transcriptional activation of an AP-1-driven luciferase reporter gene (AP-1 Luc), a readout for the β-catenin-independent signaling (6, 35), in a Ror2-dependent manner (Fig. 1C). In contrast, Wnt3a failed to activate the AP-1 at the concentration that could induce activation of the SuperTopFlash (Fig. 1D). These findings indicate that Wnt5a/Ror2 selectively activates the β-catenin-independent (AP-1), but not the β-catenin-dependent, signaling pathway, whereas Wnt3a primarily activates the β-catenin-dependent, but not the AP-1, signaling pathway in L cells.
FIG. 1.
Wnt5a induces Dvl phosphorylation via β-catenin-independent pathway in a Ror2-dependent manner. (A) Wnt5a induces Dvl phosphorylation, but not β-catenin accumulation, in a Ror2-dependent manner, while Wnt3a induces both Dvl phosphorylation and β-catenin accumulation irrespective of Ror2 expression. L cells were transfected with Ror2-Flag and treated with either Wnt5a (200 ng/ml, 1 or 2 h; left side) or Wnt3a (200 ng/ml, 2 h; right side) as indicated. Whole-cell lysates (WCL) were analyzed by immunoblotting to detect the proteins indicated. (B) Wnt5a stimulation fails to induce phosphorylation of LRP6. L cells transfected with Ror2-Flag were treated with either Wnt5a (200 ng/ml) or Wnt3a (200 ng/ml) for 2 h and analyzed by the respective antibodies against phosphorylated LRP6 (P-LRP6), LRP6, or Flag. (C) Wnt5a stimulation induces activation of the AP-1 Luc reporter in a Ror2-dependent manner. L cells were transfected with mock or Ror2 expression plasmids along with AP-1 reporter plasmids. After serum starvation for 12 h, these cells were treated with Wnt5a (400 ng/ml) or its vehicle for 24 h and subjected to luciferase assays. (D) Wnt3a stimulation induces activation of the SuperTopFlash but not the AP-1 Luc reporter. L cells were transfected with the SuperTopFlash or AP-1 Luc reporter plasmids. After serum starvation for 12 h, these cells were treated with the indicated concentration of Wnt3a for 24 h and subjected to luciferase assays.
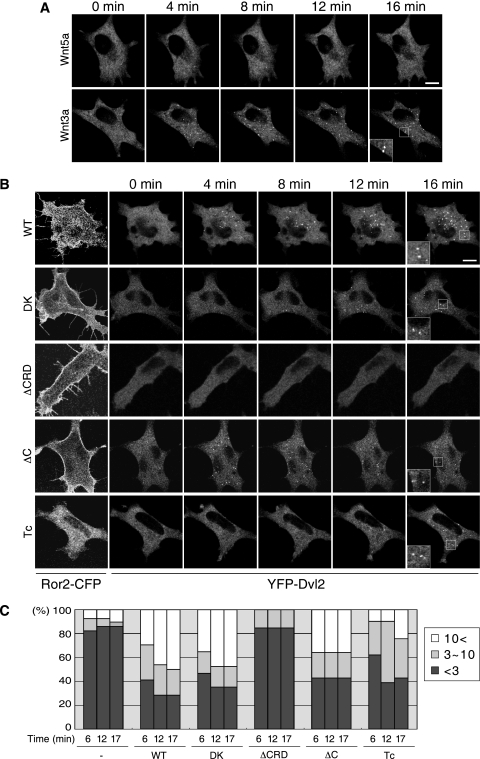
In order to examine the effects of Wnt stimulation on the distribution of Dvl2 in living cells, L cells were transfected with YFP-Dvl2. Although YFP-Dvl2 tended to form puncta because of its high-level expression as reported previously (40, 42), we selected cells with diffuse localization of YFP-Dvl2 at basal levels (Fig. 2A, 0 min). Consistent with the results of Dvl phosphorylation by Wnt5a (Fig. 1A), the distribution of YFP-Dvl2 was unaffected by Wnt5a stimulation (Fig. 2A and C; see Movie S1 in the supplemental material), even though the cells were treated with a high concentration of Wnt5a (1,000 ng/ml) or for prolonged periods up to 2 h (data not shown). In contrast, Wnt3a stimulation, at the concentration of 1,000 ng/ml, resulted in the assembly or polymerization of YFP-Dvl2, detected as a punctate pattern in the cytoplasm (Fig. 2A; see Movie S1 in the supplemental material). Coexpression of wild-type (WT) Ror2-CFP resulted in the formation of filopodia in L cells as reported previously (Fig. 2B) (28) and, importantly, YFP-Dvl2 formed puncta following Wnt5a stimulation (Fig. 2B and C; see Movie S2 [WT] in the supplemental material). The dynamic puncta formation of YFP-Dvl2 was detected within 3 min after Wnt5a stimulation and lasted for up to 2 h (data not shown). As reported previously (40, 42), these puncta are highly mobile (see Movie S2, WT, in the supplemental material) and localized at the cell membrane transiently, where Ror2-CFP was also localized (data not shown). In addition, FRAP (for fluorescence recovery after photobleaching) analysis of the puncta in these cells showed dynamic exchange of YFP-Dvl2 between puncta and its cytosolic pools (data not shown), with kinetics similar to those reported by Schwarz-Romond et al. using COS7 cells transfected with GFP-Dvl2 (40). It has been reported that Dvl2 interacts with clathrin adaptor AP-2 and is involved in internalization of Fz (36, 51). In addition, Dvl2 has been shown to associate with clathrin-coated vesicles in mouse embryos (27). We therefore examined whether the Dvl2 puncta are colocalized with endocytic compartments. L cells stably expressing Ror2 (Ror2/L cells) were transfected with YFP-Dvl2 and treated with Wnt5a. IF analysis of these cells revealed that the Dvl2 puncta are not colocalized with endocytic marker proteins, such as clathrin, AP2, EEA1, or caveolin, following Wnt5a stimulation (data not shown). We also used lipophilic dye FM4-64, which labels the membranous intracellular organelles along the endocytic pathway (45, 47). Wnt5a stimulation failed to induce any apparent colocalization of the FM4-64 stains with the Dvl2 puncta (data not shown), suggesting that Wnt5a-induced formation of Dvl2 puncta is not mediated by the association of Dvl2 with vesicles generated along endocytic pathway. Since some proteins, such as DRP1, are observed in puncta on the mitochondrial surface (50), we further examined the possible colocalization of Dvl2 puncta with mitochondria by labeling with MitoTracker Red. However, we did not detect any apparent colocalization (data not shown). Taken together with the dynamic properties of the Dvl2 puncta and the results that the M2 mutant of Dvl2, which is defective in polymerization, is also incapable of forming puncta following Wnt5a stimulation (see Fig. 4A and B), we conclude that the Dvl2 puncta detected upon Wnt5a stimulation are comparable to those reported by Schwarz-Romond et al. (39, 40), i.e., the Dvl2 puncta are formed by dynamic polymerization of Dvl2 itself but not by its stable association with the cytoplasmic vesicles. However, at present we cannot rule out the possibility that the puncta are associated with other types of vesicles.
FIG. 2.
Wnt5a induces Dvl2 polymerization in a manner dependent on the CRD but not cytoplasmic region of Ror2. (A and B) L cells were transfected with YFP-Dvl2 alone (A) or together with Ror2-CFP (Ror2 WT or its derived mutants) (B). These cells were analyzed by time-lapse fluorescence imaging, where cells were stimulated with Wnt5a (A and B) or Wnt3a (A) at zero time, and fluorescent images were collected every 15 s (see Movies S1 and S2 in the supplemental material). Shown are fluorescent images of YFP-Dvl2 at the indicated time points (A and B) and of Ror2-CFP at zero time (B) Insets show magnified images of boxed regions. Bar, 10 μm. (C) Quantification of Wnt5a-induced Dvl2 polymerization. The average number of the Dvl2 puncta per cell at 6, 12, and 17 min after stimulation was measured. The data are expressed as the percentages of cells with the indicated average number of the puncta per cell (n = 28 [control], 24 [WT], 17 [DK], 20 [ΔCRD], 14 [ΔC], 21 [Tc]).
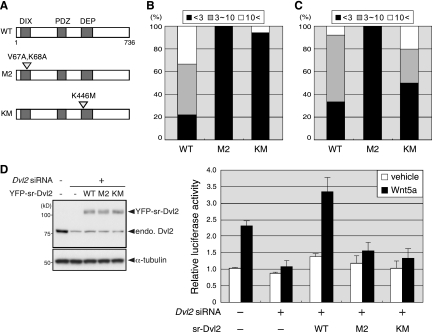
FIG. 4.
Dvl2 polymerization is required for Wnt5a-induced AP-1 activation. (A) Schematic representations of Dvl2 (WT) and its amino acid-substituted mutants (M2 and KM). (B) M2 and KM fail to polymerize in response to Wnt5a. L cells were transfected with YFP-Dvl2 or the respective mutants (M2 and KM) along with Ror2-CFP. Extents of Wnt5a-induced polymerization of the respective Dvl2 mutants were examined as described. Fig. 2, and data are expressed as in Fig. 3D (n = 9 [WT], 12 [M2], 17 [KM]). (C) Wnt3a induces polymerization of wild-type Dvl2 (WT) and KM, but not M2. L cells were transfected with YFP-Dvl2 or the respective mutants (M2 and KM). Extents of Wnt3a-induced polymerization of the respective Dvl2 proteins were examined as described Fig. 2, and data are expressed as in Fig. 3D (n = 12 [WT], 9 [M2], 10 [KM]). (D) Both KM and M2 are defective in mediating Wnt5a-induced AP-1 activation. Ror2/L cells were treated with Dvl2 siRNA for 36 h and then transfected with expression plasmids for YFP-sr-Dvl2, -Dvl2(M2), or -Dvl2(KM), along with the AP-1 reporter plasmids. After serum starvation for 12 h, WCL were analyzed by immunoblotting with anti-Dvl2 and anti-α-tubulin antibodies (left). Cells were treated with either Wnt5a or its vehicle for 24 h and subjected to luciferase assays (right).
We next examined the roles of domains or kinase activity of Ror2 in Wnt5a-induced Dvl2 polymerization. Like Ror2WT-expressing L cells, filopodium formation was observed in L cells expressing the mutant Ror2 lacking either the intrinsic kinase activity (DK) or the extracellular cysteine-rich domain (ΔCRD), while the cells expressing the Ror2ΔC or Ror2Tc, which lack the C-terminal portion or most of the cytoplasmic region, respectively, failed to form filopodia (Fig. 2B). In accordance with the results of cell migration by Wnt5a (28), Wnt5a-induced Dvl2 polymerization was observed in Ror2DK-expressing cells, but hardly in Ror2ΔCRD-expressing cells (Fig. 2B and C; see Movie S2, DK and ΔCRD, in the supplemental material). Unexpectedly, it was also observed in cells expressing either Ror2ΔC or Ror2Tc (Fig. 2B and C; see Movie S2, ΔC and Tc, in the supplemental material), indicating that Wnt5a-induced Dvl2 polymerization requires the CRD of Ror2 but not its cytoplasmic region or kinase activity. However, it should be noted that since the number of the puncta in Ror2Tc-expressing cells was reduced (Fig. 2C), the cytoplasmic region of Ror2 might have some impact on the process. Interestingly, Wnt5a-induced Dvl phosphorylation was also dependent on the CRD of Ror2, but independent of its cytoplasmic region or kinase activity (data not shown).
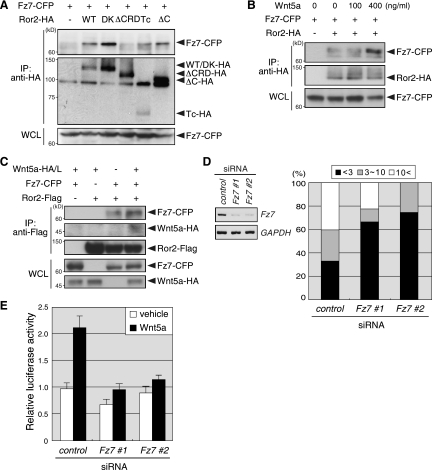
These findings suggest that Ror2 forms a receptor complex with another transmembrane protein(s) such as Fzs to mediate some of Wnt5a signalings. To investigate this possibility, we examined the effects of siRNAs for Fz2 or Fz7 on Wnt5a-induced Dvl phosphorylation, since these Fzs are involved in the β-catenin-independent Wnt/PCP signaling pathway (7, 23, 36), and the CRD of these Fzs can associate with Ror2 in 293T cells (18). Suppressed expression of Fz7, but not Fz2, drastically inhibited Wnt5a-induced Dvl phosphorylation in Ror2/L cells, and this inhibition could be restored by expression of human Fz7 (data not shown), suggesting that Fz7 mediates Wnt5a/Ror2 signaling through Dvl. As expected, Fz7 can associate with Ror2 in L cells, and this association required the CRD but not the cytoplasmic region of Ror2 (Fig. 3A). The association of Ror2 with Fz7 was enhanced by treatment with Wnt5a (Fig. 3B) or coculture with Wnt5a-expressing cells (Fig. 3C). Furthermore, using the coculture immunoprecipitation (IP) assay (49), we found that Wnt5a produced by cocultured cells was associated with Ror2 only when Ror2 was coexpressed with Fz7 (Fig. 3C), indicating that Wnt5a enhances the complex formation containing Ror2, Fz7 and Wnt5a itself. We next examined whether or not Fz7 is involved in Wnt5a-induced Dvl2 polymerization. Suppressed expression of Fz7 resulted in a substantial reduction of polymerized Dvl2 following Wnt5a stimulation of Ror2/L cells (Fig. 3D), indicating that Fz7 is indeed required for Wnt5a-induced Dvl2 polymerization. Furthermore, Wnt5a-induced activation of AP-1, which is dependent on Ror2 (Fig. 1C), was inhibited by suppressed expression of Fz7 in Ror2/L cells (Fig. 3E), indicating a critical requirement of Fz7 for Wnt5a/Ror2 signaling, leading to AP-1 activation. Collectively, these results indicate that the Ror2-Fz7 receptor complex plays an essential role in Wnt5a-induced β-catenin-independent signaling pathway by regulating Dvl.
FIG. 3.
Ror2 is associated with Fz7 to mediate Wnt5a-induced Dvl2 polymerization and AP-1 activation. (A) Association of Ror2 with Fz7 requires the CRD but not intracellular region of Ror2. L cells were transfected with Fz7-CFP and Ror2-CFP (Ror2 WT or its derived mutants) as indicated. WCL or anti-HA immunoprecipitates were analyzed by immunoblotting with anti-GFP or anti-HA antibodies to detect the proteins indicated. (B) Wnt5a stimulation enhances the association of Ror2 with Fz7. L cells were transfected with Fz7-CFP and Ror2-HA as indicated and treated with the indicated concentration of Wnt5a for 15 min. WCL or anti-HA immunoprecipitates were analyzed as in panel A. (C) Association of Wnt5a with Fz7-Ror2 receptor complex. L cells were transfected with Fz7-CFP and Ror2-Flag as indicated. These cells were cocultured with L cells transfected with Wnt5a-HA (Wnt5a-HA/L; +) or mock plasmids (−). After 48 h in culture, WCL or anti-Flag immunoprecipitates were analyzed by immunoblotting to detect the proteins indicated. (D) Fz7 is required for Wnt5a-induced Dvl2 polymerization. Ror2/L were treated with either control or Fz7 siRNA (#1 and #2) for 36 h and then transfected with YFP-Dvl2. After 24 h in culture, expression levels of Fz7 or GAPDH were analyzed by RT-PCR (left). Extents of Wnt5a-induced polymerization of YFP-Dvl2 were examined as described in Fig. 2. The data are expressed as the mean percentages of cells with the indicated average number of the puncta per cell at 6, 12, and 17 min after stimulation (n = 15 [control], 9 [Fz7 siRNA #1], 4 [Fz7 siRNA #2]) (right). (E) Fz7 is required for Wnt5a-induced AP-1 activation. Ror2/L cells were treated with Fz7 siRNA for 36 h and then transfected with the AP-1 reporter plasmids. After serum starvation for 12 h, these cells were treated with Wnt5a or its vehicle for 24 h and subjected to luciferase assays.
Previous studies demonstrate that polymerization of Dvl2 is crucial for activating the β-catenin-dependent signaling pathway. Our results show that Wnt5a induces Dvl2 polymerization in Ror2-expressing L cells, where Wnt5a can trigger the β-catenin-independent, but not the β-catenin-dependent signaling pathway, raising an attractive possibility that Dvl2 polymerization is also involved in the β-catenin-independent signaling pathway. To test this possibility, we used Dvl2 mutants, Dvl2(M2) and Dvl2(KM) (Fig. 4A). It has been proposed that Dvl2 proteins form head-to-tail polymers through two different interfaces located in the DIX domain (39). Dvl2(M2) has amino acid substitutions (V67A and K68A) within one of the interfaces, thereby preventing from polymerization (39). As expected, both Wnt5a and Wnt3a failed to induce polymerization of Dvl2(M2) in Ror2/L or L cells, respectively (Fig. 4B and C). To examine whether or not Dvl2(M2) can mediate Wnt5a signaling, Ror2/L cells were transfected with siRNA-resistant (sr)-Dvl2 following siRNA-mediated knockdown of endogenous Dvl2 (Fig. 4D). As shown, suppressed expression of Dvl2 in Ror2/L cells resulted in a drastic inhibition of Wnt5a-induced AP-1 activation, indicating an essential role of Dvl2 in Wnt5a-induced AP-1 activation. Suppressed expression of Dvl3 also inhibited Wnt5a-induced AP-1 activation, although its effect was rather partial (data not shown). Importantly, the expression of sr-Dvl2(WT), but not of sr-Dvl2(M2), restored the Wnt5a-induced AP-1 activation in the Dvl2-knockdown cells (Fig. 4D), suggesting that an intrinsic activity of polymerization is crucial for Dvl2 to mediate the β-catenin-independent signaling pathway following Wnt5a stimulation. In contrast to Dvl2(M2), Dvl2(KM) has an amino acid substitution (K446M) within the DEP domain (Fig. 4A), which corresponds to K417M mutant of Drosophila Dsh, a mutant that is defective in activating the Wnt/PCP pathway but functional for the β-catenin-dependent signaling pathway (3, 4, 34). In fact, like sr-Dvl2(M2), sr-Dvl2(KM) failed to restore the Wnt5a-induced AP-1 activation in the Dvl2-knockdown Ror2/L cells (Fig. 4D). Interestingly, Wnt5a hardly induced polymerization of Dvl2(KM) in Ror2/L cells (Fig. 4B), whereas Wnt3a could induce its polymerization in L cells (Fig. 4C), indicating that, unlike Dvl2(M2), Dvl2(KM) retains the intrinsic ability to polymerize but is unable to respond to Wnt5a stimulation, resulting in impaired activation of AP-1. Collectively, these results suggest that polymerization of Dvl2 is necessary for activating the β-catenin-independent signaling pathway following Wnt5a stimulation. Since Wnt3a can activate β-catenin-dependent signaling pathway but not AP-1 in L cells (Fig. 1D), unlike Wnt5a, Wnt3a-induced Dvl2 (WT and KM) polymerization might be involved in activation of the β-catenin-dependent signaling pathway. These results also suggest that Dvl2 polymerization per se is not sufficient for AP-1 activation.
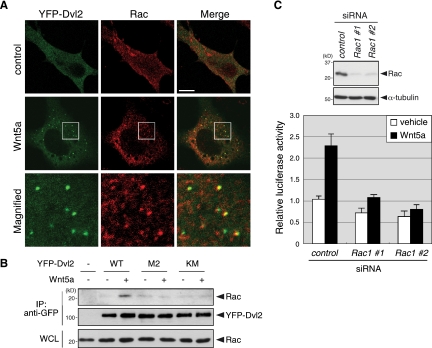
It has been proposed that Dvl2 polymers serve as a scaffold for Axin recruitment in the β-catenin-dependent signaling pathway (41), which prompted us to examine the possibility that signaling molecules involved in the β-catenin-independent signaling pathway are also recruited to Dvl2 polymers following Wnt5a stimulation. To this end, Ror2/L cells expressing YFP-Dvl2 were stained with antibodies against the Rho family of GTPases, RhoA, Rac1 and Cdc42, or JNK. Among them, only Rac1 was detected as a punctate pattern and, intriguingly, >70% of polymerized Dvl2 were colocalized with these stained puncta after Wnt5a stimulation (Fig. 5A and data not shown). However, we also detected many Rac1 puncta that did not colocalize with polymerized Dvl2. These Rac1 puncta may correspond to membrane structures, such as ruffling, lamellipodia, or endocytic or macropinocytic vesicles, where Rac is known to be localized (33, 38). Essentially identical results were obtained using two different monoclonal antibodies against Rac1 (data not shown). The colocalization of Rac1 and Dvl2 puncta was further supported by the observation that Wnt5a stimulation induced the binding between endogenous Rac1 and wild-type YFP-Dvl2, but not its M2 or KM mutant, in Ror2/L cells (Fig. 5B). Furthermore, suppressed expression of Rac1 markedly inhibited Wnt5a-induced AP-1 activation in Ror2/L cells (Fig. 5C). These results indicate that polymerized Dvl2 functions as a scaffold for Rac1 recruitment during Wnt5a signaling to mediate AP-1 activation.
FIG. 5.
Rac1 is colocalized with polymerized Dvl2 and required for Wnt5a-induced AP-1 activation. (A) Rac1 is colocalized with polymerized Dvl2. Ror2/L cells expressing YFP-Dvl2 were treated with Wnt5a or its vehicle (control) for 15 min. Cells were fixed and stained with anti-Rac1 antibody (Millipore). Magnified images of boxed regions are shown (magnified). Bar, 10 μm. (B) Wnt5a stimulation induces the association of Rac1 with wild-type Dvl2 (WT) but not its M2 and KM mutants. Ror2/L cells were transfected with expression plasmids for YFP-Dvl2 (WT, M2, or KM) as indicated and treated with Wnt5a for 15 min. WCL or anti-GFP immunoprecipitates were analyzed by immunoblotting with anti-Rac1 or anti-GFP antibodies to detect the proteins indicated. (C) Rac1 is required for Wnt5a-induced AP-1 activation. Ror2/L cells were treated with either control or Rac1 siRNA (#1 or #2) for 36 h and then transfected with the AP-1 reporter plasmids. After serum starvation for 12 h, WCL were analyzed by immunoblotting with anti-Rac1 and anti-α-tubulin antibodies (top). Cells were treated with either Wnt5a or its vehicle for 24 h and subjected to luciferase assays (bottom).
We demonstrate here that Wnt5a induces dynamic polymerization of Dvl2 via a receptor complex at least containing Ror2 and Fz7, leading to the activation of AP-1. The formation of the receptor complex and the regulation of Dvl2 require the CRD but not the cytoplasmic region of Ror2. This shows for the first time the ectodomain-dependent function of Ror2, irrespective of its cytoplasmic region, in Wnt5a signaling. Thus, Ror2 seems to mediate Wnt5a signaling through at least three distinct mechanisms, i.e., (i) the cytoplasmic region-independent mechanism as shown here, (ii) the cytoplasmic region-dependent, kinase activity-independent mechanism (28), and (iii) the kinase activity-dependent mechanism (1, 8, 19, 24). How do these distinct mechanisms work under specific physiological conditions? We have previously shown that the cytoplasmic region of Ror2, required for the association with filamin A (FLNa), but not its intrinsic kinase activity, is indispensable for Wnt5a-induced migration of L cells (28). In addition, Dvl is indispensable for Wnt5a/Ror2-induced L-cell migration (28). Therefore, the cytoplasmic region-dependent and -independent mechanisms may work together by regulating FLNa and Dvl, respectively, to mediate Wnt5a-induced cell migration. In contrast to L-cell migration, ECM degradation by osteosarcoma cells requires the intrinsic kinase activity of Ror2 (8). Since cell invasion requires both ECM degradation and cell migration, it is possible that the kinase activity-dependent and -independent mechanisms also work together by regulating ECM degradation and cell migration, respectively, thereby inducing cancer cell invasion.
By using the Dvl2 mutants with amino acid substitutions within the DIX or DEP domain, we demonstrated that the intrinsic polymerizing activity of Dvl2 is required for Wnt5a-induced AP-1 activation and that the Dvl2 mutant, defective in activating the Wnt/PCP pathway, fails to form polymers after Wnt5a stimulation. We further found that polymerized Dvl2 is colocalized with Rac and that suppressed expression of Rac1 inhibits Wnt5a-induced AP-1 activation. Collectively, our results indicate that Ror2/Fz7 receptor complex plays an important role in Wnt5a/Rac1/AP-1 pathway by regulating polymerization of Dvl2.
At present, it is unclear how the dynamics of the Dvl2 polymerization is regulated. It was reported that casein kinase I, one of candidate protein kinases responsible for Wnt5a-induced Dvl phosphorylation, negatively regulates puncta formation of Dvl2 in SN4741 dopaminergic cells (5). It has been also reported that phosphorylation of Dvl trigger negative-feedback regulation of Wnt5a/Ror2 signaling through association with Ror2 (48). These reports suggest that Wnt5a-induced phosphorylation of Dvl might regulate its polymerization negatively and thereby downregulate the Wnt5a/Ror2 signaling. Further study will be required to clarify the role of the phosphorylation of Dvl2 in its polymerizing process.
Supplementary Material
Acknowledgments
We thank Randall T. Moon (University of Washington School of Medicine, Seattle) for the SuperTopFlash.
This study was supported by Grants-in-Aid for Scientific Research, for Scientific Research on Priority Areas, for Scientific Research (B), for Young Scientists, and for the Global Center of Excellence Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by a grant from the Takeda Science Foundation.
Footnotes
Published ahead of print on 10 May 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Akbarzadeh, S., L. M. Wheldon, S. M. Sweet, S. Talma, F. K. Mardakheh, and J. K. Heath. 2008. The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PLoS One 3:e1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angers, S., and R. T. Moon. 2009. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell. Biol. 10:468-477. [DOI] [PubMed] [Google Scholar]
- 3.Axelrod, J. D., J. R. Miller, J. M. Shulman, R. T. Moon, and N. Perrimon. 1998. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 12:2610-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutros, M., N. Paricio, D. I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109-118. [DOI] [PubMed] [Google Scholar]
- 5.Bryja, V., G. Schulte, N. Rawal, A. Grahn, and E. Arenas. 2007. Wnt-5a induces Dishevelled phosphorylation and dopaminergic differentiation via a CK1-dependent mechanism. J. Cell Sci. 120:586-595. [DOI] [PubMed] [Google Scholar]
- 6.Cheyette, B. N., J. S. Waxman, J. R. Miller, K. Takemaru, L. C. Sheldahl, N. Khlebtsova, E. P. Fox, T. Earnest, and R. T. Moon. 2002. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev. Cell 2:449-461. [DOI] [PubMed] [Google Scholar]
- 7.Djiane, A., J. Riou, M. Umbhauer, J. Boucaut, and D. Shi. 2000. Role of Frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development 127:3091-3100. [DOI] [PubMed] [Google Scholar]
- 8.Enomoto, M., S. Hayakawa, S. Itsukushima, D. Y. Ren, M. Matsuo, K. Tamada, C. Oneyama, M. Okada, T. Takumi, M. Nishita, and Y. Minami. 2009. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene 28:3197-3208. [DOI] [PubMed] [Google Scholar]
- 9.Forrester, W. C., M. Dell, E. Perens, and G. Garriga. 1999. A Caenorhabditis elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature 400:881-885. [DOI] [PubMed] [Google Scholar]
- 10.Francis, M. M., S. P. Evans, M. Jensen, D. M. Madsen, J. Mancuso, K. R. Norman, and A. V. Maricq. 2005. The Ror receptor tyrosine kinase CAM-1 is required for ACR-16-mediated synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Neuron 46:581-594. [DOI] [PubMed] [Google Scholar]
- 11.Green, J. L., T. Inoue, and P. W. Sternberg. 2007. The Caenorhabditis elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development 134:4053-4062. [DOI] [PubMed] [Google Scholar]
- 12.Green, J. L., T. Inoue, and P. W. Sternberg. 2008. Opposing Wnt pathways orient cell polarity during organogenesis. Cell 134:646-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, Y., T. Hirotsu, R. Iwata, E. Kage-Nakadai, H. Kunitomo, T. Ishihara, Y. Iino, and T. Kubo. 2009. A trophic role for Wnt-Ror kinase signaling during developmental pruning in Caenorhabditis elegans. Nat. Neurosci. 12:981-987. [DOI] [PubMed] [Google Scholar]
- 14.He, F., W. Xiong, X. Yu, R. Espinoza-Lewis, C. Liu, S. Gu, M. Nishita, K. Suzuki, G. Yamada, Y. Minami, and Y. Chen. 2008. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development 135:3871-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kani, S., I. Oishi, H. Yamamoto, A. Yoda, H. Suzuki, A. Nomachi, K. Iozumi, M. Nishita, A. Kikuchi, T. Takumi, and Y. Minami. 2004. The receptor tyrosine kinase Ror2 associates with and is activated by casein kinase Iɛ. J. Biol. Chem. 279:50102-50109. [DOI] [PubMed] [Google Scholar]
- 16.Kaykas, A., J. Yang-Snyder, M. Heroux, K. V. Shah, M. Bouvier, and R. T. Moon. 2004. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat. Cell Biol. 6:52-58. [DOI] [PubMed] [Google Scholar]
- 17.Kim, C., and W. C. Forrester. 2003. Functional analysis of the domains of the Caenorhabditis elegans Ror receptor tyrosine kinase CAM-1. Dev. Biol. 264:376-390. [DOI] [PubMed] [Google Scholar]
- 18.Li, C., H. Chen, L. Hu, Y. Xing, T. Sasaki, M. F. Villosis, J. Li, M. Nishita, Y. Minami, and P. Minoo. 2008. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol. Biol. 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, Y., J. F. Ross, P. V. Bodine, and J. Billiard. 2007. Homodimerization of Ror2 tyrosine kinase receptor induces 14-3-3β phosphorylation and promotes osteoblast differentiation and bone formation. Mol. Endocrinol. 21:3050-3061. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald, B. T., K. Tamai, and X. He. 2009. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17:9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda, T., M. Nomi, M. Ikeya, S. Kani, I. Oishi, T. Terashima, S. Takada, and Y. Minami. 2001. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech. Dev. 105:153-156. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda, T., H. Suzuki, I. Oishi, S. Kani, Y. Kuroda, T. Komori, A. Sasaki, K. Watanabe, and Y. Minami. 2003. The receptor tyrosine kinase Ror2 associates with the melanoma-associated antigen (MAGE) family protein Dlxin-1 and regulates its intracellular distribution. J. Biol. Chem. 278:29057-29064. [DOI] [PubMed] [Google Scholar]
- 23.Medina, A., W. Reintsch, and H. Steinbeisser. 2000. Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech. Dev. 92:227-237. [DOI] [PubMed] [Google Scholar]
- 24.Mikels, A., Y. Minami, and R. Nusse. 2009. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J. Biol. Chem. 284:30167-30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikels, A. J., and R. Nusse. 2006. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minami, Y., I. Oishi, M. Endo, and M. Nishita. 2010. Ror-family receptor tyrosine kinases in noncanonical Wnt signaling: their implications in developmental morphogenesis and human diseases. Dev. Dyn. 239:1-15. [DOI] [PubMed] [Google Scholar]
- 27.Na, J., K. Lykke-Andersen, M. E. Torres Padilla, and M. Zernicka-Goetz. 2007. Dishevelled proteins regulate cell adhesion in mouse blastocyst and serve to monitor changes in Wnt signaling. Dev. Biol. 302:40-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishita, M., S. K. Yoo, A. Nomachi, S. Kani, N. Sougawa, Y. Ohta, S. Takada, A. Kikuchi, and Y. Minami. 2006. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J. Cell Biol. 175:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nomachi, A., M. Nishita, D. Inaba, M. Enomoto, M. Hamasaki, and Y. Minami. 2008. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J. Biol. Chem. 283:27973-27981. [DOI] [PubMed] [Google Scholar]
- 30.O'Connell, M. P., J. L. Fiori, M. Xu, A. D. Carter, B. P. Frank, T. C. Camilli, A. D. French, S. K. Dissanayake, F. E. Indig, M. Bernier, D. D. Taub, S. M. Hewitt, and A. T. Weeraratna. 2009. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene, in press. [DOI] [PMC free article] [PubMed]
- 31.Oishi, I., H. Suzuki, N. Onishi, R. Takada, S. Kani, B. Ohkawara, I. Koshida, K. Suzuki, G. Yamada, G. C. Schwabe, S. Mundlos, H. Shibuya, S. Takada, and Y. Minami. 2003. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signaling pathway. Genes Cells 8:645-654. [DOI] [PubMed] [Google Scholar]
- 32.Oishi, I., S. Takeuchi, R. Hashimoto, A. Nagabukuro, T. Ueda, Z. J. Liu, T. Hatta, S. Akira, Y. Matsuda, H. Yamamura, H. Otani, and Y. Minami. 1999. Spatio-temporally regulated expression of receptor tyrosine kinases, mRor1, mRor2, during mouse development: implications in development and function of the nervous system. Genes Cells 4:41-56. [DOI] [PubMed] [Google Scholar]
- 33.Palamidessi, A., E. Frittoli, M. Garre, M. Faretta, M. Mione, I. Testa, A. Diaspro, L. Lanzetti, G. Scita, and P. P. Di Fiore. 2008. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 134:135-147. [DOI] [PubMed] [Google Scholar]
- 34.Perrimon, N., and A. P. Mahowald. 1987. Multiple functions of segment polarity genes in Drosophila. Dev. Biol. 119:587-600. [DOI] [PubMed] [Google Scholar]
- 35.Pukrop, T., F. Klemm, T. Hagemann, D. Gradl, M. Schulz, S. Siemes, L. Trumper, and C. Binder. 2006. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc. Natl. Acad. Sci. U. S. A. 103:5454-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato, A., H. Yamamoto, H. Sakane, H. Koyama, and A. Kikuchi. 2010. Wnt5a regulates distinct signaling pathways by binding to Frizzled2. EMBO J. 29:41-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schambony, A., and D. Wedlich. 2007. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev. Cell 12:779-792. [DOI] [PubMed] [Google Scholar]
- 38.Schlunck, G., H. Damke, W. B. Kiosses, N. Rusk, M. H. Symons, C. M. Waterman-Storer, S. L. Schmid, and M. A. Schwartz. 2004. Modulation of Rac localization and function by dynamin. Mol. Biol. Cell 15:256-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarz-Romond, T., M. Fiedler, N. Shibata, P. J. Butler, A. Kikuchi, Y. Higuchi, and M. Bienz. 2007. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat. Struct. Mol. Biol. 14:484-492. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz-Romond, T., C. Merrifield, B. J. Nichols, and M. Bienz. 2005. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J. Cell Sci. 118:5269-5277. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz-Romond, T., C. Metcalfe, and M. Bienz. 2007. Dynamic recruitment of axin by Dishevelled protein assemblies. J. Cell Sci. 120:2402-2412. [DOI] [PubMed] [Google Scholar]
- 42.Smalley, M. J., N. Signoret, D. Robertson, A. Tilley, A. Hann, K. Ewan, Y. Ding, H. Paterson, and T. C. Dale. 2005. Dishevelled (Dvl-2) activates canonical Wnt signaling in the absence of cytoplasmic puncta. J. Cell Sci. 118:5279-5289. [DOI] [PubMed] [Google Scholar]
- 43.Takada, R., H. Hijikata, H. Kondoh, and S. Takada. 2005. Analysis of combinatorial effects of Wnts and Frizzleds on beta-catenin/armadillo stabilization and Dishevelled phosphorylation. Genes Cells 10:919-928. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi, S., K. Takeda, I. Oishi, M. Nomi, M. Ikeya, K. Itoh, S. Tamura, T. Ueda, T. Hatta, H. Otani, T. Terashima, S. Takada, H. Yamamura, S. Akira, and Y. Minami. 2000. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells 5:71-78. [DOI] [PubMed] [Google Scholar]
- 45.Taraska, J. W., and W. Almers. 2004. Bilayers merge even when exocytosis is transient. Proc. Natl. Acad. Sci. U. S. A. 101:8780-8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Amerongen, R., and R. Nusse. 2009. Towards an integrated view of Wnt signaling in development. Development 136:3205-3214. [DOI] [PubMed] [Google Scholar]
- 47.Wiederkehr, A., S. Avaro, C. Prescianotto-Baschong, R. Haguenauer-Tsapis, and H. Riezman. 2000. The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J. Cell Biol. 149:397-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witte, F., O. Bernatik, K. Kirchner, J. Masek, A. Mahl, P. Krejci, S. Mundlos, A. Schambony, V. Bryja, and S. Stricker. 2010. Negative regulation of Wnt signaling mediated by CK1-phosphorylated Dishevelled via Ror2. FASEB J., in press. [DOI] [PubMed]
- 49.Yamamoto, S., O. Nishimura, K. Misaki, M. Nishita, Y. Minami, S. Yonemura, H. Tarui, and H. Sasaki. 2008. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev. Cell 15:23-36. [DOI] [PubMed] [Google Scholar]
- 50.Yoon, Y., and M. A. McNiven. 2001. Mitochondrial division: new partners in membrane pinching. Curr. Biol. 11:R67-R70. [DOI] [PubMed] [Google Scholar]
- 51.Yu, A., J. F. Rual, K. Tamai, Y. Harada, M. Vidal, X. He, and T. Kirchhausen. 2007. Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev. Cell 12:129-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.