Abstract
ATM-dependent initiation of the radiation-induced G2/M checkpoint arrest is well established. Recent results have shown that the majority of DNA double-strand breaks (DSBs) in G2 phase are repaired by DNA nonhomologous end joining (NHEJ), while ∼15% of DSBs are slowly repaired by homologous recombination. Here, we evaluate how the G2/M checkpoint is maintained in irradiated G2 cells, in light of our current understanding of G2 phase DSB repair. We show that ATM-dependent resection at a subset of DSBs leads to ATR-dependent Chk1 activation. ATR-Seckel syndrome cells, which fail to efficiently activate Chk1, and small interfering RNA (siRNA) Chk1-treated cells show premature mitotic entry. Thus, Chk1 significantly contributes to maintaining checkpoint arrest. Second, sustained ATM signaling to Chk2 contributes, particularly when NHEJ is impaired by XLF deficiency. We also show that cells lacking the mediator proteins 53BP1 and MDC1 initially arrest following radiation doses greater than 3 Gy but are subsequently released prematurely. Thus, 53BP1−/− and MDC1−/− cells manifest a checkpoint defect at high doses. This failure to maintain arrest is due to diminished Chk1 activation and a decreased ability to sustain ATM-Chk2 signaling. The combined repair and checkpoint defects conferred by 53BP1 and MDC1 deficiency act synergistically to enhance chromosome breakage.
DNA double-strand breaks (DSBs) activate the DNA damage response (DDR), a coordinated process that functions to enhance survival and maintain genomic stability. The DDR includes pathways of DSB repair and a signal transduction response that activates apoptosis and cell cycle checkpoint arrest and influences DSB repair (15). DNA nonhomologous end joining (NHEJ) and homologous recombination (HR) represent the major DSB repair mechanisms, NHEJ being the major mechanism in G0/G1, while both processes function in G2 (9, 32). Ataxia telangiectasia mutated (ATM) and ATM- and Rad3-related (ATR) are related phosphoinositol 3-kinase-like kinases (PIKKs) that regulate the DNA damage signaling response. ATM is activated by DSBs, while ATR is activated at single-strand (ss) regions of DNA via a process that involves ATRIP-replication protein A (RPA)-ssDNA association. Ionizing radiation (IR) induces DSBs, base damage, and ss nicks. Since neither base damage nor ss nicks activate ATR, IR-induced signaling in the G1 and G2 phases is predominantly ATM dependent (3, 29). In S phase, ATR can be activated by both endogenous and exogenously induced lesions following replication fork stalling/collapse (8).
Recent work has shown that in G2 phase, DSBs can undergo resection via an ATM-dependent process generating ssDNA regions that can activate ATR following RPA association (11). ATR activation at resected DSBs is coupled to loss of ATM activation (11). Although ATM and ATR share overlapping substrates, there is specificity in their signaling to the transducer kinases; ATM uniquely phosphorylates Chk2, while ATR phosphorylates Chk1. Phosphorylation of either Chk1 or Chk2 causes their activation. Critical targets of Chk1/Chk2 are the Cdc25 phosphatases, which regulate the cyclin-dependent kinases (Cdks), including Cdk1, the regulator of mitotic entry (18). Collectively, these studies suggest that two components of ATM-dependent signaling to the G2/M checkpoint machinery can occur: ATM-Chk2 signaling at unresected DSBs and ATM-ATR-Chk1 signaling at resected DSBs.
Although much is known about the mechanism leading to G2/M checkpoint activation, few studies have addressed how arrest is maintained and how release coordinates with the status of DSB repair. We examine here the maintenance of checkpoint arrest during the immediate phase of DSB repair. We do not address the issue of checkpoint adaptation, a distinct phenomenon which occurs after prolonged checkpoint arrest (22). Further, we focus on the process maintaining arrest in irradiated G2-phase cells and do not consider how arrest is maintained in irradiated S-phase cells that progress into G2 phase. (Previous studies have shown that while G2/M arrest is ATM dependent at early times post-IR, at later times it becomes ATR dependent as S-phase cells progress into G2 phase [2, 33].) To focus on mechanisms maintaining ATM-dependent signaling in G2-phase cells, we use aphidicolin (APH) to prevent S-phase cells from progressing into G2 during analysis. We, thus, examine checkpoint maintenance in cells irradiated in G2 phase and do not evaluate arrest regulated by ATR following replication fork stalling. The basis for our work stems from two recent advances. First, we evaluate the impact of ATM-mediated ATR activation in the light of recent findings that resection occurs in G2 phase (11). Second, we consider the finding that NHEJ represents the major DSB repair mechanism in G2 and that a 15 to 20% subset of DSBs, representing those that are rejoined with slow kinetics in an ATM-dependent manner, undergo resection and repair by HR (3, 25). Thus, contrary to the notion that HR represents the major DSB repair pathway in G2 phase, it repairs only 15 to 20% of X- or gamma-ray-induced DSBs and represents the slow component of DSB repair in G2 phase. Given these findings, several potential models for how checkpoint arrest is maintained in G2 can be envisaged. A simple model is that the initial signal generated by IR is maintained for a defined time to allow for DSB repair. Such a model appears to explain the kinetics of checkpoint signaling in fission yeast after moderate IR (17). In mammalian cells, the duration of arrest depends on dose and DSB repair capacity (6). Thus, it is possible that the status of ongoing repair is communicated to the checkpoint machinery to coordinate timely release with the process of DSB repair. Here, we consider the impact of resection leading to ATM-ATR-Chk1 signaling versus ATM-Chk2 signaling from nonresected DSBs and how they interplay to maintain rather than initiate checkpoint arrest.
Mediator proteins, including 53BP1 and MDC1, assemble at DSBs in an ATM-dependent manner, but their roles in the DDR are unclear. Cells lacking 53BP1 or MDC1 are proficient in checkpoint initiation after moderate IR doses, leading to the suggestion that these proteins are required for amplification of the ATM signal after exposure to low doses but are dispensable after high doses, when a robust signal is generated, even in their absence (7, 16, 28, 31). Despite their apparent subtle role in ATM signaling, cells lacking these mediator proteins display significant genomic instability (19). We thus also examine whether the mediator proteins contribute to the maintenance of checkpoint arrest.
We identify two ATM-dependent processes that contribute to the maintenance of checkpoint arrest in G2-phase cells: (i) ATR-Chk1 activation at resected DSBs and (ii) a process that involves sustained signaling from ATM to Chk2 at unrepaired DSBs. Further, we show that 53BP1 and MDC1 are required for maintaining checkpoint arrest, even following exposure to high radiation doses due to roles in ATR-Chk1 activation and sustained ATM-Chk2 signaling, and that this contributes to their elevated genomic instability.
MATERIALS AND METHODS
Cell culture, irradiation, and drug treatment.
1BR3 (wild-type [WT]) hTERT, ATR-Seckel (ATR-SS) hTERT, and 2BN (XLF−/−) hTERT are immortalized human fibroblasts from normal, ATR-defective, and XLF-defective individuals, respectively. MDC1−/− and 53BP1−/− mouse embryo fibroblasts (MEFs) were a gift from J. Chen. All fibroblast cells were cultured in minimal essential medium (MEM) or Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum (FCS). Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines (LBLs) were cultured in RPMI with 15% FCS. GM2188 and DK0064 are wild-type and ATR-defective Seckel LBLs, respectively. Gamma irradiation was from a 137Cs source at a dose rate of 7.5 Gy/min. X-irradiation was carried out at a dose rate of 2 Gy/min. The ATM inhibitor (ATMi) KU55933 and the DNA-PK inhibitor NU7441 were gifts from KuDOS Pharmaceuticals (Cambridge, United Kingdom). A total of 10 μM KU55933 (ATM inhibitor) and/or 10 μM NU7441 (DNA-PK inhibitor) was added at the times indicated. A total of 2.5 μM SB218078 (Chk1/Chk2 inhibitor) was added 30 min post-IR.
siRNA knockdown.
Small interfering RNA (siRNA) transfection of A549, 1BR3 (WT) hTERT, and 2BN (XLF−/−) hTERT cells was carried out using HiPerFect (Qiagen, Hilden, Germany). siRNA oligonucleotides against scrambled control, Chk1, Chk2, 53BP1, and XLF were obtained from the Dharmacon SMARTpool siRNA (these oligonucleotides are labeled “#1” in figures). The sequence of siRNA oligonucleotides against Chk1 was 5′-AAU CGU GAG CGU UUG UUG AAC TT-3′, and Chk2 was obtained from Qiagen (Hilden, Germany) (these oligonucleotides are labeled #2 in figures).
Antibodies for immunofluorescence and immunoblotting.
Procedures used were as described previously (6) using antibodies against γ-H2AX (Upstate Biotechnology, Buckingham, United Kingdom), CENP-F (Santa Cruz, Santa Cruz, CA), pSer 10 histone H3 (Upstate Biotechnology, Buckingham, United Kingdom), Chk2 pThr68 (Cell Signaling Technology, Beverly, MA), Chk2 (Abcam, Cambridge, United Kingdom), Chk1 pSer317 (Abcam, Cambridge, United Kingdom), and β-tubulin (Abcam, Cambridge, United Kingdom). Slides were visualized using a Zeiss Axioplan microscope, and image processing was performed on Simple PCI software. Signal intensity following immunofluorescence (IF) or immunoblotting was analyzed using NIH Image-J. IR-induced intensity was calculated by subtracting the signal in nuclei without damage from that in IR-treated nuclei.
G2/M checkpoint analysis.
For G2/M checkpoint analysis, exponentially growing cells were irradiated on glass coverslips. Cells were stained with pSer10-histone H3 and DAPI (4′,6-diamidino-2-phenylindole), and pSer10-histone H3-positive and condensed chromatin cells were counted as mitotic cells. A total of 3 μM aphidicolin was routinely added to block entry of irradiated S-phase cells into G2 during analysis.
Analysis of chromosome and chromatid breaks in mitotic cells.
Exponentially growing MEFs were irradiated with 3 Gy IR, and colcemid (100 ng/ml) was added after 2 h (Sigma-Aldrich, Poole, United Kingdom). Cells were fixed for metaphase preparation 12 h post-IR using standard protocols. Slides were stained with 3% Giemsa for 3 min. Chromosome spreads were captured using a Zeiss Axioplan2 microscope and Isis software (MetaSystems, Altlussheim, Germany), and chromatid breaks were counted.
Premature chromosome condensation (PCC) analysis.
Cells were treated with 50 ng/ml calyculin A (Calbiochem) for 20 to 30 min before harvesting. Chromatid breaks and excess fragments (counted as two chromatid breaks) were scored in 100-chromosome spreads from at least three independent experiments per data point.
RESULTS
Chk1/Chk2 regulates the initiation and maintenance of checkpoint arrest.
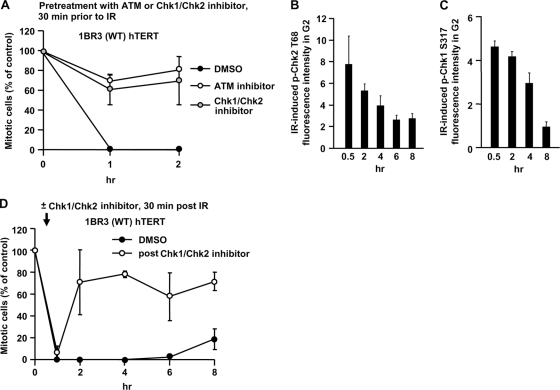
First, we verified that ATM and Chk1/Chk2 function in initiating checkpoint arrest by adding the ATM inhibitor KU55933 or the Chk1/Chk2 inhibitor SB218078 (here called ATM or Chk1/Chk2 inhibitor, respectively) 30 min prior to exposure to 3 Gy IR in 1BR3 (WT) hTERT cells. Both treatments abolished G2/M checkpoint arrest at 1 and 2 h post-IR (Fig. 1A), demonstrating that ATM and Chk1/Chk2 are required for checkpoint initiation.
FIG. 1.
Chk1/Chk2 regulates the initiation and maintenance of checkpoint arrest. (A) Checkpoint initiation requires ATM and Chk1/Chk2. Mitotic entry was examined in 1BR3 hTERT cells following 3 Gy IR in untreated cells or cells treated with the ATM inhibitor or Chk1/Chk2 inhibitor 30 min prior to IR. Cells, >400, were scored for mitotic index. Results represent the mitotic index relative to untreated cells. Maximal levels of p-Chk1 and p-Chk2 are reached within 30 min after 3 Gy. 1BR hTERT and A549 cells were exposed to 3 Gy IR, and p-Chk2 (B) and p-Chk1 (C) levels assessed by IF at various times post-IR. (D) Checkpoint maintenance requires Chk1/Chk2. The time of mitotic entry following exposure to 3 Gy IR in cells either untreated or treated with the Chk1/Chk2 inhibitor 30 min post-IR was examined. Error bars represent the standard error of the mean (SEM) from 3 experiments.
Next, we examined whether Chk1 and Chk2 are required for checkpoint maintenance (the role of ATM is examined below). In trial experiments, we observed that neither p-Chk1 nor p-Chk2 levels showed any further increase 30 min after IR, i.e., maximal levels were reached within the first 30 min (Fig. 1B and C). To assess the combined role of Chk1/Chk2 in checkpoint maintenance, we added the Chk1/Chk2 inhibitor 30 min post-IR (i.e., following maximal Chk1/Chk2 activation). Although arrest was maintained at 1 h post-IR (i.e., 30 min after Chk1/Chk2 inhibitor addition), mitotic entry commences by 2 h (Fig. 1D). This shows that Chk1 and -2 are the major components regulating checkpoint arrest and release rather than any downstream proteins, such as Cdc25. The rapid mitotic entry following Chk1/Chk2 inhibitor addition was subsequently used as a benchmark to monitor factors required for maintaining checkpoint arrest.
ATR-Chk1 activation at resected DSBs contributes to checkpoint maintenance.
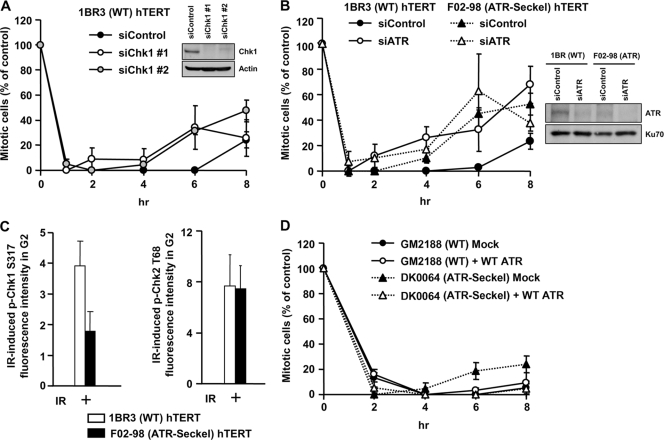
G2-phase DSBs can undergo ATM-dependent resection, leading to ATR-dependent Chk1 activation and loss of ATM activation (11). We recently observed that, contrary to the notion that HR represents the major DSB repair process in G2 phase, only 15 to 20% of IR-induced DSBs undergo resection in G2 phase (3). Therefore, since Chk1 is activated only at a fraction of IR-induced DSBs, we examined whether ATR-Chk1 contributes to IR-induced G2/M arrest. To examine checkpoint maintenance in irradiated G2-phase cells and to prevent progression of S-phase cells into G2 during analysis, we added aphidicolin (APH), an inhibitor of the replicative polymerase. Control experiments showing that APH inhibits progression of S-phase cells into late S/G2 phase are shown in Fig. S1A in the supplemental material. Additional controls showing that APH does not impact DSB repair in G2 phase are described in references 3 and 6. Moreover, IR-induced sister chromatid exchanges (SCEs) in G2 phase, an established marker for HR, are unaffected by APH treatment (see Fig. S1B in the supplemental material). To directly examine the role of Chk1 in G2/M checkpoint arrest, we used two distinct oligonucleotides for Chk1 siRNA and found that arrest was initiated normally but was not efficiently maintained (Fig. 2A). We also observed that treatment with UCN-01, a Chk1-specific inhibitor at the concentration used, impairs checkpoint maintenance and does not impact checkpoint initiation (data not shown). We also examined mitotic entry in ATR-Seckel (ATR-SS) hTERT cells, which have impaired ATR activity (21). Strikingly, although ATR-SS hTERT cells activate G2/M arrest normally following 3 Gy IR, they enter mitosis earlier (4 to 6 h) than control cells (6 to 8 h) (Fig. 2B). We show, as a control, that ATR loss reduces p-Chk1 levels but does not affect resection or p-Chk2 in G2 using CENP-F to identify G2 cells and quantifying p-Chk1 and p-Chk2 levels by IF (Fig. 2C). The specificity of the anti-p-Chk1 and anti-p-Chk2 antibodies for IF is shown in Fig. S2A to F in the supplemental material. As a further approach, we used ATR siRNA to deplete ATR in 1BR3 hTERT (WT) and ATR-SS hTERT cells. ATR siRNA-treated control cells showed a pattern of checkpoint arrest and maintenance similar to that observed with ATR-SS cells (Fig. 2B). Further, although ATR siRNA in ATR-SS cells reduced ATR expression levels, the kinetics of checkpoint entry remained similar to that observed with ATR-SS cells, suggesting that residual ATR activity in ATR-SS cells does not appreciably contribute to the arrest observed. Finally, we also employed ATR-SS lymphoblastoid cells (LBLs) for complementation analysis. Like ATR-SS hTERT cells, ATR-SS LBLs initiate checkpoint arrest normally but show premature mitotic entry (Fig. 2D). Importantly, introduction of ATR cDNA into ATR-SS LBLs conferred prolonged checkpoint arrest similar to that observed with control cells.
FIG. 2.
ATR-Chk1 signaling contributes to checkpoint maintenance. (A) Mitotic entry in 1BR3 hTERT cells following treatment with control and Chk1 siRNA and 3 Gy IR was examined. Chk1 knockdown did not significantly compromise G2 proportion based on fluorescence-activated cell sorting (FACS) analysis (data not shown). (B) Mitotic entry in 1BR3 hTERT and ATR-SS hTERT cells following treatment with control or ATR siRNA and 3 Gy IR. The right panel shows ATR expression levels. (C) ATR-SS hTERT cells show impaired Chk1 phosphorylation but normal Chk2 phosphorylation. The levels of p-Chk1 Ser317 and p-Chk2 Thr68 were quantified by IF in ATR-SS hTERT G2 cells at 30 min after IR exposure. (D) Mitotic entry in control and ATR-SS LBL cell lines following mock and ATR cDNA transfection. To verify the impact of Chk1 siRNA on Chk1 activity, we examined hydroxyurea (HU)-induced 53BP1 focus formation, which is a characterized Chk1-dependent response (26). 53BP1 foci failed to form after HU treatment in ATR-SS hTERT cells and following Chk1 siRNA, consistent with previous findings (data not shown) (30). Error bars represent the SEM from 3 experiments.
Collectively, these findings provide strong evidence that ATR-Chk1 contributes to checkpoint maintenance after 3 Gy IR. They also distinguish the initiation of G2/M checkpoint arrest, which has either no or a less stringent requirement for ATR-Chk1, from the maintenance of arrest, which is compromised when either ATR or Chk1 activity is impaired. A greater role for ATR-Chk1 in maintaining arrest is consistent with our finding that HR represents the slow component of DSB repair in G2 phase (3). Thus, while only 15 to 20% of induced DSBs undergo resection and activate Chk1, at late times post-IR, the resected DSBs represent a much higher percentage of the unrepaired DSBs.
Sustained ATM-Chk2 signaling also contributes to the maintenance of checkpoint arrest.
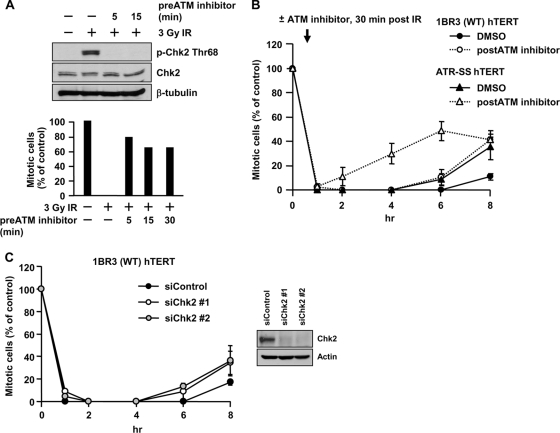
Next, we considered the contribution of Chk2 to maintaining G2/M arrest and examined whether sustained ATM-Chk2 signaling might contribute—i.e., whether unrepaired DSBs might lead to the prolongation of Chk2 activation. To investigate this, we examined the impact of the ATM inhibitor added 30 min after 3 Gy IR—i.e., when checkpoint arrest had been initiated and maximal phosphorylated Chk1/Chk2 levels had been attained. In control experiments, we show that ATM inhibitor addition 5 or 15 min prior to IR completely inhibits Chk2 phosphorylation and checkpoint arrest (Fig. 3A), indicating that ATM inhibitor addition inhibits ATM activity within 5 min. Following ATM inhibitor addition 30 min post-IR to 1BR3 (WT) hTERT cells, mitotic entry commenced at 4 to 6 h post-IR (Fig. 3B). Thus, mitotic entry occurred earlier than in control (dimethyl sulfoxide [DMSO]-treated) cells (which enter mitosis at 6 to 8 h), implicating ATM signaling not only in initiating the G2/M checkpoint but also in its full maintenance.
FIG. 3.
Sustained ATM signaling also contributes to checkpoint maintenance. (A) ATM inhibitor addition impairs checkpoint arrest and signaling within 5 min. 1BR3 hTERT cells were untreated or treated with ATM inhibitor 5, 15, or 30 min prior to IR. Cells were examined by immunoblotting using anti-Thr68-p-Chk2 at 30 min (top) and for mitotic entry at 1 h post-IR (bottom). Chk2 and β-tubulin antibodies were loading controls. (B) ATM inhibitor addition 30 min post-IR diminishes the duration of checkpoint arrest after 3 Gy IR in 1BR3 hTERT and in ATR-SS hTERT cells. (C) siRNA-Chk2 diminishes the duration of checkpoint arrest after 3 Gy IR. Error bars represent the SEM of 3 experiments.
We also examined the impact of ATM inhibitor addition 30 min post-IR on ATR-dependent Chk1 activation. Two hours after ATM inhibitor addition, the levels of RPA foci and p-Chk1 were similar to that of control cells, but by 8 h both were elevated (see Fig. S2G and H in the supplemental material). Thus, maximal resection and Chk1 activation occur within the first 30 min post-IR, but subsequent ATM inhibitor addition impairs RPA focus loss. However, despite the elevated level of p-Chk1 at 8 h, ATM inhibitor-treated cells were released prematurely from checkpoint arrest (Fig. 3B). This strongly implies that Chk2 contributes to checkpoint maintenance and that elevated Chk1 activity cannot fully substitute. As expected, ATM inhibitor addition to ATR-SS hTERT cells resulted in very early checkpoint release (at 2 to 4 h) (Fig. 3B) since this reduces ATR-Chk1 and ATM-Chk2 signaling.
We further examined the impact of Chk2 using Chk2 siRNA. Following 3 Gy IR, checkpoint arrest was initiated normally but released prematurely compared to control cells (Fig. 3C), suggesting that there is redundancy between Chk1 and Chk2 in checkpoint initiation, but both Chk1 and Chk2 contribute to its maintenance. In summary, these findings provide preliminary evidence that sustained ATM signaling to Chk2 represents an additional process that contributes to checkpoint maintenance.
Sustained ATM signaling enhances the duration of checkpoint arrest by maintaining p-Chk2 levels in control and, more strikingly, in NHEJ-defective cells.
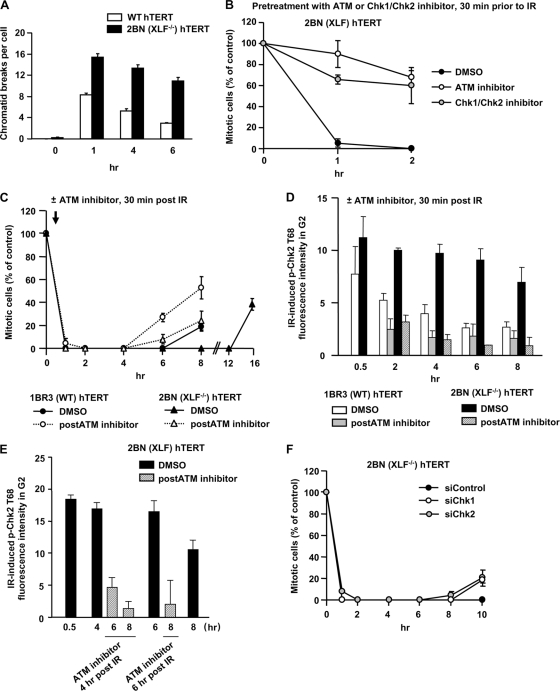
We reasoned that sustained ATM signaling might be optimally examined with a cell line in which elevated levels of unrepaired and nonresected DSBs persist. We examined 2BN hTERT cells, which are defective in the NHEJ factor XLF (1, 4, 5). 2BN (XLF−/−) hTERT cells show elevated calyculin-induced premature chromosome condensation (PCC), a procedure that monitors DSB repair in G2 phase (Fig. 4A). Thus, 2BN (XLF−/−) hTERT cells show substantially reduced DSB rejoining in G2 phase, consistent with the notion that NHEJ is the major DSB repair process in G1 and G2. Further, 2BN (XLF−/−) hTERT cells, unlike Ku-defective cells, do not show significantly increased RPA or Rad51 foci at 2 to 4 h post-IR, suggesting that they do not undergo excessively high resection (data not shown).
FIG. 4.
Sustained ATM signaling contributes to prolonged checkpoint arrest in NHEJ-defective cells. (A) Cells were exposed to 1 Gy IR, and APH was added. A total of 50 ng/ml calyculin A was added 30 min before fixation, and PCCs were scored. (B) ATM and Chk1/Chk2 are required for checkpoint initiation in 2BN (XLF−/−) hTERT cells. Mitotic entry was examined following 3 Gy IR in 2BN (XLF−/−) hTERT cells either untreated or treated with the ATM or Chk1/Chk2 inhibitor 30 min prior to IR. (C) Prolonged checkpoint arrest in 2BN (XLF−/−) hTERT cells requires sustained ATM signaling. Mitotic entry after 3 Gy IR was examined in 1BR3 and 2BN (XLF−/−) hTERT cells either with or without ATM inhibitor 30 min post-IR. (D) 1BR3 and 2BN (XLF−/−) hTERT cells were exposed to 3 Gy IR with or without ATM inhibitor addition 30 min post-IR. p-Chk2 levels were quantified in CENP-F+ (G2) cells by IF using anti-Thr68-p-Chk2 antibodies. The background signal in unirradiated cells was subtracted. At <1 h post-IR, 2BN (XLF−/−) hTERT cells harbor elevated DSBs compared to 1BR3 hTERT cells (as shown in panel A), consistent with their elevated p-Chk2 signal. By 8 h the p-Chk2 level decreased by >50% in 1BR3 hTERT cells compared with 20% in 2BN (XLF−/−) hTERT cells. (E) 2BN (XLF−/−) hTERT cells were exposed to 3 Gy IR with or without ATM inhibitor addition 4 or 6 h post-IR. p-Chk2 levels were quantified in CENP-F+ (G2) cells by IF using anti-Thr68-p-Chk2 antibodies. (F) The maintenance of checkpoint arrest in 2BN (XLF−/−) hTERT cells requires Chk1 and Chk2. Mitotic entry was examined in 2BN (XLF−/−) hTERT cells treated with control, Chk1, or Chk2 siRNA. Error bars represent the SEM of 3 experiments.
First, we demonstrated that ATM or Chk1/Chk2 inhibitor addition prior to IR abolished checkpoint arrest in 2BN (XLF−/−) hTERT cells (Fig. 4B). Next, we examined the duration of checkpoint arrest in 2BN (XLF−/−) hTERT cells. Following 3 Gy IR, 1BR3 (WT) hTERT cells enter mitosis at ∼8 h, while 2BN (XLF−/−) hTERT cells arrest for >12 h (Fig. 4C). 2BN (XLF−/−) hTERT cells exposed to 6 or 9 Gy IR arrest for >24 h (data not shown). Given the characterized role of XLF in DSB repair, these findings show that the duration of checkpoint arrest is dependent upon dose and DSB repair capacity, indicating that unrepaired DSBs cause prolonged arrest. Thus, the status of DSB repair is continuously monitored and communicated to the checkpoint machinery. We next added ATM inhibitor 30 min post-IR to 2BN (XLF−/−) hTERT cells and observed premature release at 6 to 8 h (Fig. 4C), demonstrating that sustained ATM signaling plays a significant role in maintaining arrest in a repair-defective background.
The process of sustained ATM signaling to Chk2, although arguably expected, has not been examined previously. Therefore, we determined whether sustained ATM signaling maintains p-Chk2 levels. We examined p-Chk2 levels in G2-phase cells since Chk2 activation might differ in S phase and since G1-phase cells do not undergo detectable resection (11). We achieved this by quantifying p-Chk2 by IF in G2 cells identified by CENP-F staining. 1BR3 (WT) hTERT cells were irradiated with 3 Gy IR, and ATM inhibitor was added 30 min post-IR. We observed elevated p-Chk2 following IR, which by 2 and 4 h had decayed to a greater extent in the presence of ATM inhibitor (Fig. 4D). At later times the assay was too insensitive to reliably assess p-Chk2 levels in WT cells. Nonetheless, the results demonstrate that ATM inhibitor addition after initial Chk2 activation results in diminished p-Chk2 levels, confirming that sustained ATM-to-Chk2 signaling helps to maintain p-Chk2 levels.
As anticipated, p-Chk2 levels remain elevated in 2BN (XLF−/−) hTERT compared to control cells, reflecting sustained signaling from the elevated level of unrepaired DSBs (Fig. 4D). Addition of ATM inhibitor at 30 min post-IR to 2BN (XLF−/−) hTERT cells resulted in dramatically decreased p-Chk2 levels. These findings provide strong evidence that sustained ATM signaling maintains p-Chk2 in control cells and, more strikingly, in an NHEJ-deficient background. The level of p-Chk2 at 30 min post-IR was greater in 2BN (XLF−/−) hTERT compared to control cells, which we attribute to XLF-dependent DSB repair during the first 30 min post-IR. To verify that the sustained p-Chk2 levels are not a consequence of the level of initially activated Chk2, we treated 2BN (XLF−/−) hTERT cells with ATM inhibitor at 4 or 6 h post-IR (Fig. 4E). p-Chk2 was dramatically reduced 2 h later in stark contrast to its maintenance in the absence of ATM inhibitor, demonstrating that p-Chk2 is lost rapidly when ATM signaling is abrogated.
Finally, to verify that p-Chk1 and p-Chk2 contribute to the maintenance of checkpoint arrest in a repair-deficient background, we subjected 2BN (XLF−/−) hTERT cells to Chk1 or Chk2 siRNA treatment and observed premature release compared to control siRNA treatment (Fig. 4F). We conclude that sustained ATM signaling to Chk2 represents a second process that maintains G2/M checkpoint arrest.
53BP1−/− and MDC1−/− MEFs show premature release from checkpoint arrest.
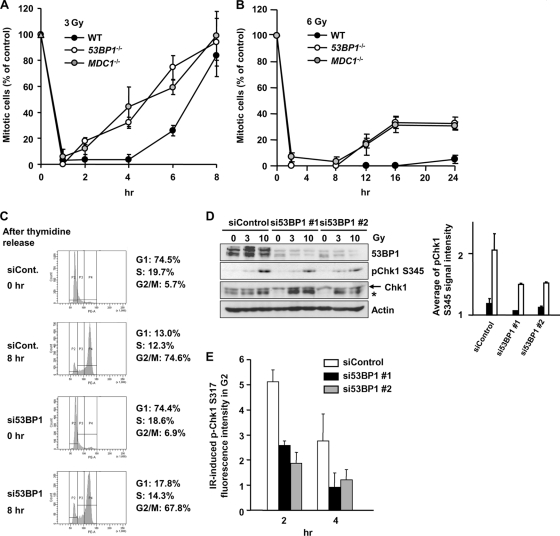
53BP1 has been reported to amplify ATM signaling, a suggestion based on the finding that it is required for the initiation of checkpoint arrest following exposure to low IR doses, when the signal is low, but is dispensable for checkpoint arrest after high doses, when the signal is more robust (7, 31). MDC1 is also required for initiation of G2/M arrest after low doses (7, 28, 31). Here, we examine whether 53BP1 and MDC1 are required for checkpoint maintenance. In 53BP1−/− and MDC1−/− MEFs, >3 Gy IR activates G2/M checkpoint arrest, but mitotic entry occurs prematurely compared to WT MEFs (Fig. 5A and B). Thus, 53BP1 and MDC1 have roles in maintaining checkpoint arrest while being dispensable for checkpoint initiation after exposure to 3 or 6 Gy IR.
FIG. 5.
MDC1−/− and 53BP1−/− MEFs show impaired G2/M checkpoint maintenance and reduced Chk1 phosphorylation. (A and B) Mitotic entry in WT, MDC1−/−, and 53BP1−/− MEFs after 3 Gy and 6 Gy IR was evaluated. APH was added after IR. (C) Cell cycle profile in control and 53BP1 siRNA cells after double thymidine block. (D) p-Chk1 is reduced in synchronized G2 53BP1 siRNA cells post-IR. Cells were synchronized in G1/S by double thymidine block and irradiated 8 h postrelease. Cells were examined by immunoblotting using anti-pChk1 Ser345 antibody. The asterisk represents a nonspecific band. Quantification of p-Chk1 from 2 experiments is shown. (E) 53BP1 siRNA cells show impaired Chk1 activation. The p-Ser317 Chk1 signal was quantified by IF in CENP-F+ (G2) A549 cells treated 3 Gy IR and 53BP1 or control siRNA. The signal in undamaged nuclei is subtracted. Error bars represent the SEM of 3 experiments.
53BP1−/− cells show diminished Chk1 activation and reduced sustained ATM signaling to Chk2.
To evaluate the mechanism by which 53BP1 functions in checkpoint maintenance, we first examined whether 53BP1 is required for Chk1 activation in irradiated G2 cells by IF. We examined, as one approach, synchronized cells. Eight hours after release from thymidine block, ∼75% of the cells were in G2 phase (Fig. 5C). Examination of p-Chk1 levels by immunoblotting, 1 h after exposure to IR at this time point, revealed an ∼50% decrease in p-Chk1 levels following treatment with 53BP1 siRNA (Fig. 5D). We also observed reduced IR-induced p-Chk1 in unsynchronized G2 cells (identified by CENP-F staining) following treatment with 53BP1 siRNA (Fig. 5E). Thus, 53BP1 is required for efficient Chk1 activation in G2 cells after IR, which likely contributes to the impaired checkpoint maintenance in 53BP1−/− MEFs.
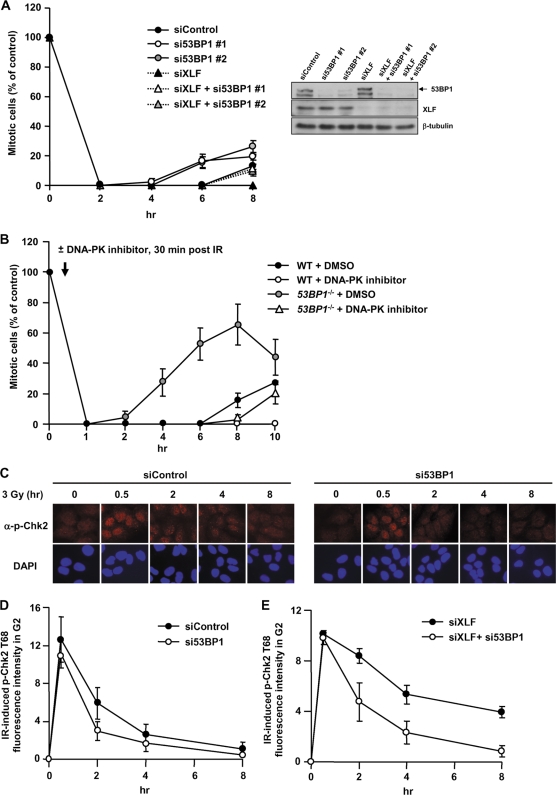
We also examined the requirement for 53BP1 in maintaining ATM-Chk2 signaling. In Fig. 4D and E, we show that sustained signaling maintains p-Chk2 levels and prolonged checkpoint arrest in XLF−/− cells. To evaluate the impact of 53BP1 on ATM-Chk2 signaling, we examined the duration of arrest following treatment with siRNA of either 53BP1 or XLF alone or combined (Fig. 6A). Similar to our findings with 2BN (XLF−/−) hTERT cells, XLF siRNA conferred prolonged arrest (>8 h) compared to cells subjected to control siRNA (released at 6 to 8 h). 53BP1 siRNA-treated cells were released prematurely, consistent with our findings with 53BP1−/− MEFs. Strikingly, cells subjected to combined 53BP1 and XLF siRNA showed prolonged checkpoint arrest compared to 53BP1 siRNA alone, but release occurred earlier than in cells treated with XLF siRNA (Fig. 6A). This suggests that 53BP1 contributes to, but is not essential for, the ability of ATM signaling to respond to the status of DSB repair.
FIG. 6.
53BP1-defective cells show impaired sustained ATM-Chk2 signaling. (A) A549 cells treated with 53BP1 siRNA, XLF siRNA, or both were exposed to 3 Gy IR, and the mitotic entry was evaluated. 53BP1 and XLF siRNA causes prolonged arrest compared to 53BP1 siRNA alone. The knockdown efficiency is shown in right panel. (B) WT or 53BP1−/− MEFs were treated with or without DNA-PK inhibitor addition 30 min after 3 Gy IR, and mitotic entry was examined. (C) Typical picture of p-Chk2 Thr68 in 53BP1 siRNA A549 cells after 3 Gy IR. α, anti. (D) Quantification of p-Chk2 levels in 53BP1 siRNA-treated A549 cells after 3 Gy IR. p-Chk2 levels were assessed by IF quantification in CENP-F+ (G2) cells. (E) Quantification of the p-Chk2 in G2 cells following XLF or 53BP1/XLF siRNA after 3 Gy IR.
Since 53BP1 siRNA may not fully deplete 53BP1 activity, we verified this finding using 53BP1−/− MEFs. To inhibit DSB repair, we treated MEFs with an inhibitor of DNA-PK, an NHEJ component (Fig. 6B). The DNA-PK inhibitor was added 30 min post-IR to ensure that we monitor the impact of impaired DSB repair on sustained versus initial signaling. We observed similar findings to those shown in Fig. 6A, namely, that DNA-PK inhibitor-treated 53BP1−/− MEFs show prolonged arrest compared to untreated cells but are released earlier than DNA-PK inhibitor-treated control cells (Fig. 6B).
Since MDC1 is required for 53BP1 focus formation after IR, we also examined whether MDC1 functions in checkpoint maintenance. While MDC1 siRNA-treated cells were released by 6 h post-IR, cells treated with MDC1 siRNA plus DNA-PK inhibitor remained arrested for 10 h (data not shown). Thus, both 53BP1- and MDC1-defective cells undergo prolonged arrest in a repair-defective background, providing strong evidence that neither 53BP1 nor MDC1 is essential for ATM-Chk2 signaling.
To substantiate that sustained signaling occurs via Chk2, we monitored p-Chk2 levels in G2-phase cells subjected to siRNA of 53BP1 or 53BP1 plus XLF. Quantification of the total cellular p-Chk2 signal revealed that, although the signal at 30 min post-IR was not significantly reduced following 53BP1 siRNA treatment, it was reduced by 2 h post-IR (Fig. 6C and D). At later times, the low signal precluded accurate assessment. Greater sensitivity was provided following XLF siRNA treatment. At 30 min post-IR, the levels of p-Chk2 were similar with or without 53BP1 siRNA but were elevated at later times in the presence of 53BP1 (Fig. 6E), demonstrating that sustained ATM-Chk2 signaling is diminished in the absence of 53BP1. However, the p-Chk2 levels remained higher following treatment with 53BP1 plus XLF siRNA compared to 53BP1 siRNA alone (compare Fig. 6D and E), confirming that 53BP1 is dispensable for ATM-Chk2 signaling. Thus, consistent with our studies on checkpoint release, ATM-Chk2 signaling occurs in the absence of 53BP1, although it is substantially diminished in magnitude compared to control cells. This implies that ATM signaling can be sustained at persistent DSBs in the absence of 53BP1.
Collectively, these findings suggest that 53BP1 has a role in both ATR-Chk1 and sustained ATM-Chk2 signaling. The requirement of 53BP1 for both arms of the signaling response is consistent with the observation that loss of 53BP1 confers earlier checkpoint release than that observed with ATR-SS hTERT cells (compare Fig. 2B and 5A), although both show similarly diminished p-Chk1 levels (compare Fig. 2C and 5E). Indeed, checkpoint release in the absence of 53BP1 was similar to that observed in ATM inhibitor-treated ATR-SS hTERT cells, which are also impaired in ATR-Chk1 and ATM-Chk2 signaling (compare Fig. 3B and 5A).
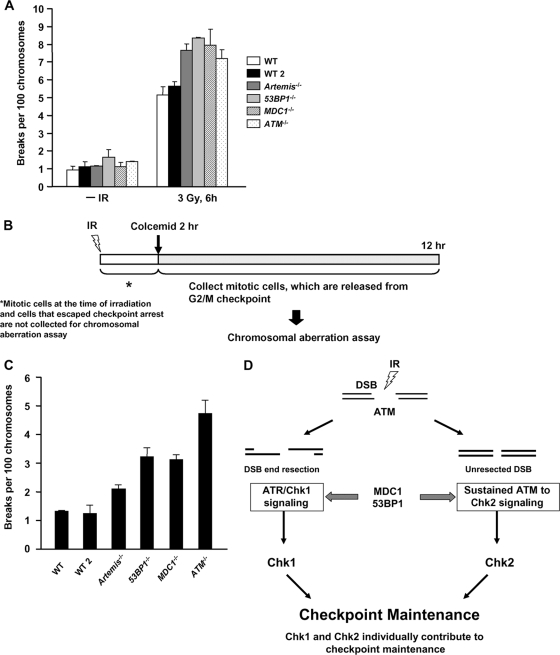
53BP1−/− and MDC1−/− MEFs show elevated chromosome breakage compared to Artemis−/− MEFs.
We have previously shown that 53BP1 and MDC1 are required for ATM-dependent DSB repair in G1 (20, 24). Using calyculin A-induced PCC analysis, we show here that 53BP1−/−, MDC1−/−, Artemis−/−, and ATM−/− MEFs have similar DSB repair defects in G2 (Fig. 7A). We next examined whether the combined checkpoint and repair defects of 53BP1−/− and MDC1−/− cells enhanced mitotic chromosome breakage by comparing breakage in mediator-defective cells (repair and checkpoint maintenance defective) with that in Artemis-defective cells, which show prolonged checkpoint arrest (i.e., checkpoint proficient and repair deficient) (6). Previous studies have shown that 53BP1−/− and MDC1−/− MEFs display elevated chromosome breakage, but chromosome aberrations per cell were measured at a single, early time point post-IR (16, 19). This procedure monitors chromosome breakage in cells that enter mitosis through checkpoint escape and may not reflect the response of the total G2 population and the contribution of checkpoint arrest in preventing chromosome breakage (12). In short, although chromosome breakage per cell may be high at early times post-IR, it may not significantly contribute to the breakage observed with the population if most cells remain arrested. We, therefore, developed an approach to assess the contribution of the entire pool of G2-phase cells to mitotic breakage. We utilized standard chromosome analysis to score chromatid breaks, but added APH to preclude the mitotic entry of irradiated S-phase cells. To examine the entire G2 population, colcemid was added from 2 to 12 h post-IR, by which time all G2 cells had entered mitosis (Fig. 7B) (6). To avoid analysis of irradiated mitotic cells as well as cells that avoid checkpoint arrest at very early times post-IR, we did not collect cells in the first 2 h posttreatment.
FIG. 7.
The combined DSB repair and signaling defects in MDC1−/− and 53BP1−/− MEFs contribute to chromosome breakage. (A) Cells were exposed to 3 Gy IR. A total of 50 ng/ml calyculin A was added 20 min before fixation. PCCs are scored per 100 chromosomes. (B) Diagram showing the procedure used. Colcemid was added from 2 to 12 h after 3 Gy IR. Mitotic cells were not collected during the first 2 h post-IR to prevent analysis of cells that “escape” checkpoint arrest. Chromatid breaks were scored. (C) Mitotic chromosomal break analysis of 2 WT, MDC1−/−, 53BP1−/−, Artemis−/−, and ATM−/− MEFs. Chromatid break numbers were normalized to 100 chromosomes to account for differences in chromosome numbers for different lines. (D) Model showing the mechanisms contributing to checkpoint maintenance and the contribution of 53BP1 and MDC1.
We observed only modestly increased breakage in Artemis−/− MEFs compared to control cells, consistent with their prolonged checkpoint arrest limiting (but not ablating) cells with DSBs entering mitosis (Fig. 7C). MDC1−/− and 53BP1−/− MEFs, in contrast, display elevated mitotic breakage that is intermediate between those of ATM−/− and WT MEFs. Since we excluded analysis of cells entering mitosis within 0 to 2 h post-IR, we likely underestimated chromosome breakage in checkpoint-defective ATM−/− MEFs. This will have little impact on 53BP1−/− MEFs since they initiate arrest normally. Taken together, the data suggest that although 53BP1 and MDC1 function in a subcomponent of DSB repair that likely contributes to their radiosensitivity, their defect in maintaining checkpoint arrest contributes to their elevated chromosome breakage.
DISCUSSION
Although the molecular steps activating G2/M arrest have been well characterized, the process by which ATM signaling maintains arrest has not been detailed. We evaluate this in the light of recent findings that (i) ATM-dependent resection can lead to ATR activation in G2 phase, conferring a switch from ATM to ATR signaling (11), and (ii) a subset (∼15%) of DSBs representing the slow component of DSB repair undergoes resection and repair by HR in G2 phase (3). We define two ATM-dependent processes that contribute to maintaining the G2/M checkpoint in irradiated G2 cells: (i) ATR-dependent Chk1 activation at resected DSBs and (ii) sustained ATM-to-Chk2 signaling at unrepaired DSBs (Fig. 7D). Further, although 53BP1 and MDC1 are dispensable for the initiation of checkpoint arrest at all but low doses, they are required for maintaining arrest, a role that contributes to their function in maintaining genomic stability. We provide insight into the role of 53BP1 by showing that 53BP1-deficient cells fail to activate Chk1 normally after IR and have a diminished ability to affect sustained ATM-Chk2 signaling.
The checkpoint maintenance mechanisms.
A subcomponent of DSBs in G2 undergoes ATM-dependent resection, generating RPA-coated ssDNA that signals via ATR recruitment to Chk1. We uniquely examine Chk1's role following resection in G2 phase by adding APH to prevent analysis of Chk1 activation at stalled replication forks. Chk1's role in maintaining ATM-dependent checkpoint arrest is demonstrated by the premature release of Chk1 siRNA and ATR-SS hTERT cells. These findings provide the first evidence in mammalian cells that ATM-dependent Chk1 activation at resected DSBs (as opposed to stalled replication forks) contributes to checkpoint maintenance. The modest impact of Chk1 is consistent with our findings that only 15 to 20% of IR-induced DSBs undergo resection and repair by HR in G2 phase. However, the DSBs that undergo resection represent the slow DSB repair component (3). Thus, resected DSBs make a greater contribution to unrepaired DSBs at later times post-IR, when the majority of NHEJ (and hence signaling to Chk2) is completed.
We also provide evidence for a mechanism involving sustained ATM-Chk2 signaling. Sustained ATM activation could occur by prolongation of initially activated ATM, by ongoing activation of ATM retained at the DSB site or by continuous recruitment of ATM to DSBs. Although further work is required to distinguish the precise mechanism, the concept of sustained ATM activation has received little consideration hitherto. Sustained ATM signaling is observed most strikingly with cells with impaired NHEJ (e.g., XLF−/− or DNA-PK inhibitor-treated cells). To dissect the process, we added ATM inhibitor at 30 min post-IR, when maximal levels of p-Chk1/Chk2 had been attained. ATM inhibitor addition to WT, XLF−/−, or DNA-PK inhibitor-treated cells showed premature checkpoint release and diminished p-Chk2 levels, demonstrating a requirement for sustained ATM-Chk2 signaling. Direct evidence for this process was also observed with the premature mitotic entry following Chk2 siRNA treatment, consistent with a previous report that Chk2−/− MEFs arrest but are released prematurely (10). Further, we show that slow decay of the initially generated p-Chk2 signal cannot account for the prolonged arrest, although it may provide a period of arrest without the need for further ATM signaling. This process may underlie the slightly longer arrest observed with ATM inhibitor-treated ATR-SS hTERT cells than with cells ablated for Chk1/Chk2 activity (i.e., WT plus Chk1/Chk2 inhibitor-treated cells), since ATR-SS hTERT cells show impaired Chk1 activation and the ATM inhibitor diminishes sustained Chk2 activation.
Overlap between Chk1 and Chk2 in checkpoint arrest.
Checkpoint maintenance differs from initiation in two ways. First, above a certain dose, sufficiently activated Chk1 or Chk2 may initiate arrest. In contrast, checkpoint release is determined by a threshold signal as DSB repair ensues. Further, if HR and NHEJ do not proceed at equal rates, the ratio of resected to nonresected DSBs will change over time (3).
Our results suggest that after 3 Gy IR, either Chk1 or Chk2 alone is sufficient to initiate arrest, while loss of either kinase impairs checkpoint maintenance. The lack of requirement for Chk2 for checkpoint initiation after 3 Gy is consistent with published findings using Chk2−/− MEFs (10). Although it is difficult to fully ablate Chk1 since it is essential, we show that following siRNA Chk1 treatment and in ATR-SS cells, checkpoint arrest is initiated normally after IR, but its maintenance is impaired. Further, both treatments abolish 53BP1 focus formation after hydroxyurea (HU) treatment, a known Chk1-dependent process. Thus, there is either no or a less stringent requirement for Chk1 and Chk2 for initiation versus checkpoint maintenance. It is possible and indeed likely that checkpoint arrest could have different requirements after exposure to lower doses, however. Checkpoint arrest in DT40 chicken cells has been reported to be Chk1 dependent after 4 Gy, consistent with the fact that most DSBs undergo resection and repair by HR in G2 in DT40 cells (23, 27). Our finding that in mammalian cells both Chk1 and Chk2 are activated and able to initiate checkpoint arrest is, thus, consistent with the notion that both HR and NHEJ contribute to DSB repair in G2 and that some but not all DSBs undergo resection. The fact that loss of either checkpoint kinase impairs the maintenance of arrest suggests that both kinases contribute to the checkpoint signal as it approaches a threshold level as DSB repair ensues, consistent with the suggestion that both HR and NHEJ contribute to DSB repair in G2. It is noteworthy that there appears to be a greater contribution of Chk1 in control cells, in agreement with the notion that resected DSBs and HR represent the slow DSB repair component (3).
The role of the mediator proteins 53BP1 and MDC1.
We demonstrate that 53BP1 and MDC1 have roles in maintaining checkpoint arrest and hence have checkpoint defects following exposure to high IR doses. In contrast, 53BP1 and MDC1 are dispensable for checkpoint initiation, except after low IR doses (7, 16, 28, 31). We show that 53BP1 impacts ATR-Chk1 activation and sustained ATM-Chk2 signaling. Interestingly, at 30 min post-IR, loss of 53BP1 had a greater impact on p-Chk1 than on p-Chk2 levels and a greater impact on maintaining p-Chk2 levels than on the initial signal. Perhaps surprisingly, we observed that 53BP1 is not essential for sustained ATM-Chk2 signaling but enhances the efficacy of the process. We have recently shown that the mediator proteins help to maintain ATM at the DSB (14, 20). We suggest that this promotes the maintenance of active ATM at the DSB, enhancing its ability to phosphorylate Chk2 and to promote resection and Chk1 activation. Importantly, we demonstrate that the mediator proteins have a significant function in maintaining ATM-Chk2 signaling, a notion that has not fully been considered hitherto.
Our study also provides insight into how the mediator proteins function to limit genomic instability. Despite their subtle DSB repair defect and the previously described subtle checkpoint defect, 53BP1−/− and MDC1−/− MEFs show marked genomic instability. Chromosome breakage represents a reliable monitor of genomic instability. We present evidence that defective checkpoint maintenance when coupled with a DSB repair defect significantly contributes to IR-induced chromosome breakage. Thus, while being apparently minor defects, they are of major significance when considering genomic instability.
Conclusion.
We have dissected mechanisms regulating the checkpoint maintenance in irradiated mammalian G2 cells. We show that (i) Chk1 activation at resected DSBs contributes to maintaining checkpoint arrest and (ii) sustained signaling from ATM to Chk2 at unrepaired DSBs can prolong arrest, most strikingly in NHEJ-defective cells. We demonstrate that the mediator proteins 53BP1 and MDC1, while impacting the checkpoint initiation only at low doses, are required to maintain arrest at all doses. They achieve this by enhancing Chk1 activation in G2 and by facilitating sustained ATM-Chk2 signaling. Thus, 53BP1−/− and MDC1−/− cells have checkpoint defects after high and low IR doses, significantly contributing to their elevated chromosome breakage.
Supplementary Material
Acknowledgments
We thank G. Smith for providing ATM inhibitor and E. Hoffman, A. Carr, and A. Lehmann for invaluable comments.
The M.L. laboratory is supported by the Deutsche Forschungsgemeinschaft (Lo 677/4-1/2), the Bundesministerium für Bildung und Forschung via Forschungszentrum Karlsruhe (02S8335 and 02S8355), and Forschungszentrum Jülich (03NUK001C). The P.A.J. laboratory is supported by the Medical Research Council, Association for International Cancer Research, Department of Health and EU grant (Integrated Project; DNA repair, LSHG-CT-2005-512113). Both laboratories are supported by EU grant FI6R-CT-2003-508842 (RiscRad).
Footnotes
Published ahead of print on 26 April 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahnesorg, P., P. Smith, and S. P. Jackson. 2006. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell 124:301-313. [DOI] [PubMed] [Google Scholar]
- 2.Beamish, H., and M. F. Lavin. 1994. Radiosensitivity in ataxia-telangiectasia: anomalies in radiation-induced cell cycle delay. Int. J. Radiat. Biol. 65:175-184. [DOI] [PubMed] [Google Scholar]
- 3.Beucher, A., J. Birraux, L. Tchouandong, O. Barton, A. Shibata, S. Conrad, A. A. Goodarzi, A. Krempler, P. A. Jeggo, and M. Lobrich. 2009. ATM and Artemis promote homologous recombination of radiation-induced DNA double-strand breaks in G2. EMBO J. 28:3413-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck, D., L. Malivert, R. de Chasseval, A. Barraud, M. C. Fondaneche, O. Sanal, A. Plebani, J. L. Stephan, M. Hufnagel, F. le Deist, A. Fischer, A. Durandy, J. P. de Villartay, and P. Revy. 2006. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell 124:287-299. [DOI] [PubMed] [Google Scholar]
- 5.Dai, Y., B. Kysela, L. A. Hanakahi, K. Manolis, E. Riballo, M. Stumm, T. O. Harville, S. C. West, M. A. Oettinger, and P. A. Jeggo. 2003. Non-homologous end joining and V(D)J recombination require an additional factor. Proc. Natl. Acad. Sci. U. S. A. 100:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deckbar, D., J. Birraux, A. Krempler, L. Tchouandong, A. Beucher, S. Walker, T. Stiff, P. A. Jeggo, and M. Lobrich. 2007. Chromosome breakage after G2 checkpoint release. J. Cell Biol. 176:748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Capetillo, O., H. T. Chen, A. Celeste, I. Ward, P. J. Romanienko, J. C. Morales, K. Naka, Z. Xia, R. D. Camerini-Otero, N. Motoyama, P. B. Carpenter, W. M. Bonner, J. Chen, and A. Nussenzweig. 2002. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat. Cell Biol. 4:993-997. [DOI] [PubMed] [Google Scholar]
- 8.Friedel, A. M., B. L. Pike, and S. M. Gasser. 2009. ATR/Mec1: coordinating fork stability and repair. Curr. Opin. Cell Biol. 21:237-244. [DOI] [PubMed] [Google Scholar]
- 9.Hefferin, M. L., and A. E. Tomkinson. 2005. Mechanism of DNA double-strand break repair by non-homologous end joining. DNA Repair (Amst.) 4:639-648. [DOI] [PubMed] [Google Scholar]
- 10.Hirao, A., A. Cheung, G. Duncan, P.-M. Girard, A. J. Elia, A. Wakeham, H. Okada, T. Sarkissian, J. A. Wong, T. Sakai, E. de Stanchina, R. G. Bristow, T. Suda, S. W. Lowe, P. A. Jeggo, S. J. Elledge, and T. E. Mak. 2002. Chk2 is a tumour suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol. Cell. Biol. 22:6521-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jazayeri, A., J. Falck, C. Lukas, J. Bartek, G. C. Smith, J. Lukas, and S. P. Jackson. 2006. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 8:37-45. [DOI] [PubMed] [Google Scholar]
- 12.Jeggo, P. A., and M. Lobrich. 2006. Contribution of DNA repair and cell cycle checkpoint arrest to the maintenance of genomic stability. DNA Repair (Amst). 5:1192-1198. [DOI] [PubMed] [Google Scholar]
- 13.Kühne, M., E. Riballo, N. Rief, K. Rothkamm, P. A. Jeggo, and M. Lobrich. 2004. A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 64:500-508. [DOI] [PubMed] [Google Scholar]
- 14.Lee, J.-H., A. A. Goodarzi, P. A. Jeggo, and T. T. Paull. 2010. 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J. 29:574-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobrich, M., and P. A. Jeggo. 2005. The two edges of the ATM sword: co-operation between repair and checkpoint functions. Radiother. Oncol. 76:112-118. [DOI] [PubMed] [Google Scholar]
- 16.Lou, Z., K. Minter-Dykhouse, S. Franco, M. Gostissa, M. A. Rivera, A. Celeste, J. P. Manis, J. van Deursen, A. Nussenzweig, T. T. Paull, F. W. Alt, and J. Chen. 2006. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol. Cell 21:187-200. [DOI] [PubMed] [Google Scholar]
- 17.Martinho, R. G., H. D. Lindsay, G. Flaggs, A. DeMaggio, M. Hoekstra, A. M. Carr, and N. J. Bentley. 1998. Analysis of Rad3 and Chk1 protein kinases defines different checkpoint responses. EMBO J. 17:7239-7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuoka, S., M. Huang, and S. J. Elledge. 1998. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282:1893-1897. [DOI] [PubMed] [Google Scholar]
- 19.Morales, J. C., Z. Xia, T. Lu, M. B. Aldrich, B. Wang, C. Rosales, R. E. Kellems, W. N. Hittelman, S. J. Elledge, and P. B. Carpenter. 2003. Role for the BRCA1 C-terminal repeats (BRCT) protein 53BP1 in maintaining genomic stability. J. Biol. Chem. 278:14971-14977. [DOI] [PubMed] [Google Scholar]
- 20.Noon, A. T., A. Shibata, N. Rief, M. Lobrich, G. S. Stewart, P. A. Jeggo, and A. A. Goodarzi. 2010. 53BP1-dependent robust, localised KAP-1 phosphorylation is essential for heterochromatic DNA double strand break repair. Nat. Cell Biol. 12:177-184. [DOI] [PubMed] [Google Scholar]
- 21.O'Driscoll, M., V. L. Ruiz-Perez, C. G. Woods, P. A. Jeggo, and J. A. Goodship. 2003. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat. Genet. 33:497-501. [DOI] [PubMed] [Google Scholar]
- 22.Pellicioli, A., S. E. Lee, C. Lucca, M. Foiani, and J. E. Haber. 2001. Regulation of Saccharomyces Rad53 checkpoint kinase during adaptation from DNA damage-induced G2/M arrest. Mol. Cell 7:293-300. [DOI] [PubMed] [Google Scholar]
- 23.Rainey, M. D., E. J. Black, G. Zachos, and D. A. Gillespie. 2008. Chk2 is required for optimal mitotic delay in response to irradiation-induced DNA damage incurred in G2 phase. Oncogene 27:896-906. [DOI] [PubMed] [Google Scholar]
- 24.Riballo, E., M. Kuhne, N. Rief, A. Doherty, G. C. Smith, M. J. Recio, C. Reis, K. Dahm, A. Fricke, A. Krempler, A. R. Parker, S. P. Jackson, A. Gennery, P. A. Jeggo, and M. Lobrich. 2004. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol. Cell 16:715-724. [DOI] [PubMed] [Google Scholar]
- 25.Rothkamm, K., I. Kruger, L. H. Thompson, and M. Lobrich. 2003. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol. 23:5706-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengupta, S., A. I. Robles, S. P. Linke, N. I. Sinogeeva, R. Zhang, R. Pedeux, I. M. Ward, A. Celeste, A. Nussenzweig, J. Chen, T. D. Halazonetis, and C. C. Harris. 2004. Functional interaction between BLM helicase and 53BP1 in a Chk1-mediated pathway during S-phase arrest. J. Cell Biol. 166:801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonoda, E., H. Hochegger, A. Saberi, Y. Taniguchi, and S. Takeda. 2006. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair (Amst.) 5:1021-1029. [DOI] [PubMed] [Google Scholar]
- 28.Stewart, G. S., B. Wang, C. R. Bignell, A. M. Taylor, and S. J. Elledge. 2003. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature 421:961-966. [DOI] [PubMed] [Google Scholar]
- 29.Stiff, T., M. O'Driscoll, N. Rief, K. Iwabuchi, M. Lobrich, and P. A. Jeggo. 2004. ATM and DNA-PK function redundantly to phosphorylate H2AX following exposure to ionising radiation. Cancer Res. 64:2390-2396. [DOI] [PubMed] [Google Scholar]
- 30.Stiff, T., S. A. Walker, K. Cerosaletti, A. A. Goodarzi, E. Petermann, P. Concannon, M. O'Driscoll, and P. A. Jeggo. 2006. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 25:5775-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, B., S. Matsuoka, P. B. Carpenter, and S. J. Elledge. 2002. 53BP1, a mediator of the DNA damage checkpoint. Science 298:1435-1438. [DOI] [PubMed] [Google Scholar]
- 32.Wyman, C., and R. Kanaar. 2006. DNA double-strand break repair: all's well that ends well. Annu. Rev. Genet. 40:363-383. [DOI] [PubMed] [Google Scholar]
- 33.Xu, B., S. T. Kim, D. S. Lim, and M. B. Kastan. 2002. Two molecularly distinct G2/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 22:1049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.