Abstract
The human β-globin genes are expressed in a developmentally controlled fashion. Studies on the molecular mechanisms underlying the stage-specific regulation of globin genes have been fueled by the clinical benefit of elevated fetal γ-globin expression in patients with sickle cell anemia and thalassemia. Recent reports suggested a role of the hematopoietic transcription factor GATA-1, its cofactor FOG-1, and the associated chromatin remodeling complex NuRD in the developmental silencing of HBG1 and HBG2 gene expression. To examine whether FOG-1 via NuRD controls HBG1 and HBG2 silencing in vivo, we created mice in which the FOG-1/NuRD complex is disrupted (A. Miccio et al., EMBO J. 29:442-456, 2010) and crossed these with animals carrying the entire human β-globin gene locus as a transgene. We found that the FOG-1/NuRD interaction is dispensable for the silencing of human HBG1 and HBG2 expression. In addition, mutant animals displayed normal silencing of the endogenous embryonic globin genes. In contrast, a significant reduction of adult-type human and murine globin gene expression was found in adult bone marrows of mutant animals. These results suggest that, unexpectedly, NuRD is required for FOG-1-dependent activation of adult-type globin gene expression but is dispensable for human γ-globin silencing in vivo.
The human β-like globin genes are expressed in a tightly controlled fashion throughout development (5, 36). During the embryonic stage of human erythropoiesis, ɛ-globin (HBE1) is the predominantly expressed gene. When hematopoiesis shifts to the fetal liver, γ-globin is produced from two almost identical genes, HBG1 and HBG2. Around the time of birth when the bone marrow becomes the primary site of hematopoiesis, HBG1 and HBG2 expression is silenced and HBB is the most highly expressed globin gene. This process is referred to as hemoglobin switching.
A major impetus for the study of hemoglobin switching derives from the long-standing observation that patients with sickle cell anemia or β-thalassemia who have linked mutations that elevate γ-globin levels experience a more benign clinical course of the disease. Progress toward the design of targeted therapies to raise γ-globin expression has been stymied by a lack of understanding of how γ-globin expression is normally silenced after birth. Several nuclear factors are thought to contribute to this complex process, including erythroid Kruppel-like factor (EKLF) and related Kruppel-like transcription factors (1, 2, 11), the stage selector protein (SSP) (20, 21, 44, 45), and the orphan nuclear receptors TR2/TR4 (37, 38). More recent studies found a major role for BCL11A in the silencing of γ-globin expression in human cells (7, 24, 26, 33, 34, 41).
The hematopoietic transcription factor GATA-1 has also been proposed to contribute to the silencing of the γ-globin genes. GATA-1 is a zinc finger protein that binds to WGATAR elements found in the regulatory regions of virtually all erythroid genes including the α- and β-globin genes (12). GATA-1 was originally identified as a positive regulator of erythroid-specific gene transcription. Later studies found that GATA-1 can also directly repress transcription of numerous genes, including GATA-2 and c-Kit (16, 23, 29, 43). In addition, it was discovered that depending on the developmental stage, GATA-1 can exert repressive activity on the β-like globin genes. For example, GATA-1 negatively regulates human HBE1 expression by interacting with one or more silencer elements within the 5′ flanking region of the gene (32). Two recent studies independently provided evidence for a direct role of GATA-1 in γ-globin silencing. In the first, a marked human globin locus was used to examine sequences required for γ-globin silencing in transgenic mice. It was found that a 352-bp region harboring a conserved GATA element at position −566 5′ of the HBG1 gene contributes to the silencing of γ-globin expression in definitive erythropoiesis (17). In the second study, a family with hereditary persistence of fetal hemoglobin (HPFH) was reported to carry a mutation at the corresponding GATA site in the HBG2 gene (6). Both reports showed that GATA-1 occupies this element in fetal erythroid cells. Thus, even in the context of globin gene expression, it appears that GATA-1 can function both as activator and repressor in a context-dependent manner. How GATA-1 exerts these distinct functions remains unresolved (7, 39).
Most of GATA-1 functions require binding to the hematopoietic-specific multitype zinc finger protein FOG-1 (40). Numerous studies revealed that FOG-1 functions during both activation and repression of gene transcription by GATA-1 (9, 10, 13, 16, 23, 40, 42). Definitive in vivo evidence for the requirement of a direct GATA-1/FOG-1 interaction derived from the study of patients with X-linked thrombocytopenia and dyserythropoietic anemia who were found to bear a point mutation (V205M) in the N-terminal zinc finger of GATA-1 (30). Importantly, substitutions at this residue were demonstrated to impair the GATA-1/FOG-1 interaction in vitro and in vivo (9, 30). Ensuing studies identified more families with similar clinical presentations that were caused by point mutations in GATA-1 that affected FOG-1 binding (8).
FOG-1 interacts with several protein complexes, including TACC3, CtBP-2, and NuRD (13, 15, 18). CtBP-2 is a corepressor, and its binding site is conserved among all members of the FOG family of proteins. While mutations at the CtBP-2 binding site affect FOG-1 function in vitro (10, 13), the in vivo relevance of this interaction remains uncertain (22). We recently reported that FOG-1 interacts with high affinity and specificity with the NuRD (nucleosome remodeling and histone deacetylase) corepressor complex in vitro and in vivo. This interaction is mediated by a short, conserved N-terminal motif that is also found in other transcriptional repressors, including Bcl11A and Bcl11B, SALL1-4, Ebf-associated zinc finger protein (EBFAZ), and Evi3 (18). Deletion of this motif leads to loss of NuRD binding and transcriptional repression in vitro (18). To examine the FOG-1/NuRD interaction in vivo, we used a knock-in approach to generate mice bearing three point mutations (R3G, R4G, and K5A) within the NuRD-binding module of FOG-1 that specifically and completely abrogate NuRD binding. Animals homozygous for the knock-in mutations (Fog1ki/ki) are anemic and display defects in platelet formation, indicating that NuRD binding by FOG-1 is essential for normal erythroid and megakaryocyte development (28). The NuRD complex contains histone deacetylases and interacts with diverse transcriptional repressors, leading to its classification as a corepressor complex. Therefore, it was unexpected to find that NuRD is also required for FOG-1-mediated transcriptional activation of select GATA-1/FOG-1 target genes (28). While this work shows that NuRD is an important coregulator for FOG-1, it leaves unanswered the question as to the distinctive features of activating versus repressive functions of FOG-1.
The studies implicating GATA-1 in the developmental silencing of γ-globin expression raised the possibility that repression occurs via FOG-1 and NuRD. Indeed, FOG-1 and the NuRD component Mi-2β were detected by chromatin immunoprecipitation (ChIP) at a region encompassing the −566 GATA element upstream of the HBG1 gene in definitive erythroid cells, suggesting that FOG-1 and NuRD control γ-globin repression directly (17). Since NuRD harbors several enzymatically active subunits (Mi-2β, HDAC-1, and HDAC-2), it stands as a potential target for pharmacological intervention to raise γ-globin levels in human patients. Moreover, small molecules that specifically inhibit the FOG-1/NuRD interaction might also derepress γ-globin expression in definitive cells. In light of these important implications, we decided to test in vivo whether FOG-1 via NuRD controls γ-globin silencing in vivo. To this end we bred the Fog1ki/ki animals with transgenic mice containing a yeast artificial chromosome (YAC) bearing the human β-globin gene cluster (hβ-YAC). The expression of all human β-like globin genes was examined at embryonic, fetal, and adult stages of development in compound Fog1ki/ki/hβ-YAC animals. We found that the FOG-1/NuRD interaction is dispensable for repression of human γ-globin in vivo. Instead, NuRD binding by FOG-1 is required for the full expression of adult-type β-like globin genes in definitive erythroid cells.
MATERIALS AND METHODS
Generation of compound Fog1ki/ki/hβ-YAC mice.
The generation of Fog1ki/ki mice was described previously (28). A20.1 hβ-YAC transgenic mice (14, 31) were kindly provided by Karin Gaensler. Fog1ki/+ and hβ-YAC mice were crossed, and genotyping performed to identify the Fog-1 mutation (described in reference 28) and the hβ-YAC transgene. For the latter, semiquantitative PCR was employed using the following primers: A20.1 F, 5′-TTTGAGGTTGCTAGTGAACACAGTT-3′, and A20.1 R, 5′-TACGTAAATACACTTGCAAAGGAGG-3′ (product length, 310 bp). Primers for the endogenous interleukin-2 (IL-2) locus (IL-2 F, 5′-CTAGGCCAGAGAATTGAAAGATCT-3′; IL-2 R, 5′-GTAGGTGGAAATTCTAGCATCATCC-3′; product length, 324 bp) served as a control. Mice were maintained in the animal facility at The Children's Hospital of Philadelphia. Experiments involving mice were approved by the Institutional Animal Care and Use Committee.
RNA analysis.
Total RNA was extracted with Trizol (Invitrogen) from fluorescence-activated cell sorter (FACS)-enriched Ter119high CD71high bone marrow (BM) and fetal liver (FL) cells. Equal numbers of sorted cells were used from Fog1ki/ki/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1+/+/hβ-YAC animals. RNA was reverse transcribed using Superscript II as per the manufacturer's instructions (Invitrogen). Quantitative reverse-transcription PCR (qRT-PCR) was performed using SYBR green (Applied Biosystems).
Primer sequences for the HBE1, HBG1, HBG2, HBD, Hbb-b1, Hba-a1, Hba-x and Hbb-bh1 genes were described previously (4). Primers for HBB and murine glycophorin A (GpA) are described in Basu et al. (3). Primers for Hbb-y gene and HBE1, HBG, and HBB primary transcripts (pT) are from Tanabe et al. (38).
Flow cytometry.
Cells were stained either with phycoerythrin (PE)-conjugated anti-mouse Ter119 (clone TER-119; BD PharMingen) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD71 (clone C2; BD PharMingen) or with allophycocyanin (APC)-conjugated anti-mouse Ter119 (clone TER-119; BD PharMingen) and PE-conjugated anti-mouse CD71 (clone C2; BD PharMingen). Peripheral blood erythroid cells were fixed and permeabilized as described previously (27), whereas fixation and permeabilization of FL and BM cells were carried out using BD Cytofix/Cytoperm solution (BD Biosciences Pharmingen, San Diego, CA). Subsequently, cells were incubated with PE-conjugated anti-human hemoglobin β antibodies (clone 37-8; Santa Cruz Biotechnology, CA) or FITC-conjugated anti-human hemoglobin γ antibodies (clone 0700-50; Fitzgerald Industries International Inc.) for 30 min at 4°C. Following washes with phosphate-buffered saline (PBS) containing 1% fetal bovine serum, cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences, MD). The data were evaluated using FlowJo, version 8.7.1 (TreeStar, OR).
For cell sorting experiments, FL and BM cells were incubated with PE-conjugated anti-mouse Ter119 (clone TER-119; BD PharMingen) and FITC-conjugated anti-mouse CD71 (clone C2; BD PharMingen). Ter119high CD71high were selected using a BD Biosciences FACSAria cell sorter with DiVa, version 6.0, software at the University of Pennsylvania Flow Cytometry and Cell Sorting core facility.
RESULTS AND DISCUSSION
Disrupting the FOG-1-NuRD interaction in transgenic mice carrying the human β-globin locus.
To examine the role of the FOG-1/NuRD complex in human globin gene expression, we bred the Fog1ki/+ animals with mice containing a single copy of a stably integrated YAC comprising ∼150 kb of the human β-globin locus (hβ-YAC), including the locus control region (LCR), the globin genes, and more than 30 kb of 3′ flanking sequences (strain A20.1 [31]). Some critical aspects of human globin gene transcription are maintained in the context of transgenic mice, including the switch from fetal to adult β-globin synthesis, which occurs around embryonic day 12.5 (E12.5) (14, 31). Timed matings were performed between compound Fog1ki/+/hβ-YAC mice to generate Fog1ki/ki/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1+/+/hβ-YAC mice. To avoid potentially confounding effects that may arise due to the presence of two copies of the hβ-YAC transgene, only offspring with a single copy were analyzed.
Disrupting the FOG-1/NuRD interaction fails to reactivate human γ-globin expression in adult mice.
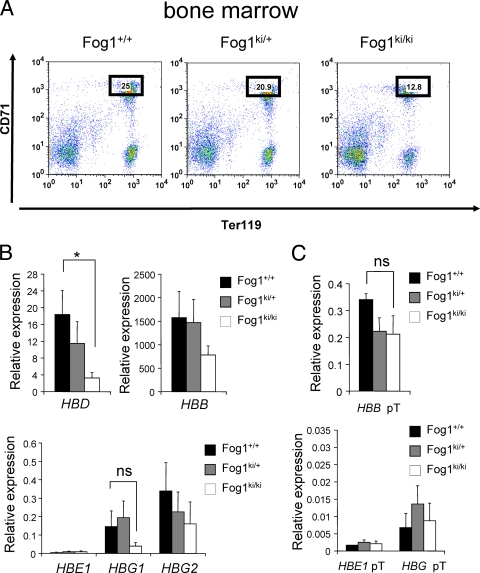
Previous work suggested that loss of GATA-1 binding to a specific site upstream of the HBG1 or HBG2 (also called Aγ-globin and Gγ-globin, respectively) promoters prevents γ-globin silencing in definitive erythroid cells in humans and transgenic mice (6, 17). To examine the role of FOG-1 and NuRD in maintaining γ-globin repression in definitive erythroblasts, we measured mRNA levels of the human β-like globin genes in bone marrow erythroid cells from 6- to 8-week-old Fog1ki/ki/hβ-YAC mice. To exclude the possibility that any differences in gene expression between Fog1ki/ki and Fog1+/+ mice are an indirect consequence of altered erythroid differentiation (28), we compared maturation stage-matched erythroid populations. Thus, bone marrow cells were stained with anti-Ter119 and anti-CD71 antibodies (Fig. 1A) (35), and Ter119high CD71high cells were selected by FACS (Fig. 1A). Cytological analyses confirmed that Fog1+/+, Fog1ki/+, and Fog1ki/ki Ter119high CD71high populations contained similar proportions of basophilic erythroblasts (>90%, as distinguished by cell size and nuclear/cytoplasmic ratio) (data not shown). mRNA was extracted from sorted cells and analyzed by quantitative real-time RT-PCR for expression of the human HBE1, HBG1, HBG2, HBD, and HBB genes (Fig. 1B). mRNA levels were normalized to those of Glycophorin A (GpA). Similar results were obtained when levels were normalized against β-actin or gapdh (data not shown). Notably, Fog1ki/ki/hβ-YAC cells expressed markedly reduced amounts of HBD (6-fold) and also tended to express lower HBB levels than Fog1ki/+/hβ-YAC or Fog1+/+/hβ-YAC erythroid cells (Fig. 1B). These findings are in agreement with a role for FOG-1 and NuRD in activating murine adult globin genes (28) (see Fig. 7A). Importantly, Fog1ki/ki/hβ-YAC erythroid cells did not express elevated levels of HBG1 or HBG2 (Fig. 1B). Instead, there was a trend toward lower HBG1 and HBG2 expression than in Fog1ki/+/hβ-YAC or Fog1+/+/hβ-YAC erythroid cells (Fig. 1B), thus indicating that the physical interaction between FOG-1 and NuRD is not required for silencing human fetal globin genes in adult mice. Similar results were obtained with 7- and 12-month-old Fog1+/+/hβ-YAC and Fog1ki/ki/hβ-YAC animals (data not shown).
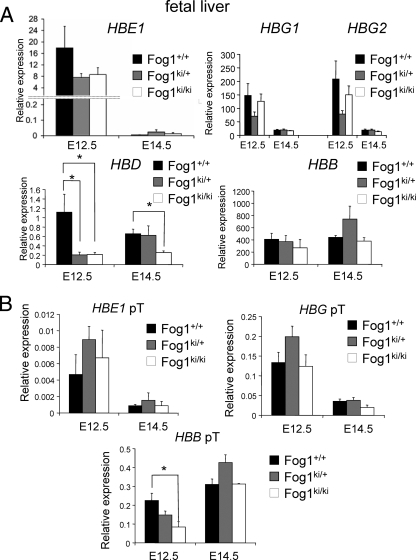
FIG. 1.
Disruption of the FOG-1/NuRD interaction fails to reactivate human γ-globin genes in Fog1ki/ki/hβ-YAC adult erythroblasts. (A) Stage-matched Ter119high CD71high bone marrow cells were obtained by FACS with anti-CD71 and anti-Ter119 antibodies. The reduced proportion of erythroid cells in Fog1ki/ki/hβ-YAC animals is consistent with the anemic phenotype observed in Fog1ki/ki mice (28). (B) Human globin mRNA levels were measured by real-time PCR in Ter119high CD71high cells with the indicated genotypes. All samples were normalized to GpA. HBG1 and HBG2 mRNA levels are comparable in Fog1+/+/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1ki/ki/hβ-YAC mice. Note the decreased HBD levels in Fog1ki/ki/hβ-YAC mice. Bars indicate standard errors of the means (n = 4). *, P < 0.05; ns, not significant. (C) Primary transcript levels of human β-globin genes in Ter119high CD71high BM erythroblasts. Error bars represent standard errors of the means (n = 3 or 4). *, P < 0.05.
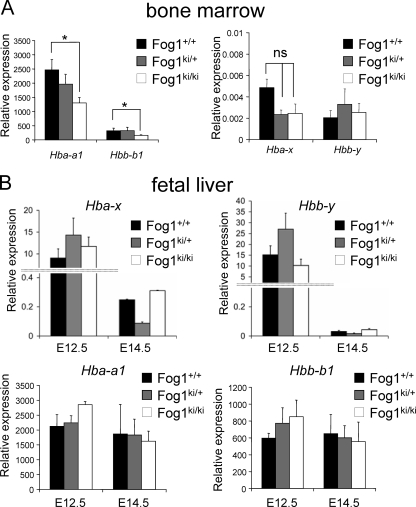
FIG. 7.
FOG-1/NuRD interaction is dispensable for silencing of murine globin genes. (A) mRNA levels of indicated murine globin genes in stage-matched bone marrow erythroblasts as measured by qRT-PCR. Error bars represent standard errors of the means (n = 4). *, P < 0.05. (B) Levels of mature mRNAs of indicated murine globin genes. Error bars represent standard errors of the means (E12.5, n = 5 to 9; E14.5, n = 4 to 11). *, P < 0.05.
Elevated γ-globin gene transcription does not always result in a corresponding accumulation of mature γ-globin transcripts, suggesting that posttranscriptional mechanisms can regulate γ-globin mRNA accumulation (5). To rule out the possibility that the FOG-1 triple mutation exerts secondary effects on mRNA processing or stability, we measured the levels of primary transcripts as they reflect more accurately the rate of transcription. No significant differences between the amounts of human HBG globin primary transcripts were observed between Fog1+/+/hβ-YAC and Fog1ki/ki/hβ-YAC cells (Fig. 1C). Moreover, no statistically significant changes of HBB primary transcripts were found in Fog1ki/ki/hβ-YAC cells (Fig. 1C). These results further corroborate that disruption of the FOG-1/NuRD interaction does not lead to reactivation of human γ-globin gene transcription.
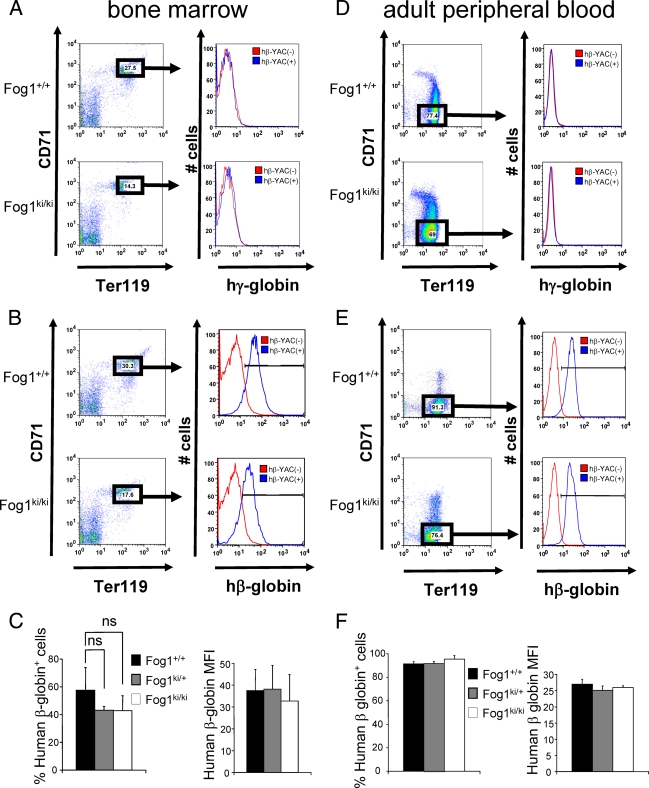
To confirm the globin gene expression studies at the protein level, we stained stage-matched Ter119high CD71high bone marrow erythroid precursors with anti-human γ-globin or anti-human β-globin antibodies. Of note, no γ-globin expression was detectable in either Fog1+/+/hβ-YAC or Fog1ki/ki/hβ-YAC erythroid cells (Fig. 2A), further confirming that the FOG-1 mutation does not lead to sustained γ-globin production. hβ-YAC-containing E12.5 fetal liver erythroblasts served as positive controls for the γ-globin antibodies (see Fig. 4A). Moreover, no statistically significant changes were found in the proportion of human β-globin-positive cells from Fog1ki/ki/hβ-YAC and Fog1ki/+/hβ-YAC mice (Fig. 2B and C). In addition, the β-globin mean fluorescence intensity (MFI) was unchanged in cells from Fog1ki/ki mice, reflecting no notable changes in the amounts of β-globin protein per cell (Fig. 2B and C). Finally, no differences in γ- or β-globin protein levels were found in mature Ter119high CD71neg red cells from peripheral blood (Fig. 2D, E, and I).
FIG. 2.
γ-Globin and β-globin protein levels in bone marrow erythroblasts and in mature peripheral red blood cells. (A to C) FACS analysis of bone marrow erythroblasts with antibodies against Ter119, CD71, and human γ-globin (hγ-globin) (A) or human β-globin (hβ-globin) (B). Ter119high CD71high erythroid cells lacking the hβ-YAC served as a negative control (red). Note that neither Fog1+/+/hβ-YAC nor Fog1ki/ki/hβ-YAC erythroblasts express γ-globin. (C) Similar proportions of Fog1+/+/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1ki/ki/hβ-YAC erythroblasts express human β-globin (left panel). The MFI levels are comparable between the three groups (right panel). Error bars represent standard errors of the means (n = 4). (D to F) Peripheral blood cells were collected from 6- to 8-week old mice. Human γ-globin (D) and β-globin (E and F) expression was evaluated in Ter119high CD71neg mature red blood cells. Human β-globin expression is comparable among the three groups both in terms of percentage of expressing cells and staining intensities (MFI). γ-Globin was undetectable (D). Red blood cells lacking the hβ-YAC served as a control. Results are averages of four to seven independent experiments. Bars indicate standard errors of the means.
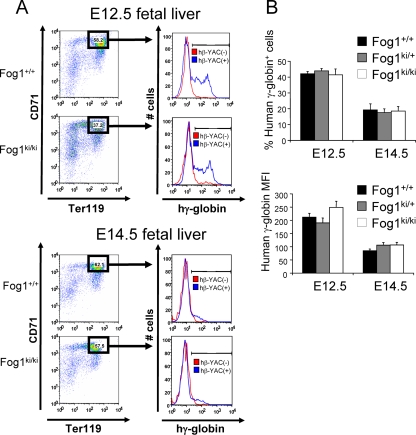
FIG. 4.
γ-Globin protein levels in E12.5 and E14.5 fetal livers. Flow cytometry profiles of E12.5 and E14.5 fetal liver cells. Representative analyses are shown in panel A. Cells from mice lacking hβ-YAC served as negative controls (red). The percentages of γ-globin-expressing cells and the corresponding mean fluorescence intensities were comparable among the different groups (B). Error bars represent standard errors of the means (E12.5, n = 5 to 9; E14.5, n = 4 to 11).
Since GATA-1 has been implicated in the repression of HBE1 expression (32), we examined whether FOG-1 via NuRD contributes to this process. We found that both mature and primary HBE1 transcript levels in bone marrow erythroid cells from Fog1ki/ki/hβ-YAC mice were low and essentially unchanged from those with wild type FOG-1 (Fig. 1B and C).
In summary, our measurements of protein, mRNA, and primary transcripts revealed no increase in γ-globin expression in Fog1ki/ki/hβ-YAC erythroid cells. This indicates that the FOG-1/NuRD interaction is dispensable for γ-globin silencing in definitive erythroid precursors. However, this interaction appears to be required for the full activation of HBD and HBB transcription.
Human hemoglobin switching occurs independently of the FOG-1/NuRD interaction.
Fog1ki/ki/hβ-YAC mice do not display elevated γ-globin levels throughout adult life. However, in light of the multitude of factors involved in hemoglobin switching and the potential redundancy among them, we considered the possibility that the differences in γ-globin silencing between Fog1+/+/hβ-YAC and Fog1ki/ki/hβ-YAC mice might be revealed only by analyzing the rate of γ-globin silencing.
In hβ-YAC-containing mice, human γ-globin gene expression is robustly downregulated at E12.5, at which time the adult HBB expression becomes highly active. At E14.5, HBB is the predominant human globin gene expressed (14, 31). To investigate if disruption of FOG-1/NuRD interaction causes a delay in human globin switching, we examined red blood cells from Fog1ki/ki/hβ-YAC fetal livers at distinct stages of development, including E12.5 and E14.5.
As the maturation of fetal liver erythroid cells is delayed in Fog1ki/ki mice (28), we analyzed human globin mRNA levels in stage-matched erythroid cells based on their Ter119/CD71 profiles as described above for bone marrow erythroid cells. Morphological evaluation of FACS-analyzed cells confirmed the homogeneity and similarity of the Ter119high CD71high-gated Fog1+/+/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1ki/ki/hβ-YAC populations (data not shown). We also considered that E12.5 and E14.5 fetal livers may contain primitive cells (19, 25), which express higher levels of human transgenic fetal globins than definitive cells (34). Therefore, we carefully determined the frequency of primitive cells in the Fog1+/+/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1ki/ki/hβ-YAC sorted fetal liver preparations. Only samples with similarly low proportions of primitive cells (<10%, as determined by cell size and nuclear/cytoplasmic ratio) (data not shown) were analyzed.
The human globin gene expression pattern was evaluated by real time RT-PCR, as described above for bone marrow erythroblasts. Ter119high CD71high cells from Fog1+/+/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1ki/ki/hβ-YAC embryos displayed similar levels of human embryonic and fetal globin transcripts (Fig. 3A, HBE1, HBG1, and HBG2) at E12.5 and E14.5, respectively. Notably, the HBE1, HBG1, and HBG2 levels decreased upon progression from embryonic day 12.5 to 14.5 in mice of all three genotypes (Fig. 3A). Moreover, the amounts of primary transcripts from embryonic and fetal globin genes correlated well with those of the corresponding mature mRNA levels (Fig. 3B). Together, these results indicate that embryonic and fetal globin genes can be silenced independently of the GATA-1/FOG-1/NuRD axis. In addition, the HBB gene was effectively activated at E12.5 and represented the predominantly transcribed human β-like globin gene at E14.5 in Fog1+/+/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1ki/ki/hβ-YAC erythroblasts (Fig. 3A). As observed in adult mice, HBD mRNA levels were significantly reduced in Fog1ki/ki/hβ-YAC and Fog1ki/+/hβ-YAC E12.5 fetal livers compared to cells with wild-type FOG-1 (5.6- and 5.9-fold, respectively) and in E14.5 erythroid Fog1ki/ki/hβ-YAC cells (2.6-fold) (Fig. 3A). The reduced HBD expression in E12.5 fetal livers from mice heterozygous for the FOG-1 mutations was surprising. However, we found that several hematological parameters were altered in Fog1ki/+ mice (28), suggesting that some genes require the full dose of FOG-1 and NuRD for maximal transcription. Also of note, E12.5 Fog1ki/ki/hβ-YAC erythroblasts displayed an ∼50% decrease of HBB primary transcripts, a change that was not detected when we measured mature HBB mRNAs (Fig. 3B). The reason for this discrepancy is unknown but might be due to posttranscriptional regulation. The transient reduction in primary HBB transcripts might reflect a delay in the onset of HBB expression, implicating NuRD in the transcriptional activation of this gene.
FIG. 3.
Human hemoglobin switching is independent of the FOG-1/NuRD interaction. (A) mRNA levels of human β-like globin genes in stage-matched Fog1+/+/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1ki/ki/hβ-YAC E12.5 and E14.5 fetal liver erythroid cells as determined by real-time RT-PCR. Results were normalized to GpA. Silencing of HBE1, HBG1, and HBG2 is unaffected in E14.5 Fog1ki/ki/hβ-YAC embryos. HBD expression is significantly reduced in both E12.5 Fog1ki/+/hβ-YAC and Fog1ki/ki/hβ-YAC and E14.5 Fog1ki/ki/hβ-YAC fetal livers. Error bars represent standard errors of the means (E12.5, n = 5 to 9; E14.5, n = 3 to 5). *, P < 0.05. (B) Primary transcripts of indicated human globin genes in maturation stage-matched Ter119high CD71high erythroblasts from E12.5 and E14.5 fetal livers as measured by real-time RT-PCR. Results were normalized to GpA. Note the reduction of HBB primary transcript in E12.5 Fog1ki/ki/hβ-YAC fetal livers. Error bars represent standard errors of the means (E12.5, n = 5 to 9; E14.5, n = 4 to 11).*, P < 0.05.
To bolster the results obtained from mRNA studies, we analyzed γ-globin protein levels in E12.5 and E14.5 Ter119high CD71high cells using FACS analysis. Approximately 43% of E12.5 erythroblasts from Fog1+/+/hβ-YAC embryos expressed fetal hemoglobin (Fig. 4A and B). Essentially the same proportion of fetal hemoglobin-positive cells was found in Fog1ki/ki/hβ-YAC-derived cells (Fig. 4A and B). Moreover, no substantial differences in MFIs were found between cells from different FOG-1 backgrounds (212 ± 24 for Fog1+/+ versus 190 ± 18 for Fog1ki/+ versus 249 ± 15 for Fog1ki/ki) (Fig. 4B). At E14.5, both the fraction of γ-globin-expressing cells as well as the corresponding MFIs were reduced by 50% in all the mice (Fig. 4B), confirming that disruption of NuRD binding does not delay the silencing of γ-globin genes.
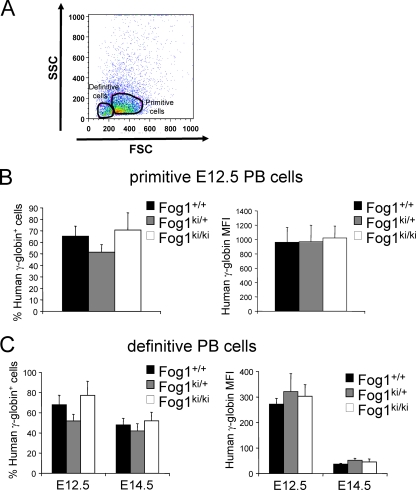
We extended our FACS-based measurements of fetal hemoglobin protein levels to peripheral red blood cells from E12.5 and E14.5 embryos. Primitive cells were distinguished from definitive ones by forward scatter, as described previously (34) (Fig. 5A). Quantitative RT-PCR analysis of globin gene expression and morphological evaluation of FACS-analyzed cells confirmed the identity of the gated primitive and definitive populations (data not shown). The fractions of primitive and definitive cells expressing γ-globin as well as the corresponding MFIs were highly comparable among the three genotypes at both E12.5 and E14.5 (Fig. 5B and C). Moreover, between E12.5 and E14.5, the MFIs decreased comparably, regardless of genotype (Fig. 5C), thus further indicating that the FOG-1 mutations do not affect fetal globin gene silencing.
FIG. 5.
γ-Globin protein levels in primitive and definitive peripheral blood (PB) cells. (A) Forward scatter (FSC) was used to separate primitive from definitive peripheral red blood cells. Human γ-globin expression was evaluated in E12.5 primitive (B) and E12.5 and E14.5 definitive (C) peripheral blood cells. Too few primitive erythroid cells were present in E14.5 PB cells to allow for an accurate analysis of γ-globin. Primitive cells express higher levels of human fetal globin than definitive cells (∼3-fold higher mean fluorescence intensity in primitive cells) (B and C, right graphs). Bars represent standard errors of the means (E12.5, n = 4 to 8; E14.5, n = 4 to 11).
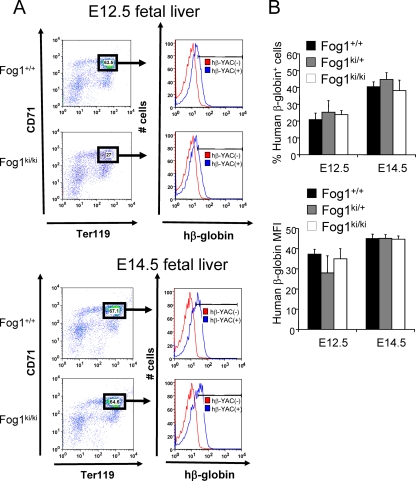
Using FACS, we also examined human β-globin protein levels in fetal liver Ter119high CD71high cells (Fig. 6A and B). At E12.5, Fog1+/+/hβ-YAC, Fog1ki/+/hβ-YAC, and Fog1ki/ki/hβ-YAC erythroblasts contained the same proportion of human β-globin-positive cells (20.85 ± 3.94 for Fog1+/+ versus 25.26 ± 6.91 for Fog1ki/+ versus 23.08 ± 2.49 for Fog1ki/ki) (Fig. 6A and B). These proportions increased similarly by day 14.5 (Fig. 6A and B). In addition, similar MFIs of β-globin-expressing cells were observed at E12.5 and E14.5 between all embryos regardless of genotypes (Fig. 6B), thus demonstrating that the human HBB gene can be activated in the absence of FOG-1/NuRD interaction.
FIG. 6.
β-Globin protein levels in E12.5 and E14.5 fetal livers. (A) Flow cytometry analysis of fetal liver cells. Fetal liver cells lacking hβ-YAC served as a negative control (red). (B) No significant differences in the frequencies of β-globin-expressing cells and the corresponding MFIs were found between cells from different FOG-1 backgrounds. Error bars represent standard errors of the means (E12.5, n = 5 to 9; E14.5, n = 4 to 11).
The FOG-1/NuRD interaction is dispensable for maintaining repression of murine embryonic globin genes in definitive erythroid cells.
Mechanisms are in place that distinguish primitive from definitive transcriptional programs. It has been reported recently that the zinc finger transcription factor BCL11A is essential for repressing murine embryonic globin genes in definitive cells (34). Since BCL11A interacts with FOG-1 and NuRD (33), the FOG/1NuRD interaction might play a role during BCL11A-mediated silencing of endogenous embryonic globin genes.
To test this possibility, we measured the levels of murine Hbb-y, Hbb-bh1, Hbb-b1, Hba-x, and Hba-a1 mRNAs in definitive erythroid cells from the bone marrow. As observed in Fog1ki/ki mice (28), Hba-a1 and Hbb-b1 globin gene expression was significantly reduced in Fog1ki/ki/hβ-YAC cells (Fig. 7A). However, Hbb-y and Hba-x mRNA levels were not affected by the FOG-1 mutation (Fig. 7A), and Hbb-bh1 mRNA was not detectable in any of the samples (data not shown). These results demonstrate that the FOG-1/NuRD interaction is dispensable for repressing primitive β-globin gene transcription in definitive erythroid cells.
Furthermore, normal levels of murine Hbb-y, Hbb-b1, Hba-x, and Hba-a1 transcripts were observed in both E12.5 and E14.5 Fog1ki/ki/hβ-YAC embryos (Fig. 7B), thus indicating that the FOG-1 mutations do not affect murine globin switching.
Overall, our data do not support a role of a GATA-1/FOG-1/NuRD axis in the developmental silencing of human and murine embryonic and fetal gene expression. Instead, NuRD is required for the full expression of both human and murine adult-type globin genes. Although the latter finding is surprising in light of NuRD's typical function as a corepressor complex, these results are consistent with previous work demonstrating a direct role for NuRD during transcriptional activation of select GATA-1/FOG-1 target genes (28).
GATA-1 has been proposed to directly function in the silencing of human γ-globin expression. Support for this model derived from ChIP experiments detecting GATA-1, FOG-1, and Mi-2ß near the HBG1 −566 and HBG2 −567 GATA sites at E18.5 of development when γ-globin is silenced (17). Our experiments using a mouse model in which the FOG-1/NuRD interaction is disrupted indicate that repression occurs independently of the FOG-1/NuRD pathway. However, this does not rule out a potential role of FOG-1 during γ-globin silencing, which might occur via other FOG-1-binding partners. Furthermore, it is possible that GATA-1 functions independently of FOG-1 in the process. Currently, it is unknown whether patients with a mutation in GATA-1 that impairs FOG-1 binding produce elevated levels of γ-globin.
It is important to note that our studies do not exclude a function for NuRD during the silencing of human γ-globin expression that occurs independently of FOG-1. NuRD interacts with diverse nuclear factors to regulate gene expression. Prominent among these is BCL11A, which has recently been implicated in the silencing of γ-globin expression (7, 24, 33, 34, 41). BCL11A contains at its N terminus a NuRD-binding motif that is identical to that found in FOG-1. Future studies aimed at exploring a broader role of the NuRD complex in γ-globin gene repression might involve the disruption of the BCL11A/NuRD interaction in vivo, similar to the experiments reported here.
Acknowledgments
We thank Wei Hong for help with the breeding of Fog1ki/ki/hβ-YAC mice, Stephan Kadauke for the critical reading of the manuscript, and the members of Blobel and Weiss laboratories for valuable discussions.
This work was supported by NIH grants DK54937 and DK58044.
Footnotes
Published ahead of print on 3 May 2010.
REFERENCES
- 1.Asano, H., X. S. Li, and G. Stamatoyannopoulos. 2000. FKLF-2: a novel Kruppel-like transcriptional factor that activates globin and other erythroid lineage genes. Blood 95:3578-3584. [PubMed] [Google Scholar]
- 2.Asano, H., X. S. Li, and G. Stamatoyannopoulos. 1999. FKLF, a novel Kruppel-like factor that activates human embryonic and fetal beta-like globin genes. Mol. Cell. Biol. 19:3571-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu, P., P. E. Morris, J. L. Haar, M. A. Wani, J. B. Lingrel, K. M. Gaensler, and J. A. Lloyd. 2005. KLF2 is essential for primitive erythropoiesis and regulates the human and murine embryonic beta-like globin genes in vivo. Blood 106:2566-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauchwitz, R., and F. Costantini. 2000. Developmentally distinct effects on human epsilon-, gamma- and delta-globin levels caused by the absence or altered position of the human beta-globin gene in YAC transgenic mice. Hum. Mol. Genet. 9:561-574. [DOI] [PubMed] [Google Scholar]
- 5.Chakalova, L., D. Carter, E. Debrand, B. Goyenechea, A. Horton, J. Miles, C. Osborne, and P. Fraser. 2005. Developmental regulation of the beta-globin gene locus. Prog. Mol. Subcell. Biol. 38:183-206. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Z., H. Y. Luo, R. K. Basran, T. H. Hsu, D. W. Mang, L. Nuntakarn, C. G. Rosenfield, G. P. Patrinos, R. C. Hardison, M. H. Steinberg, and D. H. Chui. 2008. A T-to-G transversion at nucleotide −567 upstream of HBG2 in a GATA-1 binding motif is associated with elevated hemoglobin F. Mol. Cell. Biol. 28:4386-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Z., H. Y. Luo, M. H. Steinberg, and D. H. Chui. 2009. BCL11A represses HBG transcription in K562 cells. Blood Cells Mol. Dis. 42:144-149. [DOI] [PubMed] [Google Scholar]
- 8.Ciovacco, W. A., W. H. Raskind, and M. A. Kacena. 2008. Human phenotypes associated with GATA-1 mutations. Gene 427:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crispino, J. D., M. B. Lodish, J. P. MacKay, and S. H. Orkin. 1999. Use of altered specificity mutants to probe a specific protein-protein interaction in differentiation: the GATA-1:FOG complex. Mol. Cell 3:219-228. [DOI] [PubMed] [Google Scholar]
- 10.Deconinck, A. E., P. E. Mead, S. G. Tevosian, J. D. Crispino, S. G. Katz, L. I. Zon, and S. H. Orkin. 2000. FOG acts as a repressor of red blood cell development in Xenopus. Development 127:2031-2040. [DOI] [PubMed] [Google Scholar]
- 11.Donze, D., T. M. Townes, and J. J. Bieker. 1995. Role of erythroid Kruppel-like factor in human gamma- to beta-globin gene switching. J. Biol. Chem. 270:1955-1959. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira, R., K. Ohneda, M. Yamamoto, and S. Philipsen. 2005. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol. Cell. Biol. 25:1215-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox, A. H., C. Liew, M. Holmes, K. Kowalski, J. Mackay, and M. Crossley. 1999. Transcriptional cofactors of the FOG family interact with GATA proteins by means of multiple zinc fingers. EMBO J. 18:2812-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaensler, K. M., M. Kitamura, and Y. W. Kan. 1993. Germ-line transmission and developmental regulation of a 150-kb yeast artificial chromosome containing the human beta-globin locus in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 90:11381-11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garriga-Canut, M., and S. H. Orkin. 2004. Transforming acidic coiled-coil protein 3 (TACC3) controls friend of GATA-1 (FOG-1) subcellular localization and regulates the association between GATA-1 and FOG-1 during hematopoiesis. J. Biol. Chem. 279:23597-23605. [DOI] [PubMed] [Google Scholar]
- 16.Grass, J. A., M. E. Boyer, S. Pal, J. Wu, M. J. Weiss, and E. H. Bresnick. 2003. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. U. S. A. 100:8811-8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harju-Baker, S., F. C. Costa, H. Fedosyuk, R. Neades, and K. R. Peterson. 2008. Silencing of Aγ-globin gene expression during adult definitive erythropoiesis mediated by GATA-1-FOG-1-Mi2 complex binding at the −566 GATA site. Mol. Cell. Biol. 28:3101-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong, W., M. Nakazawa, Y. Y. Chen, R. Kori, C. R. Vakoc, C. Rakowski, and G. A. Blobel. 2005. FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J. 24:2367-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isern, J., S. T. Fraser, Z. He, and M. H. Baron. 2008. The fetal liver is a niche for maturation of primitive erythroid cells. Proc. Natl. Acad. Sci. U. S. A. 105:6662-6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jane, S. M., P. A. Ney, E. F. Vanin, D. L. Gumucio, and A. W. Nienhuis. 1992. Identification of a stage selector element in the human gamma-globin gene promoter that fosters preferential interaction with the 5′ HS2 enhancer when in competition with the beta-promoter. EMBO J. 11:2961-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jane, S. M., A. W. Nienhuis, and J. M. Cunningham. 1995. Hemoglobin switching in man and chicken is mediated by a heteromeric complex between the ubiquitous transcription factor CP2 and a developmentally specific protein. EMBO J. 14:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz, S. G., A. B. Cantor, and S. H. Orkin. 2002. Interaction between FOG-1 and the corepressor C-terminal binding protein is dispensable for normal erythropoiesis in vivo. Mol. Cell. Biol. 22:3121-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letting, D. L., Y. Y. Chen, C. Rakowski, S. Reedy, and G. A. Blobel. 2004. Context-dependent regulation of GATA-1 by friend of GATA-1. Proc. Natl. Acad. Sci. U. S. A. 101:476-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lettre, G., V. G. Sankaran, M. A. Bezerra, A. S. Araujo, M. Uda, S. Sanna, A. Cao, D. Schlessinger, F. F. Costa, J. N. Hirschhorn, and S. H. Orkin. 2008. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc. Natl. Acad. Sci. U. S. A. 105:11869-11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath, K. E., P. D. Kingsley, A. D. Koniski, R. L. Porter, T. P. Bushnell, and J. Palis. 2008. Enucleation of primitive erythroid cells generates a transient population of “pyrenocytes” in the mammalian fetus. Blood 111:2409-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzel, S., C. Garner, I. Gut, F. Matsuda, M. Yamaguchi, S. Heath, M. Foglio, D. Zelenika, A. Boland, H. Rooks, S. Best, T. D. Spector, M. Farrall, M. Lathrop, and S. L. Thein. 2007. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat. Genet. 39:1197-1199. [DOI] [PubMed] [Google Scholar]
- 27.Miccio, A., R. Cesari, F. Lotti, C. Rossi, F. Sanvito, M. Ponzoni, S. J. Routledge, C. M. Chow, M. N. Antoniou, and G. Ferrari. 2008. In vivo selection of genetically modified erythroblastic progenitors leads to long-term correction of beta-thalassemia. Proc. Natl. Acad. Sci. U. S. A. 105:10547-10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miccio, A., Y. Wang, W. Hong, G. D. Gregory, H. Wang, X. Yu, J. K. Choi, S. Shelat, W. Tong, M. Poncz, and G. A. Blobel. 2010. NuRD mediates activating and repressive functions of GATA-1 and FOG-1 during blood development. EMBO J. 29:442-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munugalavadla, V., L. C. Dore, B. L. Tan, L. Hong, M. Vishnu, M. J. Weiss, and R. Kapur. 2005. Repression of c-Kit and its downstream substrates by GATA-1 inhibits cell proliferation during erythroid maturation. Mol. Cell. Biol. 25:6747-6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols, K. E., J. D. Crispino, M. Poncz, J. G. White, S. H. Orkin, J. M. Maris, and M. J. Weiss. 2000. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat. Genet. 24:266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porcu, S., M. Kitamura, E. Witkowska, Z. Zhang, A. Mutero, C. Lin, J. Chang, and K. M. Gaensler. 1997. The human beta globin locus introduced by YAC transfer exhibits a specific and reproducible pattern of developmental regulation in transgenic mice. Blood 90:4602-4609. [PubMed] [Google Scholar]
- 32.Raich, N., C. H. Clegg, J. Grofti, P. H. Romeo, and G. Stamatoyannopoulos. 1995. GATA1 and YY1 are developmental repressors of the human epsilon-globin gene. EMBO J. 14:801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankaran, V. G., T. F. Menne, J. Xu, T. E. Akie, G. Lettre, B. Van Handel, H. K. Mikkola, J. N. Hirschhorn, A. B. Cantor, and S. H. Orkin. 2008. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322:1839-1842. [DOI] [PubMed] [Google Scholar]
- 34.Sankaran, V. G., J. Xu, T. Ragoczy, G. C. Ippolito, C. R. Walkley, S. D. Maika, Y. Fujiwara, M. Ito, M. Groudine, M. A. Bender, P. W. Tucker, and S. H. Orkin. 2009. Developmental and species-divergent globin switching are driven by BCL11A. Nature 460:1093-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Socolovsky, M., H. Nam, M. D. Fleming, V. H. Haase, C. Brugnara, and H. F. Lodish. 2001. Ineffective erythropoiesis in Stat5a−/− 5b−/− mice due to decreased survival of early erythroblasts. Blood 98:3261-3273. [DOI] [PubMed] [Google Scholar]
- 36.Stamatoyannopoulos, G. 2005. Control of globin gene expression during development and erythroid differentiation. Exp. Hematol. 33:259-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanabe, O., F. Katsuoka, A. D. Campbell, W. Song, M. Yamamoto, K. Tanimoto, and J. D. Engel. 2002. An embryonic/fetal beta-type globin gene repressor contains a nuclear receptor TR2/TR4 heterodimer. EMBO J. 21:3434-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanabe, O., D. McPhee, S. Kobayashi, Y. Shen, W. Brandt, X. Jiang, A. D. Campbell, Y. T. Chen, C. Chang, M. Yamamoto, K. Tanimoto, and J. D. Engel. 2007. Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4. EMBO J. 26:2295-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripic, T., W. Deng, Y. Cheng, Y. Zhang, C. R. Vakoc, G. D. Gregory, R. C. Hardison, and G. A. Blobel. 2009. SCL and associated proteins distinguish active from repressive GATA transcription factor complexes. Blood 113:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsang, A. P., J. E. Visvader, C. A. Turner, Y. Fujiwara, C. Yu, M. J. Weiss, M. Crossley, and S. H. Orkin. 1997. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell 90:109-119. [DOI] [PubMed] [Google Scholar]
- 41.Uda, M., R. Galanello, S. Sanna, G. Lettre, V. G. Sankaran, W. Chen, G. Usala, F. Busonero, A. Maschio, G. Albai, M. G. Piras, N. Sestu, S. Lai, M. Dei, A. Mulas, L. Crisponi, S. Naitza, I. Asunis, M. Deiana, R. Nagaraja, L. Perseu, S. Satta, M. D. Cipollina, C. Sollaino, P. Moi, J. N. Hirschhorn, S. H. Orkin, G. R. Abecasis, D. Schlessinger, and A. Cao. 2008. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc. Natl. Acad. Sci. U. S. A. 105:1620-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, X., J. D. Crispino, D. L. Letting, M. Nakazawa, M. Poncz, and G. A. Blobel. 2002. Control of megakaryocyte-specific gene expression by GATA-1 and FOG-1: role of Ets transcription factors. EMBO J. 21:5225-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch, J. J., J. A. Watts, C. R. Vakoc, Y. Yao, H. Wang, R. C. Hardison, G. A. Blobel, L. A. Chodosh, and M. J. Weiss. 2004. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood 104:3136-3147. [DOI] [PubMed] [Google Scholar]
- 44.Zhou, W., D. R. Clouston, X. Wang, L. Cerruti, J. M. Cunningham, and S. M. Jane. 2000. Induction of human fetal globin gene expression by a novel erythroid factor, NF-E4. Mol. Cell. Biol. 20:7662-7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, W., Q. Zhao, R. Sutton, H. Cumming, X. Wang, L. Cerruti, M. Hall, R. Wu, J. M. Cunningham, and S. M. Jane. 2004. The role of p22 NF-E4 in human globin gene switching. J. Biol. Chem. 279:26227-26232. [DOI] [PubMed] [Google Scholar]