Abstract
Human cytomegalovirus (HCMV) UL37 proteins traffic sequentially from the endoplasmic reticulum (ER) to the mitochondria. In transiently transfected cells, UL37 proteins traffic into the mitochondrion-associated membranes (MAM), the site of contact between the ER and mitochondria. In HCMV-infected cells, the predominant UL37 exon 1 protein, pUL37x1, trafficked into the ER, the MAM, and the mitochondria. Surprisingly, a component of the MAM calcium signaling junction complex, cytosolic Grp75, was increasingly enriched in heavy MAM from HCMV-infected cells. These studies show the first documented case of a herpesvirus protein, HCMV pUL37x1, trafficking into the MAM during permissive infection and HCMV-induced alteration of the MAM protein composition.
The human cytomegalovirus (HCMV) UL37 immediate early (IE) locus expresses multiple products, including the predominant UL37 exon 1 protein, pUL37x1, also known as viral mitochondrion-localized inhibitor of apoptosis (vMIA), during lytic infection (16, 22, 24, 39, 44). The UL37 glycoprotein (gpUL37) shares UL37x1 sequences and is internally cleaved, generating pUL37NH2 and gpUL37COOH (2, 22, 25, 26). pUL37x1 is essential for the growth of HCMV in humans (17) and for the growth of primary HCMV strains (20) and strain AD169 (14, 35, 39, 49) but not strain TownevarATCC in permissive human fibroblasts (HFFs) (27).
pUL37x1 induces calcium (Ca2+) efflux from the endoplasmic reticulum (ER) (39), regulates viral early gene expression (5, 10), disrupts F-actin (34, 39), recruits and inactivates Bax at the mitochondrial outer membrane (MOM) (4, 31-33), and inhibits mitochondrial serine protease at late times of infection (28).
Intriguingly, HCMV UL37 proteins localize dually in the ER and in the mitochondria (2, 9, 16, 17, 24-26). In contrast to other characterized, similarly localized proteins (3, 6, 11, 23, 30, 38), dual-trafficking UL37 proteins are noncompetitive and sequential, as an uncleaved gpUL37 mutant protein is ER translocated, N-glycosylated, and then imported into the mitochondria (24, 26).
Ninety-nine percent of ∼1,000 mitochondrial proteins are synthesized in the cytosol and directly imported into the mitochondria (13). However, the mitochondrial import of ER-synthesized proteins is poorly understood. One potential pathway is the use of the mitochondrion-associated membrane (MAM) as a transfer waypoint. The MAM is a specialized ER subdomain enriched in lipid-synthetic enzymes, lipid-associated proteins, such as sigma-1 receptor, and chaperones (18, 45). The MAM, the site of contact between the ER and the mitochondria, permits the translocation of membrane-bound lipids, including ceramide, between the two organelles (40). The MAM also provides enriched Ca2+ microdomains for mitochondrial signaling (15, 36, 37, 43, 48). One macromolecular MAM complex involved in efficient ER-to-mitochondrion Ca2+ transfer is comprised of ER-bound inositol 1,4,5-triphosphate receptor 3 (IP3R3), cytosolic Grp75, and a MOM-localized voltage-dependent anion channel (VDAC) (42). Another MAM-stabilizing protein complex utilizes mitofusin 2 (Mfn2) to tether ER and mitochondrial organelles together (12).
HCMV UL37 proteins traffic into the MAM of transiently transfected HFFs and HeLa cells, directed by their NH2-terminal leaders (8, 47). To determine whether the MAM is targeted by UL37 proteins during infection, we fractionated HCMV-infected cells and examined pUL37x1 trafficking in microsomes, mitochondria, and the MAM throughout all temporal phases of infection. Because MAM domains physically bridge two organelles, multiple markers were employed to verify the purity and identity of the fractions (7, 8, 19, 46, 47).
(These studies were performed in part by Chad Williamson in partial fulfillment of his doctoral studies in the Biochemistry and Molecular Genetics Program at George Washington Institute of Biomedical Sciences.)
HFFs and life-extended (LE)-HFFs were grown and not infected or infected with HCMV (strain AD169) at a multiplicity of 3 PFU/cell as previously described (8, 26, 47). Heavy (6,300 × g) and light (100,000 × g) MAM fractions, mitochondria, and microsomes were isolated at various times of infection and quantified as described previously (7, 8, 47). Ten- or 20-μg amounts of total lysate or of subcellular fractions were resolved by SDS-PAGE in 4 to 12% Bis-Tris NuPage gels (Invitrogen) and examined by Western analyses (7, 8, 26). Twenty-microgram amounts of the fractions were not treated or treated with proteinase K (3 μg) for 20 min on ice, resolved by SDS-PAGE, and probed by Western analysis. The blots were probed with rabbit anti-UL37x1 antiserum (DC35), goat anti-dolichyl phosphate mannose synthase 1 (DPM1), goat anti-COX2 (both from Santa Cruz Biotechnology), mouse anti-Grp75 (StressGen Biotechnologies), and the corresponding horseradish peroxidase-conjugated secondary antibodies (8, 47). Reactive proteins were detected by enhanced chemiluminescence (ECL) reagents (Pierce), and images were digitized as described previously (26, 47).
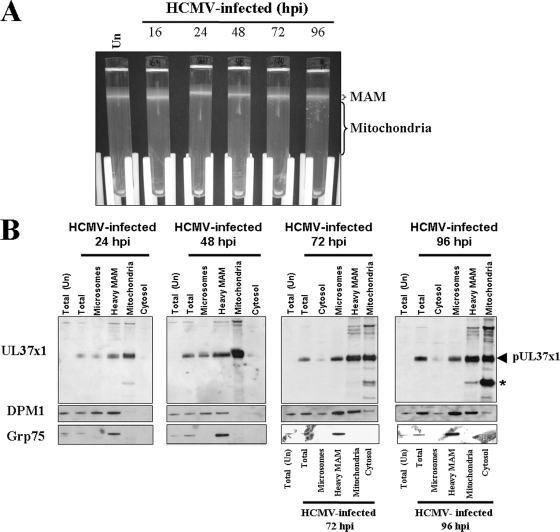
Detection of pUL37x1 in the heavy MAM fraction of HCMV-infected LE-HFFs.
To determine whether UL37 proteins traffic through the MAM of infected cells, Percoll gradients were used to fractionate MAM from mitochondria in HCMV-infected LE-HFFs throughout infection (Fig. 1 A). At 24-h postinfection (hpi), there were visible changes in mitochondrial banding. By 72 to 96 hpi, mitochondrial clumping was observed in HCMV-infected but not in uninfected LE-HFFs. These results suggest that HCMV infection increasingly alters the physical properties of mitochondria (buoyant density and shape) that dictate their banding in Percoll gradients. These results are consistent with the findings that HCMV infection, and pUL37x1/vMIA in particular, disrupts mitochondrial networks (21, 29, 31, 34, 41).
FIG. 1.
(A) HCMV infection alters mitochondrial banding in self-generated Percoll gradients. LE-HFFs were infected with HCMV (AD169, 3 PFU/cell). Four roller bottles of infected cells were harvested at 16, 24, 48, 72, and 96 hpi and fractionated in each gradient as previously described (7, 8). The positions of the MAM and mitochondrial fractions are indicated on the gradients. (B) pUL37x1 is detected in the heavy MAM fraction of HCMV-infected LE-HFFs. The microsomal, heavy MAM (6,300 × g), mitochondrial, and cytosolic fractions from uninfected cells (Un) and HCMV-infected cells were obtained from the fractionations shown in panel A at 24, 48, 72, and 96 hpi. Ten-microgram amounts of total and fractionated proteins were separated by SDS-PAGE and examined by Western blot analyses using rabbit anti-UL37x1 antiserum (DC35, 1:1,000). The fractions were reacted with antibody against an ER marker (DPM1, 1:100) or a mitochondrion/MAM marker (Grp75, 1:1,000). The order of samples used for the Grp75 blots for HCMV-infected LE-HFFs at 72 and 96 hpi is indicated below the corresponding panels. The positions of pUL37x1 (arrowhead) and of a short UL37 species (asterisk) are indicated on the UL37x1 blots.
The fractions from HCMV-infected LE-HFFs were examined for the presence of pUL37x1 (Fig. 1B). pUL37x1 is predominant during HCMV infection and was detected in microsomes, the heavy MAM fraction, and mitochondria at all times tested. A previously undetected small UL37 species (∼10 to 12 kDa) was detected in the mitochondria at late times in infection. Based upon its antibody reactivity and apparent molecular mass, this UL37 species may be pUL37S, encoded by the UL37S late transcript (1), or a specific cleavage product of pUL37x1. The identity of the MAM as an ER subdomain was verified by the presence of DPM1. These results indicate that pUL37x1 traffics to the MAM from early to late times of HCMV infection. Surprisingly, Grp75 was abundantly detected in the heavy MAM fraction of infected cells compared to its amount in mitochondrial fractions, consistent with its recent identification in a MAM macromolecular Ca2+ signaling complex (42). This suggests that HCMV infection may alter the MAM proteome.
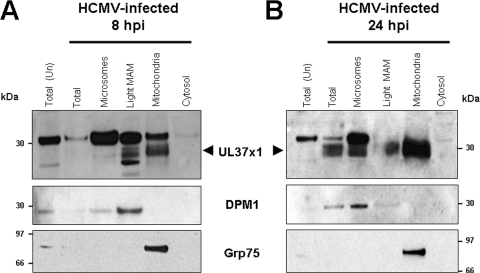
Detection of pUL37x1 in light MAM fraction at IE and early times of infection.
As Grp75 populates both MAM junctions (42) and the mitochondrial matrix (8, 26, 47), we first aimed to purify MAM fractions devoid of detectable Grp75. We thus further fractionated HCMV-infected LE-HFFs to obtain the light MAM fraction (100,000 × g), precleared (at 6,300 × g) of high-density heavy MAM material. Using this procedure, pUL37x1 was observed in microsomes, the light MAM fraction, and mitochondria at 8 and 24 hpi (Fig. 2), unequivocally demonstrating its trafficking to the MAM during infection. The presence of the ER (DPM1) marker and the absence of detectable Grp75 verified the purity and identity of the light MAM fraction. These results authenticate the presence of pUL37x1 in the MAM at IE and early times of HCMV infection.
FIG. 2.
pUL37x1 is detected in the highly purified light MAM fraction from HCMV-infected LE-HFFs at IE and early times of infection. LE-HFFs were infected with HCMV (AD169, 3 PFU/cell) and were harvested at 8 (A) or 24 (B) hpi. Fractions from 4 roller bottles each of uninfected or infected cells were obtained as described for Fig. 1 and previously described (7, 8). Twenty-microgram amounts of fractionated proteins (microsomes, light MAM fraction [100,000 × g], mitochondria, and cytosol) from cells harvested at the indicated times were resolved by SDS-PAGE and examined by Western analysis using anti-UL37x1 antiserum (DC35, 1:250), as well as antibody against an ER marker (DPM1, 1:100) or a mitochondrion/MAM marker (Grp75, 1:500). The position of pUL37x1 (arrowheads) is indicated on the UL37x1 blots.
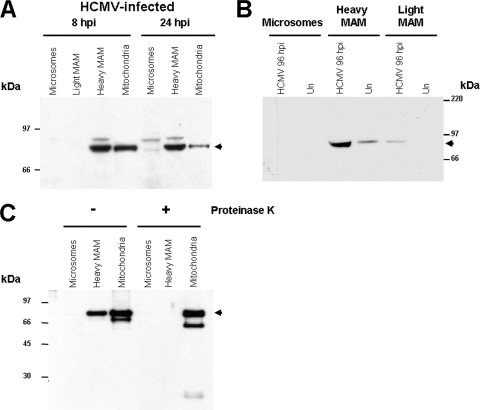
Increased abundance of Grp75 in the heavy MAM fraction during HCMV infection.
To determine whether the abundance of Grp75 in the MAM was altered during infection, the heavy MAM fraction (6,300 × g) and light MAM fraction (100,000 × g) of HCMV-infected cells were examined (Fig. 3). Within 8 hpi, the abundance of Grp75 in the heavy MAM fraction was comparable to that in purified mitochondrial fractions, and it increased above the level detected in mitochondria by 24 hpi (Fig. 3A). Moreover, the abundance of Grp75 in the heavy MAM fraction in HCMV-infected LE-HFFs at late times of infection was increased ∼3.9-fold above that from uninfected cells (Fig. 3B). This recruitment of Grp75 to the heavy MAM fraction of HCMV-infected cells was not detected in the microsomal fraction and minimally detected in the highly purified light MAM fraction.
FIG. 3.
Cytosolic Grp75 is increased in the heavy MAM fraction of HCMV-infected LE-HFFs at 8, 24, and 96 hpi. LE-HFFs were infected with HCMV, harvested at 8 or 24 hpi (A) or 96 hpi (B), and fractionated as described for Fig. 1. Proteins (20 μg) in the microsomal, heavy MAM (6,300 × g), and mitochondrial fractions were separated by SDS-PAGE and probed by Western blots using anti-Grp75 antibody (1:1,000). (C) Sensitivity of Grp75 in heavy MAM to proteinase K digestion. Twenty-microgram amounts of the microsomal, heavy MAM, or mitochondrial fraction from LE-HFFs transiently expressing gpUL37 were untreated (−) or treated (+) with proteinase K (3 μg/20 min on ice). Following digestion and inactivation with 1 μM phenylmethylsulfonyl fluoride, the proteins were resolved by SDS-PAGE, blotted, and reacted with anti-Grp75 antibody as described for panels A and B. Grp75 is indicated by arrowheads.
As Grp75 in the MAM macromolecular complex (IP3R-Grp75-VDAC) is cytosolic (42), we used proteinase K digestion to distinguish this isoform from mitochondrial-matrix-localized Grp75. Heavy MAM fractions and control mitochondrial fractions from transfected LE-HFFs expressing gpUL37 were tested first (Fig. 3C) and displayed specific protease degradation of Grp75 in the heavy MAM fractions, while the mitochondrial Grp75 was protected. The amount of Grp75 in LE-HFFs was barely detectable in microsomal fractions. These results suggest that Grp75 in the heavy MAM fraction is cytosolic and that cytosolic Grp75 progressively increases in the MAM during HCMV infection. Indeed, proteinase K digestion of the heavy MAM fraction from HCMV-infected cells verified that the Grp75 therein was cytosolic (Fig. 4 C) and did not result from contaminating mitochondria. In contrast, mitochondrial Grp75 was protected and, therefore, internally localized. As a control for these experiments, proteolytic digestion of pUL37x1 was monitored in parallel. As expected from its topology facing the cytosol (26), pUL37x1 in the heavy MAM fraction and mitochondrial fraction from HCMV-infected HFFs is sensitive to protease treatment.
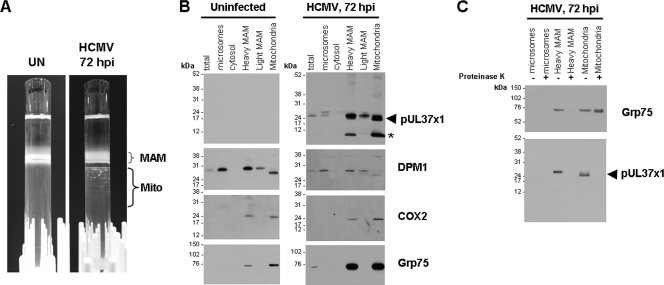
FIG. 4.
Increased abundance of cytosolic Grp75 in the heavy MAM fraction of HCMV-infected HFFs at 72 hpi. HFFs (P-13) were not infected or infected with HCMV (strain AD169, multiplicity of infection of 3) and harvested at 72 hpi. (A) The banding patterns of the MAM and mitochondria of uninfected and HCMV-infected HFFs in Percoll gradients. Uninfected and HCMV-infected HFFs (5 T175 flasks each) were fractionated in Percoll gradients as described previously (7, 8). The banding patterns of the MAM and mitochondria in the gradients are indicated. (B) Western analyses of uninfected and HCMV-infected HFFs. Ten-microgram amounts of total lysate and microsomal, cytosolic, heavy MAM, light MAM, and mitochondrial fractions were resolved by SDS-PAGE, blotted, and reacted with anti-UL37x1 antiserum (top panels) and DPM1 (1:200), COX2 (1:200), and Grp75 (1:2,500) antibodies. The positions of pUL37x1 (arrow) and a smaller UL37 species (asterisk) are indicated beside the blot. (C) Proteinase K sensitivity of cytosolic Grp75 in the heavy MAM fraction of HCMV-infected HFFs. Twenty-microgram amounts of the indicated fractions were not treated (−) or treated with proteinase K (+) as described for Fig. 3C and analyzed for the presence of Grp75 and pUL37x1.
HCMV infection alters MAM components.
To independently verify the alteration of MAM composition during HCMV infection, fractions were purified from uninfected and HCMV-infected primary HFFs at 72 hpi (Fig. 4A). The clumping of mitochondrial bands was again observed in lysates from HCMV-infected but not uninfected cells. Isolated fractions were examined for several markers, including anti-pUL37x1, anti-DPM1 (ER), anti-Grp75 (MAM and mitochondrial matrix), and anti-COX2 (inner mitochondrial membrane) markers (Fig. 4B). Similar to the results described above, pUL37x1 was detected in the microsomes, heavy and light MAM fractions, and mitochondria of infected cells at 72 hpi but not in uninfected HFFs. The presence of DPM1 verified the identities of the MAM fractions. Grp75 was abundantly detected in the heavy MAM fraction and mitochondria following HCMV infection.
This paper establishes the authentic trafficking of pUL37x1 in the full context of permissive HCMV infection. As is well documented, trafficking of some viral proteins can be significantly altered in the presence of other viral products. Thus, while our published transfection results showed the ability of HCMV UL37 proteins to traffic into the MAM, this paper establishes that they do so during permissive infection. Importantly for these studies, we observed MAM trafficking of pUL37x1 during the complete lytic cycle, suggesting the importance of its continued presence in this targeted subcellular compartment. These studies support the hypothesis that MAM localization is important for UL37 protein function in a way that transfection assays cannot. Moreover, at late times of infection, we observed a small UL37 species, possibly pUL37S, in the MAM and mitochondria. This species has not been observed in transfected cells. Finally, we documented that HCMV infection alters the abundance of cytosolic Grp75, a component of the MAM calcium signaling complex (42). HCMV infection may relocalize or increase the abundance of the macromolecular complex at the ER-mitochondrial junction to stabilize these connections during infection or to regulate ER-mitochondrion communication. Because pUL37x1 causes calcium efflux during infection (39), we anticipate that multiple components of the macromolecular complex are affected by HCMV infection. However, mitochondrial Grp75, a stress-responsive-gene product, appears to be differentially affected by HCMV infection and associated stress induction.
Acknowledgments
These studies were funded by NIH grant R01 AI057906 and NIH grant R21 AI081957, Children's Research Institute Discovery Funds, and the CNMC Board of Visitors.
Footnotes
Published ahead of print on 26 May 2010.
REFERENCES
- 1.Adair, R., G. W. Liebisch, and A. M. Colberg-Poley. 2003. Complex alternative processing of human cytomegalovirus UL37 pre-mRNA. J. Gen. Virol. 84:3353-3358. [DOI] [PubMed] [Google Scholar]
- 2.Al-Barazi, H. O., and A. M. Colberg-Poley. 1996. The human cytomegalovirus UL37 immediate-early regulatory protein is an integral membrane N-glycoprotein which traffics through the endoplasmic reticulum and Golgi apparatus. J. Virol. 70:7198-7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anandatheerthavarada, H. K., G. Biswas, J. Mullick, N. B. Sepuri, L. Otvos, D. Pain, and N. G. Avadhani. 1999. Dual targeting of cytochrome P4502B1 to endoplasmic reticulum and mitochondria involves a novel signal activation by cyclic AMP-dependent phosphorylation at ser128. EMBO J. 18:5494-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnoult, D., L. M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P. U. Park, J. Sharpe, R. J. Youle, and V. S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. U. S. A. 101:7988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biegalke, B. J. 1999. Human cytomegalovirus US3 gene expression is regulated by a complex network of positive and negative regulators. Virology 261:155-164. [DOI] [PubMed] [Google Scholar]
- 6.Borgese, N., I. Gazzoni, M. Barberi, S. Colombo, and E. Pedrazzini. 2001. Targeting of a tail-anchored protein to endoplasmic reticulum and mitochondrial outer membrane by independent but competing pathways. Mol. Biol. Cell 12:2482-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozidis, P., C. D. Williamson, and A. M. Colberg-Poley. 2007. Isolation of endoplasmic reticulum, mitochondria, and mitochondria-associated membrane fractions from transfected cells and from human cytomegalovirus-infected primary fibroblasts., unit 3.27. In J. S. Bonifacino et al. (ed.), Current protocols in cell biology. John Wiley and Sons, Inc., Hoboken, NJ. [DOI] [PubMed]
- 8.Bozidis, P., C. D. Williamson, and A. M. Colberg-Poley. 2008. Mitochondrial and secretory human cytomegalovirus UL37 proteins traffic into mitochondrion-associated membranes of human cells. J. Virol. 82:2715-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colberg-Poley, A. M., M. B. Patel, D. P. Erezo, and J. E. Slater. 2000. Human cytomegalovirus UL37 immediate-early regulatory proteins traffic through the secretory apparatus and to mitochondria. J. Gen. Virol. 81:1779-1789. [DOI] [PubMed] [Google Scholar]
- 10.Colberg-Poley, A. M., L. D. Santomenna, P. P. Harlow, P. A. Benfield, and D. J. Tenney. 1992. Human cytomegalovirus US3 and UL36-38 immediate-early proteins regulate gene expression. J. Virol. 66:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo, S., R. Longhi, S. Alcaro, F. Ortuso, T. Sprocati, A. Flora, and N. Borgese. 2005. N-Myristoylation determines dual targeting of mammalian NADH-cytochrome b5 reductase to ER and mitochondrial outer membranes by a mechanism of kinetic partitioning. J. Cell Biol. 168:735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Brito, O. M., and L. Scorrano. 2008. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456:605-610. [DOI] [PubMed] [Google Scholar]
- 13.Dolezal, P., V. Likic, J. Tachezy, and T. Lithgow. 2006. Evolution of the molecular machines for protein import into mitochondria. Science 313:314-318. [DOI] [PubMed] [Google Scholar]
- 14.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. U. S. A. 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippin, L., P. J. Magalhaes, G. Di Benedetto, M. Colella, and T. Pozzan. 2003. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J. Biol. Chem. 278:39224-39234. [DOI] [PubMed] [Google Scholar]
- 16.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. W. Han, R. J. Lutz, S. Watanabe, E. D. Cahir McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. U. S. A. 96:12536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayajneh, W. A., A. M. Colberg-Poley, A. Skaletskaya, L. M. Bartle, M. M. Lesperance, D. G. Contopoulos-Ioannidis, N. L. Kedersha, and V. S. Goldmacher. 2001. The sequence and antiapoptotic functional domains of the human cytomegalovirus UL37 exon 1 immediate early protein are conserved in multiple primary strains. Virology 279:233-240. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, T., R. Rizzuto, G. Hajnoczky, and T. P. Su. 2009. MAM: more than just a housekeeper. Trends Cell Biol. 19:81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi, T., and T. P. Su. 2007. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596-610. [DOI] [PubMed] [Google Scholar]
- 20.Jurak, I., and W. Brune. 2006. Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J. 25:2634-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karbowski, M., K. L. Norris, M. M. Cleland, S. Y. Jeong, and R. J. Youle. 2006. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443:658-662. [DOI] [PubMed] [Google Scholar]
- 22.Kouzarides, T., A. T. Bankier, S. C. Satchwell, E. Preddy, and B. G. Barrell. 1988. An immediate early gene of human cytomegalovirus encodes a potential membrane glycoprotein. Virology 165:151-164. [DOI] [PubMed] [Google Scholar]
- 23.Kuroda, R., T. Ikenoue, M. Honsho, S. Tsujimoto, J. Y. Mitoma, and A. Ito. 1998. Charged amino acids at the carboxyl-terminal portions determine the intracellular locations of two isoforms of cytochrome b5. J. Biol. Chem. 273:31097-31102. [DOI] [PubMed] [Google Scholar]
- 24.Mavinakere, M. S., and A. M. Colberg-Poley. 2004. Dual targeting of the human cytomegalovirus UL37 exon 1 protein during permissive infection. J. Gen. Virol. 85:323-329. [DOI] [PubMed] [Google Scholar]
- 25.Mavinakere, M. S., and A. M. Colberg-Poley. 2004. Internal cleavage of the human cytomegalovirus UL37 immediate-early glycoprotein and divergent trafficking of its proteolytic fragments. J. Gen. Virol. 85:1989-1994. [DOI] [PubMed] [Google Scholar]
- 26.Mavinakere, M. S., C. D. Williamson, V. S. Goldmacher, and A. M. Colberg-Poley. 2006. Processing of human cytomegalovirus UL37 mutant glycoproteins in the endoplasmic reticulum lumen prior to mitochondrial importation. J. Virol. 80:6771-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick, A. L., C. D. Meiering, G. B. Smith, and E. S. Mocarski. 2005. Mitochondrial cell death suppressors carried by human and murine cytomegalovirus confer resistance to proteasome inhibitor-induced apoptosis. J. Virol. 79:12205-12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick, A. L., L. Roback, and E. S. Mocarski. 2008. HtrA2/Omi terminates cytomegalovirus infection and is controlled by the viral mitochondrial inhibitor of apoptosis (vMIA). PLoS Pathog. 4:e1000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick, A. L., V. L. Smith, D. Chow, and E. S. Mocarski. 2003. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J. Virol. 77:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki, E., Y. Kida, K. Mihara, and M. Sakaguchi. 2005. Switching the sorting mode of membrane proteins from cotranslational endoplasmic reticulum targeting to posttranslational mitochondrial import. Mol. Biol. Cell 16:1788-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris, K. L., and R. J. Youle. 2008. Cytomegalovirus proteins vMIA and m38.5 link mitochondrial morphogenesis to Bcl-2 family proteins. J. Virol. 82:6232-6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauleau, A.-L., N. Larochette, F. Giordanetto, S. R. Scholz, D. Poncet, N. Zamzami, V. S. Golmacher, and G. Koemer. 2007. Structure-function analysis of the interaction between Bax and the cytomegalovirus-encoded protein vMIA. Oncogene 26:7067-7080. [DOI] [PubMed] [Google Scholar]
- 33.Poncet, D., N. Larochette, A. L. Pauleau, P. Boya, A. A. Jalil, P. F. Cartron, F. Vallette, C. Schnebelen, L. M. Bartle, A. Skaletskaya, D. Boutolleau, J. C. Martinou, V. S. Goldmacher, G. Kroemer, and N. Zamzami. 2004. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 279:22605-22614. [DOI] [PubMed] [Google Scholar]
- 34.Poncet, D., A. L. Pauleau, G. Szabadkai, A. Vozza, S. R. Scholz, M. Le Bras, J. J. Briere, A. Jalil, R. Le Moigne, C. Brenner, G. Hahn, I. Wittig, H. Schagger, C. Lemaire, K. Bianchi, S. Souquere, G. Pierron, P. Rustin, V. S. Goldmacher, R. Rizzuto, F. Palmieri, and G. Kroemer. 2006. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J. Cell Biol. 174:985-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reboredo, M., R. F. Greaves, and G. Hahn. 2004. Human cytomegalovirus proteins encoded by UL37 exon 1 protect infected fibroblasts against virus-induced apoptosis and are required for efficient virus replication. J. Gen. Virol. 85:3555-3567. [DOI] [PubMed] [Google Scholar]
- 36.Rizzuto, R., M. R. Duchen, and T. Pozzan. 2004. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci. STKE 2004:re1. [DOI] [PubMed] [Google Scholar]
- 37.Rizzuto, R., P. Pinton, W. Carrington, F. S. Fay, K. E. Fogarty, L. M. Lifshitz, R. A. Tuft, and T. Pozzan. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280:1763-1766. [DOI] [PubMed] [Google Scholar]
- 38.Robin, M. A., H. K. Anandatheerthavarada, G. Biswas, N. B. Sepuri, D. M. Gordon, D. Pain, and N. G. Avadhani. 2002. Bimodal targeting of microsomal CYP2E1 to mitochondria through activation of an N-terminal chimeric signal by cAMP-mediated phosphorylation. J. Biol. Chem. 277:40583-40593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharon-Friling, R., J. Goodhouse, A. M. Colberg-Poley, and T. Shenk. 2006. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc. Natl. Acad. Sci. U. S. A. 103:19117-19122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stiban, J., L. Caputo, and M. Colombini. 2008. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J. Lipid Res. 49:625-634. [DOI] [PubMed] [Google Scholar]
- 41.Suen, D. F., K. L. Norris, and R. J. Youle. 2008. Mitochondrial dynamics and apoptosis. Genes Dev. 22:1577-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabadkai, G., K. Bianchi, P. Varnai, D. De Stefani, M. R. Wieckowski, D. Cavagna, A. I. Nagy, T. Balla, and R. Rizzuto. 2006. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 175:901-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szabadkai, G., and R. Rizzuto. 2004. Participation of endoplasmic reticulum and mitochondrial calcium handling in apoptosis: more than just neighborhood? FEBS Lett. 567:111-115. [DOI] [PubMed] [Google Scholar]
- 44.Tenney, D. J., and A. M. Colberg-Poley. 1991. Expression of the human cytomegalovirus UL36-38 immediate early region during permissive infection. Virology 182:199-210. [DOI] [PubMed] [Google Scholar]
- 45.Vance, J. E. 2008. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J. Lipid Res. 49:1377-1387. [DOI] [PubMed] [Google Scholar]
- 46.Williamson, C. D., and A. M. Colberg-Poley. 2009. Access of viral proteins to mitochondria via mitochondria-associated membranes. Rev. Med. Virol. 19:147-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson, C. D., and A. M. Colberg-Poley. 2010. Intracellular sorting signals for sequential trafficking of human cytomegalovirus UL37 proteins to the endoplasmic reticulum and mitochondria. J. Virol. 84:6400-6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yi, M., D. Weaver, and G. Hajnoczky. 2004. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J. Cell Biol. 167:661-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. U. S. A. 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]