Abstract
As one of the world's most common infectious diseases, hepatitis B virus (HBV) is a serious worldwide public health problem, with HBV-associated liver disease accounting for more than half a million deaths each year. Although there is an effective prophylactic vaccine currently available to prevent infection, it has a number of characteristics that are suboptimal: multiple doses are needed to induce long-lasting immunity, immunity declines over time, it does not elicit protection in some individuals, and it is not effective therapeutically. We produced a recombinant vesicular stomatitis virus (VSV)-based vaccine vector expressing the HBV middle envelope surface protein (MS) and found that this vector was able to efficiently generate a strong HBs-specific antibody response following a single immunization in mice. A single immunization with the VSV-MS vector also induced robust CD8 T-cell activation. The CD8 T-cell response was greater in magnitude and broader in specificity than the response generated by a vaccinia virus-based vaccine vector or by recombinant protein immunization. Furthermore, a single VSV-MS immunization provided protection against virus challenge in mice. Given the similar antibody titers and superior T-cell responses elicited from a single immunization, a VSV-based HBV vaccine may have advantages over the current recombinant protein vaccine.
It is estimated that >2 billion people worldwide have been infected with hepatitis B virus (HBV), placing it among the world's most common infectious diseases (44). HBV is a serious public health concern as regions of high endemicity, including Southeast Asia, and sub-Saharan Africa, consistently report a chronic carrier prevalence of 8 to 15% (28). Areas such as Taiwan and Thailand, however, have demonstrated that implementation of infant vaccination programs can have profound effects on lowering rates of infection and chronicity (7, 8). Nevertheless, in the United States alone, where the majority of states require HBV vaccination prior to enrollment in public schools (38), there are upwards of 2 million chronically infected individuals (12). Thus, there remains a need for continued development and evaluation of HBV vaccination programs and treatment options.
Recombinant protein vaccines currently available for HBV are comprised of the small and/or large versions of the envelope surface glycoprotein (46). Despite their success, these vaccines have a number of characteristics that are suboptimal. The current vaccination protocol recommends two to three doses to induce long-lasting immunity (25). This may create delivery and compliance issues in areas lacking the appropriate medical infrastructure for administration, which includes some regions where HBV is highly endemic. Even after completion of the full HBV vaccine regimen, up to 10% of the population is unable generate a protective response to the virus (39). Furthermore, a waning antibody response following immunization may cause antibody to decline below protective titers (10 U/liter) in up to 60% of individuals who respond to vaccination (24). Due to the limitations of the current vaccine, alternative vaccination protocols continue to be explored. Generally, viral vaccine vectors efficiently express viral antigens and may be an effective strategy to enhance antigen presentation, thereby stimulating potent humoral and cell-mediated immune responses in a single dose (41). A number of viral vaccine platforms for HBV have been shown to generate protective antibody titers in animal models, including, most recently, modified vaccinia virus (VV) Ankara- and measles virus-based vaccines (21, 31).
In the present study, we utilize the negative-strand RNA virus vesicular stomatitis virus (VSV), as it offers a variety of features that make it an ideal viral vaccine vector. The VSV genome is simple and more fully understood than other potential vaccine platforms, such as vaccinia virus, and can be grown to high titers in mammalian cell lines approved for vaccine production. Additionally, VSV infection in humans is rare, reducing the risk of preexisting immunity, which may interfere with vaccine efficacy (10). Despite the fact that neutralizing antibodies are generated against the VSV glycoprotein following a single vaccination, a variety of VSV serotypes are available if boosting is required (33). Finally, VSV vectors can be delivered intranasally in a needle-free manner. Recombinant VSV vectors have been developed for a variety of viruses, including HIV, influenza virus, and hepatitis C virus (HCV) (15, 32, 36). The majority of these vectors have provided protection against secondary challenge in animal models after a single dose. Taken together, the unique characteristics of VSV may lead to the development of a prophylactic vaccine that could be delivered in a single-dose and needle-free manner, which would provide certain advantages over the current vaccine. We have generated a VSV vector expressing HBV middle envelope surface protein (MS) antigen that is properly localized and secreted. Here we examine the potential of this vector as an alternative prophylactic HBV vaccine.
MATERIALS AND METHODS
Plasmids and recombinant viruses.
The open reading frame for the middle version of the HBV envelope glycoprotein (MS; ayw subtype) was amplified by PCR from pCMV-S2S using primers 5′-CGTCGACATGCAGTGGAATTCCACAACC-3′ and 5′-GCTAGCTTAAATGTATACCCAAAGACA-3′, introduc-ing upstream SalI and downstream NheI sites for directional cloning. The MS PCR product was cleaved with SalI and NheI and cloned into the fifth position of the pVSVXN2 plasmid after its cleavage with XhoI and NheI. The middle version of the HBV envelope protein was selected as the antigen for these studies because it potentially contained a greater number of T-cell epitopes than the small version. Furthermore, unlike the large version of the protein, it does not contain the HBV receptor-binding domain. Therefore, if it were incorporated into the VSV virion, it would not change the tropism or pathogenesis of the vector.
Recombinant VSV containing MS (VSV-MS) was recovered as previously described (22). Briefly, BHK-21 cells grown to 50% confluence were infected with recombinant vaccinia virus expressing T7 RNA polymerase (multiplicity of infection [MOI] of 10) and incubated for 1 h in serum-free Dulbecco's modified Eagle's medium (DMEM). Vaccinia virus-infected cells were then cotransfected with the generated plasmid expressing the recombinant VSV antigenome with expression vectors for the VSV N, P, and L proteins under the control of a T7 promoter. Supernatants were collected 48 h posttransfection, filtered through a 0.2-μm-pore filter to remove vaccinia virus, and passaged onto fresh BHK-21 cells. The medium was collected immediately after cytopathic effects were observed (∼2 days) and filtered through a 0.1-μm-pore filter. Recombinant VSV-MS was then plaque purified and expanded, the titer was determined, and the VSV-MS was stored at −80°C until use. Recombinants were thawed and diluted to the correct titration immediately prior to use.
A plasmid (pCMV-S2S) expressing middle envelope protein of HBV (ayw subtype) was used for DNA vaccinations (14, 26). Vaccinia virus (VV) immunizations and challenges were completed using a recombinant VV expressing the small (S) envelope protein of HBV (VV-S; subtype adw2) (40).
Detection of MS.
BHK-21 cells were infected with VSV-MS (MOI of 10) for 6 h, with uninfected and VSV-infected cells used as controls. Medium was collected, cells were washed with phosphate-buffered saline (PBS) and lysed with 2× SDS sample buffer. Proteins were separated on a 10% SDS gel, transferred to a nitrocellulose membrane, probed with anti-HBs antibody (Santa Cruz Biotechnology), and detected with secondary antibody using chemiluminesence. Secreted HBV MS protein was detected in collected media (data not shown) using a commercially available HBsAg enzyme-linked immunosorbent assay (ELISA) kit (International Immunodiagnostics).
Indirect immunofluorescence.
For immunofluorescence microscopy, BHK-21 cells were grown on glass coverslips and infected with VSV-MS (MOI of 10), with uninfected and VSV-infected cells as controls. Following a 6-h infection, cells were fixed with 2% paraformaldehyde and permeabilized with 0.5% Triton X-100 for 20 min at 37°C. After blocking for 1 h in PBS containing 10% fetal bovine serum, cells were incubated with mouse anti-HBsAg antibody (Santa Cruz Biotechnology; 1:50) for 30 min at 37°C. As a control for VSV infection, a second set of unpermeabilized cells from the same infection was soaked in PBS-glycine for 10 min at 25°C and then incubated with mouse anti-VSV-G antibody (1:200) for 30 min at 37°C. All cells were washed with PBS following incubation with primary antibody and then incubated with Alexa Fluor 488-labeled goat anti-mouse secondary antibody (Invitrogen; 1:1,000) for 30 min at 37°C. After washing, cells were mounted onto microscope slides with ProLong Gold antifade reagent containing DAPI (4′,6-diamidino-2-phenylindole; Invitrogen). A Nikon Microphot-FX microscope equipped with a SPOT digital camera was used to capture images at a 60× magnification.
Immunization/challenge protocols.
Female CB6F1 mice 8 to 10 weeks of age were purchased from Charles River (Wilmington, MA) and housed at the Yale University School of Medicine animal facilities. All experiments were performed in accordance with Yale Institutional Animal Care and Use Committee-approved procedures. Mice were lightly anesthetized with 30% isoflurane (Baxter) diluted in propylene glycol (vol/vol) prior to all immunizations. Single intranasal (i.n.) inoculations of 106 PFU were administered in a 25-μl volume for VSV or VSV-MS. For direct comparison of antibody responses, recombinant HBs antigen (rHBsAg) (Biospacific; 10 μg), HBV recombinant protein vaccine (Engerix-B; 20 μg), or VSV-MS (106 PFU) was administered intramuscularly (i.m.) in a 100-μl volume. In order to optimize CD8 T-cell counts for polyfunctional analysis, mice were administered an intramuscular prime of DNA-MS (100 μg) in 100 μl PBS. Mice then received boosts of DNA-MS (i.m., 100 μg in 100 μl), VSV-MS (i.n., 106 PFU in 25 μl), or VV-S (intraperitoneal [i.p.], 106 PFU in 100 μl) 3 weeks postprime.
Two challenge protocols were used for the present study. In the first, mice received an i.n. inoculation of 106 PFU VSV or VSV-MS in 25 μl of PBS. They were then challenged 8 weeks later with 106 PFU VV or VV-S administered i.n. in 25 μl PBS. In the second protocol, mice again received an i.n. inoculation of 106 PFU VSV or VSV-MS in 25 μl PBS or an i.m. inoculation with recombinant HBs antigen (Biospacific; 10 μg) in 100 μl PBS. Following 8 weeks, mice were challenged using a hydrodynamic transfection protocol, whereby a total of 10 μg pT-HBV1.3 was injected into the tail veins in a volume of PBS equal to 9% of their body mass (45). A group of VSV-MS-immunized mice were also treated with anti-CD8 antibody (BioXCell; 500 μg), administered i.p. in 250 μl, 1 day prior to hydrodynamic transfection and again on day 2 posttransfection.
ELISPOT assay.
A gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) set (BD Biosciences) was used to quantify T-cell activation following immunization. Briefly, 96-well plates were coated overnight with purified anti-mouse IFN-γ antibody (1:200). After capture antibody had been removed, plates were blocked for 2 h using DMEM supplemented with 10% fetal bovine serum, 100 μg/ml penicillin, and 2 mM l-glutamine. Splenocytes were purified from mice 7 days postimmunization/challenge by passage through 70-μm strainers (BD Falcon) and treatment with ACK lysing buffer (Lonza). After being washed with Hanks' balanced salt solution (Gibco), cells were suspended in DMEM supplemented with 10% fetal bovine serum, 100 μg/ml penicillin, and 2 mM l-glutamine and seeded at 2 × 105 cells/well. The cells were stimulated overnight at 37°C with HBV (ayw serotype)- or VSV-specific peptides (Table 1) at a concentration of 20 μg/ml, with media and phorbol myristate acetate (PMA)/ionomycin-treated cells used as negative and positive controls, respectively. Cells were washed from plates using PBS-Tween (0.05% [vol/vol]) and incubated with provided biotinylated anti-mouse IFN-γ antibody (1:250) for 2 h at 25°C. After being washed, streptavidin-horseradish peroxidase (HRP) (1:1,000) was added to wells and incubated for 1 h at 25°C. Following the final washes, 3-amino-9-ethyl-carbazole (AEC) chromogen-substrate (BD Biosciences) was added to the wells and allowed to develop at 25°C for 20 to 40 min. The reaction was stopped with distilled water (dH2O), and the plates were allowed to air dry before spot-forming cells (SFC) were enumerated.
TABLE 1.
CD8 T-cell epitopes
| Epitope | Protein | Positions | Sequencea | MHC | Reference(s) |
|---|---|---|---|---|---|
| 191 | HBV S | 191-202 | IPQSLDSWWTSL | Ld | 1, 20 |
| 353 | HBV S | 353-360 | VWLSVIWM | Kb | 34 |
| 364 | HBV S | 364-372 | WGPSLYSIL | Dd | 37 |
| 371 | HBV S | 371-378 | ILSPFLPL | Kb | 35, 37 |
| N1 | VSV N | 74-83 | MPYLIDFGL | Ld | 30 |
| N2 | VSV N | 52-59 | RGYVYQGL | Kb | 43 |
HBV ayw serotype.
Flow cytometry.
Splenocytes for intracellular cytokine staining (ICS) were prepared 7 days postimmunization/postchallenge as described for the ELISPOT assay above. Intrahepatic lymphocytes (IHL) were purified from mice 7 days postchallenge by passage through 70-μm-pore strainers (BD Falcon) and 30 min of treatment at 37°C with 0.5 mg/ml collagenase D (Roche). After washing with RPMI (Gibco), cells were resuspended in 44% Percoll in RPMI (vol/vol) and layered over 56% Percoll in PBS (vol/vol). The gradient was centrifuged for 30 min at 850 × g, the interphase was collected and then washed with PBS, and cells were suspended in DMEM supplemented with 10% fetal bovine serum, 100 μg/ml penicillin, and 2 mM l-glutamine. The cells were then stimulated for 6 h with HBV- and VSV-specific peptides diluted to a final concentration of 1 μg/ml in DMEM supplemented with 20 IU interleukin-2 (IL-2) (BD Biosciences), 2 μg/ml brefeldin A (BD Biosciences), and 2 μg/ml of antibody to the costimulatory molecules CD28 and CD49d (BD Biosciences). The cells were then washed in staining buffer (PBS containing 1% fetal bovine serum) and stained for CD8 and CD3 surface expression for 30 min on ice using fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD8 antibody (BD Biosciences) and peridinin chlorophyll protein (PerCP)-conjugated anti-mouse CD3 antibody (BD Biosciences). The surface-stained cells were washed, fixed, and permeabilized using a commercially available Cytofix/Cytoperm kit (BD Biosciences). The cells were then stained for 30 min on ice for intracellular cytokine expression using phycoerythrin (PE)-conjugated anti-mouse IFN-γ antibody (BD Biosciences), allophycocyanin (APC)-conjugated anti-mouse IL-2 antibody (BD Biosciences), and Pacific Blue-conjugated anti-mouse tumor necrosis factor alpha (TNF-α) antibody (eBioscience). After washing, cells were resuspended in staining buffer and data were acquired using a multicolor LSRII flow cytometer (BD Biosciences). Data were then analyzed using FlowJo software version 6.4 (Treestar).
HBV RNA and DNA analysis.
Viral RNA and replicative DNA intermediates were detected by Northern and Southern blot analyses of total genomic liver RNA and DNA, respectively. Total genomic DNA was purified from the liver as described previously (20), and Southern blot analysis was performed by agarose gel electrophoresis of 30 μg total liver DNA digested with HindIII. For Northern blot analysis, total liver RNA was isolated using an RNeasy minikit (Qiagen) according to the manufacturer's instructions and 20 μg denatured RNA was separated by agarose (containing 5% formaldehyde) gel electrophoresis. Nucleic acids were transferred to nylon membranes overnight using the capillary transfer method with 10× SSC (0.15 M NaCl with 0.015 M sodium citrate). They were then cross-linked to the membrane by UV irradiation and hybridized to probes prepared from 3.2-kb HBV DNA with 32P-labeled dCTP and a Roche random-primed DNA labeling kit, and signal was detected using a PhosophorImager (Fuji).
Immunohistochemistry.
Liver tissue was fixed in 10% buffered formalin phosphate (Fisher Scientific) and paraffin embedded. HBV core was then detected by immunohistochemical staining performed by Yale University Research Histology using anti-core polyclonal rabbit antibody (Dako).
sALT activity assay.
The level of serum alanine aminotransferase (sALT) was determined with Infinity ALT reagent (Thermo Electron) using a Spectramax spectrophotometer (Molecular Devices) and enzyme standards (Verichem Laboratories).
ELISA.
Serum HBeAg, HBsAg, and anti-HBsAg levels were determined by ELISA following the manufacturer's protocol (International Immunodiagnostics).
RESULTS
Construction and characterization of recombinant VSV expressing HBV MS protein.
The HBV middle surface (MS) envelope glycoprotein (ayw serotype) open reading frame was PCR amplified and cloned into a plasmid DNA vector encoding the VSV genome (Fig. 1 A). After sequencing confirmed that the plasmid contained no PCR-generated mutations, a recombinant VSV, designated VSV-MS, was recovered. An MS-specific antibody was used to characterize protein expression in VSV-MS-infected BHK-21 cells. A band consistent with the molecular weight of glycosylated MS was detected in VSV-MS-infected cells (Fig. 1B). Indirect immunofluorescent staining was then used to determine MS protein localization in VSV-MS-infected cells. Protein expression was localized in a pattern consistent with endoplasmic reticulum, as would be expected for a secreted protein (Fig. 1C). Control VSV-infected cells exhibited no immunoreactivity with the HBs antibody but did express the VSV-G protein, confirming infection (Fig. 1C). Secreted MS protein was detected in the media of VSV-MS-infected BHK-21 cells by ELISA, confirming that MS generated in infected cells is correctly processed for secretion (data not shown). Sucrose gradient density experiments were used to determine if MS is incorporated into VSV virions. Secreted MS did not migrate at the same density as VSV proteins, indicating that it is not incorporated into the virions (Fig. 1D). Therefore, when expressed from VSV, MS protein is correctly processed for secretion independent of virion assembly and release.
FIG. 1.
Characterization of MS expression from recombinant VSV. (A) Gene encoding HBV middle envelope (MS) inserted in the fifth position of the VSV genome, diagrammed from the 3′-to-5′ orientation of the negative-stranded viral RNA genome. (B) BHK cells were harvested 6 h postinfection with VSV or VSV-MS and assayed for MS expression by Western blotting (WB) with anti-HBsAg monoclonal antibody. Uninf, uninfected. (C) Alternatively, 6 h postinfection, BHK cells were fixed with 2% paraformaldehyde prior to permeabilization and staining. MS localization was detected using primary mouse anti-HBsAg (α-HBS) and secondary Alexa Fluor 488-labeled goat anti-mouse antibodies. α-VSV-G, anti-VSV-G. (D) Medium from VSV-MS-infected BHK cells was layered over a continuous sucrose gradient (5 to 25%) and centrifuged at 30,000 × g for 20 min. Gradient fractions were collected, and migration densities were determined for HBsAg by ELISA and VSV-M protein by WB quantification using polyclonal anti-VSV antibody. O.D., optical density.
Immunization with VSV-MS elicits a specific CD8 T-cell response.
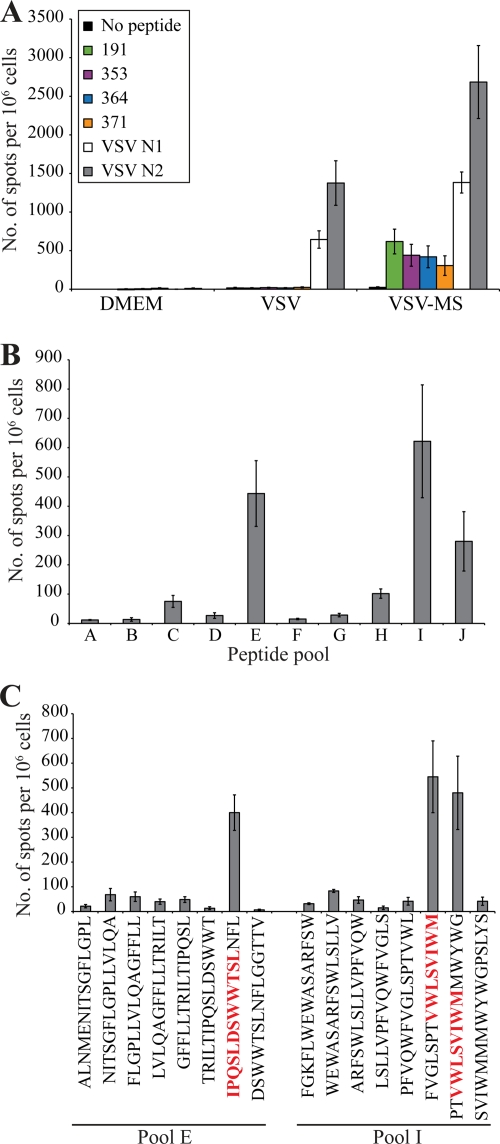
We conducted IFN-γ ELISPOT assays to determine whether VSV-MS immunization generates functional MS-specific CD8 T cells. At 7 days postimmunization, splenocytes were isolated from CB6F1 (H-2bxd) mice receiving an intranasal inoculation of 1 × 106 PFU of empty VSV or VSV-MS. The CD8 T-cell response was analyzed following an overnight stimulation with two VSV N peptides and four peptides corresponding to known HBV-specific H-2b- and H-2d-restricted epitopes (Table 1). As expected, both VSV-MS- and empty-VSV-immunized mice generated CD8 T-cell responses to the two VSV N peptides (Fig. 2 A). However, only VSV-MS-immunized mice generated CD8 T-cell responses to the four HBs peptides (Fig. 2A).
FIG. 2.
Specific CD8+ T-cell responses are elicited following a single immunization with VSV-MS. CB6F1 mice were immunized with DMEM (n = 4), control VSV vectors (n = 5), or VSV-MS (n = 5). (A) Seven days postimmunization, splenocytes were harvested and analyzed using an IFN-γ ELISPOT assay. The number of cells responding to stimulation with HBV S- or VSV-specific peptides is represented as quantification of the number of spot-forming cells (SFC) per 106 splenocytes. (B) A peptide library containing 76 15-mer peptides spanning the HBV envelope was pooled into 9 groups of 8 peptides and 1 group of 4 peptides and used in an IFN-γ ELISPOT assay conducted 7 days postimmunization with VSV-MS (n = 3). (C) The 9 peptides from responsive pools E and I were then tested individually in an IFN-γ ELISPOT assay to determine the immunogenic epitopes. All error bars represent standard error (SE).
The four HBs peptides used for ELISPOT analysis were selected based on prior studies demonstrating their immunogenicity (Table 1). Due to the fact that these epitopes were identified using different immunization strategies, the possibility that VSV-MS immunization induced T-cell responses to unidentified epitopes within MS could not be ruled out. Therefore, a peptide library spanning the entire MS protein was used to identify possible novel epitopes against which VSV-MS vaccination may generate a T-cell response. The library was comprised of 76 15-mer peptides, each overlapping by 5 amino acids. Peptides were divided into 9 pools containing 8 peptides (pools A to I) and 1 pool of 4 peptides (pool J). These pools were then used to stimulate splenocytes purified from mice 7 days post-intranasal immunization with 1 × 106 PFU VSV-MS. The strongest responses were elicited to pools E and I, although there was also a somewhat lower response to pool J (Fig. 2B). The splenocytes were then stimulated with the individual peptides from pools E and I in order to identify which epitopes elicited the responses. Within pool E, the one peptide that elicited a response contained the previously identified HBs epitope, 191-202, representing positions 191 to 202 (Fig. 2C). Similarly, the two peptides within pool I eliciting responses contained the previously identified 353-360 epitope (Fig. 2C). The individual peptides from pool J were not tested as three out of the four peptides within the pool contained known HBs epitopes 364-372 and 371-378, which were likely responsible for the weak response observed following stimulation with pool J. Therefore, VSV-MS elicits a specific CD8 T-cell response in CB6F1 mice that is directed to the four known epitopes within the antigen.
VSV-MS elicits better CD8 T-cell and antibody responses than recombinant protein and Engerix-B vaccination.
Recombinant protein vaccines currently available for HBV are comprised of the small and/or large versions of the envelope surface glycoprotein adsorbed to an adjuvant. Although the current vaccines are designed to elicit high HBV-specific antibody titers, they are unable to generate strong T-cell responses (24). In order to compare the immune response elicited by VSV-MS vaccination to that elicited by the current vaccine, mice were immunized with an available HBV vaccine, Engerix-B, comprised of 20 μg HBV envelope protein (adw serotype) adsorbed to 500 μg aluminum hydroxide. Additionally, to compare the immune response to recombinant protein vaccination without adjuvant, 10 μg recombinant HBV envelope protein (ayw serotype) was used to immunize a separate group of mice. Not only was VSV-MS immunization superior to recombinant protein vaccination, both with and without adjuvant, at eliciting a specific CD8 T-cell response 7 days postadministration (Fig. 3 A), but it also generated comparable or greater antibody titers 4 weeks postimmunization (Fig. 3B). Therefore, VSV-MS immunization simultaneously generates CD8 T-cell and antibody responses that cannot be achieved with recombinant protein vaccination alone.
FIG. 3.
VSV-MS elicits stronger cellular and humoral immune responses than recombinant MS protein and Engerix-B vaccination. (A) CB6F1 mice were immunized with VSV-MS (i.m., n = 5; i.n., n = 5), recombinant S protein (rHBsAg) (n = 5), or Engerix-B (n = 5). Seven days postimmunization, splenocytes were isolated and analyzed for CD8, CD3, and IFN-γ expression by flow cytometry. The values shown represent CD8+ and CD3+ gated cells. (B) CB6F1 mice were immunized with VSV-MS (i.m., n = 5; i.n., n = 5), recombinant S protein (rHBsAg) (n = 5), or Engerix-B (n = 10). Quantitative HBsAb ELISAs were conducted on serum collected 4 weeks postimmunization to determine specific antibody titers. All values are presented as the average from each group; error bars represent SE.
VSV-MS immunization elicits a more robust T-cell phenotype than other vaccine platforms.
DNA and viral vaccine platforms have been successful at generating T-cell responses to the HBV glycoprotein, thus making them promising vaccine candidates. Because poxviruses are the most widely used and best-characterized viral vaccine vectors (41), a vaccinia virus platform was used for comparison to VSV-MS. Mice were immunized with 1 × 106 PFU i.n. (empty VSV and VSV-MS) or i.p. (recombinant vaccinia virus expressing S; VV-S). ELISPOT assays were then conducted 7 days postimmunization to measure the HBV S-specific T-cell response. Although both VSV-MS- and VV-S-immunized mice generated responses to the HBs peptides that were above the background level observed in control mice, these responses were greater in VSV-MS-immunized mice (Fig. 4 A). Therefore, compared to VV-S, VSV-MS elicits a T-cell response greater in magnitude and broader in specificity.
FIG. 4.
VSV-MS generates a CD8+ T-cell response superior to that of VV-S. CB6F1 mice were immunized with DMEM (n = 2), VSV (n = 3), VSV-MS (n = 7), or VV-S (n = 6). (A) An IFN-γ ELISPOT assay was conducted 7 days postimmunization using four HBV S-specific peptides and two VSV-specific peptides. (B) Three weeks after a DNA-MS prime, CB6F1 mice were boosted with DMEM (n = 3), DNA-MS (n = 3), VV-S (n = 3), and VSV-MS (n = 4). Seven days postimmunization, splenocytes were isolated and analyzed for CD8, IFN-γ, TNF-α, and IL-2 expression by flow cytometry. CD8+ T cells were gated, and IFN-γ-producing cells are presented as the percent average from each group. Error bars represent SE. (C) Boolean gating analysis was used to identify sets of CD8+ T cells expressing each possible combination of cytokines (IFN-γ, TNF-α, and IL-2). Data are presented as the average from each group with triple-, double-, and single-cytokine-producing cells represented as the percentage of total cytokine-producing CD8+ T cells.
The ability of CD8 T cells to generate polyfunctional responses following viral infection has been correlated with improved viral clearance (9). In order to determine the ability of VSV-MS to generate polyfunctional T cells in comparison to other potential vaccine platforms, mice were primed with 100 μg DNA plasmid expressing MS (DNA-MS) i.m. Mice were then boosted 3 weeks postprime with 1 × 106 PFU VSV-MS (i.n.), 1 × 106 PFU VV-S (i.p.), or 100 μg DNA-MS (i.m.). Intracellular cytokine staining for IFN-γ, IL-2, and TNF-α was performed 7 days postboost, and Boolean gating analysis was used to identify polyfunctional CD8 T cells. Consistent with the ELISPOT analysis after primary immunization (Fig. 4A), secondary immunization with VSV-MS elicited a higher percentage of MS-specific CD8 T cells expressing IFN-γ alone than VV-S immunization (Fig. 4B). This enhanced response was seen across all four peptides and was independent of the sequence heterogeneity and differences in immunogenicity between the ayw and adw2 peptides (data not shown). Furthermore, VSV-MS immunization generated a higher percentage of HBV S-specific CD8 T cells simultaneously expressing two or more cytokines than immunization with VV-S (Fig. 4C). Therefore, VSV-MS elicits a T-cell response greater in magnitude and functional quality than other vaccine platforms.
VSV-MS provides protection against challenge after a single dose.
Due to the fact that mice cannot be naturally infected with HBV, two models were used to determine the protective effects of VSV-MS vaccination. The first model was designed based on previous studies demonstrating that mice lose up to 15% of their body weight 5 to 7 days post-intranasal VV infection (42). Mice were, therefore, administered an intranasal prime dose of 1 × 106 PFU empty VSV or VSV-MS. Both groups of mice received an intranasal challenge of 1 × 106 PFU VV-S 60 days postprime and were then weighed for 14 days postchallenge to monitor changes in weight. Mice that received an empty-VSV prime lost up to 10% body weight 6 days postchallenge, while VSV-MS-primed mice were protected from VV-S-associated weight loss (Fig. 5 A). ELISPOT analysis 14-days postchallenge revealed a specific CD8 T-cell recall response was induced following VV-S challenge in VSV-MS-immunized mice (Fig. 5B). Thus, VSV-MS immunization protects mice from pathogenesis associated with heterologous virus challenge in a manner that is likely CD8 T-cell mediated, as MS-specific antibodies will not neutralize VV infection.
FIG. 5.
VSV-MS provides protection against vaccinia virus challenge. C57BL/6 mice were primed 60 days prior to heterologous challenge. The following prime/challenge strategies were used: VSV/VV-S (n = 4), VSV-MS/VV-S (n = 4), and VSV-MS/VV (n = 2). (A) Body weight was measured for 14 days following challenge. **, P < 0.002; and *, P < 0.02 (weight loss in VSV-MS-immunized/VV-S-challenged mice compared to that in VSV-immunized/VV-S-challenged mice). (B) Splenocytes were harvested 14 days postchallenge and stimulated using HBV S- and VSV-specific peptides in an ELISPOT assay to measure recall responses. All values are presented as the average from each group, and error bars indicate the SE.
To further examine the protective effects of VSV-MS vaccination in the context of HBV replication, a second model was established using the hydrodynamic transfection technique. Mice were first immunized with empty VSV or VSV-MS. Two months later, all mice received intravenous injections of pT-HBV1.3 under hydrodynamic conditions, allowing for transfection of hepatocytes in vivo. Mice were analyzed either 4 or 7 days posttransfection to determine the effects of VSV-MS immunization on HBV replication and host immune responses. Northern and Southern blots conducted using hepatocellular RNA and DNA, respectively, revealed that levels of both HBV RNA and DNA in immunized animals were greatly diminished compared to those in unimmunized animals by day 4 posttransfection and HBV RNA and DNA reached undetectable levels by day 7 posttransfection (Fig. 6 A). Liver sections from transfected mice stained for HBV core protein showed that compared to unimmunized mice, VSV-MS-immunized mice had 4-fold-fewer core-positive hepatocytes by day 4 posttransfection. While core-positive hepatocytes continued to increase at least until day 7 posttransfection in unimmunized mice, the number of core-positive hepatocytes diminished almost entirely among VSV-MS-immunized mice (Fig. 6B).
FIG. 6.
VSV-MS provides protection against hydrodynamic HBV challenge. CB6F1 mice were immunized with VSV-MS (n = 8) or empty VSV (n = 7). Sixty days postimmunization, HBV replication was induced by hydrodynamic injection of HBV 1.3 plasmid (10 μg), and analysis was performed on days 4 (n = 10) and 7 (n = 5) postchallenge. (A) Liver-associated HBV DNA and RNA were measured using Southern blot (SB) and Northern blot (NB) analyses, respectively. RC, relaxed circular DNA; SS, single-stranded DNA. (B) HBcAg-positive hepatocytes per 10× field were quantified after liver sections were stained with anti-HBcAg antibody. Values represent the average of two fields per sample with SE. Serum analysis for HBeAg (C) and HBsAg (D) was completed by ELISA on days 0, 1, 4, and 7 post-hydrodynamic challenge. O.D., optical density. (E) HBsAb titers were determined using a quantitative ELISA, and (F) serum alanine aminotransferase (sALT) activity was measured using Infinity ALT reagent. All values are presented as the average from each group, and error bars represent SE.
HBV antigens were detected in the sera of transfected mice by ELISA. Although HBeAg levels were similarly elevated in unimmunized and immunized animals on day 1 posttransfection, over the course of 7 days, serum HBeAg dropped to nearly undetectable levels in VSV-MS-immunized mice while continuing to rise in unimmunized animals (Fig. 6C). Clearance of HBeAg from the sera of immunized mice is likely mediated by T-cell killing of transfected hepatocytes, as immunization with VSV-MS does not elicit HBe-specific antibodies. Levels of serum HBsAg, however, remained nearly undetectable in VSV-MS-immunized animals through at least day 7, although they increased until day 4 in unimmunized animals (Fig. 6D). This is likely a result of HBsAg neutralization due to the high titers of HBs-specific antibody generated by VSV-MS immunization (Fig. 6E). Immunization with VSV-MS, therefore, controls and clears HBV replication within 7 days following hydrodynamic transfection challenge.
In addition to a decline in serum HBeAg, measures of host immune response also suggest that HBV clearance may be at least partially mediated by a cellular immune response in animals immunized with VSV-MS. Consistent with previous reports, successful transfection resulted in significantly increased sALT activity on day 1 posttransfection. However, while sALT activity returned to baseline in unimmunized mice, it remained significantly elevated (P = 0.03) in immunized mice (Fig. 6F). Increased sALT activity in immunized animals may be an indication of T-cell-mediated hepatocellular damage. This is further supported by hematoxylin and eosin (H&E) staining, which revealed necroinflammatory lesions of increased number and size in liver sections from immunized compared to unimmunized mice by day 4 posttransfection (data not shown).
To further determine the role of CD8 T cells in the hydrodynamic transfection model of protection, we compared VSV-MS and recombinant protein (HBsAg) immunizations. Following hydrodynamic transfection with pT-HBV1.3 at 8 weeks postimmunization, mice were analyzed to determine the effects on HBV replication and host immune responses. Similar to the previous challenge experiment, measurement of HBeAg levels in the serum showed elevation in both unimmunized and immunized mice day 1 posttransfection (Fig. 7 A). Although serum HBeAg dropped to nearly undetectable levels in VSV-MS-immunized mice, it remained elevated in rHBsAg-immunized animals over the course of 7 days (Fig. 7A). Providing further evidence that this clearance is CD8 T-cell mediated, 7 days posttransfection, VSV-MS-immunized mice had higher percentages of T cells specific for the four MS epitopes in both the spleen and in the liver than rHBsAg-immunized mice (Fig. 7B). The high percentage of HBV-specific CD8 T cells present in the liver of VSV-MS-immunized mice suggests that clearance of HBV from transfected hepatocytes is T-cell mediated. This is also supported by the fact that on day 7 posttransfection, liver-associated HBV DNA remains in rHBsAg-immunized mice at levels similar to those of unimmunized controls, while it is undetectable in VSV-MS-immunized mice (Fig. 7C). In addition, the depletion of CD8 T cells in VSV-MS-immunized mice 1 day prior to hydrodynamic transfection suppressed the protective effects of immunization. With CD8 T cells depleted in the livers of anti-CD8 antibody-treated mice, HBV DNA was not cleared by day 7 posttransfection (Fig. 7D). Therefore, VSV-MS may provide protection against HBV infection by eliciting both antibody and memory T-cell responses.
FIG. 7.
VSV-MS-mediated protection correlates with CD8 T-cell recall response. CB6F1 mice were immunized with VSV-MS (n = 5) or recombinant MS protein (rHBsAg) (n = 4); a control group of unimmunized (Unimm.) mice was also included (n = 4). Sixty days postimmunization, HBV replication was induced by hydrodynamic injection of HBV 1.3 plasmid (10 μg), and analysis was performed on day 7 postchallenge. (A) Serum analysis for HBeAg was completed by ELISA on days 0, 1, 4, and 7 post-hydrodynamic challenge. (B) Splenocytes and intrahepatic lymphocytes (IHL) were isolated and analyzed for CD8, CD3, and IFN-γ expression by flow cytometry. CD8+ CD3+ T cells were gated, and IFN-γ-producing cells are presented as the percent average from each group for unpooled samples; error bars represent SE. (C) Liver-associated HBV DNA was measured using Southern blot analysis. RC, relaxed circular DNA; SS, single-stranded DNA. (D) CD8 T cells were depleted by anti-CD8 (αCD8) antibody injection 1 day prior to hydrodynamic challenge and again on day 2 postchallenge, and analysis was performed on day 7 postchallenge. Liver-associated HBV DNA was measured using Southern blot analysis. IHL were pooled and analyzed for CD8+ CD3+ T cells by flow cytometry.
DISCUSSION
Here, we demonstrate that a VSV-based HBV vaccine vector induces an immune response capable of protecting against challenge after a single dose. Viral vaccine vectors, such as VSV, have certain inherent advantages compared to recombinant protein vaccines. Recombinant protein HBV vaccines induce high antibody titers, and VSV is able to induce comparable or greater antibody titers. This is likely due to the secretion of HBV MS from VSV-MS infected cells, as this allows for endocytosis by antigen-presenting cells. Unlike recombinant protein vaccines, however, viral vectors contain pathogen-associated molecular patterns. Such patterns engage Toll-like receptors and other pattern recognition receptors, activating the innate immune response and, thus, enhancing the adaptive immune response. Viral vectors also allow genes encoding HBV proteins to be introduced directly into host cells. As encoded proteins are expressed within host cells, the endogenous class I antigen presentation pathway can be exploited, resulting in strong CD8 T-cell responses. Although the currently available vaccine likely relies entirely on antibody responses to provide protection against exposure to HBV, both of our challenge models suggest that VSV-mediated protection is dependent upon memory T cells. The ability of VSV vectors to elicit robust cellular and humoral immune responses may provide advantages over the currently available recombinant protein HBV vaccines.
In addition to their ability to elicit potent immune responses, VSV vectors possess a number of characteristics that make them attractive potential vaccine platforms. First, although VSV naturally infects insects and livestock, it is nonpathogenic or only mildly pathogenic in humans (10). Furthermore, infection with VSV in humans is rare, thus reducing the risk of preexisting immunity, which may interfere with vaccine efficacy. Additionally, attenuated forms of VSV have been developed which eliminate the pathogenesis of the virus while maintaining the immunogenicity of the vector (6, 11, 13, 29). Clinical trials are approved for VSV-based HIV vaccines, and given the ideal characteristics of VSV as a vaccine vector, present a promising platform for an alternative prophylactic HBV vaccine.
Although the current HBV vaccine has proven to be highly successful in many regions of the world, there are a number of potential uses for a new VSV-based prophylactic vaccine. Because our study and others have shown that VSV is able to elicit protection after a single dose in animal models, the use of a VSV-based HBV vaccine in humans may be advantageous in situations where single-dose immunization eliminates logistical difficulties associated with the current HBV vaccine series. Furthermore, as up to 10% of those who receive the full HBV vaccine series do not develop protective antibody titers, the availability of a vaccine that elicits protective immunity by induction of endogenous antigen presentation pathways may expand the population of protected individuals. Additionally, a VSV-based HBV vaccine vector may also provide improved postexposure prophylaxis (PEP). It has been demonstrated that VSV-based vaccines protect nonhuman primates from lethal challenge with Ebola virus (16, 17, 19), even when administered postexposure (18). Also, it has been recently shown that recombinant VSV immunization can provide postexposure protection against lethal influenza virus challenge in mice if administered within 24 h after influenza virus administration (2). Furthermore, the mechanism of this protection was demonstrated to be a combination of innate, cellular, and humoral responses. This evidence along with the rapid induction of CD8 T-cell responses we observe in mice postvaccination suggests that VSV-MS may provide postexposure protection against HBV. This might be particularly important for exposed individuals with no history of HBV vaccination, as PEP by recombinant protein vaccination may induce antibody responses too long after exposure to offer protection and alternative immunoglobulin treatment poses a number of risks. As a VSV-based HBV vaccine vector may fulfill a variety of functions that are absent or suboptimal with the use of current HBV vaccines, it should continue to be explored as an alternative.
Finally, VSV vectors could also be studied for their potential use as therapeutic vaccines. Ideally, immunization with a therapeutic vaccine would stimulate the immune system to generate a specific CD8 T-cell response capable of controlling infection. Therapeutic vaccination is particularly attractive for HBV, as chronic infection is established due to a weak and narrowly focused CD8 T-cell response (3, 27). While therapeutic vaccines are a promising concept, they have proven to be challenging to design effectively. To date, treatment of chronic HBV infection with therapeutic vaccines has shown limited clinical efficacy, likely due to defects of HBV-specific T cells in these patients (4). VSV vectors may be particularly effective as therapeutic vaccines due to their ability to elicit robust T-cell responses to multiple T-cell epitopes. Furthermore, vaccination with VSV has been demonstrated to be effective as a therapeutic strategy for cervical carcinoma and other papillomavirus-associated cancers in animal models, an effect that was shown to be CD8 T-cell dependent (5, 23). As the current recombinant protein vaccines for HBV are limited in their capabilities for both prophylactic and therapeutic uses, further study of the potential of VSV-based HBV vaccines as viable alternatives for providing such functions is warranted.
Acknowledgments
Support for this work was provided by NIH grant CA131133 and a Yale Cancer Center/American Cancer Society Institutional Research Award (IRG-58-012-050). M.A.C. was also supported by training grant T32GM007324.
We thank Dorothy van Rhijn for providing reagents and Brian Yordy and Ashley Viehmann for assistance with generating the vectors.
Footnotes
Published ahead of print on 26 May 2010.
REFERENCES
- 1.Ando, K., T. Moriyama, L. G. Guidotti, S. Wirth, R. D. Schreiber, H. J. Schlicht, S. N. Huang, and F. V. Chisari. 1993. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J. Exp. Med. 178:1541-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barefoot, B. E., K. Athearn, C. J. Sample, and E. A. Ramsburg. 2009. Intramuscular immunization with a vesicular stomatitis virus recombinant expressing the influenza hemagglutinin provides post-exposure protection against lethal influenza challenge. Vaccine 28:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnaba, V., A. Franco, A. Alberti, C. Balsano, R. Benvenuto, and F. Balsano. 1989. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J. Immunol. 143:2650-2655. [PubMed] [Google Scholar]
- 4.Bertoletti, A., and A. Gehring. 2009. Therapeutic vaccination and novel strategies to treat chronic HBV infection. Expert Rev. Gastroenterol. Hepatol. 3:561-569. [DOI] [PubMed] [Google Scholar]
- 5.Brandsma, J. L., M. Shlyankevich, Y. Su, D. Zelterman, J. K. Rose, and L. Buonocore. 2009. Reversal of papilloma growth in rabbits therapeutically vaccinated against E6 with naked DNA and/or vesicular stomatitis virus vectors. Vaccine 14:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braxton, C. L., S. H. Puckett, S. B. Mizel, and D. S. Lyles. 2010. Protection against lethal vaccinia virus challenge using an attenuated matrix protein mutant vesicular stomatitis virus vaccine vector expressing poxvirus antigens. J. Virol. 84:3552-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, M. H., C. J. Chen, M. S. Lai, H. M. Hsu, T. C. Wu, M. S. Kong, D. C. Liang, W. Y. Shau, and D. S. Chen. 1997. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 336:1855-1859. [DOI] [PubMed] [Google Scholar]
- 8.Chongsrisawat, V., P. Yoocharoen, A. Theamboonlers, P. Tharmaphornpilas, P. Warinsathien, S. Sinlaparatsamee, S. Paupunwatana, K. Chaiear, S. Khwanjaipanich, and Y. Poovorawan. 2006. Hepatitis B seroprevalence in Thailand: 12 years after hepatitis B vaccine integration into the national expanded programme on immunization. Trop. Med. Int. Health 11:1496-1502. [DOI] [PubMed] [Google Scholar]
- 9.Ciuffreda, D., D. Comte, M. Cavassini, E. Giostra, L. Buhler, M. Perruchoud, M. H. Heim, M. Battegay, D. Genne, B. Mulhaupt, R. Malinverni, C. Oneta, E. Bernasconi, M. Monnat, A. Cerny, C. Chuard, J. Borovicka, G. Mentha, M. Pascual, J. J. Gonvers, G. Pantaleo, and V. Dutoit. 2008. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur. J. Immunol. 38:2665-2677. [DOI] [PubMed] [Google Scholar]
- 10.Clarke, D. K., D. Cooper, M. A. Egan, R. M. Hendry, C. L. Parks, and S. A. Udem. 2006. Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector. Springer Semin. Immunopathol. 28:239-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke, D. K., F. Nasar, M. Lee, J. E. Johnson, K. Wright, P. Calderon, M. Guo, R. Natuk, D. Cooper, R. M. Hendry, and S. A. Udem. 2007. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J. Virol. 81:2056-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen, C., A. A. Evans, W. T. London, J. Block, M. Conti, and T. Block. 2008. Underestimation of chronic hepatitis B virus infection in the United States of America. J. Viral Hepat. 15:12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper, D., K. J. Wright, P. C. Calderon, M. Guo, F. Nasar, J. E. Johnson, J. W. Coleman, M. Lee, C. Kotash, I. Yurgelonis, R. J. Natuk, R. M. Hendry, S. A. Udem, and D. K. Clarke. 2008. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and G gene truncation reduces neurovirulence and enhances immunogenicity in mice. J. Virol. 82:207-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, H. L., R. Schirmbeck, J. Reimann, and R. G. Whalen. 1995. DNA-mediated immunization in mice induces a potent MHC class I-restricted cytotoxic T lymphocyte response to the hepatitis B envelope protein. Hum. Gene Ther. 6:1447-1456. [DOI] [PubMed] [Google Scholar]
- 15.Ezelle, H. J., D. Markovic, and G. N. Barber. 2002. Generation of hepatitis C virus-like particles by use of a recombinant vesicular stomatitis virus vector. J. Virol. 76:12325-12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geisbert, T. W., K. M. Daddario-Dicaprio, J. B. Geisbert, D. S. Reed, F. Feldmann, A. Grolla, U. Stroher, E. A. Fritz, L. E. Hensley, S. M. Jones, and H. Feldmann. 2008. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine 26:6894-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geisbert, T. W., K. M. Daddario-Dicaprio, M. G. Lewis, J. B. Geisbert, A. Grolla, A. Leung, J. Paragas, L. Matthias, M. A. Smith, S. M. Jones, L. E. Hensley, H. Feldmann, and P. B. Jahrling. 2008. Vesicular stomatitis virus-based Ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 4:e1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geisbert, T. W., K. M. Daddario-DiCaprio, K. J. Williams, J. B. Geisbert, A. Leung, F. Feldmann, L. E. Hensley, H. Feldmann, and S. M. Jones. 2008. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J. Virol. 82:5664-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisbert, T. W., J. B. Geisbert, A. Leung, K. M. Daddario-DiCaprio, L. E. Hensley, A. Grolla, and H. Feldmann. 2009. Single-injection vaccine protects nonhuman primates against infection with Marburg virus and three species of Ebola virus. J. Virol. 83:7296-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidotti, L. G., B. Matzke, H. Schaller, and F. V. Chisari. 1995. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69:6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchings, C. L., S. C. Gilbert, A. V. Hill, and A. C. Moore. 2005. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J. Immunol. 175:599-606. [DOI] [PubMed] [Google Scholar]
- 22.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U. S. A. 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao, J. B., J. Publicover, J. K. Rose, and D. DiMaio. 2008. Single-dose, therapeutic vaccination of mice with vesicular stomatitis virus expressing human papillomavirus type 16 E7 protein. Clin. Vaccine Immunol. 15:817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu, M., S. Menne, D. Yang, Y. Xu, and M. Roggendorf. 2007. Immunomodulation as an option for the treatment of chronic hepatitis B virus infection: preclinical studies in the woodchuck model. Expert Opin. Invest. Drugs 16:787-801. [DOI] [PubMed] [Google Scholar]
- 25.Mast, E. E., C. M. Weinbaum, A. E. Fiore, M. J. Alter, B. P. Bell, L. Finelli, L. E. Rodewald, J. M. Douglas, Jr., R. S. Janssen, J. W. Ward, and Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. 2006. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part II. Immunization of adults. MMWR Recomm. Rep. 55:1-33; quiz, CE1-CE4. [PubMed] [Google Scholar]
- 26.Michel, M. L., H. L. Davis, M. Schleef, M. Mancini, P. Tiollais, and R. G. Whalen. 1995. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc. Natl. Acad. Sci. U. S. A. 92:5307-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nayersina, R., P. Fowler, S. Guilhot, G. Missale, A. Cerny, H. J. Schlicht, A. Vitiello, R. Chesnut, J. L. Person, A. G. Redeker, and F. V. Chisari. 1993. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J. Immunol. 150:4659-4671. [PubMed] [Google Scholar]
- 28.Plymoth, A., S. Viviani, and P. Hainaut. 2009. Control of hepatocellular carcinoma through hepatitis B vaccination in areas of high endemicity: perspectives for global liver cancer prevention. Cancer Lett. 286:15-21. [DOI] [PubMed] [Google Scholar]
- 29.Publicover, J., E. Ramsburg, and J. K. Rose. 2005. A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. J. Virol. 79:13231-13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsburg, E. A., J. M. Publicover, D. Coppock, and J. K. Rose. 2007. Requirement for CD4 T cell help in maintenance of memory CD8 T cell responses is epitope dependent. J. Immunol. 178:6350-6358. [DOI] [PubMed] [Google Scholar]
- 31.Reyes-del Valle, J., G. Hodge, M. B. McChesney, and R. Cattaneo. 2009. Protective anti-hepatitis B virus responses in rhesus monkeys primed with a vectored measles virus and boosted with a single dose of hepatitis B surface antigen. J. Virol. 83:9013-9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 33.Rose, N. F., A. Roberts, L. Buonocore, and J. K. Rose. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schirmbeck, R., W. Bohm, N. Fissolo, K. Melber, and J. Reimann. 2003. Different immunogenicity of H-2 Kb-restricted epitopes in natural variants of the hepatitis B surface antigen. Eur. J. Immunol. 33:2429-2438. [DOI] [PubMed] [Google Scholar]
- 35.Schirmbeck, R., N. Dikopoulos, M. Kwissa, F. Leithauser, K. Lamberth, S. Buus, K. Melber, and J. Reimann. 2003. Breaking tolerance in hepatitis B surface antigen (HBsAg) transgenic mice by vaccination with cross-reactive, natural HBsAg variants. Eur. J. Immunol. 33:3342-3352. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz, J. A., L. Buonocore, A. Roberts, A. Suguitan, Jr., D. Kobasa, G. Kobinger, H. Feldmann, K. Subbarao, and J. K. Rose. 2007. Vesicular stomatitis virus vectors expressing avian influenza H5 HA induce cross-neutralizing antibodies and long-term protection. Virology 366:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sette, A. D., C. Oseroff, J. Sidney, J. Alexander, R. W. Chesnut, K. Kakimi, L. G. Guidotti, and F. V. Chisari. 2001. Overcoming T cell tolerance to the hepatitis B virus surface antigen in hepatitis B virus-transgenic mice. J. Immunol. 166:1389-1397. [DOI] [PubMed] [Google Scholar]
- 38.Shuler, C. M., A. E. Fiore, R. Neeman, B. P. Bell, W. Kuhnert, S. Watkins, K. Kilgour, and K. E. Arnold. 2009. Reduction in hepatitis B virus seroprevalence among U.S.-born children of foreign-born Asian parents—benefit of universal infant hepatitis B vaccination. Vaccine 27:5942-5947. [DOI] [PubMed] [Google Scholar]
- 39.Sjogren, M. H. 2005. Prevention of hepatitis B in nonresponders to initial hepatitis B virus vaccination. Am. J. Med. 118(Suppl. 10A):34S-39S. [DOI] [PubMed] [Google Scholar]
- 40.Smith, G. L., M. Mackett, and B. Moss. 1983. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature 302:490-495. [DOI] [PubMed] [Google Scholar]
- 41.Souza, A. P., L. Haut, A. Reyes-Sandoval, and A. R. Pinto. 2005. Recombinant viruses as vaccines against viral diseases. Braz. J. Med. Biol. Res. 38:509-522. [DOI] [PubMed] [Google Scholar]
- 42.Turner, G. S. 1967. Respiratory infection of mice with vaccinia virus. J. Gen. Virol. 1:399-402. [DOI] [PubMed] [Google Scholar]
- 43.Van Bleek, G. M., and S. G. Nathenson. 1990. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature 348:213-216. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 2009. Hepatitis B vaccines. Wkly. Epidemiol. Rec. 84:405-419. [PubMed] [Google Scholar]
- 45.Yang, P. L., A. Althage, J. Chung, and F. V. Chisari. 2002. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc. Natl. Acad. Sci. U. S. A. 99:13825-13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuckerman, J. N. 2006. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J. Med. Virol. 78:169-177. [DOI] [PubMed] [Google Scholar]