Abstract
One essential downstream signaling pathway of receptor tyrosine kinases (RTKs), such as vascular endothelial growth factor receptor (VEGFR) and the Tie2 receptor, is the phosphoinositide-3 kinase (PI3K)-phosphoinositide-dependent protein kinase 1 (PDK1)-Akt/protein kinase B (PKB) cascade that plays a critical role in development and tumorigenesis. However, the role of PDK1 in cardiovascular development remains unknown. Here, we deleted PDK1 specifically in endothelial cells in mice. These mice displayed hemorrhage and hydropericardium and died at approximately embryonic day 11.5 (E11.5). Histological analysis revealed defective vascular remodeling and development and disrupted integrity between the endothelium and trabeculae/myocardium in the heart. The atrioventricular canal (AVC) cushion and valves failed to form, indicating a defect in epithelial-mesenchymal transition (EMT), together with increased endothelial apoptosis. Consistently, ex vivo AVC explant culture showed impeded mesenchymal outgrowth. Snail protein was reduced and was absent from the nucleus in AVC cells. Delivery of the Snail S6A mutant to the AVC explant effectively rescued EMT defects. Furthermore, adenoviral Akt delivery rescued EMT defects in AVC explant culture, and deletion of PTEN delayed embryonic lethality of PDK1 endothelial deletion mice by 1 day and rendered normal development of the AVC cushion in the PDK1-deficient heart. Taken together, these results have revealed an essential role of PDK1 in cardiovascular development through activation of Akt and Snail.
Polypeptide growth factors, such as insulin, insulin-like growth factor 1 (IGF-I), vascular endothelial growth factor (VEGF), and angiopoietin 1 (Ang1), exert biological functions through binding to their transmembrane receptors that belong to a large family of receptor tyrosine kinases (RTKs) (4). Consequently, the receptor molecules form homo- or heterodimers, and the intracellular tyrosines at the carboxyl termini of the receptors become phosphorylated (37). Numerous distinct adaptor/regulatory proteins, through their Src homologous 2 (SH2) domains, bind to the phosphotyrosines and transduce the signal to downstream pathways, among which are two essential and well-characterized signaling cascades—the mitogen-activated protein kinase (MAPK) and phosphoinositide-3 kinase (PI3K)-phosphoinositide-dependent protein kinase 1 (PDK1)-Akt signaling pathways (4, 13, 37).
The regulatory subunit of PI3K, p85, possesses the SH2 domain and can, therefore, bind to phosphotyrosines on the RTKs and subsequently render activation of the catalytic subunit of PI3K, p110 (7, 8). Active p110 phosphorylates phosphoinositide biphosphate (PIP2), turning it into PIP3 that recruits PDK1 and Akt to the cellular membrane, where Akt is phosphorylated at threonine 308 (T308 for Akt1) by PDK (5, 23, 30). The serine 473 (S473) of Akt (Akt1) is phosphorylated by mTOR complex 2 (mTORC2) and other kinases (17, 36). Phosphorylation of Akt at these two amino acids brings it to full activation. In PDK1-deficient embryonic stem (ES) cells, T308 phosphorylation was abolished and most of the Akt activity was lost, although the S473 phosphorylation was intact (40).
Akt plays an important role in multiple biological processes, such as cell survival, growth, glucose metabolism, and angiogenesis (2, 12, 14-16, 22, 23, 39, 41-43). In mammals, there are three Akt isoforms, termed Akt 1, -2, and -3. Previously, we generated Akt1- and Akt3-deficient mice and studied their roles in mouse development (2, 15, 39, 42, 43). We found that the Akt1 and -3 double knockout (KO) (DKO) mice were embryonically lethal at around embryonic day 12 (E12) and manifested developmental defects in multiple tissues, including the cardiovascular system (14, 15, 43). These studies suggest that the Akt signaling pathway is involved in cardiovascular development.
Other than Akt isoforms, PDK1 also activates another group of AGC family kinases, such as p70 ribosomal S6 kinase (S6K) (32), serum, and glucocorticoid-induced protein kinase (SGK) (26), p90 ribosomal S6 kinase (RSK) (21), and atypical isoforms of protein kinase C (PKC) (31). Comprehensive and intensive mouse genetic studies performed mainly by Alessi and coworkers have confirmed the regulation of these AGC kinases by PDK1 (3, 9, 10, 27-29, 40).
PDK1 knockout mice were severely growth retarded and died at around E9.0, indicating an essential role of PDK1 in development (27). However, its function and downstream targets in cardiovascular development are still elusive. To study this, we deleted PDK1 specifically in endothelial cells through Cre recombinase-mediated excision (25). The results have revealed an essential role of PDK1 in vascular remodeling and integrity and in cardiac development through activation of Akt and its downstream target of Snail.
MATERIALS AND METHODS
Mice.
Mice were housed in accordance with the regulations on mouse welfare and ethics of Nanjing University in groups with 12-h dark-light cycles and free access to food and water. All procedures were conducted with the approval of the relevant authority. PDK1-floxed mice were provided by Dario Alessi (University of Dundee), and PTEN-floxed mice were as previously described (29, 38). These mice were maintained in B6 genetic background. Primers for genotyping were as follows: PDK1-p99, 5′ ATC CCA AGT TAC TGA GTT GTG TTG GAA G 3′, and PDK1-p100, 5′ TGT GGA CAA ACA GCA ATG AAC ATA CAC GC 3′.
Antibodies.
Antibodies for immunofluorescence staining and for Western blotting are as follows: anti-smooth muscle antigen (anti-SMA) (Thermo MS-114-P0), platelet endothelial cell adhesion molecule 1 (PECAM-1)/CD31 (BD Pharmingen 550274), endomucin (eBioscience 14-5851), total Akt (CST 9272), pAKT Thr308 (EPITOMICS 2214-1) and Ser473 (CST 9271), PDK1 (Epitomics 1624-1), S6K (CST 9202), pS6K Ser240/244 (CST 2215), pS6K Thr389 (CST 9205), pS6K Ser235/236 (CST 2211), PRAS40 (CST 2610), pPRAS40 Thr246 (CST 2640), pPKC Thr514 (for pS6K Thr229, CST 9379), pan-actin (Thermo MS-1295-P0), Snail (MN Bioworld BS1853), MF20 (DSHB), fluorescein (fluorescein isothiocyanate [FITC])-conjugated Affinipure goat anti-mouse IgG (Jackson ImmunoResearch 113-095-166), Cy3-conjugated AffiniPure goat anti-rat IgG (Jackson ImmunoResearch 112-165-167), Alexa Fluor 488-conjugated goat anti-rabbit IgG (Molecular Probes), and horseradish peroxidase (HRP)-linked secondary antibodies (Thermos Scientific product no. 31460 and product no. 31430).
Western blotting.
Yolk sacs and embryos were collected and snap-frozen in liquid nitrogen until use. Tissue lysates were prepared in lysis buffer (20 mM Tris, 150 mM NaCl, 10% glycerol, 20 mM glycerophosphate, 1% NP-40, 5 mM EDTA, 0.5 mM EGTA, 1 mM Na3VO4, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM benzamidine, 1 mM dithiothreitol [DTT], 50 mM NaF, 4 μM leupeptin, at pH 8.0). Samples were resolved by 10% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). Membranes were blocked with TBST (50 mM Tris, 150 mM NaCl, 0.5 mM Tween 20, pH 7.5) and incubated with primary antibodies overnight. The membranes were then incubated with a secondary anti-rabbit or anti-mouse HRP-conjugated antibody, and the signals were detected with enhanced chemiluminescence (ECL). Membranes were blotted for pan-actin for equal loading control after membrane stripping.
Histology and immunofluorescence staining.
The hematoxylin-eosin (HE) and immunofluorescence (IF) protocols were as described previously (42). Briefly, E9.5 to -11 embryos or yolk sacs were first washed with cold phosphate-buffered saline (PBS) and then fixed in 4% paraformaldehyde (PFA) 1 or 2 h at 4°C. The samples were processed successively by (i) a 30-min wash in PBS at 4°C; (ii) a 15-min incubation each in 30%, 50%, 75%, and 85% ethanol and then 2 × 10-min incubation in 95% and 100% ethanol at room temperature (RT); (iii) 3 × 10-min incubation in xylene at RT; (iv) 20-min incubation in paraffin/xylene (1:1) at 65°C; and (v) 3 × 30-min incubation in fresh paraffin at 65°C. The processed samples were then embedded in paraffin and sectioned (6 μm thick), and the sections were stained with HE following standard protocol. For whole-mount IF staining, embryos and yolk sacs were fixed as described above in 4% PFA at 4°C for 1 h and then rinsed twice with PBS. The samples were blocked overnight at 4°C in 3% goat serum (Boster; AR0009). IF staining was conducted using anti-PECAM (CD31) antibody or anti-SMA antibody at 4°C overnight. The samples were washed three times with PBS and incubated with Cy3-conjugated AffiniPure goat anti-rat IgG or FITC-conjugated AffiniPure goat anti-mouse IgG. Fluorescence microscopy images were obtained with a research fluorescence microscope (Olympus) equipped with a digital camera. Images were collected and recorded using Adobe Photoshop 5.0 on an IBM R52 computer. For atrioventricular canal (AVC) ex vivo explant culture IF staining, the samples were fixed with 4% PFA, rinsed with PBS, permeabilized with 0.5% Triton X-100, pH 7.0, and blocked overnight at 4°C in 3% bovine serum albumin (BSA)/0.5% Tween 20 in PBS. The samples were incubated with anti-SMA antibody (dilution 1:500) for 2 h at 4°C and washed with 0.2% BSA/0.5% Tween 20 in PBS. The slides were then incubated with FITC-conjugated AffiniPure goat anti-mouse IgG (1:500). To-Pro-3 (642/661 Molecular Probes T3605) was applied to the samples to display the nucleus. Images were obtained and recorded as described above. Confocal microscope images were obtained using an inverted microscope (IX10; 1X40 Olympus) equipped with a scanning laser system (Fluo-View; Olympus). En face and Z-plane sections were obtained using FluoView software (Olympus).
AVC ex vivo explant culture.
Rat tail collagen type I (Sigma) was prepared according to the manufacturer's instructions. The solution was dispensed in 24-well dishes and allowed to solidify inside a 37°C, 5% CO2 incubator. Subsequently, collagen gels were washed several times with media containing M199, 5% fetal bovine serum (FBS), and selenium (ITS) plus antibiotics and drained overnight. AVC tissue was dissected in sterile PBS plus antibiotics from E9.5 embryos. AVC explants were placed on drained collagen gels, with the endocardium facing down and allowed to attach for 12 h at 37°C, 5% CO2. Media (0.5 ml/well) were added, and the cultures were reincubated for up to 3 days.
TUNEL assay.
The terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was performed as previously reported (43). Briefly, the sections were treated with proteinase K (20 μg/ml) and incubated with terminal deoxynucleotidyl transferase (TdT) and biotinylated dUTP.
Statistics.
The t test was used for statistical analysis.
RESULTS
Generation of endothelium-specific PDK1 deletion mice.
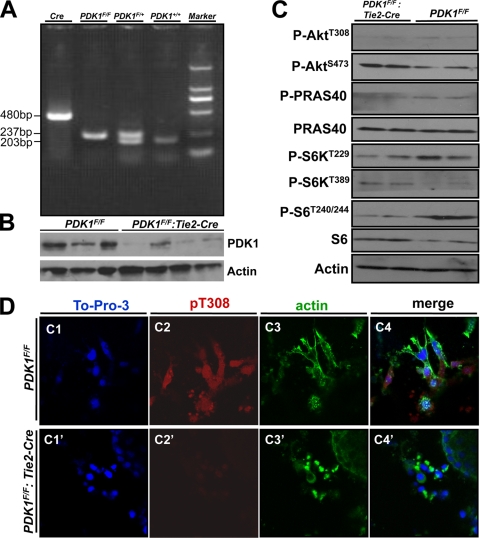
PDK1-floxed mice were as previously reported and were maintained in a C57/B6 genetic background (29). To delete PDK1 specifically in endothelial cells, female PDK1-floxed mice (PDK1F/F) were crossed with male Tie-2 Cre mice to obtain female PDK1F/+ mice and male PDK1F/+:Tie-2 Cre mice (25). Mating between the mice of these two genotypes gives rise to PDK1F/F:Tie-2 Cre mice that have the PDK1 deletion in endothelial cells. Mice were genotyped by PCR, and PDK1 loss was confirmed by Western blotting (Fig. 1 A and B). The result showed that PDK1 levels were dramatically decreased in endothelial PDK1 deletion mice (Fig. 1B). We also examined the phosphorylation levels of PDK1 downstream targets, such as Akt T308 and S473 and S6K T229 and T389, as well as Akt substrate PRAS40 and S6K substrate S6 in embryos of the control and of endothelial PDK1 deletion. The results showed that the phosphorylation levels of Akt T308 and S6K T229 (PDK1 phosphorylation sites) were significantly reduced in embryos of PDK1 deletion compared to the control (Fig. 1C). Although Akt T473 and S6K T389 phosphorylation levels were higher in PDK1-deficient embryos than those in control, the activity of Akt and S6K became lower because the phosphorylation levels of their substrates, PRAS40 and S6K, were reduced in PDK1-deficient embryos compared to the control (Fig. 1C). Furthermore, we performed immunofluorescence staining to show that the T308 phosphorylation of Akt, a residue phosphorylated by PDK1, was nearly absent in the PDK1 deletion endothelial cells (Fig. 1D). Collectively, these results indicate that PDK1 has been deleted correctly in endothelial cells, which in turn reduced total PDK1 activity in the whole embryo.
FIG. 1.
Endothelium-specific deletion of PDK1 in mice. (A) Genotyping of mice by PCR. (B) Western blotting of PDK1 in yolk sac. Note that in PDK1F/F:Tie2 Cre mice, the levels of PDK1 are dramatically decreased (as the deletion of PDK1 is only in endothelial cells; all the other cells in yolk sac still have PDK1). (C) Western blotting of E10.5 embryos. (D, panels C1 to C4 and C1′ to C4′) Immunofluorescence staining to display the absence of T308 phosphorylation (pT308) in PDK1 deletion endothelial cells. The tissues are atrioventricular canal (AVC) specimens cultured on slides. Magnification, ×40.
Cardiovascular dysfunction and embryonic lethality of endothelial PDK1 deletion mice.
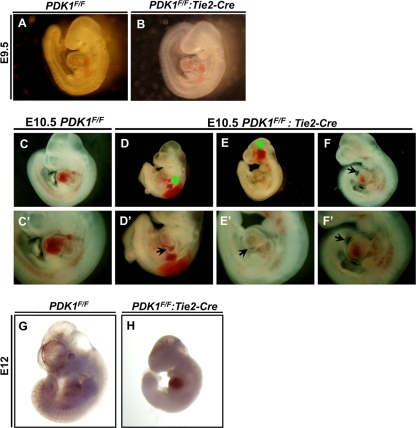
At E9.5, endothelial PDK1 deletion mice were nearly as normal as the controls (Fig. 2 A and B). At E10.5, the endothelial PDK1 deletion mice were growth retarded and displayed hemorrhage and hydropericardium as well as hearts with abnormal morphology (Fig. 2C to F and C′ to F′). The development of these mice stopped, and the mice died at around E11.5 (Fig. 2G and H). These phenotypes indicate that endothelial deletion of PDK1 affects cardiovascular integrity and function, resulting in embryonic mortality.
FIG. 2.
Microscopic analysis of embryos between E9.5 and E12. (A and B) At E9.5, the PDK1 deletion embryo was comparable to the control. (C to F and C′ to F′) E10.5 embryos. Panels C′ to F′ are higher magnifications of panels C to F. Panels C and C′ are control. PDK1 deletion mice were growth retarded (D to F and D′ to F′) and showed hemorrhage (indicated by green arrows in panels D and E), abnormal epicardium (indicated by arrow in panel D′) and heart morphology (indicated by arrow in panel E′), and hydropericardium (indicated by arrows in panels F and F′). (G and H) PDK1 deletion embryo stopped development and was pale at approximately E12 (H).
Abnormal vascular remodeling in endothelial PDK1 deletion mice.
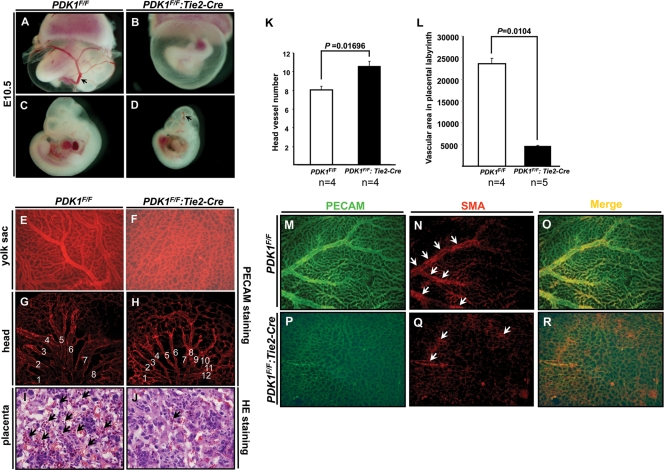
At E10.5, big vessels were absent in the yolk sacs of endothelial PDK1 deletion mice (Fig. 3 A and B). The PDK1 deletion embryo also displayed head hemorrhage (Fig. 3D). Further analysis of the yolk sac by whole-mount PECAM staining showed that the primary capillary plexus was present in PDK1 deletion mice, but these capillary plexuses failed to fuse into big vessels, indicating a vascular remodeling defect (Fig. 3E and F). Consistently, a similar remodeling abnormality was found in the head of the PDK1 deletion embryo (Fig. 3G and 3H). Compared to the control, the PDK1 deletion embryo showed more but thinner vessel branches (Fig. 3G, H, and K).
FIG. 3.
Vascular analysis. (A to D) Microscopic analysis of E10.5 yolk sac and embryos. (B) Big vessels were missing in PDK1 deletion yolk sac. PDK1 deletion embryo showed small size and head hemorrhage (indicated by arrow in panel D). (E to H) Whole-mount PECAM staining of yolk sacs and embryos shown in panels A to D. (F) PDK1 deletion yolk sac lacked big vessels, a sign of remodeling defects. (H) Consistently, PDK1 deletion embryo showed vascular remodeling abnormality in head. The thinner vessels failed to fuse into bigger vessels. Numbers indicate big vascular branches. (I and J) HE staining to display vasculature in placental labyrinth at E10.5. Arrows indicate the capillary vessels/villi. While these capillary vessels were abundant in control, they were hardly seen in PDK1 deletion placenta. (K and L) Quantification of head vessels shown in panels G and H and villus areas shown in panels I and J. (M to R) Analysis of yolk sac shown in panels E and F by immunofluorescence staining. Arrows indicate SMA-positive cells (smooth muscle cells). (N and Q) These cells were fewer and scattered in PDK1 deletion yolk sac.
We also found defective vasculature in the placental labyrinth of PDK1 deletion mice (Fig. 3I and J). While the capillary vessels/villi could be discerned clearly in the control, they were hardly observed with the endothelial PDK1 deletion placenta (Fig. 3I, J, and L).
Smooth muscle cells are involved in vascular remodeling (1, 11). We therefore analyzed these cells in wild-type and PDK1 deletion mice by immunofluorescence staining. In the wild-type control, the smooth muscle cells (SMA positive) were present mainly along the big vessels, indicating normal smooth muscle investment (Fig. 3M to O). In contrast, the SMA-positive cells were fewer and scattered in PDK1 deletion mice (Fig. 3P to R).
Taken together, these results indicate that the vasculature in both the embryonic and extraembryonic tissues of PDK1 deletion mice has developmental and remodeling abnormalities.
Loss of endocardiac integrity and impaired myocardial development in endothelial PDK1 deletion heart.
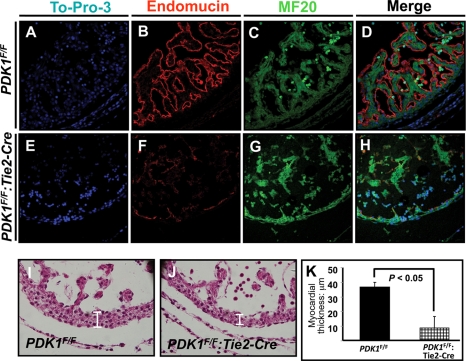
As displayed in Fig. 2, endothelial PDK1 deletion mice showed hydropericardium, a sign of cardiac dysfunction. We then investigated the cardiac structure by endomucin and MF20 staining to display endothelium and myocardium/trabeculae. As shown in Fig. 4 A to D, the trabeculae were well connected to myocardium of the ventricular wall and the endothelial cells were tidily lining the trabeculae and myocardium in the control. In contrast, the structural integrity between the endothelium and trabeculae/myocardium was disrupted in the PDK1 deletion heart (Fig. 4E to H). Both the endothelium and the trabeculae became sparse and disordered, and the trabeculae lost connection with the myocardium of the ventricular wall (Fig. 4E to H). In addition, the myocardium of PDK1 deletion mice became thinner than that of the control (Fig. 4I to K). As PDK1 was deleted only in endothelial cells, the reduced thickness of the myocardium could be a secondary effect.
FIG. 4.
Histological study of E10.5 heart. (A to H) To-Pro-3-stained nucleus, endomucin-stained endothelium, and MF20-stained trabeculae and myocardium in ventricular wall. In the control (A to D), trabeculae were connected to ventricular wall myocardium and both were lined by endothelial cells. (E to H) The integrity between endothelium and trabeculae/myocardium was disrupted in PDK1 deletion heart. The trabeculae also became irregular. (I to K) Comparison of myocardial thicknesses. The myocardium in PDK1 deletion heart was thinner than that of control.
Abnormal development of atrioventricular canal and valve in endothelial PDK1 deletion mice.
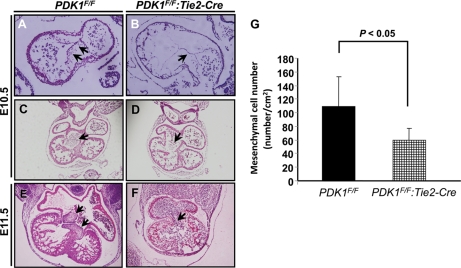
At E10.5, mesenchymal cells were found accumulated along the AVC and the cushion developed normally in the control heart (Fig. 5 A and C). However, the mesenchymal cells were dramatically decreased, and the cushion was undeveloped in the PDK1 deletion heart (Fig. 5B, D, and G). At E11.5, the valves were established in the control, while they could hardly be discerned in the PDK1 deletion mice (Fig. 5E and F). These results indicated that the developmental process of epithelial-mesenchymal transition (EMT) was impaired in the PDK1 deletion AVC.
FIG. 5.
Histological analysis of atrioventricular canal (AVC), cushion, and valve. (A and B) HE staining. AVC mesenchymal cells are indicated by arrows. PDK1 deletion AVC had fewer mesenchymal cells. (C and D) AV cushion. While in the control the AV cushion was well formed (indicated by arrow in panel C), it was hard to discern in the PDK1 deletion heart (indicated by arrow in panel D). (E and F) The valves were present in control (indicated by arrows in panel E), while they were undeveloped in PDK1 deletion heart (indicated by arrow in panel F). Panel G shows quantification of mesenchymal cells in AVC at E10.5.
Deficit of EMT in AVC explant culture from PDK1 deletion heart.
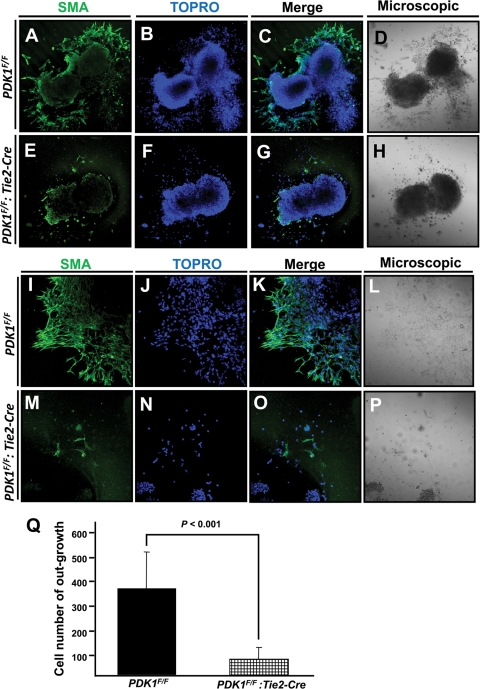
To further investigate the EMT process during AVC development, we set up an ex vivo explant AVC culture system (6). In the control, cushion mesenchymal cells migrated normally outside the AVC, while few such cells could be seen in the PDK1 deletion AVC (Fig. 6 A to P). We also counted the numbers of anti-smooth muscle antigen (anti-SMA)-positive mesenchymal cells of outgrowth in each AVC sample and found a substantial reduction in PDK1 deletion samples compared to the control (Fig. 6Q). These results confirmed the defective EMT and valve development in the PDK1 deletion heart and demonstrated that PDK1 is essential for AVC EMT and valve development.
FIG. 6.
Immunofluorescence staining of ex vivo AVC explant culture, with SMA-stained mesenchymal cells and To-Pro-3-stained nucleus. (A to H) Low-magnification (×10) pictures to display AVC explant and staining. (A to D) In control, mesenchymal cells from EMT migrated outside the AVC. (E to H) In contrast, fewer migrating mesenchymal cells were seen in PDK1 deletion AVC explant culture. (I to P) Higher magnification (×20) of AVC explant staining. (M to P) PDK1 deletion AVC explant showed severely defective EMT and had no apparent outgrowth. (Q) Quantification of cells in outgrowth of AVC ex vivo culture samples.
Loss of PDK1 in endothelial cells caused apoptosis.
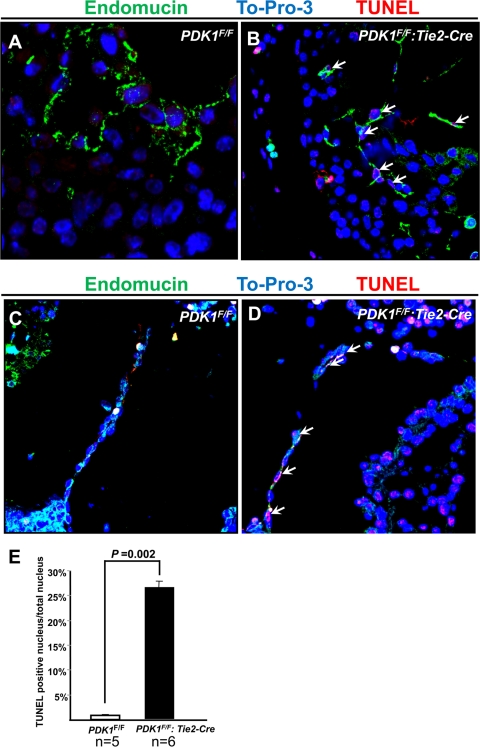
The multiple developmental defects in endothelial PDK1 deletion mice indicated that PDK1 was indispensable for endothelial function. We therefore studied endothelial proliferation and survival of PDK1 deletion mice. A bromodeoxyuridine (BrdU) experiment was performed with control and PDK1 deletion mice, and the results were slightly distinguishable between these two genotypes (data not shown). Next, we carried out a TUNEL assay to assess endothelial apoptosis. As shown in Fig. 7 A to E, while nearly no apoptotic cells were detected in the control, the TUNEL-positive endothelial cells could be found in both the endothelium and AVC of the PDK1 deletion heart (Fig. 7A to E).
FIG. 7.
TUNEL assay of E11 hearts, with endomucin-stained endothelium, To-Pro-3-stained nucleus, and TUNEL-stained apoptotic cells. (A and B) Left ventricle. PDK1 deletion ventricle showed increased apoptotic endothelial cells (indicated by arrows in panel B). (C and D) AVC. Similar to results shown in panels A and B, increased apoptosis was seen in PDK1 deletion AVC (arrows indicate apoptotic endothelial cells). Panel E shows quantification. Magnification, ×60.
Altered Snail levels and cellular translocation in PDK1-deficient AVC and EMT defects rescued by constitutively active Snail.
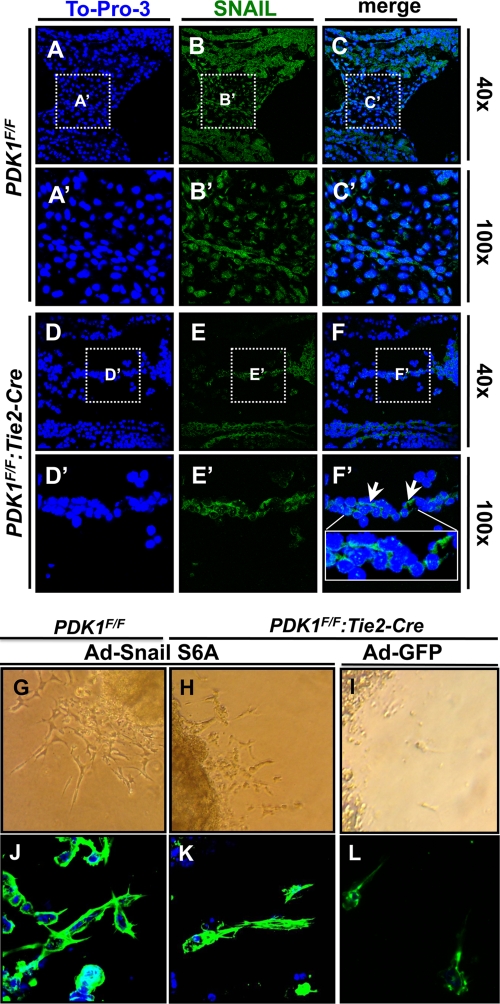
During EMT, epithelial cells lose adhesive cell-cell junctions and marker gene expression, such as for E-cadherin and endomucin. Instead, the mesenchymal cells generated from the epithelial cells become motile and express marker genes of SMA and Snail, a transcription factor and master gene for EMT that suppresses expression of adhesive molecules but activates transcription of mesenchymal genes (24). Previously, it was reported that activation of Akt induced Snail expression and EMT in squamous cell carcinoma cell lines (20).We, therefore, examined the levels of Snail in the AVC of control and PDK1-deficient mice. As shown in Fig. 8, while Snail was widely observed and localized mainly in the nucleus in the control, its expression was absent from a large number of cells and its localization was changed from nucleus to cytosol (Fig. 8A to F and A′ to F′). It was also reported that Snail can be dually regulated by glycogen synthase kinase 3β (GSK3β) through direct phosphorylation, which promotes Snail degradation and translocation from the nucleus to cytosol, resulting in reduced EMT (45). A variant of Snail (Snail S6A that is constitutively active) that abolishes this phosphorylation significantly promotes EMT (45). GSK3β is a well-established Akt substrate, and phosphorylation of GSK3β by Akt causes its inactivation. In the case of PDK1 deletion, Akt activity is low, which may increase GSK3β activity and Snail phosphorylation, leading to reduced EMT. We therefore delivered adenoviral Snail S6A to the AVC explants and found that, while empty adenovirus (Ad-green fluorescent protein [GFP]) failed to activate EMT, Ad-Snail S6A (constitutively active) effectively rescued the EMT defects in the explants of PDK1 deficiency (Fig. 8G to L).
FIG. 8.
Staining of Snail in AVC and delivery of Snail S6A to AVC explants. Panels A′ to F′ are higher magnifications of boxed areas shown in panels A to F. Snail was widely expressed and localized mainly in nucleus in control AVC, while in PDK1 deletion AVC, its expression was dramatically reduced and it was localized only in cytosol (indicated by arrows in panel F′). (G to L) Delivery of Snail S6A (Ad-Snail S6A) to AVC explants. While empty adenovirus (Ad-GFP) showed little effect on EMT (I), delivery of Snail S6A (Ad-Snail S6A) rescued EMT defects of endothelial PDK1 deletion AVC explants (G and H). (J to L) Immunostaining of cells migrating from explants. Levels of cell morphology are comparable between control and endothelial PDK1-deficient explants.
Rescue of EMT and AVC cushion defects by Akt delivery and inactivation of PTEN.
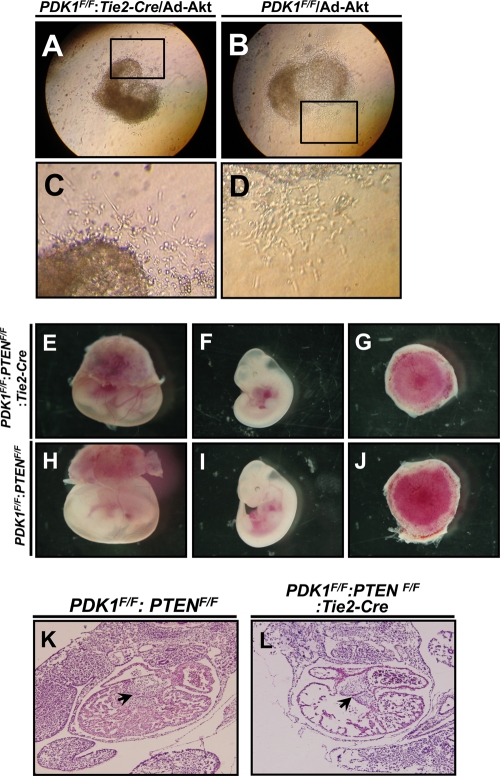
To investigate whether the EMT defects in the AVC explant of PDK1 deletion mice were due to Akt inactivation, we delivered adenovirus expressing constitutively active Akt to the AVC explant culture. Three out of five AVC cultures showed a complete rescue of EMT defect in PDK1-deficient AVC (Fig. 9 A to D).
FIG. 9.
Adenoviral Akt delivery to AVC explants and analysis of heart development in PDK1/PTEN DKO mice. (A to D) Delivery of adenoviral Akt rescued cell migration deficiency of PDK1 deletion AVC explant. (E to J) Microscopic analysis of embryo development. Note the normal morphology of yolk sac, embryo, and placenta in DKO mice in panels E to G. (K and L) HE staining of heart sections. The levels of valve development are comparable between control and DKO mice (indicated by arrows).
PTEN negatively regulates PDK1-Akt activation (23). Therefore, we next studied the effects of PTEN loss on cardiovascular development of PDK1 deletion mice by crossing PTEN-floxed and PDK1-floxed mice together with Tie-2 Cre mice (PDK1F/F:PTENF/F:Tie2-Cre). Because endothelium-specific deletion of PDK1 resulted in embryonic mortality at around E11.5 (Fig. 2G and H), we dissected the embryos at this stage. Out of four PTENF/F:PDK1F/F:Tie2-Cre mice analyzed, two appeared normal, and histological analysis revealed a level of development of the AVC cushion comparable to that of the control (Fig. 9E to L). At E12.5, we observed six viable embryos out of 18 double knockout mice. Therefore, the loss of PTEN can postpone embryonic lethality by 1 day. Collectively, these data showed that increased Akt activity could rescue the heart phenotype of PDK1-deficient mice, and the loss of PTEN may enhance Akt activity through increased phosphorylation of S473 of Akt in the absence of PDK1.
DISCUSSION
In this study, we have revealed the crucial function of PDK1 in endothelial cells and cardiovascular development through activation of Akt and Snail. Loss of PDK1 in endothelial cells caused impaired vascular integrity, remodeling, and development and embryonic lethality at around E11.5. In the heart, endothelial deletion of PDK1 affected endocardiac development, including EMT in the AVC and valve formation. As a result of poor development of the endothelium, the structure of the trabeculae and myocardium was disrupted. Thus, our study has demonstrated that PDK1 plays an indispensable role in the development of the cardiovascular system.
VEGF is essential for endothelial proliferation, survival, and migration (1, 11). After binding to its receptor of VEGFR, VEGF activates two essential intracellular signaling pathways of MAPK and PI3K-PDK1-Akt. Similarly, both angiopoietin 1 (Ang1) and its receptor, Tie2, are required for vascular remodeling and vessel integrity. Recently, it was reported that Akt is the major downstream effector of Ang1-Tie2 signaling (18, 35). Therefore, the developmental and functional defects in the absence of PDK1 in endothelial cells could be a consequence resulting from the disruption of VEGF and Ang1 signaling. This is consistent with a previous report using PDK1 KO ES cells to study cell migration (34). It was shown that PDK1 plays an essential role in regulating endothelial cell migration in response to VEGF-A stimulation (34).
Recently, it was shown that PI3K KO mice displayed defects in angiogenesis and vascular integrity (19, 44). In these mice, the activation of Akt was impaired, which is the possible cause for vascular abnormalities (19, 44). Previously, we studied the Akt1 and Akt3 double knockout (DKO) mice (41, 43). These mice were embryonically lethal at around E12 and displayed developmental defects in multiple organs and tissues, including the cardiovascular system (43). Endothelium-specific PDK1 deletion in mice also caused embryonic mortality at around E11.5 and cardiovascular defects. These similarities between the endothelial PDK1 deletion and Akt1 and -3 DKO mice suggest that, first, Akt proteins are the major downstream players of PI3K-PDK1 signaling among the AGC kinases in the endothelium and, second, the phenotype in Akt 1 and -3 DKO mice could be attributed to disrupted endothelial development and function. Data presented here support this idea because delivery of active Akt to the AVC explant of PDK1-deficient mice rescued EMT defects, and enhancement of Akt activation by PTEN deletion resulted in normal AVC development and survival of PDK1-deficient mice at approximately E12. In the future, it will be of great interest to study whether overexpression of Akt could rescue the phenotype of endothelial PDK1 deletion mice. However, this might be a big challenge as transgenic mice with Akt overexpression in endothelial cells were embryonically lethal and showed excessive aberrant vasculature (33). On the other hand, this suggests that balanced and refined regulation of PDK1-Akt signaling is crucial for cardiovascular development, as either a higher intensity (in the case of Akt transgenic mice) or less activity (in the case of PDK1 and Akt KO mice) of Akt signaling gives rise to developmental abnormalities.
In this study, we have demonstrated that deletion of PDK1 in endothelial cells had nearly no impact on cell proliferation. Instead, we found that PDK1 played a role in promoting cell survival, as loss of PDK1 caused endothelial apoptosis. One of the important functions of Akt is antiapoptosis and prosurvival (12). Therefore, the increased endothelial cell death might be due to inactivation of Akt.
Our study was consistent with a previous report showing that Akt activation promoted Snail expression (20). We found that Snail was ubiquitously expressed in the AVC of the control but was absent from a large amount of cells in the PDK1 deletion AVC. In addition, its cellular localization was changed from the nucleus in the control to the cytosol in PDK1 deletion cells. As Snail is a transcription factor and a master gene for EMT that activates mesenchymal gene expression while suppressing the transcription of adhesive molecules of epithelial cells, the changes of Snail expression patterns may reflect the deficit of EMT in the PDK1 deletion AVC. GSK3β was reported to phosphorylate Snail, resulting in Snail translocation to the cytosol and degradation (45). Because GSK3β has been the best-characterized substrate of the Akt kinase and the phosphorylation of GSK3β by Akt suppresses GSK3β activity, the distribution and cellular localization of Snail in PDK1 deletion cells could be due to loss of activity of PDK1-Akt signaling. Our results have demonstrated that Snail S6A (constitutively active) rescues EMT defects in PDK1 deficiency and confirmed this hypothesis.
Another intriguing finding of this study is that the loss of PTEN can rescue the phenotype of PDK1 deficiency. Mechanistically, PTEN loss in the absence of PDK1 may compensate Akt activity through enhancement of Akt S473 phosphorylation. We found a dramatic increase of Akt S473 phosphorylation in cardiomyocyte-specific PTEN/PDK1 double deletion mice (data not shown). Hence, our study suggests that Akt S473 phosphorylation might be PI3K dependent.
The data presented here could be promising for therapeutic applications. In the future, it will be helpful to modulate Akt signaling to improve cardiovascular complications in human patients.
Acknowledgments
We are grateful to Dario Alessi at the University of Dundee, United Kingdom, for providing the PDK1-floxed mice and Xiao Yang in Beijing for providing the PTEN-floxed mice.
This work was supported by the National Key Basic Research Program of China (2006CB943503) and the National Science Foundation of China (30500264, 30671040, and 30770893) with grants to Zhongzhou Yang and Xinli Li.
Footnotes
Published ahead of print on 10 May 2010.
REFERENCES
- 1.Adams, R., and K. Alitalo. 2007. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8:464-478. [DOI] [PubMed] [Google Scholar]
- 2.Baudry, A., Z. Yang, and B. Hemmings. 2006. PKBa is required for adipose differentiation of mouse embryonic fibroblasts. J. Cell Sci. 119:889-897. [DOI] [PubMed] [Google Scholar]
- 3.Bayascas, J., S. Wullschleger, K. Sakamoto, J. Garcia-Martinez, C. Clacher, D. Komander, D. van Aalten, K. Boini, F. Lang, C. Lipina, I. Logie, C. Sutherland, J. Chudek, J. van Diepen, P. Voshol, J. Lucocq, and D. Alessi. 2008. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol. Cell. Biol. 28:3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blume-Jensen, P., and T. Hunter. 2001. Oncogenic kinase signalling. Nature 411:355-365. [DOI] [PubMed] [Google Scholar]
- 5.Brazil, D., Z. Yang, and B. Hemmings. 2004. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 29:233-242. [DOI] [PubMed] [Google Scholar]
- 6.Camenisch, T., J. Schroeder, J. Bradley, S. Klewer, and J. McDonald. 2002. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat. Med. 8:850-855. [DOI] [PubMed] [Google Scholar]
- 7.Cantley, L. 2008. Growth and survival signaling by lipid kinases. FASEB J. 22:263.3. [Google Scholar]
- 8.Cantley, L. 2002. The phosphoinositide 3-kinase pathway. Science 296:1655-1657. [DOI] [PubMed] [Google Scholar]
- 9.Collins, B., M. Deak, J. Arthur, L. Armit, and D. Alessi. 2003. In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. EMBO J. 22:4202-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, B., M. Deak, V. Murray-Tait, K. Storey, and D. Alessi. 2005. In vivo role of the phosphate groove of PDK1 defined by knockin mutation. J. Cell Sci. 118:5023-5034. [DOI] [PubMed] [Google Scholar]
- 11.Coultas, L., K. Chawengsaksophak, and J. Rossant. 2005. Endothelial cells and VEGF in vascular development. Nature 438:937-945. [DOI] [PubMed] [Google Scholar]
- 12.Datta, S., A. Brunet, and M. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 13.Davis, R. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 14.Dummler, B., and B. Hemmings. 2007. Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 35:231-235. [DOI] [PubMed] [Google Scholar]
- 15.Dummler, B., O. Tschopp, D. Hynx, Z. Yang, S. Dirnhofer, and B. Hemmings. 2006. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol. Cell. Biol. 26:8042-8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fayard, E., L. Tintignac, A. Baudry, and B. Hemmings. 2005. Protein kinase B/Akt at a glance J. Cell Sci. 118:5675-5678. [DOI] [PubMed] [Google Scholar]
- 17.Feng, J., J. Park, P. Cron, D. Hess, and B. Hemmings. 2004. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279:41189-41196. [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara, S., K. Sako, T. Minami, K. Noda, H. Kim, T. Kodama, M. Shibuya, N. Takakura, G. Koh, and N. Mochizuki. 2008. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat. Cell Biol. 10:513-526. [DOI] [PubMed] [Google Scholar]
- 19.Graupera, M., J. Guillermet-Guibert, L. Foukas, L. Phng, R. Cain, A. Salpekar, W. Pearce, S. Meek, J. Millan, and P. Cutillas. 2008. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 453:662-666. [DOI] [PubMed] [Google Scholar]
- 20.Grille, S., A. Bellacosa, J. Upson, A. Klein-Szanto, F. Roy, S. Lee-Kwon, M. Donowitz, P. Tsichlis, and L. Larue. 2003. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 63:2172-2178. [PubMed] [Google Scholar]
- 21.Hauge, C., and M. Frodin. 2006. RSK and MSK in MAP kinase signalling. J. Cell Sci. 119:3021. [DOI] [PubMed] [Google Scholar]
- 22.Hay, N. 2005. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8:179-183. [DOI] [PubMed] [Google Scholar]
- 23.Hemmings, B., D. Restuccia, and N. Tonks. 2009. Targeting the kinome II. Curr. Opin. Cell Biol. 21:135-139. [DOI] [PubMed] [Google Scholar]
- 24.Kalluri, R., and E. Neilson. 2003. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 112:1776-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kisanuki, Y. Y., R. E. Hammer, J.-I. Miyazaki, S. C. Williams, J. A. Richardson, and M. Yanagisawa. 2001. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230:230-242. [DOI] [PubMed] [Google Scholar]
- 26.Lang, F., C. Bohmer, M. Palmada, G. Seebohm, N. Strutz-Seebohm, and V. Vallon. 2006. (Patho) physiological significance of the serum-and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 86:1151. [DOI] [PubMed] [Google Scholar]
- 27.Lawlor, M., A. Mora, P. Ashby, M. Williams, V. Murray-Tait, L. Malone, A. Prescott, J. Lucocq, and D. Alessi. 2002. Essential role of PDK1 in regulating cell size and development in mice. EMBO J. 21:3728-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McManus, E., B. Collins, P. Ashby, A. Prescott, V. Murray-Tait, L. Armit, J. Arthur, and D. Alessi. 2004. The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J. 23:2071-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mora, A., A. Davies, L. Bertrand, I. Sharif, G. Budas, S. Jovanović, V. Mouton, C. Kahn, J. Lucocq, G. Gray, A. Jovanović, and, D. R. Alessi. 2003. Deficiency of PDK1 in cardiac muscle results in heart failure and increased sensitivity to hypoxia. EMBO J. 22:4666-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mora, A., D. Komander, D. van Aalten, and A. DR. 2004. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell Dev. Biol. 15:161-170. [DOI] [PubMed] [Google Scholar]
- 31.Newton, A. 2003. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 370:361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobukuni, T., S. Kozma, and G. Thomas. 2007. hvps34, an ancient player, enters a growing game: mTOR complex1/S6K1 signaling. Curr. Opin. Cell Biol. 19:135-141. [DOI] [PubMed] [Google Scholar]
- 33.Phung, T., K. Ziv, D. Dabydeen, G. Eyiah-Mensah, M. Riveros, C. Perruzzi, J. Sun, R. Monahan-Earley, I. Shiojima, and J. Nagy. 2006. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 10:159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Primo, L., L. di Blasio, C. Roca, S. Droetto, R. Piva, B. Schaffhausen, and F. Bussolino. 2007. Essential role of PDK1 in regulating endothelial cell migration. J. Cell Biol. 176:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saharinen, P., L. Eklund, J. Miettinen, R. Wirkkala, A. Anisimov, M. Winderlich, A. Nottebaum, D. Vestweber, U. Deutsch, and G. Koh. 2008. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol. 10:527-537. [DOI] [PubMed] [Google Scholar]
- 36.Sarbassov, D., D. Guertin, S. Ali, and D. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science 307:1098-1101. [DOI] [PubMed] [Google Scholar]
- 37.Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211-225. [DOI] [PubMed] [Google Scholar]
- 38.Teng, Y., A. Sun, X. Pan, G. Yang, L. Yang, M. Wang, and X. Yang. 2006. Synergistic function of Smad4 and PTEN in suppressing forestomach squamous cell carcinoma in the mouse. Cancer Res. 66:6972. [DOI] [PubMed] [Google Scholar]
- 39.Tschopp, O., Z. Yang, D. Brodbeck, B. Dummler, M. Hemmings-Mieszczak, T. Watanabe, T. Michaelis, J. Frahm, and B. Hemmings. 2005. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132:2943-2954. [DOI] [PubMed] [Google Scholar]
- 40.Williams, M., J. Arthur, A. Balendran, J. van der Kaay, V. Poli, P. Cohen, and D. Alessi. 2000. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 10:439-448. [DOI] [PubMed] [Google Scholar]
- 41.Yang, Z., O. Tschopp, A. Baudry, B. Dummler, D. Hynx, and B. Hemmings. 2004. Physiological functions of protein kinase B/Akt. Biochem. Soc. Trans. 32:350-354. [DOI] [PubMed] [Google Scholar]
- 42.Yang, Z., O. Tschopp, M. Hemmings-Mieszczak, J. Feng, D. Brodbeck, E. Perentes, and B. Hemmings. 2003. Protein kinase Bα/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 278:32124-32131. [DOI] [PubMed] [Google Scholar]
- 43.Yang, Z. Z., O. Tschopp, N. Di-Poi, E. Bruder, A. Baudry, B. Dummler, W. Wahli, and B. Hemmings. 2005. Dosage-dependent effects of Akt1/protein kinase Bα (PKBα) and Akt3/PKBγ on thymus, skin, and cardiovascular and nervous system development in mice. Mol. Cell. Biol. 25:10407-10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan, T., H. Choi, A. Matsui, C. Benes, E. Lifshits, J. Luo, J. Frangioni, and L. Cantley. 2008. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 105:9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, B., J. Deng, W. Xia, J. Xu, Y. Li, M. Gunduz, and M. Hung. 2004. Dual regulation of Snail by GSK-3β mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 6:931-940. [DOI] [PubMed] [Google Scholar]