Abstract
We studied the function of lipid rafts in generation and signaling of T-cell receptor microclusters (TCR-MCs) and central supramolecular activation clusters (cSMACs) at immunological synapse (IS). It has been suggested that lipid raft accumulation creates a platform for recruitment of signaling molecules upon T-cell activation. However, several lipid raft probes did not accumulate at TCR-MCs or cSMACs even with costimulation and the fluorescence resonance energy transfer (FRET) between TCR or LAT and lipid raft probes was not induced at TCR-MCs under the condition of positive induction of FRET between CD3ζ and ZAP-70. The analysis of LAT mutants revealed that raft association is essential for the membrane localization but dispensable for TCR-MC formation. Careful analysis of the accumulation of raft probes in the cell interface revealed that their accumulation occurred after cSMAC formation, probably due to membrane ruffling and/or endocytosis. These results suggest that lipid rafts control protein translocation to the membrane but are not involved in the clustering of raft-associated molecules and therefore that the lipid rafts do not serve as a platform for T-cell activation.
Lipid rafts are specialized liquid-ordered membrane microdomains that are enriched in cholesterol and sphingolipids. Many studies using various methodologies have shown that lipid rafts exist as leaflets less than 200 nm in size and float on the plasma membrane (6, 10, 24, 28, 32). They have been implied to play a role in protein sorting and cell activation as a platform by recruiting various signaling molecules such as Src family kinases, G proteins, and adaptor molecules. Because of size limitation, all of the raft-associated molecules could not be accommodated on the same lipid raft, and heterogeneity of lipid rafts both in size and in the repertoire of resident molecules has been suggested (22). The functional importance of lipid rafts in signal transduction has been particularly appreciated in T-cell activation through the T-cell receptor (TCR). Some of the initial observations in this area included the findings that cross-linking of the raft-associated ganglioside GM1 induces T-cell activation (12) and that a mutant of LAT, a membrane adaptor protein, that was unable to localize to rafts failed to induce activation signals (33). Since then, increasing data have demonstrated that lipid raft accumulation creates a platform to stabilize the signaling complex for T-cell activation (13, 29).
T cells are activated upon recognition of peptide-major histocompatibility complex (MHC) complexes expressed on antigen-presenting cells (APC). An immunological synapse (IS) is formed at the interface between the T cell and the APC where a specialized segregated structure of T-cell surface receptors is generated. This supramolecular activation cluster (SMAC) contains the TCR in the central region (cSMAC) and lymphocyte function-associated antigen 1 (LFA-1) in the peripheral region (pSMAC). The accumulation of lipid rafts at this interface, particularly in the cSMAC, has been suggested to create a transient structure to mediate signal transduction (13, 17). In addition, CD28-mediated costimulation has been suggested to enhance lipid raft accumulation and TCR activation (29). However, the idea that lipid rafts accumulated in the cSMAC serve as the platform for T-cell activation has been controversial; the accumulation of the lipid raft was only partial in the contact area (3), or the concentration of lipid raft was constant even in the area of T-cell activation (5, 8, 28, 32). These variations could be partly attributed to differences in experimental approaches such as the cell systems being analyzed, stimulation conditions, and detection methods, including imaging and biochemical fractionation. The idea that the cSMAC is the site responsible for inducing signals for T-cell activation has been recently revised based on analysis of the dynamic assembly of signaling complexes upon TCR stimulation. Analysis of T-cell activation using a planar membrane system containing glycosylphosphatidylinositol (GPI)-anchored MHC-peptide complexes and the LFA-1 ligand intercellular adhesion molecule 1 (ICAM-1) revealed that small clusters containing approximately a hundred TCRs, kinases, and adaptors, which we termed TCR microclusters (MCs), were generated at the initial contact sites. This was followed by translocation of the MCs to the center of the interface to generate a cSMAC (31). Since protein phosphorylation, including that of ZAP-70, was induced in the TCR-MCs and Ca2+ mobilization was induced in parallel with the formation of TCR-MCs, these MCs appear to be the very first and minimum unit for generating TCR activation signals (31). Furthermore, a major costimulatory receptor, CD28, forms clusters which are also colocalized in TCR-MCs to regulate costimulatory signals (30).
Among these TCR proximal signaling molecules, LAT is a well-studied raft-associated membrane adaptor protein that is indispensable for TCR activation. LAT is phosphorylated by ZAP-70 and then behaves as a signal scaffold, recruiting various signaling adaptors and effector molecules such as phospholipase Cγ (PLCγ), SLP-76, and Grb2/Gads. Because mutation of LAT palmitoylation sites (C26,29A) resulted in its dislocation from lipid rafts and defective signaling, it was concluded that the association with lipid rafts is essential for the function of LAT (33). However, a recent study showed that this mutant LAT has impaired trafficking to the plasma membrane in the Jurkat T-cell line (27), raising the question of whether the impaired signaling resulting from this LAT mutation was due to dislocation from the raft or defective trafficking to the membrane.
Here, we analyzed the role of lipid rafts in T-cell activation, particularly their relationship with immunological synapse formation (9). Provided that lipid raft functions as a platform for T-cell activation, the new idea that TCR-MCs serve as the signal unit for activation would predict that lipid raft could be accumulated in or interact with TCR-MCs (29).
Utilizing several lipid raft probes, which retain the capability of raft localization but lack signaling capacity, we found that the full-length LAT generated MCs, but none of the raft probes formed visible clusters at TCR-MCs or cSMAC, even in conjunction with CD28-mediated costimulation. Furthermore, no significant interaction between lipid rafts and TCR-MCs was revealed by fluorescence resonance energy transfer (FRET) analysis. Conversely, the non-raft-localizing LAT mutant showed MC formation upon TCR stimulation. These results suggest that lipid rafts do not serve as a platform for TCR signaling but rather regulate the traffic/recruitment of proteins to the plasma membrane. Furthermore, our data indicate that the previous observation of lipid raft accumulation at the cSMAC may reflect membrane ruffling and endocytosis rather than active formation of signal platform.
MATERIALS AND METHODS
Cell culture.
Freshly isolated AND TCR-transgenic (AND-Tg) T cells were stimulated with irradiated spleen cells from B10BR mice and 3 μM moth cytochrome c [MCC(88-103)] peptide in RPMI (Sigma) with 10% fetal calf serum and 1% Pen/Strep (Invitrogen, Carlsbad, CA). Twenty-four hours after stimulation, the gene of interest was introduced into the activated cells by retrovirus-mediated gene transfer. The cells were kept in RPMI medium containing interleukin-2 (IL-2) until imaging by confocal microscopy at 72 to 96 h after the gene transfer. AND-Tg T-cell hybridoma cells were grown in RPMI with 10% fetal calf serum and 1% Pen/Strep (30).
Construction and transduction.
Expression constructs for all the genes were generated by subcloning into the retrovirus vector pMX. CD3ζ-Halo; CD3ζ-cyan fluorescent protein (CFP); mLAT-green fluorescent protein (GFP); mLAT-CFP; mLAT(ΔCP)-GFP; mLAT(C29A)-GFP; mLAT(C26,29A)-GFP; hLAT-GFP; hLAT(Y132F)-GFP; hLAT(3YF)-GFP, which changed Tyr171, Tyr191, and Tyr226 to Ala; Lck-GFP; Lck(N10)-GFP; Lck(wt)-YPet; and Lck(N10)-YPet were generated by recombinant PCR with mouse cDNA, Halo tag pHT2 vector (Promega), pEGFP-N1 (BD Clontech), and pYPet-His (Addgene plasmid 14031). LAXhLAT is a kind gift from Weiguo Zhang (Duke University, Durham, NC). Lck(N10)-YPet and Lck(wt)-YPet have a linker (GGGAAGGGAA) for increasing FRET efficiency. To produce pseudovirus, the recombinant plasmid was transfected into Phoenix packaging cells using Lipofectamine 2000.
Planar bilayer system.
GPI-anchored mouse MHC class II molecule I-Ek, ICAM-1, and CD80 were transfected into and purified from Chinese hamster ovary (CHO) or baby hamster kidney cells. The purified GPI-anchored proteins were incorporated into dioleoyl phosphatidylcholine liposomes (Avanti Polar Lipids). Planar bilayers containing I-Ek and ICAM-1 were formed in a flow cell chamber system (Bioptechs). Planar bilayers were loaded overnight at 37°C with 50 μM MCC in citrate buffer, pH 4.5, blocked for 1 h at 37°C with 5% nonfat dried milk in phosphate-buffered saline (PBS), and opened in the assay medium. All experiments in the planar bilayers were conducted with HEPES-buffered saline containing 1% bovine serum albumin, 2 mM MgCl2, and 1 mM CaCl2.
Live cell imaging by confocal microscopy.
Gene-transferred cells were incubated and loaded with 4,000×-diluted tetramethylrhodamine (TMR) ligand to give fluorescence to Halo tag or stained with 0.5 μM cholera toxin B subunit (CTXB)-Alexa 488 on ice so as not to accumulate intracellularly. The prepared cells were mounted on the stage of a Leica TCS SP5 microscope with a 60× oil-immersion objective.
FRET analysis.
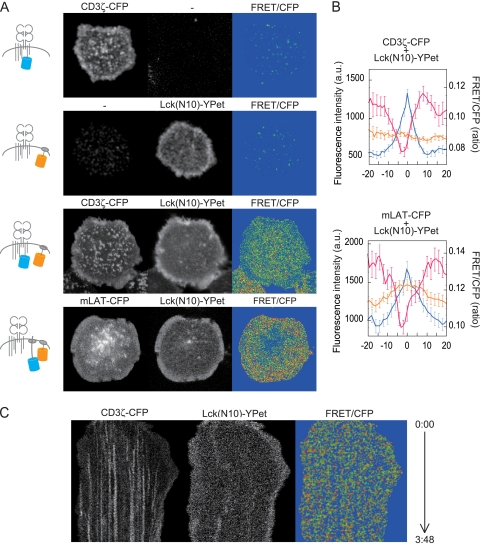
We used the CFP-YPet FRET pair for efficient FRET analysis (18, 19). We took 405-nm-excited 430- to 500-nm images as the CFP image, 405-nm-excited 500- to 590-nm images as the FRET-containing image, and 488-nm-excited 500- to 590-nm images as the YPet image with 12-bit resolution. FRET images were obtained by subtracting CFP leakage and YPet directly excited by 405 nm from the FRET-containing image. All the AND-Tg T-cell hybridoma cells expressing the pair CD3ζ-CFP and Lck(N10)-YPet, mLAT-CFP and Lck(N10)-YPet, ZAP-CFP and CD3ζ-YPet, or CD3ζ-CFP and Lck(wt)-YPet showed sufficiently strong FRET intensity (see Fig. 2). FRET efficiency was also checked by donor fluorescence recovery after acceptor bleaching (see Fig. S2 in the supplemental material).
FIG. 2.
FRET analysis for the interaction between CD3ζ or LAT and a lipid raft probe, Lck(N10). (A) Images of CFP, YPet, and FRET efficiency (FRET/CFP) of AND-TCR hybridoma cells expressing either CD3ζ-CFP or Lck(N10)-YPet or either pair CD3ζ-CFP/Lck(N10)-YPet or pair LAT-CFP/Lck(N10)-YPet at 2 min. The diagrams in the left column depict the labeled molecules under each experimental condition. (B) The intensity profiles at the peaks of CD3ζ-CFP or LAT-CFP were arranged according to peak position. The intensities were averages from a 10- × 41-pixel area of each peak. Data are means ± standard errors of the means of 40 to 50 peaks from 3 cells. Blue line, CFP; orange, YPet; pink, FRET/CFP. a.u., arbitrary units. (C) Kymograph analysis for time-lapse measurement of FRET/CFP between CD3ζ-CFP and Lck(N10)-YPet. Images were taken every 2.6 s after cellular attachment, and 5- × 348-pixel images were extracted and arranged in temporal order.
Imaging of the interface between T cell and APC.
B cells were isolated from spleen and lymph nodes of B10BR mice, activated with 1 μg/ml lipopolysaccharide (LPS), and loaded with 5 μM MCC for 24 h. On the next day, the cells were incubated with AND-Tg T cells and the conjugates were analyzed by fluorescent microscopy. FM4-64 was purchased from Molecular Probes and used at 10 mM in HEPES-buffered saline.
Sucrose density gradient ultracentrifugation.
AND-Tg T-cell hybridoma cells expressing mLAT-GFP were lysed in a buffer with 1% Triton X-100, and the lysates were ultracentrifuged on a sucrose density gradient as described previously (33). The fractions were analyzed by Western blotting using anti-LAT antibody (Upstate 06-807).
RESULTS
TCR clustering independent of lipid raft clusters.
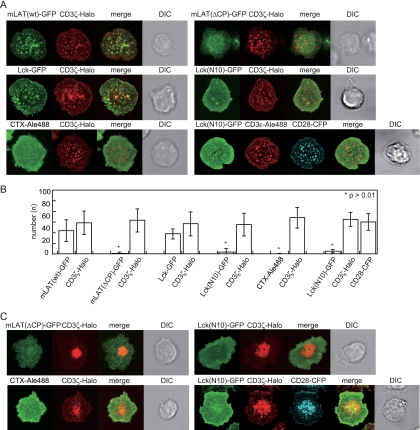
To address the question of whether TCR-MC formation is accompanied by lipid raft clustering, we prepared several lipid raft marker proteins and analyzed their movement on the planar bilayer system. Murine LAT fused to GFP [mLAT(wt)-GFP] and CD3ζ fused to Halo tag (CD3ζ-Halo) (21) were coexpressed in antigen-specific T cells from AND-Tg mice. On the bilayer, these T cells exhibited attachment, spreading, generation of TCR-MCs, and activation as reported previously (31). mLAT(wt)-GFP also formed clusters that colocalized with the TCR-MCs (Fig. 1A) similarly to the phosphorylated LAT (4). A LAT mutant, mLAT(ΔCP), which lacks most of the cytoplasmic domain but retains four amino acids, including two palmitoylation sites for lipid raft association, was fused to GFP [mLAT(ΔCP)-GFP] and used as a lipid raft probe (23). Surprisingly, mLAT(ΔCP)-GFP did not show any clustering upon TCR stimulation (Fig. 1A).
FIG. 1.
Images of lipid raft-associated proteins and lipid raft probes at TCR-MCs. (A) AND-Tg T cells expressing mLAT(wt)-GFP, mLAT(ΔCP)-GFP, Lck-GFP, or Lck(N10)-GFP together with CD3ζ-Halo, or cells expressing CD3ζ-Halo and stained with CTXB-Alexa 488, were loaded with TMR ligand and placed on the planar bilayer containing ICAM-I and I-Ek with 10 μM MCC peptide. Images were collected 2 min after attachment to the bilayer. The cells expressing Lck(N10)-GFP and CD28-CFP were fixed at 2 min on the CD80-containing bilayer and stained with anti-CD3ɛ-biotin and streptavidin-Alexa 566. The large bright area in cells with mLAT(ΔCP)-GFP reflects its presence within cytoplasmic organelles as detected in the x-z image (see Fig. S1 in the supplemental material). (B) The numbers of GFP and Halo tag clusters were counted objectively using software with Gaussian fitting and counting capability. Data are means ± standard deviations of 15 to 30 cells. (C) The cells used in panel A were incubated for 10 min, and images were collected. DIC, differential interference contrast.
The Src family kinase Lck is another lipid raft-associating molecule critical for T-cell activation (14, 35). A deletion mutant of Lck containing only the N-terminal 10 amino acids (aa) [Lck(N10)], which includes the two myrisoylation sites responsible for the lipid raft localization, was examined. Similarly to LAT, the full-length Lck(wt)-GFP was clustered at TCR-MCs, whereas the Lck(N10)-GFP showed no clustering (Fig. 1A). Both LAT and Lck are intracellular molecules, and their mutant raft probes reside in the inner leaflet of raft. Therefore, we examined a classical lipid raft marker, the cholera toxin B subunit (CTXB) conjugated with Alexa 488, which binds to the outer leaflet of raft. We obtained results showing that CTXB did not show any cluster upon stimulation similar to those with Lck(N10)-GFP (Fig. 1A). Since the above experiments were performed in the absence of costimulation, we next used CD80-containing bilayers for the addition of CD28-mediated costimulation (30). However, the result with Lck(N10)-GFP was the same; no clusters were detected (Fig. 1A). These findings were quantified and subjected to statistical analysis as shown in Fig. 1B after determining the number of clusters from 30 cells for each molecule.
Lipid raft clustering at the cSMAC was also examined at later time points (10 min) using mLAT(ΔCP)-GFP, Lck(N10)-GFP, and CTXB-Alexa 488. None of the raft makers showed accumulation at the cSMAC upon stimulation regardless of the presence of CD28 costimulation (Fig. 1C; see Movie S1 in the supplemental material). These results indicate that lipid rafts do not form visible clusters at either the TCR-MCs or the cSMAC, at least within the limitation of detection by conventional fluorescent microscopy.
Reduced interaction of lipid rafts with TCR-MCs.
It became clear from these results that lipid rafts do not form clusters similar to TCR-MCs. However, if the lipid raft is a functional microdomain for cell activation, it does not necessarily have to form clusters that are similar in size to the TCR-MCs. To examine the possibility that lipid rafts interact with signaling molecules on a smaller scale, we performed FRET analysis between TCR or LAT and a lipid raft probe using AND-TCR hybridoma cell lines. The data on acceptor bleaching confirmed the specific induction of FRET (see Fig. S2 in the supplemental material).
CD3ζ-CFP or Lck(N10)-YPet alone showed no FRET/CFP (Fig. 2A). FRET efficiency (FRET/CFP) between TCR and a lipid raft probe was then analyzed in the cells expressing both molecules, and unexpectedly it was significantly decreased at the TCR-MCs (Fig. 2A). The FRET/CFP between LAT-CFP and Lck(N10)-YPet was also significantly reduced at the LAT clusters (Fig. 2A). Averaged intensity profiles of CD3ζ-CFP or LAT-CFP peaks were arranged by peak position and plotted (Fig. 2B). To investigate the earlier stage of TCR-MC formation, we took time-lapse images of FRET/CFP between CD3ζ-CFP and Lck(N10)-YPet just after T-cell attachment to the bilayer (Fig. 2C; see Movie S2 in the supplemental material). We found that the FRET/CFP showed no increase even at the initial stage of activation, while it was clearly reduced at the TCR-MCs.
We utilized other molecular pairs for FRET—CD3ζ-CFP and Lck(wt)-YPet, ZAP-70-CFP and CD3ζ-YPet, or CD3ζ-CFP and CD3ζ-YPet as FRET controls—to confirm that the observed low FRET/CFP was not an artifact caused by the accumulated CFP (see Fig. S2 in the supplemental material). In the case of CD3ζ-CFP and Lck(wt)-YPet and of ZAP-70-CFP and CD3ζ-YPet, both CFP and YPet accumulated at the TCR-MCs and the FRET/CFP was increased at these peaks, indicating that, as expected, FRET was induced between two separate molecules within the TCR-MC. On the other hand, the FRET between CD3ζ-YPet and CD3ζ-CFP did not increase even though both CD3ζ-YPet and CD3ζ-CFP were accumulated at the same place, indicating that simple accumulation of CFP did not induce FRET and that the FRET depends on the protein pair. The data with the FRET reduction at TCR-MCs clearly indicate that the frequency of the interaction between CD3ζ or LAT and the lipid raft probes is relatively reduced at the TCR-MCs and indicate that the lipid raft does not contribute to cluster formation as a component of the signaling platform.
Raft association confers membrane localization.
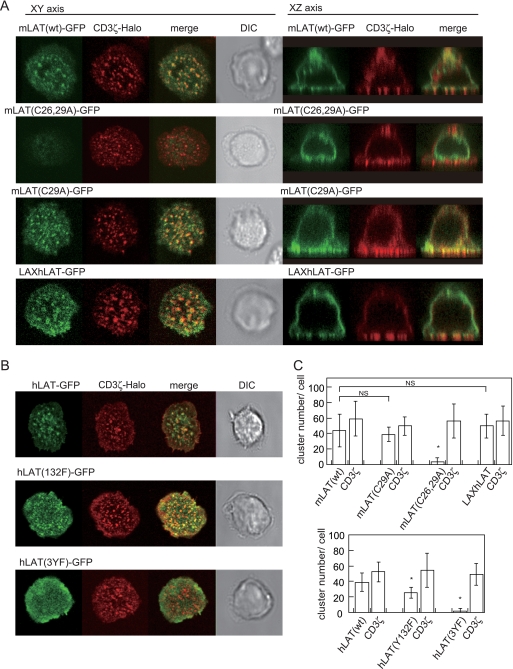
Next, we investigated the effect of the lipid raft on clustering of raft-associated molecules by analyzing LAT mutants. Consistent with previous reports (27), an mLAT bearing mutations in both palmitoylation sites [mLAT(C26,29A)] does not associate with lipid rafts (see Fig. S3 in the supplemental material). The protein is mostly located intracellularly and is hardly detectable on the cell surface (Fig. 3A). Thus, lipid raft localization confers membrane translocation onto the plasma membrane from intracellular compartments. We then analyzed a mutant, mLAT(C29A), possessing the replacement in only a single palmitoylation site, which was reported to have decreased association with lipid rafts but otherwise retains mostly normal functions (33) (Fig. S3). Interestingly, mLAT(C29A)-GFP was mainly expressed on the plasma membrane and formed clusters at the TCR-MCs upon stimulation. The number of mLAT(C29A)-GFP clusters was not significantly different from that of mLAT(wt)-GFP clusters (Fig. 3A and C), suggesting that MC formation is independent of raft localization. To further confirm this observation, another LAT mutant, LAXhLAT-GFP, a fusion of LAX(1-67aa) and hLAT(34-233aa) and GFP, was analyzed. Because LAX is not associated with lipid rafts, LAXhLAT-GFP was expressed in the non-lipid raft fraction on the plasma membrane (34) (see Fig. S3 in the supplemental material). As expected, LAXhLAT-GFP exhibited normal MC formation, similarly to mLAT (Fig. 3A and C), thus confirming that TCR-MCs are generated in the absence of detectable lipid raft clustering.
FIG. 3.
Expression and microcluster formation of LAT mutants on a planar bilayer. (A) AND-Tg T cells expressing CD3ζ-Halo and mLAT(wt)-GFP, mLAT(C29A)-GFP, mLAT(C26,29A)-GFP, or LAXhLAT-GFP were loaded with TMR ligand and placed on the bilayer for 2 min. (B) AND-Tg T cells expressing CD3ζ-Halo and hLAT(wt)-GFP, hLAT(Y132F)-GFP, or hLAT(3YF)-GFP were placed on the bilayer for 2 min. (C) Numbers of clusters of GFP or Halo tag were counted objectively by software as described for Fig. 1. Data are means ± standard deviations of 15 to 30 cells. DIC, differential interference contrast.
We further examined the relationship of LAT structure with its localization. The role of tyrosine phosphorylation in MC formation was examined using hLAT mutants. It has been shown elsewhere that hLAT(Y132F) and hLAT(3YF) fail to associate with PLCγ and Grb2/Gads, respectively (16). The hLAT(Y132F)-GFP showed MC formation similar to that of the wild type (WT), whereas hLAT(3YF) showed no cluster formation (Fig. 3B and C), although both molecules were retained normally in the lipid raft fraction (data not shown). These data suggest that LAT induces MC formation through Grb2/Gad-dependent protein interactions but independently of PLCγ.
Endocytosis of lipid rafts at the cSMAC.
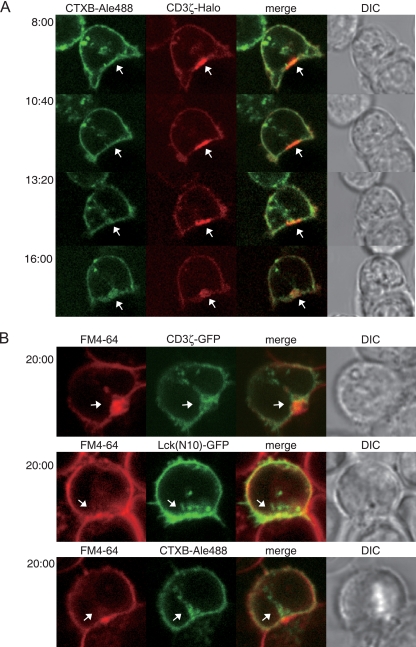
Despite our results showing that lipid raft clustering does not contribute to the formation or maintenance of TCR-MCs, previous imaging analyses, partially relying on CTXB staining, revealed the accumulation of lipid rafts at the T-cell-APC interface. This observation has been a strong basis of the idea that lipid rafts function as a signaling platform. Therefore, we examined the behavior of lipid raft markers under the same cell-cell interaction conditions. AND-Tg T cells expressing CD3ζ-Halo were stained with CTXB-Alexa 488 and incubated with peptide-loaded activated B cells. Ten minutes later, when the cSMAC could be detected, real-time video imaging was performed. CTXB-Alexa 488 did not show stable accumulation at the immunological synapse (IS) or colocalization in the cSMAC. However, after several minutes, CTXB-Alexa 488 began to be internalized and also to accumulate at the cSMAC (Fig. 4 A; see also Movie S3 in the supplemental material). A similar result was obtained with Lck(N10)-GFP (see Fig. S4 in the supplemental material). We found that CTX accumulation occurred much later than did cSMAC formation (Fig. 4A). Consistently, the percentage of cSMACs with colocalized CTXB accumulation increased from 45.5% ± 1.6% (n = 27) to 74.1% ± 7.2% (n = 47) during 15 to 30 min after coculture. This delayed accumulation of the lipid raft probes suggests that the lipid raft accumulation is dispensable for cSMAC formation. We assumed that the accumulated lipid raft probes were the result of membrane ruffling and endocytosis (8). This idea was proven using the amphiphilic dye FM4-64, which is a well-characterized fluorescent endocytosis probe. AND-Tg T cells expressing CD3ζ-GFP or Lck(N10)-GFP or stained with CTXB-Alexa 488 were incubated with antigen-loaded activated B cells in the presence of 10 mM FM4-64. Twenty minutes after incubation, FM4-64 accumulated at the cSMAC, similarly to the lipid raft probes (Fig. 4B), suggesting that lipid raft accumulation most likely reflects endocytosis at the cSMAC (20).
FIG. 4.
Endocytosis of lipid raft probes and CD3ζ at the interface between T cells and APC. (A) AND-Tg T cells expressing CD3ζ-Halo and stained with CTXB-Alexa 488 were loaded with TMR ligand and incubated with 5 μM MCC peptide-loaded LPS-activated B cells. Video images were collected 8 min after starting the incubation. Images taken at each time point revealed a different time course of CD3ζ-Halo and CTXB-Alexa 488 accumulation. (B) AND-Tg T cells expressing Lck(N10)-GFP or CD3ζ-GFP or stained with CTXB-Alexa 488 were incubated with the activated B cells for 20 min in the presence of 10 μM FM 4-64. White arrows indicate cSMAC (A) and endocytosed molecules (B). DIC, differential interference contrast.
DISCUSSION
We addressed here a controversial problem of whether lipid raft serves as a platform for T-cell activation by investigating the relationship between TCR-MCs and lipid raft. After it became clear that TCR-MC is responsible for T-cell activation as the unit for recruitment of signaling molecules, it has been an obvious question whether TCR-MCs are based on or supported by a lipid raft cluster.
To address this controversy, we performed imaging analyses using several lipid raft probes to ask whether these probes are colocalized (or corporate) with TCR-MCs and/or cSMACs. Our data indicate that TCR-MCs are generated as a signalsome to induce T-cell activation by recruiting signaling proteins mainly through protein-protein interactions and not through lipid raft clustering. We have shown this conclusion from three different analyses: (i) failure of colocalization of several lipid raft probes with TCR-MCs upon activation, (ii) no specific increase of FRET between raft probes and TCR or LAT in TCR-MCs, and (iii) active signaling capacity and TCR-MC generation of LAT mutants without lipid raft localization. Instead of a signaling platform, our results indicate that lipid raft has a critical function in transporting raft-localizing signaling components to the plasma membrane and probably delivers them to the active receptor engagement site, TCR-MC.
We have utilized raft probes which reside in both inner and outer leaflets of lipid raft. The former are the mutants of LAT [LAT(ΔCP)] and Lck [Lck(N10)], which are intracellular proteins defective in assembly with signaling molecules, and the latter was cholera toxin for binding to GM1. All three probes revealed raft localization biochemically by sucrose gradient fractionation and failed to show any cluster formation or accumulation in TCR-MC. Since both full-length LAT and Lck were accumulated into TCR-MC, we could examine the structure of LAT required for TCR-MC accumulation. We found that the tyrosine phosphorylations at Tyr 171, 191, and 226, which are known to be sites for association with Grb2/Gads, are critical for accumulation in TCR-MCs, suggesting that such protein interaction rather than the association with lipid raft is essential for T-cell activation. Similarly, Lck also needs protein-protein interaction with CD4 at the TCR activation site as shown in previous reports that revealed relocation of CD4 to the activation site and CD4-dependent relocation of Lck (11, 15); although the precise structural requirement of Lck to be localized into the TCR-MC has to be determined, Sohn et al. (25) have reported detection of FRET between the B-cell antigen receptor (BCR) and a Lyn-based lipid raft probe. They showed a transient increase of FRET between Igα and the lipid raft probe and proposed that it reflects a transition in the BCR conformation prior to BCR phosphorylation (25). Since our experimental setup using the planar bilayer system is similar, if the TCR-CD3s were activated before TCR-MC generation, the FRET should have increased immediately after contact with antigen. However, we did not obtain any positive FRET at the TCR-MCs from the initial time course. Our FRET data with the reduction at TCR-MCs clearly indicate that the frequency of the interaction between CD3ζ or LAT and the lipid raft probes is relatively reduced at the TCR-MCs and indicate that the lipid raft does not contribute to cluster formation as a component of the signaling platform.
The evidence that lipid raft localization is not correlated with TCR-MC formation or T-cell activation is also derived from the results with LAT mutants. LAT(C29A) and LAXhLAT mutants were not found in lipid raft fraction; nevertheless, both mutants were accumulated into TCR-MCs and had the capacity to induce T-cell activation. This result with the LAXhLAT chimera also strengthens the evidence for the critical role of TCR-MC for T-cell activation.
Mice with the mLAT(Y136F) mutation [equivalent to hLAT(Y132F)] had a dramatic reduction in thymocyte numbers, whereas peripheral T cells exhibited enhanced proliferation and Th2 development, resulting in allergic disease (1, 26). These findings suggest that mLAT Tyr-136 as the PLCγ-binding site may have both positive and negative effects on T-cell activation, proliferation, or survival. Although this result was surprising from the point of the critical requirement of the LAT-Gads/SLP76-PLCγ assembly for TCR proximal signaling, the observation that the assembly of PLCγ was dispensable for signaling clusters nucleated by SLP-76 (2) is consistent with our result. Our data show that hLAT(Y132F) could generate clusters in the absence of any PLCγ interaction and thus may function as a positive regulator at TCR-MC but that MCs may induce negative downstream signals that prevent lymphoproliferative disorders.
Our results showing that lipid raft probes do not form visible clusters at the TCR-MCs or the cSMAC, within the limitation of detection by confocal and total internal reflection fluorescent (TIRF) microscopy, and that specific FRETs between raft probes and TCR-MCs were not detected upon stimulation strongly suggest that lipid raft does not play a critical role or serve as a platform for T-cell activation. However, it remains possible that the membrane lipid order at the TCR activation region might be changed upon T-cell activation as Gaus reported in the system using a dye detecting lipid order (7), because our raft probes or FM dye cannot detect membrane lipid order condition. Our data demonstrate that the association of signaling molecules with lipid raft is advantageous for intracellular trafficking to the plasma membrane. Conversely, the endocytosis of these signaling molecules from the membrane may also be induced via raft-mediated vesicle transport, which may retain signaling activity for sustained T-cell activation.
Supplementary Material
Acknowledgments
We thank S. Yamasaki, K. Hirose, and A. Altman for helpful discussion; Y. Murahashi for assistance with numerical analysis of FRET data; and H. Yamaguchi for secretarial assistance.
This work was supported by a Grant-in-Aid for Scientific Research (A.H.-T. and T.S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Published ahead of print on 24 May 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aguado, E., S. Richelme, S. Nunez-Cruz, A. Miazek, A. M. Mura, M. Richelme, X. J. Guo, D. Sainty, H. T. He, B. Malissen, and M. Malissen. 2002. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science 296:2036-2040. [DOI] [PubMed] [Google Scholar]
- 2.Bunnell, S. C., A. L. Singer, D. I. Hong, B. H. Jacque, M. S. Jordan, M. C. Seminario, V. A. Barr, G. A. Koretzky, and L. E. Samelson. 2006. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol. Cell. Biol. 26:7155-7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burack, W. R., K. H. Lee, A. D. Holdorf, M. L. Dustin, and A. S. Shaw. 2002. Cutting edge: quantitative imaging of raft accumulation in the immunological synapse. J. Immunol. 169:2837-2841. [DOI] [PubMed] [Google Scholar]
- 4.Campi, G., R. Varma, and M. L. Dustin. 2005. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202:1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglass, A. D., and R. D. Vale. 2005. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121:937-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggeling, C., C. Ringemann, R. Medda, G. Schwarzmann, K. Sandhoff, S. Polyakova, V. N. Belov, B. Hein, C. von Middendorff, A. Schonle, and S. W. Hell. 2009. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457:1159-1162. [DOI] [PubMed] [Google Scholar]
- 7.Gaus, K., E. Chklovskaia, B. Fazekas de St. Groth, W. Jessup, and T. Harder. 2005. Condensation of the plasma membrane at the site of T lymphocyte activation. J. Cell Biol. 171:121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glebov, O. O., and B. J. Nichols. 2004. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat. Cell Biol. 6:238-243. [DOI] [PubMed] [Google Scholar]
- 9.Grakoui, A., S. K. Bromley, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science 285:221-227. [DOI] [PubMed] [Google Scholar]
- 10.Harder, T., C. Rentero, T. Zech, and K. Gaus. 2007. Plasma membrane segregation during T cell activation: probing the order of domains. Curr. Opin. Immunol. 19:470-475. [DOI] [PubMed] [Google Scholar]
- 11.Holdorf, A. D., K. H. Lee, W. R. Burack, P. M. Allen, and A. S. Shaw. 2002. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat. Immunol. 3:259-264. [DOI] [PubMed] [Google Scholar]
- 12.Janes, P. W., S. C. Ley, and A. I. Magee. 1999. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147:447-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janes, P. W., S. C. Ley, A. I. Magee, and P. S. Kabouridis. 2000. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 12:23-34. [DOI] [PubMed] [Google Scholar]
- 14.Kabouridis, P. S., A. I. Magee, and S. C. Ley. 1997. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 16:4983-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krummel, M. F., M. D. Sjaastad, C. Wulfing, and M. M. Davis. 2000. Differential clustering of CD4 and CD3zeta during T cell recognition. Science 289:1349-1352. [DOI] [PubMed] [Google Scholar]
- 16.Lin, J., and A. Weiss. 2001. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J. Biol. Chem. 276:29588-29595. [DOI] [PubMed] [Google Scholar]
- 17.Monks, C. R., B. A. Freiberg, H. Kupfer, N. Sciaky, and A. Kupfer. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395:82-86. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen, A. W., and P. S. Daugherty. 2005. Evolutionary optimization of fluorescent proteins for intracellular FRET. Nat. Biotechnol. 23:355-360. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang, M., J. Sun, S. Chien, and Y. Wang. 2008. Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc. Natl. Acad. Sci. U. S. A. 105:14353-143538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parton, R. G., and A. A. Richards. 2003. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4:724-738. [DOI] [PubMed] [Google Scholar]
- 21.Reck-Peterson, S. L., A. Yildiz, A. P. Carter, A. Gennerich, N. Zhang, and R. D. Vale. 2006. Single-molecule analysis of dynein processivity and stepping behavior. Cell 126:335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw, A. S. 2006. Lipid rafts: now you see them, now you don't. Nat. Immunol. 7:1139-1142. [DOI] [PubMed] [Google Scholar]
- 23.Shogomori, H., A. T. Hammond, A. G. Ostermeyer-Fay, D. J. Barr, G. W. Feigenson, E. London, and D. A. Brown. 2005. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J. Biol. Chem. 280:18931-18942. [DOI] [PubMed] [Google Scholar]
- 24.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. (Erratum, 2:216, 2001.) [DOI] [PubMed] [Google Scholar]
- 25.Sohn, H. W., P. Tolar, and S. K. Pierce. 2008. Membrane heterogeneities in the formation of B cell receptor-Lyn kinase microclusters and the immune synapse. J. Cell Biol. 182:367-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommers, C. L., C. S. Park, J. Lee, C. Feng, C. L. Fuller, A. Grinberg, J. A. Hildebrand, E. Lacana, R. K. Menon, E. W. Shores, L. E. Samelson, and P. E. Love. 2002. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 296:2040-2043. [DOI] [PubMed] [Google Scholar]
- 27.Tanimura, N., S. Saitoh, S. Kawano, A. Kosugi, and K. Miyake. 2006. Palmitoylation of LAT contributes to its subcellular localization and stability. Biochem. Biophys. Res. Commun. 341:1177-1183. [DOI] [PubMed] [Google Scholar]
- 28.Varma, R., and S. Mayor. 1998. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394:798-801. [DOI] [PubMed] [Google Scholar]
- 29.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283:680-682. [DOI] [PubMed] [Google Scholar]
- 30.Yokosuka, T., W. Kobayashi, K. Sakata-Sogawa, M. Takamatsu, A. Hashimoto-Tane, M. L. Dustin, M. Tokunaga, and T. Saito. 2008. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity 29:589-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokosuka, T., K. Sakata-Sogawa, W. Kobayashi, M. Hiroshima, A. Hashimoto-Tane, M. Tokunaga, M. L. Dustin, and T. Saito. 2005. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 6:1253-1262. [DOI] [PubMed] [Google Scholar]
- 32.Zacharias, D. A., J. D. Violin, A. C. Newton, and R. Y. Tsien. 2002. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296:913-916. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, W., R. P. Trible, and L. E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9:239-246. [DOI] [PubMed] [Google Scholar]
- 34.Zhu, M., E. Janssen, K. Leung, and W. Zhang. 2002. Molecular cloning of a novel gene encoding a membrane-associated adaptor protein (LAX) in lymphocyte signaling. J. Biol. Chem. 277:46151-46158. [DOI] [PubMed] [Google Scholar]
- 35.Zlatkine, P., B. Mehul, and A. I. Magee. 1997. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J. Cell Sci. 110:673-679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.