Abstract
Human rhinoviruses (HRVs) were discovered as common cold pathogens over 50 years ago. Recent advances in molecular viral diagnostics have led to an appreciation of their role in more-significant respiratory illnesses, including bronchiolitis in infancy, childhood pneumonia, and acute exacerbations of chronic respiratory diseases such as asthma, chronic obstructive lung disease, and cystic fibrosis. Until a few years ago, only two groups of HRVs (A and B) had been recognized. However, full and partial sequencing of HRVs led to the discovery of a third species of HRV (HRV-C) that has distinct structural and biologic features. Risk factors and pathogenic mechanisms for more-severe HRV infections are being defined, and yet fundamental questions persist about mechanisms relating this common pathogen to allergic diseases and asthma. The close relationship between HRV infections and asthma suggests that antiviral treatments could have a major impact on the morbidity associated with this chronic respiratory disease.
Virology and clinical textbooks and virtually all web-based information sources generally describe human rhinoviruses (HRVs) as the most frequent cause of the common cold. In this light, HRVs are important pathogens because common colds are so widespread and annoying, and from an economic perspective, supplying symptomatic-relief medications is a huge industry. A 2003 study estimated that expenditures on common cold medications in the United States exceeded 4 billion dollars, including 1.1 billion dollars spent on inappropriate prescriptions for antibiotics (18). Finding a cure for the common cold has been a much-publicized goal ever since HRVs were first discovered in the 1950s and 1960s. The basic biology of the replication cycle was soon described, and most HRVs were found to replicate best at 33° to 35°C (88, 99), which is consistent with their role as upper airway pathogens. HRVs were found to have tremendous diversity, and approximately 100 serotypes in two groups (A and B) had been identified by 1990, when it was presumed that all, or nearly all, HRVs had been identified. These HRVs were found to bind to one of two main receptors: ICAM-1 (major group) (30) and the low-density-lipoprotein receptor (most of the minor group) (35). Despite advances in understanding HRV genetics and the picornavirus replication cycle, efforts toward developing specific antivirals have fallen short due to modest efficacy, side effects, drug interactions, and economic considerations (16). Consequently, enthusiasm for pursuing a common cold cure has waned, and the pharmaceutical industry has refocused attention on viruses that cause more-significant illnesses. This failure is a bit embarrassing. As summarized by White and Fenner, “In this era of organ transplantation, genetic engineering, and other dramatic demonstrations of the wonders of medical science, the man in the street perceives a certain irony in the inability of modern medicine to make the slightest impact on that most trivial of all human ailments, the common cold” (119).
In addition to relaying a sense of irony, this statement also sums up an opinion held by many that HRV infection causes a trivial illness, and among viruses, HRV is afforded little respect by clinicians and even by many virologists. However, this opinion may be based on incomplete information and some historic assumptions about HRV that have since been disproven. In fact, there is now convincing evidence that HRVs (i) can replicate in the lower airways, (ii) play a critical role in causing exacerbations of asthma and other chronic lung diseases, (iii) can cause bronchiolitis and pneumonia, and (iv) are a common cause of hospitalization. In addition, since the discovery of a unique HRV strain in 2006, over 50 additional strains of HRV have been identified, the majority of which are members of the newly designated HRV-C species, with distinct biologic features, including receptor specificity. In this review, recent information concerning the role of HRVs as important respiratory pathogens, especially related to asthma and lower airway illnesses, will be discussed.
HRV AS A LOWER AIRWAY PATHOGEN
Most HRVs replicate best at 33° to 35°C, and so it was long assumed that infections were limited to the upper airway, where the temperature of mucosal surfaces was known to be relatively cool. There are several lines of evidence, however, that HRV frequently infects the lower airways. First, although lung parenchyma is at core temperature (37°C), airways are considerably cooler, and temperatures in large and medium-sized airways are ideal for HRV replication (76). In tissue culture, HRVs grow at least as well (81) or even better (69) in primary cultures of epithelial cells from the lower versus the upper airways. Following experimental infection of the upper airway, the presence of HRVs has been demonstrated in lower airway fluids and cells in most volunteers, using a variety of techniques (Fig. 1) (23, 82, 87). Furthermore, the amounts of HRV in sputum often exceed what is found in upper airway secretions (36, 61, 82). During natural infections, HRV has also been detected in bronchial biopsies from infants with recurrent respiratory symptoms (71) and in tracheal secretions of children with tracheostomies, which allow for direct sampling of secretions from the lower airway without contamination from upper airway secretions (102).
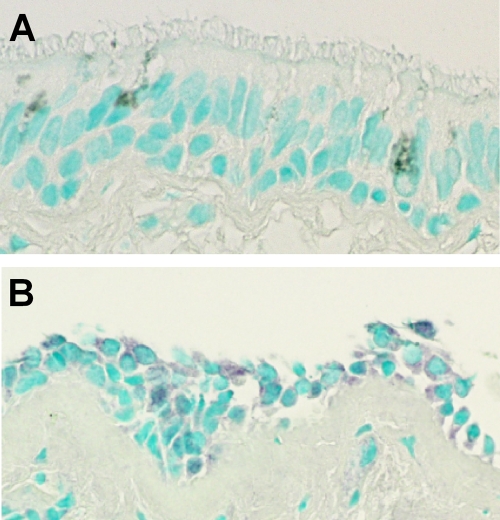
FIG. 1.
HRV infection of bronchial epithelium. Before (A) and after (B) experimental infection of volunteers with HRV-16 in the nose, subjects underwent bronchoscopy, and biopsies of lower airway tissues (bronchial epithelium) were obtained. Immunohistochemistry was performed, using a monoclonal antibody (R16-7) specific for the VP0/VP2 capsid protein of HRV-16. Patchy cytoplasmic staining (purple) was observed in biopsies after viral inoculation (B). Cell nuclei are stained pale green. Modified from the American Journal of Respiratory and Critical Care Medicine (82) with permission of the publisher.
The development of PCR technology dramatically changed the ability to detect respiratory viruses, and with this advance have emerged renewed interest and appreciation of the role of respiratory infections in lower respiratory illnesses. This is especially important for HRVs, many of which are difficult to culture, including HRV-C strains that have yet to be successfully grown in cell culture. From studies using PCR and other molecular diagnostics, there is considerable clinical and epidemiologic evidence linking HRV infections to bronchitis (large-airway infections), bronchiolitis (small-airway infections), and pneumonia (alveolar-airspace infections), especially in children (Table 1) (95). As an example, the New Vaccine Surveillance Network, which is a collaborative effort involving the Centers for Disease Control, Vanderbilt University, and the University of Rochester, conducted a population-based study of children hospitalized for respiratory illnesses. The virus most often detected was HRV, and risk factors included young age and the presence of asthma (80). In a study of specimens obtained from hospitalized children after tests were negative for respiratory syncytial virus (RSV) and influenza, HRVs were detected in 75%, using molecular diagnostics (95). In adults, HRV infections have been linked to exacerbations of asthma, outbreaks of “flu-like” illnesses in nursing homes, and pneumonia in immunocompromised patients (Table 1 and reference 34).
TABLE 1.
Role of HRV in lung diseasesa
| Clinical syndrome | Population cohort | % virus-infected subjects |
% HRV-infected subjectsb |
References | ||
|---|---|---|---|---|---|---|
| Sick | Well | Sick | Well | |||
| Acute illness | ||||||
| Bronchiolitis | Outpatient with wheezing aged <2 years | 66-68 | 25-31 | 33-45 | 11-26 | 21, 58, 66 |
| Hospitalized or emergency department patient aged <2 years | 86-93 | 10-30 | 7, 42, 72 | |||
| Pneumonia | Hospitalized childrenc | 55-65 | 24-45 | 48, 61, 112 | ||
| Immunocompromised adultsc | 17-19 | 4-8 | 20, 39 | |||
| Exacerbation of chronic lung disease | ||||||
| Asthma | Outpatient children | 62-81 | 12-41 | 52-54 | 12-28 | 44, 47 |
| Hospitalized children | 61-63 | 18-23 | 48-57 | 2-19 | 33, 51 | |
| Outpatient adults | 44-76 | 3-13 | 18-48 | 0-13 | 2, 32, 52, 85 | |
| Hospitalized adults | 26-48 | 18 | 10-35 | 4 | 29, 108 | |
| COPD | Older adults | 22-64 | 12-19 | 3-36 | 0-4 | 54, 77, 96, 100, 108 |
| Cystic fibrosis | Children | 28 | 16 | 105 | ||
Studies in the table are limited to those using molecular techniques for detection of HRV. Some of the studies mentioned in the article used diagnostic techniques that did not differentiate between HRV and enteroviruses and reported detection rates for picornaviruses. Some studies did not include control data for virus detection in well children.
Data for infections with HRV as a single viral pathogen.
Bacterial and viral pathogens codetected in some infections.
HRV infections in infancy and the development of asthma.
Wheezing illnesses are closely associated with viral respiratory infections in all age groups (Fig. 2). In infancy, illnesses such as bronchiolitis share many clinical features with acute asthma, including wheezing, rapid breathing, prolonged expiratory phase typical of small airway inflammation, and respiratory compromise. About a third of infants who have an acute wheezing illness go on to develop recurrent wheezing, leading to speculation that viral respiratory illnesses in early life promote asthma. This is a plausible hypothesis because the lungs and the immune system are growing and developing during infancy and might be especially vulnerable during this period of time (26). It is also possible that the relationship is not causal and that virus-induced wheezing episodes instead reveal a preexisting tendency for asthma secondary to impaired lung physiology or antiviral responses. A third possibility, which combines elements of the first two, is a “two-hit hypothesis” in which viral infections promote asthma mainly in predisposed children (65, 104). Understanding the host-pathogen interactions that determine the severity of respiratory illnesses and long-term sequelae would be of great help in identifying at-risk individuals and in designing new and more-effective treatments and preventive strategies.
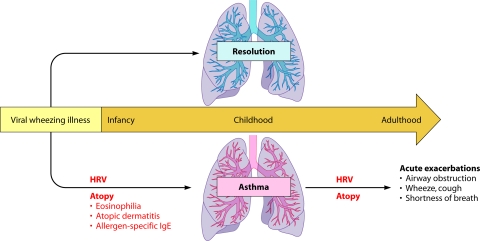
FIG. 2.
Relationship between HRV infections and asthma. Infants who develop virus-induced wheezing episodes are at increased risk for subsequent asthma, but even so, most acute wheezing illnesses in infancy resolve with no long-term sequelae. Indicators of heightened risk for developing asthma include wheezing episodes caused by HRV infections and the development of atopic features such as atopic dermatitis, allergen-specific IgE specific for foods or aeroallergens (e.g., house dust, mites, or cat or dog dander), and blood eosinophilia. Once asthma has been established, HRV infections are the most common cause of acute exacerbations, especially in children. As in infancy, atopy is an important risk factor for acute episodes of virus-induced wheezing.
Long-term studies have demonstrated that infants hospitalized with bronchiolitis have a 2- to 3-fold increase in the risk of developing asthma later in childhood. This risk is further increased by a strong family history of allergic diseases or the development of allergic diseases, particularly if this occurs during early childhood. The type of virus-induced wheezing episode also appears to influence the risk of subsequent asthma. Wheezing illnesses caused by RSV, parainfluenza viruses, or influenza appear to have similar long-term prognoses.
Several studies suggest that infants who wheeze with HRV may be at even greater risk for subsequent asthma. For example, a case control study conducted in Finland demonstrated that infants hospitalized with HRV-induced wheezing were found to have a particularly high risk for subsequent asthma, and this relationship persisted at least through the teen years (37, 56). This finding is further supported by the results of two birth cohort studies. The Childhood Origins of Asthma (COAST) study is a high-risk birth cohort study in which families with at least one parent with allergies or asthma were enrolled prenatally and both the development of immunity and respiratory illnesses were prospectively evaluated (65). Through the use of PCR-based diagnostics, viral etiologies were identified in 90% of wheezing illnesses. Notably, moderate-to-severe HRV infections (with and without wheezing) during infancy were a significant risk factor (odds ratio = 10) for persistent wheezing at age 3 years (66). Moreover, HRV wheezing illnesses in the first 3 years of life were significantly associated with the development of asthma at age 6 years (40). The combination of allergic sensitization and HRV-induced wheezing by age 3 years was associated with the highest risk of developing asthma. Similarly, in an Australian birth cohort study, Kusel and colleagues enrolled 198 babies and compared viral respiratory illnesses in the first year of life to subsequent respiratory outcomes (59). Wheezing illnesses with either HRV or RSV in infancy were associated with asthma at age 5 years. Interestingly, these associations were significant only in the children with early-onset (by age 2 years) allergic sensitization. Collectively, these results highlight the role of virus-induced wheezing, particularly HRV-induced wheezing, in infancy in determining the risk for subsequent asthma. Children who develop allergic diseases in early life are at especially high risk for developing asthma after virus-induced wheezing.
HRV and exacerbations of asthma.
For decades, clinicians had suspected that respiratory infections were a major cause of asthma exacerbations, which are characterized by acute onset of obstruction of the small airways leading to clinical manifestations such as wheezing and shortness of breath. Beginning in the mid-1990s, using PCR-based viral diagnostics, viral respiratory infections were detected in up to 85% of exacerbations of asthma in children and in about half of exacerbations in adults (Table 1). Furthermore, approximately two-thirds of the infections associated with asthma exacerbations were caused by HRV. Similar findings have been reported for children and adults hospitalized for acute asthma. Exacerbations of asthma are seasonal, and in temperate climates there are strong peaks in asthma morbidity in September, shortly after children return to school, and also in the spring (45). These spring and fall peaks in hospitalizations correspond closely to patterns of HRV isolation within the community (46), suggesting a causal relationship. In contrast, influenza and RSV infections are more likely to be associated with acute asthma symptoms in the wintertime.
It is interesting that individuals with asthma do not necessarily have more colds, and neither the severity nor the duration of virus-induced upper respiratory symptoms is enhanced during experimental HRV infections in adults with asthma (14, 15). In contrast to findings for the upper airway, a prospective study of colds in couples consisting of one asthmatic and one normal individual demonstrated that colds cause greater duration and severity of lower respiratory symptoms in subjects with asthma (14). These findings suggest that asthma-related differences in the expression of respiratory viral infections are specific to the lower airway.
Together, these studies provide evidence of a strong relationship between viral infections, particularly those due to HRV, and acute exacerbations of asthma. It has yet to be resolved, however, whether viral infections alone are sufficient to initiate an acute asthma attack or if additional cofactors are needed. Notably, HRV inoculation of subjects with asthma does not usually provoke acute asthma symptoms (15, 19). Moreover, there is evidence that viral infections may exert synergistic effects together with other stimuli to provoke asthma symptoms. For example, the effects of colds on asthma exacerbations are greatest in allergic individuals (94) and may be amplified by exposure to allergens (29) and possibly by exposure to greater levels of air pollutants (110).
In addition to provoking asthma, HRV infections can increase lower airway obstruction in individuals with other chronic airway diseases such as chronic obstructive lung disease (COPD) and cystic fibrosis (Table 1). In addition, HRV can cause pneumonitis in immunocompromised individuals, and this has been well documented in solid-organ or bone marrow transplant recipients. Collectively, these observations have demonstrated the importance of the common cold to exacerbations of asthma and other chronic diseases, promoted a renewed interest in the mechanisms by which respiratory viruses can lead to asthma exacerbations, and been directive in helping to define host and environmental factors that contribute to susceptibility to viral illnesses and the risk of subsequent wheezing (24, 90).
HRV PATHOGENESIS
HRV infections can be transmitted by either aerosol droplets or contact with infected secretions. Once infection is established, respiratory symptoms are the result of two processes: destruction of normal airway tissue due to direct effects of the virus and proinflammatory immune responses to the infection. Virus-induced damage to the epithelium can disturb airway physiology through a number of different pathways. For example, epithelial edema and shedding together with mucus production can cause airway obstruction and wheezing. In addition, viral replication initiates innate immune responses within the epithelial cell, including induction of interferons, antiviral effectors, and chemokines that recruit inflammatory cells into the airway (22, 55, 63, 93, 117). There is evidence that HRV, like many other viruses, has developed mechanisms to inhibit antiviral responses by cleaving proteins such as RIG-I and IPS-1 that are involved in virus recognition pathways (3, 17). The recent development of rodent models of infection with HRV and related picornaviruses will provide systems to test theories about immunopathogenesis in vivo (4, 84, 97). Studies to identify antiviral responses that determine susceptibility to HRV are now under way in a number of laboratories.
With viruses such as HRV that infect relatively few cells in the airway (1, 81), inflammatory responses contribute to respiratory symptoms. The majority of the cells recruited to the airway during acute colds are neutrophils, with smaller numbers of mononuclear cells and, in some studies, eosinophils. Products of neutrophil activation are likely involved in obstructing the airways and causing lower airway symptoms. For example, the release of the potent secretagogue elastase from activated neutrophils can upregulate goblet cell secretion of mucus (9). In addition, changes in neutrophils in nasal secretions have been related to respiratory symptoms and virus-induced increases in airway hyperresponsiveness (enhanced bronchoconstriction in response to irritants), a key feature of asthma (25, 31). These findings suggest that one strategy to reduce the severity of viral illnesses might be to moderate neutrophilic inflammatory responses. Mononuclear cells are recruited into the upper and lower airways during the early stages of a viral respiratory infection and serve to limit the extent of infection and to clear virus-infected epithelial cells. This is consistent with reports of severe viral lower respiratory infections in immunocompromised patients (70).
RISK FACTORS FOR MORE-SEVERE HRV ILLNESSES
Risk factors for more-severe HRV illnesses have been identified by observational studies of natural infection, experimental inoculation, and in vitro models. These factors are related to environmental exposures as well as personal characteristics related to allergies and asthma, genetics, lifestyle, diet, age, immune responses, and genetics (Table 2).
TABLE 2.
Factors linked to more-severe HRV or common cold infections
| Host characteristics | Immunologic status | Environment and lifestyle |
|---|---|---|
| Chronic lung disease | Low level of interferon responses | Exposed to tobacco smoke |
| Asthma | IFN-α | |
| COPD | IFN-β | Exposed to pollutants (NO2) |
| Cystic fibrosis | IFN-γ | |
| IFN-λ | Diet | |
| Age | Includes vitamin D | |
| Preschool child | Allergy | Includes probiotics |
| Elderly | Eosinophilia | |
| Stress | ||
| Genetics | Epithelial integrity | |
| Gender (young boys) | Immunocompromise |
Allergy and antiviral responses.
Several studies have addressed the possibility that allergies and asthma may be associated with more-severe viral respiratory illnesses and impaired antiviral responses. In infants, studies evaluating whether “atopic” features such as allergy, atopic dermatitis (a chronic inflammatory disorder of the skin leading to rash and intense itching), or a family history of allergy increases the risk of acute virus-induced wheezing have yielded conflicting results (103). Conversely, most (83, 107, 111) but not all (101) studies have concluded that bronchiolitis does not promote allergy. What is clear is that children who have other atopic features are prone to developing additional wheezing episodes, and in fact, atopy is one of the strongest risk factors for developing childhood asthma after virus-induced wheezing episodes in infancy (73, 104).
Respiratory allergy is also a major risk factor for wheezing with HRV infections later on in childhood and in adults. In studies conducted in an emergency department, individual risk factors for developing wheezing included detection of a respiratory virus, most commonly HRV, the presence of allergen-specific IgE, and evidence of eosinophilic inflammation (94). These findings provide strong evidence of a synergistic relationship between respiratory allergies, eosinophilia, and acute virus-induced wheezing.
Several mechanisms have been proposed to explain these virus-allergen interactions. Viral infections could damage the barrier function of the airway epithelium, leading to enhanced absorption of allergens and/or irritants across the airway wall and enhanced inflammation (98). Conversely, intact epithelial layers are difficult to infect with HRV in vitro, and replication is enhanced by damaging the epithelium or by removing the top layer of cells (41, 68). These findings suggest that allergens and pollutants that damage airway epithelium could increase susceptibility to infection and/or lead to more-severe infections (Fig. 3).
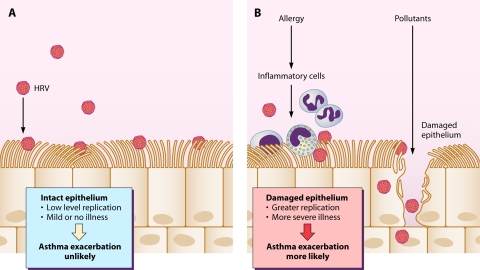
FIG. 3.
Proposed effects of epithelial integrity on severity of HRV infections and exacerbations of asthma. (A) Intact airway epithelium is resistant to HRV infection. If the epithelium is healthy, exposure to HRV is less likely to initiate an infection, and if infection does occur, replication is at a low level and illness severity is mild. (B) Allergies and pollutants can damage the epithelium through a variety of mechanisms; allergy is associated with chronic cellular inflammation that can disrupt epithelial cells, while pollutants can have direct toxic effects on epithelial cells. When the epithelial layer is injured, exposure to HRV leads to enhanced viral replication and more-severe illness. In patients with asthma, severe colds are more likely than mild or asymptomatic infections to provoke acute exacerbations of asthma.
In addition to interactions involving epithelial integrity, there are a number of immunologic interactions between allergy and antiviral immunity. For example, allergic inflammation can inhibit innate immune interferon responses under some conditions (114). Furthermore, HRV infection stimulates Toll-like receptor 3 (TLR-3)-dependent secretion of thymic stromal lymphopoietin (TSLP), a cytokine that can enhance allergic inflammation. Cytokines such as interleukin-4 (IL-4) that are overproduced in asthma synergistically enhance TSLP secretion (49), which provides a potential mechanism for HRV infections to enhance preexisting allergic airway inflammation in asthma. Mucus-secreting goblet cells are present in greater numbers in the airways of subjects with asthma; there is evidence that HRV replication is enhanced in these cells (60). Finally, animal models suggest that biased innate immune responses to respiratory viruses in early life may establish overproduction of key cytokines such as IL-13, leading to suboptimal antiviral responses, increased risk for respiratory allergies, and changes in airway structure to promote asthma (5).
Interferons and HRV infections.
Immunologic risk factors for more-severe respiratory illnesses have been identified in various experimental models. Animal models of respiratory viral infection strongly suggest that interferon responses affect the outcome of respiratory infections and that the stage of lung or immune system development may also be a crucial factor (57, 78, 106). The latter principal is further supported by rodent models of Sendai virus infection, in which infection of mice in early life induces recurrent airway obstruction, chronic airway remodeling, and airway hyperresponsiveness (57, 116).
Interferons have also been related to outcomes of HRV illnesses in studies of volunteers inoculated with a safety-tested strain of HRV-16. For example, weak blood cell interferon responses at the time of birth are a risk factor for more-severe respiratory illnesses (13) and wheezing illnesses (21). Strong gamma interferon (IFN-γ) responses to virus in blood mononuclear cells were associated with reduced viral shedding (91), and stronger T-helper-1-like responses in sputum cells (higher IFN-γ/IL-5 mRNA ratio) during induced colds were associated with milder cold symptoms and more rapid clearance of the virus (27). In addition, children with recurrent respiratory infections were reported to have low IFN-α responses (38).
Interestingly, there is evidence that mononuclear-cell production of IFN-α and IFN-γ may be impaired in asthma (89). It has also been reported that HRV-induced epithelial cell production of IFN-β and IFN-λ is also impaired in asthma, raising the possibility that asthma is associated with a global defect in interferon production (12, 118). This theory is controversial, however, because two recent studies were unable to confirm an asthma-specific deficiency in interferon responses in epithelial cells (6, 69), and so far studies of experimentally inoculated volunteers have not found significant differences in HRV shedding related to asthma. Additional studies of patients with asthma and naturally acquired colds will be needed to resolve these differences.
Other factors.
Other epidemiologic and biologic factors that have been related to the frequency and severity of viral respiratory infections include stress (11), smoking (115), genetic factors (103), and diet. Vitamin D has attracted attention recently because of studies linking low vitamin D levels to both asthma risk and increased numbers of respiratory illnesses (8, 28). In addition, probiotics such as Lactobacillus have been touted as promoting normal development of immunity (92), and a recent interventional study suggested that use of probiotics may reduce the frequency of common cold symptoms (67).
HRV-C
HRVs were classified into 100 serotypes based on growth in tissue culture and inhibition by specific antisera, and these canonical strains were classified into groups A and B based on similarity of partial genetic sequences and responses to certain antiviral medications. Data from studies using molecular diagnostics in the United States (52, 53, 62, 64, 79), Australia (74, 75), Hong Kong (10), China (43), and Germany (95) demonstrate that there are at least 50 more HRV strains than had been previously appreciated. Analysis of full-genome sequences of these newly identified HRVs indicates that the majority belong to a unique species now designated HRV-C (86). Like several other newly discovered respiratory viruses, HRV-Cs do not grow in standard tissue culture, and this characteristic explains why their discovery required the development of molecular diagnostics. Analysis of a limited number of genomes suggests that HRV-C shares structural motifs in the 5′ untranslated region, such as the cloverleaf and internal ribosome entry site, but probably binds to unique cellular receptors (86). More definitive information awaits the development of tissue culture systems for HRV-C.
The clinical significance of HRV-C is the subject of intense study. In one study, HRV-Cs were more often associated with severe exacerbations of asthma than other species (79), and there is a case report of an HRV-C virus causing systemic infection (109). HRV epidemiology is complex; up to 20 strains of HRV circulate through a community during a single season. Furthermore, the prevailing HRV strains differ significantly from place to place and from season to season. Given these findings, along with the large number of HRV strains, long-term population-based studies will be required to define the relative virulence of HRV-C.
OPPORTUNITIES FOR TREATMENT
Over 500 patents for common cold cures have been filed, but an effective treatment remains elusive. Vitamin C, zinc, echinacea, and other nutritional approaches have been tried without convincing evidence of success. Vaccination was considered impractical, given the large number of serotypes, although antibodies to VP4 that can neutralize multiple serotypes have recently been generated (50). Newer approaches based on the viral replication cycle or specific host antiviral responses have included alpha interferon and molecules that block viral attachment (soluble ICAM-1, pirodavir, and pleconaril) and a key viral protease (rupintrivir). Each of these programs was derailed by some combination of cost, pharmacokinetics, toxicity, drug interactions, and/or marginal efficacy (113). Perhaps the fact that HRV-C strains are common yet have distinct biology contributed to the suboptimal efficacy of previous HRV antivirals in clinical trials. Including the HRV-C strains in drug discovery programs should help to improve the clinical performance of anti-HRV medications.
CONCLUSIONS
The development of molecular diagnostics has led to breakthroughs in understanding the role of HRV infections in causing lower airway illnesses, particularly in young children and individuals with asthma and other chronic lung diseases. The discovery of a new species of HRV and complete genome sequencing of the known HRV-A and HRV-B viruses have provided new insights into HRV phylogeny and biology. Risk factors for more-severe HRV illnesses have been identified, and the close relationship between allergic diseases and virus-induced asthma suggests that treatment directed at either the viral infection or the underlying allergies could reduce the risk for wheezing illnesses. Furthermore, defining the specific mechanisms that link allergic inflammation and antiviral responses could lead to the development of new therapeutic strategies. Collectively, new experimental insights and clinical findings relating to HRV should stimulate renewed interest in developing strategies for the prevention and treatment of the common cold.
Acknowledgments
This work was supported by NIH grants P01AI50500 and P01HL070831.
Biography
 James E. Gern (M.D.) is a Professor of pediatrics and medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He received his M.D. degree from the University of South Florida and completed a pediatrics residency at Tufts University in Boston and an allergy/immunology fellowship at Johns Hopkins University. The focus of Dr. Gern's research program has been to determine the role of viral infections in the initiation of asthma and the mechanisms by which rhinovirus infections cause acute exacerbations of asthma. He is the Principal Investigator for the University of Wisconsin Asthma and Allergic Diseases Clinical Research Center, and the program is entitled “Mechanisms of Rhinovirus-Induced Exacerbations of Asthma.” In addition, he leads efforts in two birth cohort studies (Urban Environment and Childhood Asthma and Childhood Origins of Asthma) to identify lifestyle and environmental factors (including viral infections) that influence early immune system development to modify the risks for allergic diseases and asthma.
James E. Gern (M.D.) is a Professor of pediatrics and medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He received his M.D. degree from the University of South Florida and completed a pediatrics residency at Tufts University in Boston and an allergy/immunology fellowship at Johns Hopkins University. The focus of Dr. Gern's research program has been to determine the role of viral infections in the initiation of asthma and the mechanisms by which rhinovirus infections cause acute exacerbations of asthma. He is the Principal Investigator for the University of Wisconsin Asthma and Allergic Diseases Clinical Research Center, and the program is entitled “Mechanisms of Rhinovirus-Induced Exacerbations of Asthma.” In addition, he leads efforts in two birth cohort studies (Urban Environment and Childhood Asthma and Childhood Origins of Asthma) to identify lifestyle and environmental factors (including viral infections) that influence early immune system development to modify the risks for allergic diseases and asthma.
Footnotes
Published ahead of print on 7 April 2010.
REFERENCES
- 1.Arruda, E., T. R. Boyle, B. Winther, D. C. Pevear, J. M. Gwaltney, and F. G. Hayden. 1995. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J. Infect. Dis. 171:1329-1333. [DOI] [PubMed] [Google Scholar]
- 2.Atmar, R. L., E. Guy, K. K. Guntupalli, J. L. Zimmerman, V. D. Bandi, B. D. Baxter, and S. B. Greenberg. 1998. Respiratory tract viral infections in inner-city asthmatic adults. Arch. Intern. Med. 158:2453-2459. [DOI] [PubMed] [Google Scholar]
- 3.Barral, P. M., D. Sarkar, P. B. Fisher, and V. R. Racaniello. 2009. RIG-I is cleaved during picornavirus infection. Virology 391:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett, N. W., R. P. Walton, M. R. Edwards, J. Aniscenko, G. Caramori, J. Zhu, N. Glanville, K. J. Choy, P. Jourdan, J. Burnet, T. J. Tuthill, M. S. Pedrick, M. J. Hurle, C. Plumpton, N. A. Sharp, J. N. Bussell, D. M. Swallow, J. Schwarze, B. Guy, J. W. Almond, P. K. Jeffery, C. M. Lloyd, A. Papi, R. A. Killington, D. J. Rowlands, E. D. Blair, N. J. Clarke, and S. L. Johnston. 2008. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat. Med. 14:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benoit, L. A., and M. J. Holtzman. 2010. New immune pathways from chronic post-viral lung disease. Ann. N. Y. Acad. Sci. 1183:195-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochkov, Y. A., K. M. Hanson, S. Keles, R. A. Brockman-Schneider, N. N. Jarjour, and J. E. Gern. 2010. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 3:69-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvo, C., F. Pozo, M. Garcia-Garcia, M. Sanchez, M. Lopez-Valero, P. Perez-Brena, and I. Casas. 16 February 2010, posting date. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatr. [Epub ahead of print.] doi: 10.1111/j.1651-2227.2010.01714.x. [DOI] [PMC free article] [PubMed]
- 8.Camargo, C. A., Jr., S. L. Rifas-Shiman, A. A. Litonjua, J. W. Rich-Edwards, S. T. Weiss, D. R. Gold, K. Kleinman, and M. W. Gillman. 2007. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am. J. Clin. Nutr. 85:788-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardell, L. O., C. Agusti, K. Takeyama, P. Stjarne, and J. A. Nadel. 1999. LTB(4)-induced nasal gland serous cell secretion mediated by neutrophil elastase. Am. J. Respir. Crit. Care Med. 160:411-414. [DOI] [PubMed] [Google Scholar]
- 10.Cheuk, D. K., I. W. Tang, K. H. Chan, P. C. Woo, M. J. Peiris, and S. S. Chiu. 2007. Rhinovirus infection in hospitalized children in Hong Kong: a prospective study. Pediatr. Infect. Dis. J. 26:995-1000. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, S. 1995. Psychological stress and susceptibility to upper respiratory infections. Am. J. Respir. Crit. Care Med. 152:S53-S58. [DOI] [PubMed] [Google Scholar]
- 12.Contoli, M., S. D. Message, V. Laza-Stanca, M. R. Edwards, P. A. Wark, N. W. Bartlett, T. Kebadze, P. Mallia, L. A. Stanciu, H. L. Parker, L. Slater, A. Lewis-Antes, O. M. Kon, S. T. Holgate, D. E. Davies, S. V. Kotenko, A. Papi, and S. L. Johnston. 2006. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat. Med. 12:1023-1026. [DOI] [PubMed] [Google Scholar]
- 13.Copenhaver, C. C., J. E. Gern, Z. Li, P. A. Shult, L. A. Rosenthal, L. D. Mikus, C. J. Kirk, K. A. Roberg, E. L. Anderson, C. J. Tisler, D. F. DaSilva, H. J. Heimke, K. Gentile, R. E. Gangnon, and R. F. Lemanske, Jr. 2004. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am. J. Respir. Crit. Care Med. 170:175-180. [DOI] [PubMed] [Google Scholar]
- 14.Corne, J. M., C. Marshall, S. Smith, J. Schreiber, G. Sanderson, S. T. Holgate, and S. L. Johnston. 2002. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 359:831-834. [DOI] [PubMed] [Google Scholar]
- 15.DeMore, J. P., E. H. Weisshaar, R. F. Vrtis, C. A. Swenson, M. D. Evans, A. Morin, E. Hazel, J. A. Bork, S. Kakumanu, R. Sorkness, W. W. Busse, and J. E. Gern. 2009. Similar colds in subjects with allergic asthma and nonatopic subjects after inoculation with rhinovirus-16. J. Allergy Clin. Immunol. 124:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Palma, A. M., I. Vliegen, C. E. De, and J. Neyts. 2008. Selective inhibitors of picornavirus replication. Med. Res. Rev. 28:823-884. [DOI] [PubMed] [Google Scholar]
- 17.Drahos, J., and V. R. Racaniello. 2009. Cleavage of IPS-1 in cells infected with human rhinovirus. J. Virol. 83:11581-11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fendrick, A. M., A. S. Monto, B. Nightengale, and M. Sarnes. 2003. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch. Intern. Med. 163:487-494. [DOI] [PubMed] [Google Scholar]
- 19.Fleming, H. E., F. F. Little, D. Schnurr, P. C. Avila, H. Wong, J. Liu, S. Yagi, and H. A. Boushey. 1999. Rhinovirus-16 colds in healthy and in asthmatic subjects: similar changes in upper and lower airways. Am. J. Respir. Crit. Care Med. 160:100-108. [DOI] [PubMed] [Google Scholar]
- 20.Garbino, J., P. M. Soccal, J. D. Aubert, T. Rochat, P. Meylan, Y. Thomas, C. Tapparel, P. O. Bridevaux, and L. Kaiser. 2009. Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax 64:399-404. [DOI] [PubMed] [Google Scholar]
- 21.Gern, J. E., G. D. Brooks, P. Meyer, A. M. Chang, K.-L. Shen, M. D. Evans, C. Tisler, D. DaSilva, K. A. Roberg, L. D. Mikus, L. A. Rosenthal, C. J. Kirk, P. A. Shult, A. Bhattacharya, Z. Li, R. Gangnon, and R. F. Lemanske, Jr. 2006. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J. Allergy Clin. Immunol. 117:72-78. [DOI] [PubMed] [Google Scholar]
- 22.Gern, J. E., D. A. French, K. A. Grindle, R. A. Brockman-Schneider, S. Konno, and W. W. Busse. 2003. Double-stranded RNA induces the synthesis of specific chemokines by bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 28:731-737. [DOI] [PubMed] [Google Scholar]
- 23.Gern, J. E., D. M. Galagan, N. N. Jarjour, E. C. Dick, and W. W. Busse. 1997. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am. J. Respir. Crit. Care Med. 155:1159-1161. [DOI] [PubMed] [Google Scholar]
- 24.Gern, J. E., and R. F. Lemanske, Jr. 2006. Viral respiratory infections and asthma, p. 153-185. In S. J. Szefler and S. Pedersen (ed.), Childhood asthma: breaking down barriers. Marcel Dekker, New York, NY.
- 25.Gern, J. E., M. S. Martin, K. A. Anklam, K. Shen, K. A. Roberg, K. T. Carlson-Dakes, K. Adler, S. Gilbertson-White, R. Hamilton, P. A. Shult, C. J. Kirk, D. F. Da Silva, S. A. Sund, M. R. Kosorok, and R. F. Lemanske, Jr. 2002. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr. Allergy Immunol. 13:386-393. [DOI] [PubMed] [Google Scholar]
- 26.Gern, J. E., L. A. Rosenthal, R. L. Sorkness, and R. F. Lemanske, Jr. 2005. Effects of viral respiratory infections on lung development and childhood asthma. J. Allergy Clin. Immunol. 115:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gern, J. E., R. Vrtis, K. A. Grindle, C. Swenson, and W. W. Busse. 2000. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am. J. Respir. Crit. Care Med. 162:2226-2231. [DOI] [PubMed] [Google Scholar]
- 28.Ginde, A. A., J. M. Mansbach, and C. A. Camargo, Jr. 2009. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 169:384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green, R. M., A. Cusotvic, G. Sanderson, J. Hunter, S. L. Johnston, and A. Woodcock. 2002. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ 324:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greve, J. M., G. Davis, A. M. Meyer, C. P. Forte, S. C. Yost, C. W. Marlor, M. E. Kamarck, and A. Mcclelland. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839-847. [DOI] [PubMed] [Google Scholar]
- 31.Grünberg, K., M. C. Timmers, H. H. Smits, E. P. A. De Klerk, E. C. Dick, W. J. M. Spaan, P. S. Hiemstra, and P. J. Sterk. 1997. Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin. Exp. Allergy. 27:36-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harju, T. H., M. Leinonen, J. Nokso-Koivisto, T. Korhonen, R. Raty, Q. He, T. Hovi, J. Mertsola, A. Bloigu, P. Rytila, and P. Saikku. 2006. Pathogenic bacteria and viruses in induced sputum or pharyngeal secretions of adults with stable asthma. Thorax 61:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heymann, P. W., H. T. Carper, D. D. Murphy, T. A. Platts-Mills, J. Patrie, A. P. McLaughlin, E. A. Erwin, M. S. Shaker, M. Hellems, J. Peerzada, F. G. Hayden, T. K. Hatley, and R. Chamberlain. 2004. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J. Allergy Clin. Immunol. 114:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hicks, L. A., C. W. Shepard, P. H. Britz, D. D. Erdman, M. Fischer, B. L. Flannery, A. J. Peck, X. Lu, W. L. Thacker, R. F. Benson, M. L. Tondella, M. E. Moll, C. G. Whitney, L. J. Anderson, and D. R. Feikin. 2006. Two outbreaks of severe respiratory disease in nursing homes associated with rhinovirus. J. Am. Geriatr. Soc. 54:284-289. [DOI] [PubMed] [Google Scholar]
- 35.Hofer, F., M. Gruenberger, H. Kowalski, H. Machat, M. Huettinger, E. Kuechler, and D. Blaas. 1994. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. U. S. A. 91:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horn, M. E. C., S. E. Reed, and P. Taylor. 1979. Role of viruses and bacteria in acute wheezy bronchitis in childhood: a study of sputum. Arch. Dis. Child. 54:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyvärinen, M. K., A. Kotaniemi-Syrjanen, T. M. Reijonen, K. Korhonen, and M. O. Korppi. 2005. Teenage asthma after severe early childhood wheezing: an 11-year prospective follow-up. Pediatr. Pulmonol. 40:316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isaacs, D., J. R. Clarke, D. A. Tyrrell, A. D. Webster, and H. B. Valman. 1981. Deficient production of leucocyte interferon (interferon-alpha) in vitro and in vivo in children with recurrent respiratory tract infections. Lancet ii:950-952. [DOI] [PubMed] [Google Scholar]
- 39.Ison, M. G., F. G. Hayden, L. Kaiser, L. Corey, and M. Boeckh. 2003. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin. Infect. Dis. 36:1139-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson, D. J., R. E. Gangnon, M. D. Evans, K. A. Roberg, E. L. Anderson, T. E. Pappas, M. C. Printz, W. M. Lee, P. A. Shult, E. Reisdorf, K. T. Carlson-Dakes, L. P. Salazar, D. F. DaSilva, C. J. Tisler, J. E. Gern, and R. F. Lemanske, Jr. 2008. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 178:667-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakiela, B., R. Brockman-Schneider, S. Amineva, W. M. Lee, and J. E. Gern. 2008. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 38:517-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jartti, T., P. Lehtinen, T. Vuorinen, and O. Ruuskanen. 2009. Bronchiolitis: age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr. Infect. Dis. J. 28:311-317. [DOI] [PubMed] [Google Scholar]
- 43.Jin, Y., X. H. Yuan, Z. P. Xie, H. C. Gao, J. R. Song, R. F. Zhang, Z. Q. Xu, L. S. Zheng, Y. D. Hou, and Z. J. Duan. 2009. Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections. J. Clin. Microbiol. 47:2895-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston, N. W., S. L. Johnston, J. M. Duncan, J. M. Greene, T. Kebadze, P. K. Keith, M. Roy, S. Waserman, and M. R. Sears. 2005. The September epidemic of asthma exacerbations in children: a search for etiology. J. Allergy Clin. Immunol. 115:132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston, N. W., S. L. Johnston, G. R. Norman, J. Dai, and M. R. Sears. 2006. The September epidemic of asthma hospitalization: school children as disease vectors. J. Allergy Clin. Immunol. 117:557-562. [DOI] [PubMed] [Google Scholar]
- 46.Johnston, S. L., P. K. Pattemore, G. Sanderson, S. Smith, M. J. Campbell, L. K. Josephs, A. Cunningham, B. S. Robinson, S. H. Myint, M. E. Ward, D. A. J. Tyrrell, and S. T. Holgate. 1996. The relationship between upper respiratory infections and hospital admissions for asthma: a time trend analysis. Am. J. Respir. Crit. Care Med. 154:654-660. [DOI] [PubMed] [Google Scholar]
- 47.Johnston, S. L., P. K. Pattemore, G. Sanderson, S. Smith, F. Lampe, L. Josephs, P. Symington, S. O'Toole, S. H. Myint, D. A. Tyrrell, and S. T. Holgate. 1995. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ 310:1225-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juvén, T., J. Mertsola, M. Waris, M. Leinonen, O. Meurman, M. Roivainen, J. Eskola, P. Saikku, and O. Ruuskanen. 2000. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr. Infect. Dis. J. 19:293-298. [DOI] [PubMed] [Google Scholar]
- 49.Kato, A., S. Favoreto, Jr., P. C. Avila, and R. P. Schleimer. 2007. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J. Immunol. 179:1080-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katpally, U., T. M. Fu, D. C. Freed, D. R. Casimiro, and T. J. Smith. 2009. Antibodies to the buried N terminus of rhinovirus VP4 exhibit cross-serotypic neutralization. J. Virol. 83:7040-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khetsuriani, N., N. N. Kazerouni, D. D. Erdman, X. Lu, S. C. Redd, L. J. Anderson, and W. G. Teague. 2007. Prevalence of viral respiratory tract infections in children with asthma. J. Allergy Clin. Immunol. 119:314-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kistler, A., P. C. Avila, S. Rouskin, D. Wang, T. Ward, S. Yagi, D. Schnurr, D. Ganem, J. L. DeRisi, and H. A. Boushey. 2007. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J. Infect. Dis. 196:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kistler, A. L., D. R. Webster, S. Rouskin, V. Magrini, J. J. Credle, D. P. Schnurr, H. A. Boushey, E. R. Mardis, H. Li, and J. L. DeRisi. 2007. Genome-wide diversity and selective pressure in the human rhinovirus. Virol. J. 4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ko, F. W., M. Ip, P. K. Chan, J. P. Fok, M. C. Chan, J. C. Ngai, D. P. Chan, and D. S. Hui. 2007. A 1-year prospective study of the infectious etiology in patients hospitalized with acute exacerbations of COPD. Chest 131:44-52. [DOI] [PubMed] [Google Scholar]
- 55.Korpi-Steiner, N. L., M. E. Bates, W. M. Lee, D. J. Hall, and P. J. Bertics. 2006. Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J. Leukoc. Biol. 80:1364-1374. [DOI] [PubMed] [Google Scholar]
- 56.Kotaniemi-Syrjänen, A., R. Vainionpaa, T. M. Reijonen, M. Waris, K. Korhonen, and M. Korppi. 2003. Rhinovirus-induced wheezing in infancy—the first sign of childhood asthma? J. Allergy Clin. Immunol. 111:66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar, A., R. L. Sorkness, M. R. Kaplan, and R. F. Lemanske, Jr. 1997. Chronic, episodic, reversible airway obstruction after viral bronchiolitis in rats. Am. J. Respir. Crit. Care Med. 155:130-134. [DOI] [PubMed] [Google Scholar]
- 58.Kusel, M. M., N. H. de Klerk, P. G. Holt, T. Kebadze, S. L. Johnston, and P. D. Sly. 2006. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr. Infect. Dis. J. 25:680-686. [DOI] [PubMed] [Google Scholar]
- 59.Kusel, M. M., N. H. de Klerk, T. Kebadze, V. Vohma, P. G. Holt, S. L. Johnston, and P. D. Sly. 2007. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 119:1105-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lachowicz-Scroggins, M. E., H. A. Boushey, W. E. Finkbeiner, and J. H. Widdicombe. 15 January 2010, posting date. Interleukin-13 induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. [Epub ahead of print.] doi: 10.1165/rcmb.2009-0244OC. [DOI] [PMC free article] [PubMed]
- 61.Lahti, E., V. Peltola, M. Waris, R. Virkki, K. Rantakokko-Jalava, J. Jalava, E. Eerola, and O. Ruuskanen. 2009. Induced sputum in the diagnosis of childhood community-acquired pneumonia. Thorax 64:252-257. [DOI] [PubMed] [Google Scholar]
- 62.Lamson, D., N. Renwick, V. Kapoor, Z. Liu, G. Palacios, J. Ju, A. Dean, K. St. George, T. Briese, and W. I. Lipkin. 2006. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J. Infect. Dis. 194:1398-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lau, C., X. Wang, L. Song, M. North, S. Wiehler, D. Proud, and C. W. Chow. 2008. Syk associates with clathrin and mediates phosphatidylinositol 3-kinase activation during human rhinovirus internalization. J. Immunol. 180:870-880. [DOI] [PubMed] [Google Scholar]
- 64.Lee, W. M., C. Kiesner, T. Pappas, I. Lee, K. Grindle, T. Jartti, B. Jakiela, R. F. Lemanske, P. A. Shult, and J. E. Gern. 2007. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS. One 2:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemanske, R. F., Jr. 2002. The childhood origins of asthma (COAST) study. Pediatr. Allergy Immunol. 13(Suppl. 15):38-43. [DOI] [PubMed] [Google Scholar]
- 66.Lemanske, R. F., Jr., D. J. Jackson, R. E. Gangnon, M. E. Evans, Z. Li, P. Shult, C. J. Kirk, E. Reisdorf, K. A. Roberg, E. L. Anderson, K. T. Carlson-Dakes, K. J. Adler, S. Gilbertson-White, T. E. Pappas, D. F. DaSilva, C. J. Tisler, and J. E. Gern. 2005. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J. Allergy Clin. Immunol. 116:571-577. [DOI] [PubMed] [Google Scholar]
- 67.Leyer, G. J., S. Li, M. E. Mubasher, C. Reifer, and A. C. Ouwehand. 2009. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics 124:e172-e179. [DOI] [PubMed] [Google Scholar]
- 68.Lopez-Souza, N., G. Dolganov, R. Dubin, L. A. Sachs, L. Sassina, H. Sporer, S. Yagi, D. Schnurr, H. A. Boushey, and J. H. Widdicombe. 2004. Resistance of differentiated human airway epithelium to infection by rhinovirus. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L373-L381. [DOI] [PubMed] [Google Scholar]
- 69.Lopez-Souza, N., S. Favoreto, H. Wong, T. Ward, S. Yagi, D. Schnurr, W. E. Finkbeiner, G. M. Dolganov, J. H. Widdicombe, H. A. Boushey, and P. C. Avila. 2009. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J. Allergy Clin. Immunol. 123:1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malcolm, E., E. Arruda, F. G. Hayden, and L. Kaiser. 2001. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J. Clin. Virol. 21:9-16. [DOI] [PubMed] [Google Scholar]
- 71.Malmström, K., A. Pitkaranta, O. Carpen, A. Pelkonen, L. P. Malmberg, M. Turpeinen, M. Kajosaari, S. Sarna, H. Lindahl, T. Haahtela, and M. J. Makela. 2006. Human rhinovirus in bronchial epithelium of infants with recurrent respiratory symptoms. J. Allergy Clin. Immunol. 118:591-596. [DOI] [PubMed] [Google Scholar]
- 72.Mansbach, J. M., A. J. McAdam, S. Clark, P. D. Hain, R. G. Flood, U. Acholonu, and C. A. Camargo, Jr. 2008. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad. Emerg. Med. 15:111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez, F. D., A. L. Wright, L. M. Taussig, C. J. Holberg, M. Halonen, W. J. Morgan, for the Group Health Medical Associates. 1995. Asthma and wheezing in the first six years of life. N. Engl. J. Med. 332:133-138. [DOI] [PubMed] [Google Scholar]
- 74.McErlean, P., L. A. Shackelton, E. Andrews, D. R. Webster, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2008. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C). PLoS. One 3:e1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McErlean, P., L. A. Shackelton, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2007. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J. Clin. Virol. 39:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McFadden, E. R., Jr., B. M. Pichurko, H. F. Bowman, E. Ingenito, S. Burns, N. Dowling, and J. Solway. 1985. Thermal mapping of the airways in humans. J. Appl. Physiol. 58:564-570. [DOI] [PubMed] [Google Scholar]
- 77.McManus, T. E., A. M. Marley, N. Baxter, S. N. Christie, H. J. O'Neill, J. S. Elborn, P. V. Coyle, and J. C. Kidney. 2008. Respiratory viral infection in exacerbations of COPD. Respir. Med. 102:1575-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mikus, L. D., L. A. Rosenthal, R. L. Sorkness, and R. F. Lemanske, Jr. 2001. Reduced interferon-gamma secretion by natural killer cells from rats susceptible to postviral chronic airway dysfunction. Am. J. Respir. Cell Mol. Biol. 24:74-82. [DOI] [PubMed] [Google Scholar]
- 79.Miller, E. K., K. M. Edwards, G. A. Weinberg, M. K. Iwane, M. R. Griffin, C. B. Hall, Y. Zhu, P. G. Szilagyi, L. L. Morin, L. H. Heil, X. Lu, and J. V. Williams. 2009. A novel group of rhinoviruses is associated with asthma hospitalizations. J. Allergy Clin. Immunol. 123:98-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller, E. K., X. Lu, D. D. Erdman, K. A. Poehling, Y. Zhu, M. R. Griffin, T. V. Hartert, L. J. Anderson, G. A. Weinberg, C. B. Hall, M. K. Iwane, and K. M. Edwards. 2007. Rhinovirus-associated hospitalizations in young children. J. Infect. Dis. 195:773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mosser, A. G., R. A. Brockman-Schneider, S. P. Amineva, L. Burchell, J. B. Sedgwick, W. W. Busse, and J. E. Gern. 2002. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J. Infect. Dis. 185:734-743. [DOI] [PubMed] [Google Scholar]
- 82.Mosser, A. G., R. Vrtis, L. Burchell, W. M. Lee, C. R. Dick, E. Weisshaar, D. Bock, C. A. Swenson, R. D. Cornwell, K. C. Meyer, N. N. Jarjour, W. W. Busse, and J. E. Gern. 2005. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am. J. Respir. Crit. Care Med. 171:645-651. [DOI] [PubMed] [Google Scholar]
- 83.Murray, M., M. S. C. Webb, C. Ocallaghan, A. S. Swarbrick, and A. D. Milner. 1992. Respiratory status and allergy after bronchiolitis. Arch. Dis. Child. 67:482-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newcomb, D. C., U. S. Sajjan, D. R. Nagarkar, Q. Wang, S. Nanua, Y. Zhou, C. L. McHenry, K. T. Hennrick, W. C. Tsai, J. K. Bentley, N. W. Lukacs, S. L. Johnston, and M. B. Hershenson. 2008. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am. J. Respir. Crit. Care Med. 177:1111-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nicholson, K. G., J. Kent, and D. C. Ireland. 1993. Respiratory viruses and exacerbations of asthma in adults. BMJ 307:982-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmenberg, A. C., D. Spiro, R. Kuzmickas, S. Wang, A. Djikeng, J. A. Rathe, C. M. Fraser-Liggett, and S. B. Liggett. 2009. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 324:55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Papadopoulos, N. G., P. J. Bates, P. G. Bardin, A. Papi, S. H. Leir, D. J. Fraenkel, J. Meyer, P. M. Lackie, G. Sanderson, S. T. Holgate, and S. L. Johnston. 2000. Rhinoviruses infect the lower airways. J. Infect. Dis. 181:1875-1884. [DOI] [PubMed] [Google Scholar]
- 88.Papadopoulos, N. G., G. Sanderson, J. Hunter, and S. L. Johnston. 1999. Rhinoviruses replicate effectively at lower airway temperatures. J. Med. Virol. 58:100-104. [DOI] [PubMed] [Google Scholar]
- 89.Papadopoulos, N. G., L. A. Stanciu, A. Papi, S. T. Holgate, and S. L. Johnston. 2002. Rhinovirus-induced alterations on peripheral blood mononuclear cell phenotype and costimulatory molecule expression in normal and atopic asthmatic subjects. Clin. Exp. Allergy 32:537-542. [DOI] [PubMed] [Google Scholar]
- 90.Papadopoulos, N. G., P. Xepapadaki, P. Mallia, G. Brusselle, J. B. Watelet, M. Xatzipsalti, G. Foteinos, C. M. van Drunen, W. J. Fokkens, C. D'Ambrosio, S. Bonini, A. Bossios, J. Lotval, P. van Cauwenberge, S. T. Holgate, G. W. Canonica, A. Szczeklik, G. Rohde, J. Kimpen, A. Pitkaranta, M. Makela, P. Chanez, J. Ring, and S. L. Johnston. 2007. Mechanisms of virus-induced asthma exacerbations: state-of-the-art. A GA2LEN and InterAirways document. Allergy 62:457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parry, D. E., W. W. Busse, K. A. Sukow, C. R. Dick, C. A. Swenson, and J. E. Gern. 2000. Rhinovirus-induced peripheral blood mononuclear cell responses and outcome of experimental infection in allergic subjects. J. Allergy Clin. Immunol. 105:692-698. [DOI] [PubMed] [Google Scholar]
- 92.Prescott, S. L., and B. Bjorksten. 2007. Probiotics for the prevention or treatment of allergic diseases. J. Allergy Clin. Immunol. 120:255-262. [DOI] [PubMed] [Google Scholar]
- 93.Proud, D., R. B. Turner, B. Winther, S. Wiehler, J. P. Tiesman, T. D. Reichling, K. D. Juhlin, A. W. Fulmer, B. Y. Ho, A. A. Walanski, C. L. Poore, H. Mizoguchi, L. Jump, M. L. Moore, C. K. Zukowski, and J. W. Clymer. 2008. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am. J. Respir. Crit. Care Med. 178:962-968. [DOI] [PubMed] [Google Scholar]
- 94.Rakes, G. P., E. Arruda, J. M. Ingram, G. E. Hoover, J. C. Zambrano, F. G. Hayden, T. A. Platts-Mills, and P. W. Heymann. 1999. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am. J. Respir. Crit. Care Med. 159:785-790. [DOI] [PubMed] [Google Scholar]
- 95.Renwick, N., B. Schweiger, V. Kapoor, Z. Liu, J. Villari, R. Bullmann, R. Miething, T. Briese, and W. I. Lipkin. 2007. A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J. Infect. Dis. 196:1754-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rohde, G., A. Wiethege, I. Borg, M. Kauth, T. T. Bauer, A. Gillissen, A. Bufe, and G. Schultze-Werninghaus. 2003. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax 58:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosenthal, L. A., S. P. Amineva, R. J. Szakaly, R. F. Lemanske, Jr., J. E. Gern, and R. L. Sorkness. 2009. A rat model of picornavirus-induced airway infection and inflammation. Virol. J. 6:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakamoto, M., S. Ida, and T. Takishima. 1984. Effect of influenza virus infection on allergic sensitization to aerosolized ovalbumin in mice. J. Immunol. 132:2614-2617. [PubMed] [Google Scholar]
- 99.Schroth, M. K., E. Grimm, P. Frindt, D. M. Galagan, S. Konno, R. Love, and J. E. Gern. 1999. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 20:1220-1228. [DOI] [PubMed] [Google Scholar]
- 100.Seemungal, T. A., R. Harper-Owen, A. Bhowmik, D. J. Jeffries, and J. A. Wedzicha. 2000. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur. Respir. J. 16:677-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sigurs, N., R. Bjarnason, F. Sigurbergsson, and B. Kjellman. 2000. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 161:1501-1507. [DOI] [PubMed] [Google Scholar]
- 102.Simons, E., M. K. Schroth, and J. E. Gern. 2005. Analysis of tracheal secretions for rhinovirus during natural colds. Pediatr. Allergy Immunol. 16:276-278. [DOI] [PubMed] [Google Scholar]
- 103.Singh, A. M., P. E. Moore, J. E. Gern, R. F. Lemanske, Jr., and T. V. Hartert. 2007. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am. J. Respir. Crit. Care Med. 175:108-119. [DOI] [PubMed] [Google Scholar]
- 104.Sly, P. D., A. L. Boner, B. Bjorksten, A. Bush, A. Custovic, P. A. Eigenmann, J. E. Gern, J. Gerritsen, E. Hamelmann, P. J. Helms, R. F. Lemanske, F. Martinez, S. Pedersen, H. Renz, H. Sampson, E. von Mutius, U. Wahn, and P. G. Holt. 2008. Early identification of atopy in the prediction of persistent asthma in children. Lancet 372:1100-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smyth, A. R., R. L. Smyth, C. Y. W. Tong, C. A. Hart, and D. P. Heaf. 1995. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch. Dis. Child. 73:117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sorkness, R. L., W. L. Castleman, A. Kumar, M. R. Kaplan, and R. F. Lemanske, Jr. 1999. Prevention of chronic postbronchiolitis airway sequelae with IFN-γ treatment in rats. Am. J. Respir. Crit. Care Med. 160:705-710. [DOI] [PubMed] [Google Scholar]
- 107.Stein, R. T., D. Sherrill, W. J. Morgan, C. J. Holberg, M. Halonen, L. M. Taussig, A. L. Wright, and F. D. Martinez. 1999. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354:541-545. [DOI] [PubMed] [Google Scholar]
- 108.Tan, W. C., X. Xiang, D. Qiu, T. P. Ng, S. F. Lam, and R. G. Hegele. 2003. Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am. J. Med. 115:272-277. [DOI] [PubMed] [Google Scholar]
- 109.Tapparel, C., A. G. L'Huillier, A. L. Rougemont, M. Beghetti, C. Barazzone-Argiroffo, and L. Kaiser. 2009. Pneumonia and pericarditis in a child with HRV-C infection: a case report. J. Clin. Virol. 45:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tarlo, S. M., I. Broder, P. Corey, M. Chan-Yeung, A. Ferguson, A. Becker, C. Rogers, M. Okada, and J. Manfreda. 2001. The role of symptomatic colds in asthma exacerbations: influence of outdoor allergens and air pollutants. J. Allergy Clin. Immunol. 108:52-58. [DOI] [PubMed] [Google Scholar]
- 111.Thomsen, S. F., S. van der Sluis, L. G. Stensballe, D. Posthuma, A. Skytthe, K. O. Kyvik, D. L. Duffy, V. Backer, and H. Bisgaard. 2009. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am. J. Respir. Crit. Care Med. 179:1091-1097. [DOI] [PubMed] [Google Scholar]
- 112.Tsolia, M. N., S. Psarras, A. Bossios, H. Audi, M. Paldanius, D. Gourgiotis, K. Kallergi, D. A. Kafetzis, A. Constantopoulos, and N. G. Papadopoulos. 2004. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin. Infect. Dis. 39:681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turner, R. B. 2005. New considerations in the treatment and prevention of rhinovirus infections. Pediatr. Ann. 34:53-57. [DOI] [PubMed] [Google Scholar]
- 114.Tversky, J. R., T. V. Le, A. P. Bieneman, K. L. Chichester, R. G. Hamilton, and J. T. Schroeder. 2008. Human blood dendritic cells from allergic subjects have impaired capacity to produce interferon-alpha via Toll-like receptor 9. Clin. Exp. Allergy 38:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Venarske, D. L., W. W. Busse, M. R. Griffin, T. Gebretsadik, A. K. Shintani, P. A. Minton, R. S. Peebles, R. Hamilton, E. Weisshaar, R. Vrtis, S. B. Higgins, and T. V. Hartert. 2006. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J. Infect. Dis. 193:1536-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walter, M. J., J. D. Morton, N. Kajiwara, E. Agapov, and M. J. Holtzman. 2002. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J. Clin. Invest. 110:165-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang, Q., D. R. Nagarkar, E. R. Bowman, D. Schneider, B. Gosangi, J. Lei, Y. Zhao, C. L. McHenry, R. V. Burgens, D. J. Miller, U. Sajjan, and M. B. Hershenson. 2009. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J. Immunol. 183:6989-6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wark, P. A., S. L. Johnston, F. Bucchieri, R. Powell, S. Puddicombe, V. Laza-Stanca, S. T. Holgate, and D. E. Davies. 2005. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 201:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.White, D. O., and F. J. Fenner. 1994. Medical virology. Academic Press, San Diego, CA.