Abstract
Marek's disease virus (MDV) causes a devastating disease in chickens characterized by the development of lymphoblastoid tumors in multiple organs and is transmitted from the skin of infected chickens. We have previously reported that the US2, UL44 (glycoprotein C [gC]), and UL13 genes are essential for horizontal transmission of MDV in gain-of-function studies using an a priori spread-deficient virus that was based on an infectious clone from the highly virulent RB-1B virus (pRB-1B). To precisely determine the importance of each individual gene in the process of chicken-to-chicken transmission, we used the transmission-restored clone that readily transmits horizontally and mutated each individual gene in loss-of-function experiments. Two independent US2-negative mutants transmitted horizontally, eliminating US2 as being essential for the process. In contrast, the absence of gC expression or mutating the invariant lysine essential for UL13 kinase activity abolished horizontal spread of MDV between chickens.
Marek's disease (MD) is caused by the oncogenic alphaherpesvirus Gallid herpesvirus 2 (GaHV-2), better known as MD virus (MDV). The most prominent sign of MD is the development of lymphoproliferative disease in chickens characterized by solid tumors in the viscera and other organs (3, 19). Natural infection begins through inhalation of virus, after which MDV is taken to the lymphoid organs and primary cytolytic infection in B and then T lymphocytes ensues. Following lytic infection, latency is established mainly in activated CD4+ T cells, which may be transformed with differing efficiencies, depending on the genotype of the infected chicken, and result in lymphoma formation. Irrespective of the transformation event, infection of feather follicle epithelial cells in the skin by migrating lymphocytes leads to the production of infectious particles that are shed into the environment, providing a continuous source of infectious virus. While the majority of the work on MDV has been focused on the transformation and reactivation of MDV during infection, little is known about horizontal transmission of virus from one chicken to another.
We recently identified genes important for horizontal transmission of MDV. We originally used a transmission-deficient virus derived from a bacterial artificial chromosome (BAC) clone of the very virulent RB-1B strain (pRB-1B-5) (35). Following sequencing of the complete BAC (40), specific genes suspected to be important for transmission were identified. We were able to restore horizontal transmission by repair of specific genes (17). We concluded that a combination of three genes, the unique short (US) 2, unique long (UL) 44 or glycoprotein (g) C, and UL13 protein kinase genes, was essential for horizontal transmission. Repair of each gene individually did not restore spread, nor did various combinations of two genes. In this report, we further defined which genes are essential by using loss-of-function studies utilizing mutant viruses in which US2, UL13, or gC was inactivated in the transmission-competent virus (17). Mutant viruses were engineered using an infectious clone and markerless Red recombination exactly as previously described (17) using primers shown in Table 1. Following confirmation of the correct modifications by restriction fragment length polymorphism (RFLP), PCR, and sequencing analyses, mutant viruses lacking the mini-F BAC sequences after Cre-Lox excision were reconstituted in chicken embryo cell cultures and propagated in chicken kidney cell cultures as previously described (17). Groups of P2a chickens (n = 10), which are highly susceptible to the development of MD (5), were experimentally infected with 1,000 PFU of the mutant viruses intra-abdominally and placed in glove box isolators with 10 age-matched, uninfected contact chickens. All experimental procedures were conducted in compliance with approved Institutional Animal Care and Use Committee (IACUC) protocols (Cornell University protocol numbers 2002-0085 and 2008-0018).
TABLE 1.
Primers used for mutating Marek's disease virus genes in transmission-competent pRB-1B
| Mutanta | Directionb | Sequencec |
|---|---|---|
| ΔUS2 | Forward | CAGTTATTAACAATAAAAAAGATTATTGGTGGAGGTGAAGTAGAATTCAGATCTGCTAGATAGGGATAACAGGGTAATCGATTT |
| Reverse | GCATACATTATACGAAGTTATCTAGCAGATCTGAATTCTACTTCACCTCCACCAATAATCGCCAGTGTTACAACCAATTAACC | |
| US2M1 stop | Forward | CCCAGTTATTAACAATAAAAAAGATTATTGGTGGAGGTGAAGTAAGGTGTGTCCATGATAACTATTAGGGATAACAGGGTAATCGATTT |
| Reverse | ATCGCATTCATCTAGAAGTGTGACTATAGTTATCATGGACACACCTTACTTCACCTCCACCAATAGCCAGTGTTACAACCAATTAACC | |
| UL13 K170M | Forward | CGGAGTAGTTAAAATATTTAAGAAGACGGACATAGCCGTCATGAAGTATTGGAATGTTTTAATAGGGATAACAGGGTAATCGAT |
| Reverse | ATGTCATAAGTAACTCAGTTTTAAAACATTCCAATACTTTCATGACGGCTATGTCCGTCTTCTGCCAGTGTTACAACCAATTAAC | |
| UL13 M170K | Forward | CGGAGTAGTTAAAATATTTAAGAAGACGGACATAGCCGTCAAAAAAGTATTGGAATGTTTTAATAGGGATAACAGGGTAATCGAT |
| Reverse | ATGTCATAAGTAACTCAGTTTTAAAACATTCCAATACTTTTTTGACGGCTATGTCCGTCTTCTGCCAGTGTTACAACCAATTAAC | |
| UL13 K270A | Forward | TTCTAAACGTGTCTTGTGGGTTGACTCATTTGGATATCGCATGTGGGAATATCTTTGGCCAGTGTTACAACCAATTAACC |
| Reverse | AGGACCCTCGGTGACGTTAACAAAGATATTCCCACATGCGATATCCAAATGAGTCATAGGGATAACAGGGTAATCGATTT | |
| gCM1 stop | Forward | CCAAACGTAACCCTCTACATATCTTCCCTCTAGCTCACGCCGCGTGTTTTACGAGCTTTGTAGGGATAACAGGGTAATCGATTT |
| Reverse | AAAAAGAGTCCAGTCCACCCCAAAGCTCGTAAAACACGCGGCGTGAGCTAGAGGGAAGATGCCAGTGTTACAACCAATTAACC | |
| gCstopM1 | Forward | CCAAACGTAACCCTCTACATATCTTCCCTCATGCTCACGCCGCGTGTGTTACGAGCTTTGTAGGGATAACAGGGTAATCGATTT |
| Reverse | AAAAAGAGTCCAGTCCACCCCAAAGCTCGTAACACACGCGGCGTGAGCATGAGGGAAGATGCCAGTGTTACAACCAATTAACC |
Gene mutation.
Directionality of the primer.
Underlined sequence indicates the template binding region of the primers for PCR amplification with pEPKanS. Bold and italicized letters indicate the mutated sequences introduced into the genome.
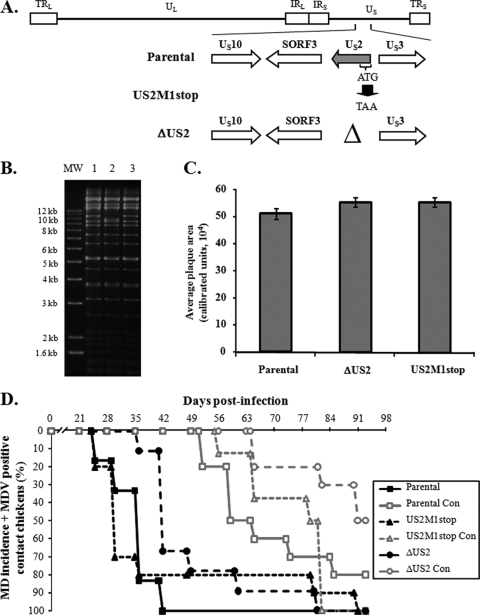
The US2 protein is located in the virion tegument (4) and is conserved in alphaherpesviruses. The MDV US2 ortholog has been shown to be nonessential for MDV replication in vitro and in vivo (33), and our earlier study (17) led to the first report suggesting US2 may have a functional role in MDV pathogenesis (i.e., horizontal transmission). In order to conclusively determine the role of US2 in this process, we generated two mutant viruses in which either the entire US2 open reading frame (ORF) was deleted (ΔUS2) or the start codon of US2 was mutated to a stop codon (US2M1stop) in the transmission-competent virus (Fig. 1A). RFLP analysis showed that no spurious mutations were evident, apart from the expected deletion of US2 in the ΔUS2 clone (Fig. 1B). The average plaque area of each virus was determined exactly as previously described (18) and found to be similar for both mutant and parental viruses (Fig. 1C). Figure 1D shows MD incidence in chickens experimentally infected with each respective virus (black lines) and contact chickens (gray lines) exposed to the experimentally infected chickens. Over the course of 13 weeks, both mutant viruses and the parental virus induced MD incidences of 100%. Both ΔUS2 and US2M1stop were also able to spread from chicken to chicken, with the ΔUS2 virus being slightly slower in causing MD in contact chickens. By 13 weeks postinfection (p.i.), only 40% of contact chickens developed MD in this group. One chicken showed no clinical signs or gross lesions at termination, but it had MDV viremia, which was determined by previously described qPCR assays (17). From these results, we concluded that US2 is nonessential for horizontal transmission, contrary to what we had originally reported (17). This is in agreement with results using another infectious MDV clone in which the US2 gene was removed during BAC construction and reconstituted virus was able to horizontally spread (30).
FIG. 1.
Generation of US2 mutant MDVs and evaluation of their ability to induce MD and horizontally transmit to contact chickens. (A) Two US2 mutant viruses were generated, one in which the complete US2 ORF was deleted (ΔUS2) and another where the ATG start codon was mutated to a TAA stop codon (US2M1stop). Also shown are genes flanking the US2 ORF in the US region of the MDV genome. (B) RFLP analysis of DNA obtained from parental virus (lane 1) and ΔUS2 (lane 2) and US2M1stop (lane 3) BAC clones using BamHI restriction patterns. Deletion of US2 reduces the size of the 10,354-bp fragment of the parental virus (lane 1) to 9,544 bp (lane 2). No extraneous alterations are evident in both clones. The molecular size marker (MW) used is the 1-kb Plus DNA ladder from Invitrogen, Inc. (Carlsbad, CA). (C) The average plaque area ± standard error of the mean (SEM) for each respective virus was determined from 75 plaques exactly as previously described (18). No significant differences were seen between viruses using Student's t tests. (D) MD incidence of P2a chickens inoculated at 1 day of age with reconstituted BAC clones described in the text and contact (Con) chickens housed with experimentally infected chickens over the course of 13 weeks of infection. MD incidence was determined by identification of gross lesions in dead or euthanized chickens. Chickens not succumbing to MD over the course of the experiment were terminated at 92 days p.i. Blood was collected from all remaining birds and tested for MDV genomic copies using qPCR exactly as previously described (17). For determination of horizontal transmission, contact chickens positive for MDV genomic copies in the blood were included, since the presence of MDV genomes indicated spread.
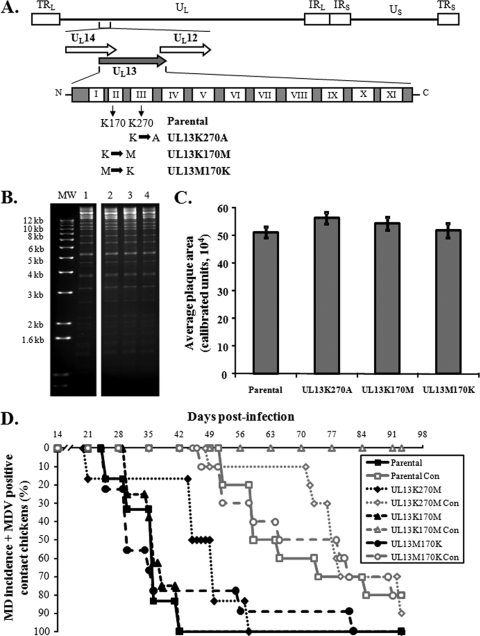
Another gene identified to be important for horizontal transmission was the UL13 serine/threonine protein kinase (17). The UL13 gene is highly conserved, not only among the alphaherpesviruses but in all members of the Herpesviridae. In the case of herpes simplex virus 1 (HSV-1), UL13 was shown to be present in the tegument of enveloped virus and has been shown to autophosphorylate and phosphorylate a large number of viral and host proteins (6, 20, 21, 29, 31, 36). Morrison et al. (28) showed that UL13 protein kinase activity promotes dissociation of tegument by phosphorylation of tegument proteins, while Moffat and coworkers (26) showed that the varicella zoster virus (VZV) ortholog of UL13 (ORF47) is required for efficient infection of T lymphocytes and skin in the SCID-hu mouse model. The MDV UL13 amino acid sequence contains the 11 (I to XI) conserved catalytic domains found in all protein kinases (11, 37), and the protein is catalytically active (37). The transmission-deficient MDV previously analyzed contained a frameshift mutation within the UL13 ORF leading to a truncated protein that encoded only the first two conserved domains (I and II) (2, 17). We hypothesized that this truncated protein lacked kinase activity and that UL13 kinase activity was important for transmission of MDV. The invariant lysine in the catalytic domain, positioned at amino acid 170 in the MDV UL13 protein, was shown to be essential for kinase activity in other UL13 orthologs (7, 10, 12, 20, 22, 34, 41). Therefore, we mutated lysine 170 of MDV UL13 domain II to a methionine (UL13K170M) and generated a revertant virus of this mutant by replacing the methionine in the original sequence (UL13M170K) (Fig. 2A). As an additional control, we mutated the lysine at position 270 in domain III (UL13K270A). RFLP analysis showed that there were no discernible differences between the parental, mutant, and revertant clones (Fig. 2B). Also, there were no significant differences in average plaque areas for each virus derived from the cloned DNA (Fig. 2C). Figure 2D shows that both mutant viruses caused 100% MD in experimentally infected chickens by 13 weeks p.i. However, when transmission from chicken to chicken was evaluated, the UL13 kinase mutant (UL13K170M) did not spread to contact chickens. In contrast, both the revertant of UL13K170M (UL13M170K) and the UL13K270A viruses were able to horizontally transmit to contact chickens with efficiencies and kinetics similar to those of the parental virus. A second experiment was conducted to confirm the lack of spread with the UL13 kinase mutant, and, again, no transmission to contact chickens was observed (data not shown). The data strongly suggest that MDV UL13 protein kinase activity is essential for horizontal transmission of MDV. It has been previously shown that UL13 protein kinase activity promotes dissociation of tegument by phosphorylation of tegument proteins for HSV-1 (28). We hypothesize that MDV UL13 may perform a similar function during natural infection and therefore virus shed from the infected chickens that lack UL13 kinase activity are defective in cell entry. Studies are in progress to define at what point transmission from animal to animal is deficient.
FIG. 2.
Generation of UL13 mutant MDVs and evaluation of their ability to induce MD and horizontally transmit to contact chickens. (A) Schematic diagram showing the UL13 ORF flanked by overlapping UL14 and UL12 ORFs and reversed in the figure for simplicity. Also shown are the 11 (I to XI) kinase domains contained within the conserved UL13 protein. Two UL13 mutants were generated from the transmission-competent BAC clone (parental) as described in the text. (B) RFLP analysis of DNA obtained from parental virus (lane 1) and UL13K270M (lane 2), UL13K170M (lane 3), and UL13M170K (lane 4) BAC clones using BamHI restriction patterns. No extraneous alterations are evident in all clones. The molecular size marker (MW) used is the 1-kb Plus DNA ladder (Invitrogen). (C) Same as in Fig. 1C. No significant differences in plaque sizes were seen between each virus using Student's t tests. (D) Same as in Fig. 1D. All contact chickens housed with the UL13K170M (kinase mutant) were negative for MD lesions following necropsy and negative for MDV genomic copies in the blood using qPCR assays.
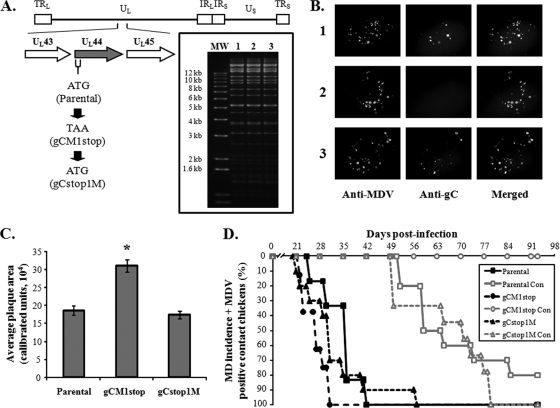
Alphaherpesvirus gC orthologs have multiple functions. They play major roles in the primary attachment of cell-free virus to heparin- and chondroitin-like glycosaminoglycans on the surface of cells (25, 38), and involvement of gC in a late step of virus egress from cultured cells has been shown for the members of the Varicellovirus genus, pseudorabies virus (PRV-1) and equine herpesvirus 1 (EHV-1) (25, 32). Additionally, the gC proteins of HSV-1, HSV-2, PRV-1, bovine herpes virus 1 (BHV-1), and EHV-1 are able to bind complement component C3 (1, 8, 9, 13, 14, 15). It had been suspected that gC was important for horizontal transmission of MDV, but formal proof was missing, as the constructed and tested gC mutant virus used had reduced ability to replicate in experimental chickens (27). Identification of a frameshift mutation within the UL44 ORF in the original pRB-1B-5 BAC (35) and the subsequent repair of this mutation that restored horizontal transmission in combination with the repair of US2 and UL13 strongly suggested that gC was essential for horizontal transmission (17). To definitively determine if gC is essential for animal-to-animal spread, we mutated the start codon of gC to a stop codon (gCM1stop) in the transmission-competent virus (Fig. 3A). We also generated a revertant of the gCM1stop clone in which the start codon was repaired (gCstop1M). RFLP analysis showed that there were no discernible differences between the parental, mutant, or revertant BAC clones (Fig. 3A, inset). While the gCM1stop virus plaques were negative for gC expression, both parental and revertant (gCstop1M) viruses were reactive with the gC antibody (Fig. 3B) using an MDV gC-specific monoclonal antibody in immunofluorescence (IF) assays as previously described (18). Measurement of plaque areas of each virus showed that the gCM1stop virus lacking gC expression produced plaques approximately twice as large as the parental and revertant viruses (Fig. 3C), consistent with previous results (42). Each of the recombinant viruses induced MD in chickens infected by intra-abdominal inoculation with similar efficiencies and kinetics (Fig. 3D, black lines). However, the gCM1stop virus was unable to spread from infected to sentinel chickens, while the revertant (gCstop1M) spread efficiently (Fig. 3D, gray lines). In two follow-up experiments, the gC-null virus was repeatedly unable to spread, while its revertant transmitted like the parental virus (data not shown), clearly showing that functional gC is required for horizontal transmission of MDV. It is suspected that the functional role of MDV gC during natural infection involves binding complement. The MDV gC protein contains homologous regions (K. W. Jarosinski, unpublished observation) that have been shown to be important for complement binding of other herpesvirus gC proteins (16, 39) and protecting virus from complement-mediated destruction (23, 24). Studies are in progress to address this possibility.
FIG. 3.
Generation of UL44 (gC) mutant MDV and evaluation of their ability to induce MD and horizontally transmit to contact chickens. (A) Schematic diagram showing the location of UL44 (gC) in relation to UL43 and UL45 in the MDV genome. The start codon of gC was mutated to a stop codon (gCM1stop) in the transmission-competent BAC clone (parental). A revertant of the mutant was also produced in which the stop codon was replaced with a start codon (gCstop1M). Inset shows RFLP analysis of DNA obtained from parental virus (lane 1) and gCM1stop (lane 2) and gCstop1M (lane 3) BAC clones using BamHI restriction patterns. No extraneous alterations are evident in the clones. The molecular size marker (MW) used is the 1-kb Plus DNA ladder (Invitrogen). (B) IF assays of respective plaques for each virus using a polyclonal anti-MDV chicken antibody and an anti-gC monoclonal antibody with Alexa Fluor 568 and 488 secondary antibodies, respectively, as previously described (17, 18). Numbers are the same as in panel A. (C) Same as in Fig. 1C and Fig. 2C. The gC-null virus (gCM1stop) produced plaques approximately twice as large as parental and revertant viruses, and this was significantly different (P < 0.001) using Student's t tests and is indicated with an asterisk (*). (D) Same as in Fig. 1D and Fig. 2D. All contact chickens housed with the gCM1stop (gC-null) clone were negative for MD lesions following necropsy and negative for MDV genomic copies in the blood using qPCR assays.
In conclusion, the studies presented here used loss-of-function analyses to definitely determine if expression of US2, gC, and UL13 protein kinase activity individually were essential for horizontal transmission of MDV in chickens. We were able to conclusively show that US2 is not essential whereas both gC and UL13 protein kinase activity, individually, are essential for horizontal transmission of MDV in chickens.
Acknowledgments
We thank Neil Margulis and Najet Chbab for help in construction of clones.
This work was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2003-02234 to N.O. and USDA-National Institute of Food and Animals—Agriculture and Food Research Initiative grant number 2009-01745 to K.W.J.
Footnotes
Published ahead of print on 19 May 2010.
REFERENCES
- 1.Allen, G. P., and L. D. Coogle. 1988. Characterization of an equine herpesvirus type-1 gene encoding a glycoprotein (gp13) with homology to herpes simplex virus glycoprotein C. J. Virol. 62:2850-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blondeau, C., N. Chbab, C. Beaumont, K. Courvoisier, N. Osterrieder, J. F. Vautherot, and C. Denesvre. 2007. A full UL13 open reading frame in Marek's disease virus (MDV) is dispensable for tumor formation and feather follicle tropism and cannot restore horizontal spread in rRB-1B in vivo. Vet. Res. 38:419-433. [DOI] [PubMed] [Google Scholar]
- 3.Calnek, B. W. 2001. Pathogenesis of Marek's disease virus infection, p. 25-55. In K. Hirai (ed.), Marek's disease. Springer, Berlin, Germany. [DOI] [PubMed]
- 4.Clase, A. C., M. G. Lyman, T. del Rio, J. A. Randall, C. M. Calton, L. W. Enquist, and B. W. Banfield. 2003. The pseudorabies virus Us2 protein, a virion tegument component, is prenylated in infected cells. J. Virol. 77:12285-12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, R. K. 1968. Studies on genetic resistance to Mareks disease. Avian Dis. 12:9-28. [PubMed] [Google Scholar]
- 6.Cunningham, C., A. J. Davison, A. Dolan, M. C. Frame, D. J. McGeoch, D. M. Meredith, H. W. M. Moss, and A. C. Orr. 1992. The UL13 virion protein of herpes-simplex virus type-1 is phosphorylated by a novel virus-induced protein-kinase. J. Gen. Virol. 73:303-311. [DOI] [PubMed] [Google Scholar]
- 7.de Wind, N., J. Domen, and A. Berns. 1992. Herpesviruses encode an unusual protein-serine/threonine kinase which is nonessential for growth in cultured cells. J. Virol. 66:5200-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenberg, R. J., M. P. Deleon, H. M. Friedman, L. F. Fries, M. M. Frank, J. C. Hastings, and G. H. Cohen. 1987. Complement component C3B binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 3:423-435. [DOI] [PubMed] [Google Scholar]
- 9.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein-C of herpes-simplex virus-1 acts as a receptor for the C3B complement component on infected cells. Nature 309:633-635. [DOI] [PubMed] [Google Scholar]
- 10.Gershburg, E., and J. S. Pagano. 2002. Phosphorylation of the Epstein-Barr virus (EBV) DNA polymerase processivity factor EA-D by the EBV-encoded protein kinase and effects of the l-riboside benzimidazole 1263W94. J. Virol. 76:998-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanks, S. K., A. M. Quinn, and T. Hunter. 1988. The protein-kinase family—conserved features and deduced phylogeny of the catalytic domains. Science 241:42-52. [DOI] [PubMed] [Google Scholar]
- 12.He, Z. W., Y. S. He, Y. Kim, L. L. Chu, C. Ohmstede, K. K. Biron, and D. M. Coen. 1997. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 71:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huemer, H. P., C. Larcher, and N. E. Coe. 1992. Pseudorabies virus glycoprotein III (gIII) derived from virions and infected cells binds to the 3rd component of complement. Virus Res. 23:271-280. [DOI] [PubMed] [Google Scholar]
- 14.Huemer, H. P., C. Larcher, van Drunen Littel-van den Hurk, and L. A. Babiuk. 1993. Species selective interaction of Alphaherpesvirinae with the “unspecific” immune system of the host. Arch. Virol. 130:353-364. [DOI] [PubMed] [Google Scholar]
- 15.Huemer, H. P., N. Nowotny, B. S. Crabb, H. Meyer, and P. H. Hubert. 1995. gp13 (EHV-gC): a complement receptor induced by equine herpesviruses. Virus Res. 37:113-126. [DOI] [PubMed] [Google Scholar]
- 16.Hung, S. L., S. Srinivasan, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 1992. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J. Virol. 66:4013-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarosinski, K. W., N. G. Margulis, J. P. Kamil, S. J. Spatz, V. K. Nair, and N. Osterrieder. 2007. Horizontal transmission of Marek's disease virus requires US2, the UL13 protein kinase, and gC. J. Virol. 81:10575-10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarosinski, K. W., N. Osterrieder, V. K. Nair, and K. A. Schat. 2005. Attenuation of Marek's disease virus by deletion of open reading frame RLORF4 but not RLORF5a. J. Virol. 79:11647-11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarosinski, K. W., B. K. Tischer, S. Trapp, and N. Osterrieder. 2006. Marek's disease virus: lytic replication, oncogenesis and control. Expert Rev. Vaccines 5:761-772. [DOI] [PubMed] [Google Scholar]
- 20.Kato, A., M. Yamamoto, T. Ohno, M. Tanaka, T. Sata, Y. Nishiyama, and Y. Kawaguchi. 2006. Herpes simplex virus 1-encoded protein kinase UL13 phosphorylates viral Us3 protein kinase and regulates nuclear localization of viral envelopment factors UL34 and UL31. J. Virol. 80:1476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1998. Eukaryotic elongation factor 1δ is hyperphosphorylated by the protein kinase encoded by the UL13 gene of herpes simplex virus 1. J. Virol. 72:1731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon, T. K., J. Lynch, J. Hay, W. Ruyechan, and C. Grose. 2001. Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J. Virol. 75:8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubinski, J., L. Y. Wang, D. Mastellos, A. Sahu, J. D. Lambris, and H. M. Friedman. 1999. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J. Exp. Med. 190:1637-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubinski, J. M., M. Jiang, L. Hook, Y. Chang, C. Sarver, D. Mastellos, J. D. Lambris, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 2002. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J. Virol. 76:9232-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mettenleiter, T. C. 1989. Glycoprotein gIII deletion mutants of pseudorabies virus are impaired in virus entry. Virology 171:623-625. [DOI] [PubMed] [Google Scholar]
- 26.Moffat, J. F., L. Zerboni, M. H. Sommer, T. C. Heineman, J. I. Cohen, H. Kaneshima, and A. M. Arvin. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc. Natl. Acad. Sci. U. S. A. 95:11969-11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan, R. W., A. Anderson, J. Kent, and M. Parcells. 1996. Characterization of Marek's disease virus RB1B-based mutants having disrupted glycoprotein C or glycoprotein D homolog genes, p. 207-212. In Current research on Marek's disease: proceedings of the 5th International Symposium on Marek's Disease. American Association of Avian Pathologists, Kennett Square, PA.
- 28.Morrison, E. E., Y. F. Wang, and D. M. Meredith. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol. 72:7108-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng, T. I., W. O. Ogle, and B. Roizman. 1998. U(L)13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology 241:37-48. [DOI] [PubMed] [Google Scholar]
- 30.Niikura, M., J. B. Dodgson, R. F. Silva, and H. H. Cheng. 2008. Comparative sequence analysis of infectious Marek's disease virus bacterial artificial chromosome clones generated from virulent strains, p. 15. In The 8th International Marek's Disease Symposium. James Cook University, Townsville, Australia.
- 31.Ogle, W. O., T. I. Ng, K. L. Carter, and B. Roizman. 1997. The U(L)13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406-413. [DOI] [PubMed] [Google Scholar]
- 32.Osterrieder, N. 1999. Construction and characterization of an equine herpesvirus 1 glycoprotein C negative mutant. Virus Res. 59:165-177. [DOI] [PubMed] [Google Scholar]
- 33.Parcells, M. S., A. S. Anderson, J. L. Cantello, and R. W. Morgan. 1994. Characterization of Marek's disease virus insertion and deletion mutants that lack US1 (ICP22 homolog), US10, and/or US2 and neighboring short-component open reading frames. J. Virol. 68:8239-8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park, J., D. Lee, T. Seo, J. Chung, and J. Choe. 2000. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) open reading frame 36 protein is a serine protein kinase. J. Gen. Virol. 81:1067-1071. [DOI] [PubMed] [Google Scholar]
- 35.Petherbridge, L., A. C. Brown, S. J. Baigent, K. Howes, M. A. Sacco, N. Osterrieder, and V. K. Nair. 2004. Oncogenicity of virulent Marek's disease virus cloned as bacterial artificial chromosomes. J. Virol. 78:13376-13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purves, F. C., and B. Roizman. 1992. The UL13 gene of herpes-simplex virus-1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein-alpha-22. Proc. Natl. Acad. Sci. U. S. A. 89:7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy, S. M., D. Sui, P. Wu, and L. Lee. 1999. Identification and structural analysis of a MDV gene encoding a protein kinase. Acta Virol. 43:174-180. [PubMed] [Google Scholar]
- 38.Rue, C. A., and P. Ryan. 2002. Characterization of pseudorabies virus glycoprotein C attachment to heparan sulfate proteoglycans. J. Gen. Virol. 83:301-309. [DOI] [PubMed] [Google Scholar]
- 39.Rux, A. H., W. T. Moore, J. D. Lambris, W. R. Abrams, C. Peng, H. M. Friedman, G. H. Cohen, and R. J. Eisenberg. 1996. Disulfide bond structure determination and biochemical analysis of glycoprotein C from herpes simplex virus. J. Virol. 70:5455-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spatz, S. J., Y. Zhao, L. Petherbridge, L. P. Smith, S. J. Baigent, and V. Nair. 2007. Comparative sequence analysis of a highly oncogenic but horizontal spread-deficient clone of Marek's disease virus. Virus Genes 35:753-766. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka, M., Y. Nishiyama, T. Sata, and Y. Kawaguchi. 2005. The role of protein kinase activity expressed by the UL13 gene of herpes simplex virus 1: the activity is not essential for optimal expression of UL41 and ICP0. Virology 341:301-312. [DOI] [PubMed] [Google Scholar]
- 42.Tischer, B. K., D. Schumacher, D. Chabanne-Vautherot, V. Zelnik, J. F. Vautherot, and N. Osterrieder. 2005. High-level expression of Marek's disease virus glycoprotein C is detrimental to virus growth in vitro. J. Virol. 79:5889-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]