Abstract
Cystic fibrosis (CF) is the most common lethal recessive genetic disease in the Caucasian population. It is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene that is normally expressed in ciliated airway epithelial cells and the submucosal glands of the lung. Since the CFTR gene was first characterized in 1989, a major goal has been to develop an effective gene therapy for CF lung disease, which has the potential to ameliorate morbidity and mortality. Respiratory syncytial virus (RSV) naturally infects the ciliated cells in the human airway epithelium. In addition, the immune response mounted against an RSV infection does not prevent subsequent infections, suggesting that an RSV-based vector might be effectively readministered. To test whether the large 4.5-kb CFTR gene could be expressed by a recombinant RSV and whether infectious virus could be used to deliver CFTR to ciliated airway epithelium derived from CF patients, we inserted the CFTR gene into four sites in a recombinant green fluorescent protein-expressing RSV (rgRSV) genome to generate virus expressing four different levels of CFTR protein. Two of these four rgRSV-CFTR vectors were capable of expressing CFTR with little effect on viral replication. rgRSV-CFTR infection of primary human airway epithelial cultures derived from CF patients resulted in expression of CFTR protein that was properly localized at the luminal surface and corrected the chloride ion channel defect in these cells.
Cystic fibrosis (CF) is an autosomal recessive genetic disease that occurs with an incidence of 1 in every 3,400 live Caucasian births in the United States and is one of the most common fatal hereditary diseases in the world (47). CF is caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that encodes a low-conductance ATP- and cyclic AMP (cAMP)-dependent chloride ion (Cl−) channel. More than 1,500 mutations that can lead to various degrees of CF have been found in CFTR. The most common mutation found in individuals of European descent is a deletion of 3 nucleotides in the CFTR gene resulting in the loss of phenylalanine at position 508 of the CFTR protein (ΔF508). This mutation results in the translation of a protein that folds improperly, causing it to be degraded upon exit from the endoplasmic reticulum. Since 90% of the mortality caused by CF results from lung pathology, restoring functional CFTR to the airways of CF patients remains a goal of gene replacement therapeutics for the disease. In the lung, CFTR is expressed by the respiratory epithelium that lines the lumen of the airways, where it is localized to the apical membrane of ciliated cells and the submucosal gland ductal epithelium (20, 40, 48). CFTR is responsible for the movement of Cl− ions across the apical membranes of the airway epithelium and, in combination with sodium ion (Na+) transport, it dictates the volume of airway surface liquid that facilitates mucus transport and mucociliary clearance. Lack of functional CFTR in the cell membrane decreases Cl− ion secretion; a net increase in the intracellular Cl− ion concentration is then followed by increased uptake of sodium (Na+) ions by epithelial sodium channels (ENaCs). This additional intracellular ion concentration results in a net increase in water uptake into the cell (68). In patients with CF, the fundamental consequence of CFTR dysfunction in the airway is dehydration of the airway surface liquid (ASL) and an increase in the viscosity of the mucus secretions that coat the respiratory tract. This thickened mucus leads to plugging of the airways, in addition to decreased airway clearance, resulting in an increased susceptibility to both bacterial and viral airway pathogens.
Early in vitro experiments using the available recombinant adenoviruses (AdV) and adeno-associated viruses (AAV) showed some efficacy in airway cell transduction (29, 67); however, the human clinical trials were less promising due to the low efficiency of CFTR delivery to the appropriate cells and short-lived CFTR expression, primarily as a consequence of the innate and adaptive immune responses (28, 34, 39, 90). Further studies revealed that CAR, the coxsackievirus and AdV receptor, and heparan sulfate, the AAV receptor, are both expressed on the basolateral surface of the human airway, likely providing another explanation for the poor transduction efficiency of airway cells by these vectors when introduced apically (7, 62, 77, 92). More recently, AAV serotypes that transduce the airway epithelium at a much higher rate have been identified, and additional improvements have been made by mutagenesis, capsid shuffling, and directed evolution (24, 36, 52-54, 78, 89). Lentiviral vectors for the delivery of CFTR to CF patients have also been examined, and improvements have been made, but efficiency and safety concerns persist (33, 41, 57, 72, 76, 85). Here, we suggest a potential viral vector to treat CF that naturally targets the airways.
In vitro studies in which CF cells and CFTR-corrected CF cells have been mixed in measured ratios have determined that CFTR expression in 6 to 10% of respiratory cells returns Cl− transport to levels similar to those measured in non-CF epithelial cell cultures (2, 42). However, this low level of correction may not repair some of the other associated defects, such as sodium hyperabsorption and mucus dehydration (40). Similar studies performed by mixing airway epithelial cells from CF and non-CF patients to create mixed well-differentiated human airway epithelial cell (HAE) cultures indicated that if 20% of the cells expressed endogenous levels of CFTR, this correlated with 70% of the Cl− channel response measured in cultures made with 100% non-CF cells (25). More recently, infection of HAE cultures with a recombinant parainfluenza virus type 3 (PIV3) vector engineered to express CFTR was shown to fully correct the Cl− transport defect in HAE cultures. In these studies, CFTR delivery to 25% of the surface airway epithelial cells was required to restore airway surface liquid volume and mucus transport to normal non-CF levels (93). Collectively, these in vitro experiments, in relevant airway cell models, suggest that an effective vector for CFTR delivery would need to target at least 25% of the airway surface epithelial cells.
Respiratory syncytial virus (RSV) is a single-stranded negative-sense RNA virus that infects the ciliated cells of the airway epithelium of the human respiratory tract (94). Most individuals become infected with RSV during the first and second years of life; however, due to incomplete immunity, individuals can be reinfected by RSV throughout their lifetimes. In most cases, infection results in only mild, self-limited, common cold-like symptoms, although a proportion of primary infections do involve lower respiratory tract disease. Serious illness, which typically involves bronchiolitis or pneumonia, is usually restricted to young infants or the frail elderly. Although RSV infects CF patients at the same frequency that it infects their age-matched siblings, CF patients tend to develop more frequent lower respiratory tract illness. It has been shown that CF patients require more frequent hospitalization due to RSV infection when they are young, but this decreases with age, as it does for healthy children (32, 87). Since RSV can infect the lungs of CF patients, it appears that it can not only navigate through the physical barriers of the normal respiratory tract, but can also make its way through the sticky and mucus-rich environment of the CF lung. In addition, RSV has other features that suggest it might have advantages as a gene therapy vector for the delivery of CFTR to the airways of CF patients. RSV has a tropism for the luminal ciliated cells of the airway, which are a relevant target for CFTR gene therapy (40, 48), and RSV has been shown to lack the overt cytopathology of other respiratory viruses, suggesting that it will not rapidly destroy the cells that it infects (94). RSV also has the ability to reinfect, implying that multiple sequential administrations of an RSV-based vector would be possible.
Here, we tested the utility of RSV as a CFTR gene transfer vector. The CFTR gene was inserted into four different sites in the RSV genome to obtain a range of expression levels. The vector was then evaluated for the ability to deliver CFTR to the ciliated cells in an in vitro model of the human airway (HAE). We show that RSV delivered CFTR to ciliated cells and resulted in sufficient transduction efficiency and functional CFTR expression to fully correct the Cl− transport bioelectric defect in primary HAE cultures derived from CF patients. These data support continued efforts to explore the utility of RSV-based vectors as potential gene delivery vectors for the treatment of CF lung disease.
MATERIALS AND METHODS
Viruses and cells.
Recombinant green fluorescent protein (GFP)-expressing RSV (rgRSV) (38) was used as the vector backbone in this study. All viruses were rescued and grown in HeLa cells in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). All cells were grown in a humidified incubator supplied with 5% CO2.
Recombinant virus construction and rescue.
The full-length RSV cDNA construct, RW30, used as the backbone for CFTR gene insertion, was derived from the original RSV cDNA (18) with the following modifications: (i) the highly efficient T7 promoter that initiates the transcript with GGG at its 5′ terminus is followed by a 5′ hammerhead ribozyme sequence (1) so that the positive-sense, antigenomic T7 RNA transcript can be produced efficiently but the ribozyme removes itself and the GGG, leaving the exact, native 5′ terminus; (ii) the Renilla recombinant green fluorescent protein (RrFP), a PCR product from pFBRrFP (Stratagene) without mammalian optimization, is inserted as the first viral gene with a KpnI site following its open reading frame (ORF); (iii) unique restriction sites are inserted in the intergenic regions flanking each of the membrane-associated protein genes; (iv) a BsiWI restriction site is inserted following the L gene that required 3 nucleotide changes, as provided by Rachel Fearns; and (v) the 3′ hepatitis D virus antigenomic ribozyme (59) replaces the hammerhead ribozyme so that the transcript will have an exact 3′ native terminus. These insertions and mutations were produced in small plasmids by inverted PCR mutagenesis (14) and moved into the full-length cDNA by standard subcloning techniques. The positions and sequences of these alterations are listed in Table 1.
TABLE 1.
Modifications to the full-length RSV D53 cDNA to produce RW30
| Position | Sequence |
|---|---|
| 5′ hammerhead ribozyme | GGGAAA-CGCGTCTGATGAGGCCGTTAGGCCGAAACTCCTCTCCGGAGTC-ACGCGAAAAAa |
| BssHII in the P-M IGb | AGGAAAGGGTGCGCGCTGGGGCAAATc |
| Sequence flanking the RrFP gene, | |
| including KpnI | AATTTAACTCTAGACCCGGGTTACCACCATGGTG-RrFP-GTTTAACGGTACCCGGGAGAGTTAATAAAAAACCAAAAAAATGGGGCAAATAAGAATTTGATAAGTACCACTTAAATTTAACTCCCTTGGTTAGAGATGGGC |
| PvuI in the M-SH IG | ATACACATGGCGATCGACATGGGGCA |
| ApaI in the SH-G IG | TTAAAAATTAGGGCCCAACAATGAAC |
| SacII in the G-F IG | CCTTGACCAACCGCGGAGAATCAAAA |
| XhoI in the F-M2 IG | TTGCATGCCACTCGAGCTTACCATCT |
| BsiWI in the trailer | AATTAAAAATCGTACGATTTTTTAAA |
| 3′ hepatitis delta virus ribozyme | GGGTCGGCATGGCATCTCCACCTCCTCGCGGTCCGACCTGGGCATCCGAAGGAGGACGCACGTCCACTCGGATGGCTAAG |
The sequence between the hyphens is the 5′ hammerhead ribozyme. The first three Gs (italicized) of the transcript are the final 3 Gs of the optimal T7 promoter and are included in the transcript. The 5 italicized and underlined nucleotides are complementary to the first 5 nucleotides of the mature RNA antigenome.
IG, intergenic region, between the GE of the previous gene and the GS of the next gene.
Boldface nucleotides indicate inserted restriction sites flanked by the native sequences to enable their location in the genome sequence.
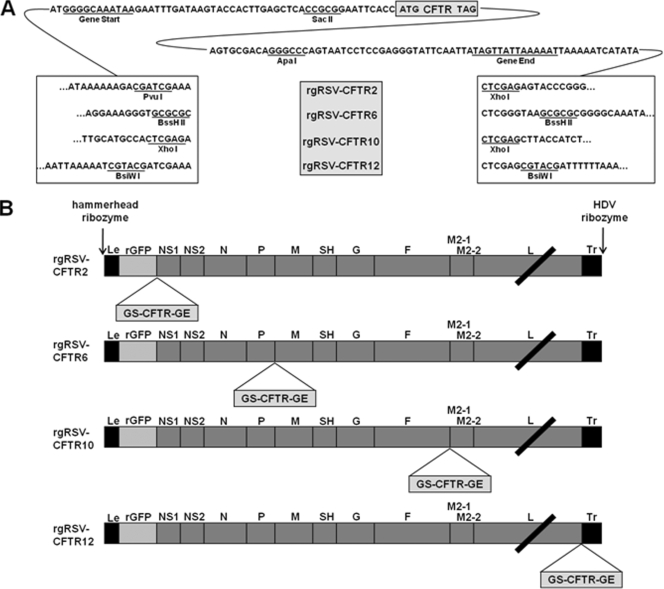
The human CFTR cDNA (John Riordan, University of North Carolina [UNC], Chapel Hill, NC) was first flanked by RSV gene start (GS) and gene end (GE) sequences, as well as unique restriction sites to enable the insertion of the gene unit into RW30. A series of plasmids were created containing the GS and GE sequences from the RSV F gene, internally flanked by SacII and ApaI restriction sites and externally flanked by PvuI and XhoI, BssHII, XhoI, or BsiWI restriction sites. The CFTR gene was inserted into these plasmids using SacII and ApaI. The resulting CFTR gene units were inserted at the BssHII, XhoI, or BsiWI restriction site in RW30, creating rgRSV-CFTR6, rgRSV-CFTR10, and rgRSV-CFTR12, respectively (Fig. 1). rgRSV-CFTR2 was created by first inserting the CFTR gene unit into a partial rgRSV plasmid using PvuI and XhoI. The CFTR gene unit and a genome segment containing the NS1, NS2, N, and P genes were then cloned into the complete rgRSV plasmid using the KpnI and BssHII sites. The names of the resulting plasmids include a number that describes the position occupied by the CFTR gene relative to the 3′ end of the genome.
FIG. 1.
rgRSV-CFTR constructs. The CFTR gene, flanked by the RSV GS and GE, was inserted into the rgRSV cDNA at 4 positions. (A) Sequences flanking the CFTR gene unit in the four constructs. (B) Schematic of CFTR locations within rgRSV-CFTR genomes. The constructs are named for the position of the CFTR gene relative to the viral promoter at the 3′ end of the genome.
All viruses were rescued in HeLa cells using the reverse genetics system described previously (83). Briefly, a plasmid containing a full-length cDNA of the virus genome, along with support plasmids expressing the RSV N, P, L, and M2-1 genes, was transfected into 6-well plates of 40% confluent HeLa cells. Initial replication of the plasmids was driven by a T7 polymerase supplied by coinfection of the cells with vaccinia virus MVA-T7. Once virus replication and spread were evident, detected by the presence and clustering of GFP-containing cells, the virus was harvested. Cells were scraped from the plate, pipetted, and vortexed before pelleting of the cells by centrifugation. The virus-containing supernatant was then passaged onto successively larger plates to obtain higher-titered virus stocks. All viruses were amplified, and the titers were determined in HeLa cells.
Western blotting.
HeLa cells at 60% confluence in 100-mm dishes were inoculated with rgRSV, rgRSV-CFTR2, rgRSV-CFTR6, rgRSV-CFTR10, and rgRSV-CFTR12 at a multiplicity of infection (MOI) of 0.5 or mock inoculated. The cells were lysed at 72 h postinoculation (p.i.) using 500 μl lysis buffer (1% Triton X-100, 150 mM NaCl, 50 mM Tris, and an EDTA-free protease inhibitor cocktail [Roche]). Laemmli sample buffer containing 5% β-mercaptoethanol was added to equal volumes of lysate. Samples were incubated at 37°C for 30 min, electrophoresed on a 10% SDS-PAGE gel (49), and transferred to an Immobilon-P membrane (Millipore), which was blocked overnight at 4°C with 5% dry milk in phosphate-buffered saline plus Tween 20 (PBST). The membrane was probed with CFTR monoclonal antibody (MAb) 596 (John Riordan, UNC) diluted 1:1,000. A goat anti-mouse peroxidase-labeled secondary antibody (Chemicon) was used at a dilution of 1:10,000. The blot was developed with LumiLight chemiluminescent substrate (Roche). Lysate from Calu3 cells, prepared in the same manner, was used as a positive control for CFTR.
Growth curve.
Duplicate wells of HeLa cells at 40% confluence in 12-well plates were inoculated with rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12 at an MOI of 0.1. At the indicated time points, cells from duplicate wells inoculated with each virus were scraped, collected, and vortexed. The cells were removed by centrifugation at 2,000 × g for 5 min. The virus-containing supernatant was transferred to a new tube and frozen on dry ice. The titers of all samples were determined on HeLa cells by counting GFP-expressing cells at 24 h postinoculation.
Generation of CF HAE.
Primary human tracheobronchial epithelial cells from CF (ΔF508 homozygous) and non-CF donors were obtained from tracheobronchial tissues by the UNC CF Center Tissue Culture Core under UNC Institutional Review Board (IRB)-approved protocols. The cells were plated at a density of 250,000 cells per well on permeable type IV collagen-coated Millicells (0.4-μm pore size, 12-mm diameter; Millipore). Cultures were allowed to differentiate at an air-liquid interface for 4 to 6 weeks to form well-differentiated cultures with morphological characteristics that resembled those of the human cartilaginous pseudostratified ciliated epithelium in vivo, as previously described (31, 61).
Inoculation of CF HAE with rgRSV-CFTR.
Prior to virus inoculation, the apical surfaces of HAE were rinsed 3 times with PBS to remove accumulated apical secretions. The apical surfaces of quadruplicate cultures were then inoculated with 200 μl of rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) or mock inoculated and incubated for 2 h at 37°C. After removal of the inoculum, the HAE were returned to a humidified incubator until assays were performed.
Quantification of infection of CF HAE cultures.
CF HAE inoculated with rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) were photographed with a Leica DMIRB inverted fluorescence microscope 24 h postinoculation, and en face percentages of GFP-positive cells were quantified with ImageJ software (NIH) as previously described (93).
Quantitation of CFTR and RSV N mRNA following CF HAE inoculation.
Total RNA was isolated from quadruplicate cultures of CF HAE 48 h after inoculation with rgRSV, rgRSV-CFTR10, rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) or mock inoculation using acid phenol-guanidine thiocyanate, followed by DNase digestion and further purification using the Qiagen RNeasy Mini Kit. The first-strand cDNA was synthesized with oligo(dT) and SuperScript II reverse transcriptase (Invitrogen). Quantitative PCR was performed using a Roche LightCycler with the Roche FastStart DNA Master SYBR green I kit according to the manufacturer's protocols. Levels of CFTR mRNA were normalized to the level of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) measured using LightCycler software version 4.0. Levels of RSV N mRNA in cultures inoculated with rgRSV-CFTR10 and rgRSV-CFTR12 were compared to the level in rgRSV-inoculated cultures, which was set to 1.
Immunofluorescent staining.
To immunolocalize CFTR in CF HAE inoculated with rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12 or mock inoculated, cells were gently scraped with a pipette tip and then resuspended in PBS before being pelleted onto glass slides with Cytospin and air dried. The cells were fixed with 4% paraformaldehyde, and then CFTR was detected with anti-human CFTR mouse MAb 596 and Alexafluor 568-conjugated goat anti-mouse antibody. Cell nuclei were counterstained with Hoechst 33342 (Invitrogen). Images were taken with a Leica SP2 laser scanning confocal microscope and processed with Adobe Photoshop CS2.
Ion transport measurements.
CF HAE inoculated with rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) or mock inoculated were mounted in Ussing chambers for measurement of transepithelial resistance (Rt) and transepithelial potential difference (PD) under open-circuit conditions using a Physiologic Instruments voltage clamp (San Diego, CA) 48 h postinoculation. The electrical potential difference across the tissue was continuously recorded, and a constant current pulse (2 to 10 μA) was applied across the tissue at 1-min intervals to calculate tissue resistance. From these measurements, the equivalent short-circuit current (Isc) was calculated. Additional details of Ussing chamber techniques have been published previously (35). Ussing chamber experiments were performed in bilateral Krebs bicarbonate Ringer solution (KBR) gassed with 95% O2, 5% CO2. Drugs (amiloride [10−4 M], forskolin [10−5 M], and CFTR172 [10−5 M]) were added sequentially to the indicated final concentration from concentrated stock solutions (Sigma-Aldrich) to lumenal and/or basal surfaces. CFTR172 (10−5 M), a potent small-molecule inhibitor of CFTR, was synthesized according to appropriate standards and used as previously described (56). Untreated non-CF HAE cultures were tested in parallel as controls.
ASL measurements.
At 48 h after inoculation of the CF HAE cultures with rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) or mock inoculation, 25 μl of PBS containing 0.2% Texas Red-dextran (Invitrogen) was added to the apical surface, giving the cultures an initial ASL height of 20 to 30 μm. Images of the ASL were acquired using laser scanning confocal microscopy (Zeiss model 510). The ASL height was determined by averaging the heights obtained from the XZ scans of four culture regions over time. Additional details concerning ASL measurement have been published previously (82).
RESULTS
Construction and rescue of recombinant rgRSV vectors expressing CFTR.
A CFTR gene unit containing the 4.5-kb CFTR gene flanked by a set of RSV transcription signals was inserted into the full-length rgRSV cDNA in four different positions, creating the plasmid templates for rgRSV-CFTR2, rgRSV-CFTR6, rgRSV-CFTR10, and rgRSV-CFTR12, which were numbered according to the CFTR gene position in the viral genome (Fig. 1A and B). Virus was rescued from multiple clones of each rgRSV-CFTR cDNA by transfection into HeLa cells. Similar to rgRSV, rgRSV-CFTR10 and rgRSV-CFTR12 began to spread by 4 days posttransfection, as visualized by fluorescence microscopy. rgRSV-CFTR2 and rgRSV-CFTR6 required an additional 2 to 4 days to spread and were more cytotoxic (i.e., they caused more cell death, and the cells in the culture were visibly less healthy) during this period and subsequent early virus passages compared to the other viruses, including the parental rgRSV. This increase in cytopathology could account for the extra time required to rescue these viruses. By the third passage, however, these viruses caused cytopathology similar to that of rgRSV-CFTR10 and rgRSV-CFTR12.
rgRSV can express full-length, mature CFTR.
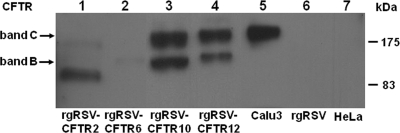
To test whether these viruses expressed CFTR, we inoculated HeLa cells at an MOI of 0.5, harvested cell lysates at 72 h p.i., and assayed for CFTR by Western blotting. As a positive control, we used lysate from the human lung cell line Calu3, which endogenously expresses a high level of mature CFTR. HeLa cells inoculated with rgRSV-CFTR10 and rgRSV-CFTR12 expressed mature CFTR protein, band C (Fig. 2, lanes 3 and 4), with more CFTR produced by rgRSV-CFTR10 than by rgRSV-CFTR12. The immunoreactive lower band detected in HeLa cells inoculated with rgRSV-CFTR10 and rgRSV-CFTR12 likely represents CFTR in its immature, underglycosylated state. In contrast, rgRSV-CFTR2 and rgRSV-CFTR6 failed to produce full-length, mature CFTR protein. Four clones of each of these viruses were tested, and none were capable of expressing full-length, mature CFTR. Representative clones of rgRSV-CFTR2 and rgRSV-CFTR6 are shown in Fig. 2 (lanes 1 and 2). No CFTR immunoreactivity was detectable in mock-inoculated HeLa cells or in HeLa cells inoculated with rgRSV alone. These data indicate that mature CFTR can be expressed from the rgRSV genome but that the location of the CFTR gene in the genome may impact the integrity of the CFTR gene unit. In addition, the difference in CFTR expression levels between rgRSV-CFTR10 and rgRSV-CFTR12 is consistent with the polarity of RSV expression. Genes further from the 3′ promoter, the only viral transcription promoter, are expressed at lower levels because a proportion of the polymerase falls off the template at each intergenic region.
FIG. 2.
CFTR expression from rgRSV-CFTR. A Western blot was performed to detect CFTR protein in HeLa cells infected with rgRSV-CFTR vectors. Band C (∼190 kDa) represents full-length, mature CFTR. Band B (∼150 kDa) represents immature CFTR. Endogenous CFTR from Calu3 cells was used as a positive control (lane 5). Lysates from mock-infected HeLa cells and rgRSV-inoculated HeLa cells were negative controls (lanes 6 and 7). Shown is a representative blot from four experiments.
The observations that rgRSV-CFTR2 and rgRSV-CFTR6 produced little to no full-length CFTR are likely related to the increased cytopathic effect (CPE) of these vectors during rescue. rgRSV-CFTR2 and rgRSV-CFTR6 would have been expected to express substantially higher levels of CFTR than rgRSV-CFTR10 and rgRSV-CFTR12 because they bear the CFTR gene unit in positions closer to the RSV promoter. It is conceivable that these high levels of CFTR were cytotoxic during the rescue and early passages of these viruses. The loss of cytotoxicity upon serial passage might be explained by the selection of adventitious mutations that block expression of a functional CFTR protein. This may explain why multiple clones of the rescued rgRSV-CFTR2 and rgRSV-CFTR6 lost both their cytopathic phenotype and their ability to express complete CFTR. Due to their lack of CFTR expression, rgRSV-CFTR2 and rgRSV-CFTR6 were not further evaluated in this study.
Growth kinetics of rgRSV-CFTRs.
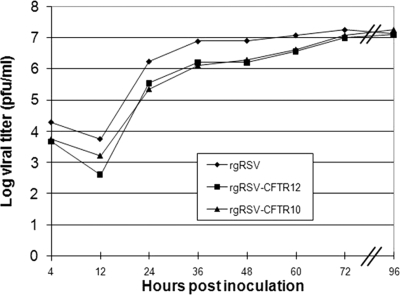
We compared the growth kinetics of rgRSV-CFTR10 and rgRSV-CFTR12 to those of rgRSV to determine whether the 4.5-kb CFTR gene insertion or the position at which it was inserted affected virus replication. Cells were infected with each virus at an MOI of 0.1, and the virus yield was determined over 4 days. Although the virus yield from rgRSV-CFTR10 and rgRSV-CFTR12 seemed to be delayed by 4 h compared to that from rgRSV, the shapes of all the curves were similar, particularly during the early phase (Fig. 3). By 72 h, the yields for all viruses were similar, indicating that the insertion of CFTR delayed virus replication somewhat but had little effect on the final virus yield. Because the growth kinetics of rgRSV-CFTR10 were similar to those of rgRSV-CFTR12, we concluded that the insertion of the CFTR gene, not its location in the genome, was responsible for the delay in virus yield. We hypothesize that the 23% increase in the rgRSV genome length that results from the insertion of CFTR was causing a delay in virus replication, most likely due to the additional time needed for the RSV polymerase to traverse the longer genome, leading to a delay in virus yield.
FIG. 3.
Growth analysis of rgRSV-CFTR vectors. Shown are growth curves for rgRSV-CFTR10 and rgRSV-CFTR12 compared to parental rgRSV. Duplicate wells of HeLa cells were inoculated at an MOI of 0.1 with either rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12. Virus was harvested at the indicated times and titrated.
rgRSV can deliver CFTR to CF HAE cultures.
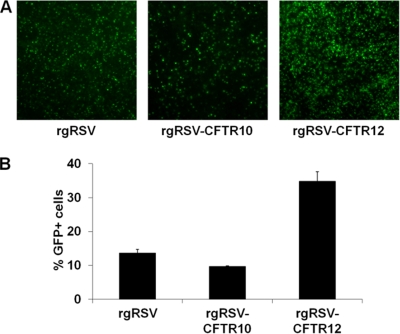
To determine whether RSV can deliver CFTR to well-differentiated airway cells, quadruplicate HAE cultures derived from airway tissue of CF patients (CF HAE) with a ΔF508 homozygous genotype were inoculated with 200 μl of rgRSV (2 × 105 PFU), rgRSV-CFTR10 (2 × 105 PFU), or rgRSV-CFTR12 (1 × 106 PFU) or mock inoculated. These inocula resulted in multiplicities of infection of ∼0.2 for rgRSV and rgRSV-CFTR10 and ∼1.0 for rgRSV-CFTR12 for ciliated cells in the cultures. At 24 h p.i., GFP-positive cells were visible in all infected cultures (Fig. 4 A). The transduction efficiencies for rgRSV, rgRSV-CFTR10, and rgRSV-CFTR12 were 14%, 10%, and 35%, respectively (Fig. 4B).
FIG. 4.
rgRSV-CFTR infection of CF HAE. Quadruplicate CF HAE cultures were inoculated with 2.0 × 105 PFU, 2.0 × 105 PFU, and 1.0 × 106 PFU of rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12, respectively. (A) At 24 h postinoculation, GFP expression was detectable in cultures infected with rgRSV, rgRSV-CFTR10, and rgRSV-CFTR12, indicating infection (representative images are shown). (B) The percentages of GFP-positive cells at 24 h p.i. were quantified, and the results are displayed as means and standard deviations. The results depicted are representative of two experiments, each including quadruplicate cultures for each experimental condition.
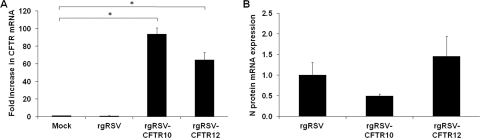
To quantify CFTR expression levels, we measured CFTR mRNA 48 h p.i. from the quadruplicate infected CF HAE cultures described above and compared it to endogenous CFTR mRNA from mock-infected cultures. Since the amounts of CFTR mRNA are the same in ΔF508 CF tissues and non-CF tissues, the endogenous levels of CFTR mRNA in these CF HAE cultures would be representative of that in non-CF cultures. Our data showed that the endogenous CFTR mRNA levels in these cultures were not altered by rgRSV infection. However, CF HAE inoculated with rgRSV-CFTR10 and rgRSV-CFTR12 had 94-fold and 64-fold higher levels of CFTR mRNA, respectively, compared to the levels in CF HAE inoculated with rgRSV or mock infected (Fig. 5 A). The relative levels of CFTR mRNA expression by these two vectors correlated more closely with the genome position of the CFTR gene and the amount of CFTR protein produced by each virus in HeLa cells (Fig. 2) than with the proportion of CF HAE transduced by each virus (Fig. 4B).
FIG. 5.
Expression of CFTR mRNA in CF HAE cultures infected with rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) or mock infected. Total mRNA was collected from infected and mock-infected cultures 48 h postinoculation (n = 4). (A) The amount of CFTR mRNA present in infected cultures was compared to that in mock-treated CF HAE cultures. (B) The amount of viral N protein mRNA present in cultures infected with the rgRSV-CFTR vectors was compared to that in rgRSV-infected cultures. All results are shown as means and standard deviations. The asterisks indicate a statistically significant difference (P value < 0.05) as determined by an unpaired Student t test. The results depicted are representative of two experiments, each including quadruplicate cultures for each experimental condition.
To correlate the amount of CFTR mRNA generated by the vectors with virus replication, we also quantified the relative levels of N protein mRNA in these cultures (Fig. 5B). RSV N protein mRNA levels correlated closely with infection efficiency (Fig. 4A), indicating similar amounts of N protein mRNA production and, therefore, similar levels of replication for each virus. These data suggest that the levels of replication of these viruses do not account for the difference in levels of CFTR mRNA expression. By comparison of the amount of CFTR mRNA with that of the N mRNA being expressed by each virus, the rgRSV-CFTR10 vector produced approximately 4-fold more CFTR mRNA per infected cell than the rgRSV-CFTR12 vector.
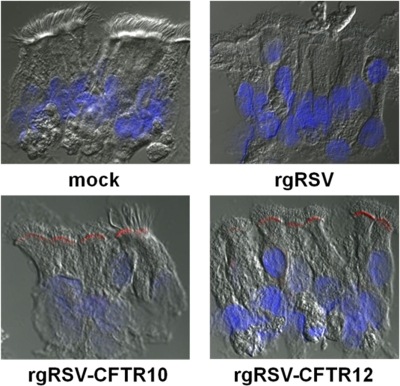
To determine whether the CFTR protein produced by rgRSV-CFTR10 and rgRSV-CFTR12 was correctly localized at the apical surfaces of the ciliated CF HAE cells, we immunoprobed CF HAE 48 h p.i. for CFTR protein. These studies confirmed that CFTR protein was produced in ciliated cells by both vectors and was detected at the apical surface (Fig. 6), thus recapitulating the localization of endogenous CFTR in non-CF ciliated HAE, as previously reported (20, 48).
FIG. 6.
CFTR expression and localization in rgRSV-CFTR-infected CF HAE cultures. At 48 h postinoculation, CF HAE cultures that were mock infected or infected with rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12, as indicated, were sectioned and stained for CFTR with MAb 596, followed by a goat anti-mouse Ig-Alexafluor 568-conjugated secondary antibody (red). Nuclei were stained with Hoechst 33342 (blue). Representative images are shown.
CFTR delivered to CF cells by rgRSV-CFTRs is functional.
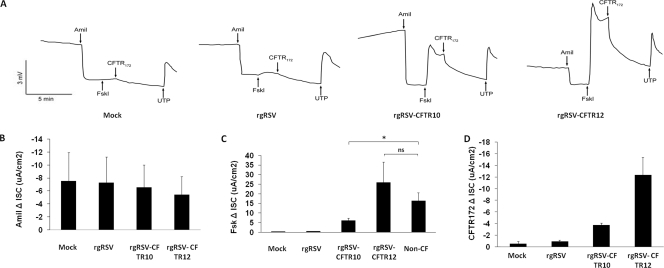
To determine whether the CFTR produced by the RSV vectors was functional as a Cl− channel, CF HAE cultures were inoculated with rgRSV, rgRSV-CFTR10, and rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) or were mock inoculated and assessed for ion channel activity in Ussing chambers. Two days after virus inoculations, cultures were mounted in Ussing chambers and ion transport was assessed using previously established methods (35). Briefly, electric current across the tissue (Isc) in response to applied electric pulses was measured, while a series of drugs was applied in the following order: amiloride, a potent inhibitor of Na+ ion channels (specifically, ENaCs), was used to prime the cells for maximal CFTR activity; forskolin, an activator of adenylate cyclase, was used to maximally stimulate CFTR channel activity; CFTR172, a potent inhibitor of CFTR, was added to confirm the specificity of any forskolin-induced change in Isc; and UTP, a stimulator of Ca2+-activated Cl− channels, was added to activate Cl− channels other than CFTR as a positive control. Representative traces from Ussing chamber experiments are shown in Fig. 7 A. CF HAE infected with rgRSV-CFTR12 exhibited a lower Isc before treatment with amiloride and therefore a reduced ΔIsc after amiloride treatment, suggesting that the Na+ channel hyperactivity exhibited by ENaCs in these cells was reduced somewhat by this treatment (Fig. 7A and B). However, due to the lack of other factors involved in the control of ENaC function, this trend will need to be tested further to determine if this decrease in ENaC hyperactivity is significant. CF HAE inoculated with rgRSV-CFTR10 and rgRSV-CFTR12, however, did show increases in Isc after forskolin treatment (Fig. 7A and C). These increases returned to baseline following treatment with CFTR172 (Fig. 7A and D), indicating that they can be attributed to the presence of functional CFTR channels provided by rgRSV-CFTRs. When the magnitudes of the forskolin-induced changes in Isc produced by rgRSV-CFTR10-, rgRSV-CFTR12-, and rgRSV-infected cells were quantified and compared in parallel experiments to that produced in non-CF HAE, infection with rgRSV-CFTR12 resulted in a 25.84 ± 10.76-μA/cm2 increase, which is not statistically different than that seen in non-CF HAE cultures (16.34 ± 4.26 μA/cm2) (Fig. 7C). Although infection with rgRSV-CFTR10 also resulted in increased forskolin-activated ion channel activity (6.12 ± 1.23 μA/cm2), the increase was not as great as that in non-CF HAE or CF HAE infected with rgRSV-CFTR12 (P values of 0.04 and 0.006, respectively). This suggested that full CFTR correction occurred in response to rgRSV-CFTR12, but not rgRSV-CFTR10 (which had been administered at a lower dose and infected fewer cells, as shown in Fig. 4).
FIG. 7.
Ion transport properties of CF HAE cultures following inoculation with rgRSV-CFTRs. CF HAE cultures were inoculated with rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) or were mock infected and were measured for ion transport properties in Ussing chambers at 48 h p.i. (A) Representative traces of the ISC responses (y axis) in mock-, rgRSV-, and rgRSV-CFTR-infected cultures in response to sequential treatment with amiloride (Amil) to inhibit ENaCs, forskolin (Fskl) to activate CFTR activity, CFTR172 to inhibit CFTR activity, and UTP to stimulate Ca2+-activated Cl− channels as a positive control. (B to D) Maximum changes in ISC (ΔISC) in response to individual treatments. (B) ΔISC of CF HAE after amiloride treatment. (C) Forskolin-induced ΔISC of CF HAE, indicating the presence of functional CFTR. (D) ΔISC of CF HAE following treatment with CFTR172. These results showed that the magnitude of forskolin-activated changes in CF HAE infected with the RSV-CFTRs was comparable to that in non-CF HAE (n = 7). All results are shown as means and standard deviations. “ns” indicates a difference that is not statistically significant (P value > 0.05). The asterisk indicates a difference that is statistically significant (P value < 0.05), as determined by an unpaired Student t test. The results depicted are representative of two experiments, each including quadruplicate cultures for each experimental condition.
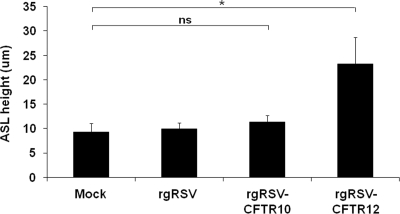
Treatments to measure ASL were performed on CF HAE cultures 48 h after the cultures were inoculated with rgRSV, rgRSV-CFTR10, and rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) or were mock inoculated, in order to determine if the RSV-supplied CFTR could improve the maintenance of ASL height. Texas Red-dextran-labeled PBS was applied to each culture, and the height of this overlay was measured 48 h later. In CF HAE that had been mock infected, over half of the fluid was absorbed. In contrast, cultures infected with rgRSV-CFTR12 were able to maintain their ASL height, indicating that the CFTR supplied by the rgRSV-CFTR12 vector was functional as an ion channel and could also perform its downstream role of equilibrating liquid absorption from the ASL.
DISCUSSION
Shortly after the identification of the gene encoding CFTR and the demonstration that mutations in this gene were responsible for CF, gene therapy approaches began to be explored for the treatment of CF lung disease, as this was the primary cause of morbidity in the CF patient. Clinical trials using viral vectors (AdV and AAV) and non-virus-based vectors (e.g., liposomes) to deliver CFTR cDNA to the airways of CF patients were determined to be safe but showed little efficacy due to poor transduction of the lumenal epithelial cells of the airways (4, 11, 28, 39, 62, 63, 69, 84). However, recently, significant changes have been made to AAV vectors to improve their targeting of the airway epithelium. It will be interesting to see if these new AAV vectors will result in increased ASL in CF airway cultures, as we have shown here for RSV, as we consider ASL measurements to be the optimal measurement for determining whether the CF phenotype has been corrected (24, 52, 53). The pseudotyping of lentiviruses and the creation of nonintegrating lentiviruses may also make them a more suitable vector for targeting the airway (3, 5, 12, 46, 70, 72, 86). We have chosen to take a different approach to vector construction by exploiting a natural respiratory virus, RSV, which has a natural tropism for the ciliated cells of the airway lumen. Ciliated cells express CFTR and likely dictate the majority of airway surface liquid volume regulation (20, 40, 48), suggesting that they are a logical cellular target for the replacement of CFTR function.
To test our hypothesis that RSV could deliver sufficient CFTR to ciliated cells to correct the CF bioelectric defect, we inserted the CFTR gene into four different positions in the RSV genome to produce different amounts of CFTR. All four viruses were rescued, but CFTR was expressed only from the two expected to produce the smallest amounts of CFTR, suggesting that higher levels of CFTR were cytotoxic. Overexpression of CFTR in fibroblasts has previously been shown to slow cellular proliferation and possibly to cause an unfolded-protein stress response, resulting in cell death (88). Overexpression of a protein by RSV is not, by itself, cytotoxic, since other transgenes, such as GFP, can be expressed at a high level from the first genome position without toxicity. rgRSV-CFTR10 and rgRSV-CFTR12 were passaged four times in HeLa cells without losing CFTR expression, indicating that they are genetically stable despite the inclusion of a large gene that is not necessary for virus replication.
Our data show that ∼3.5 times more ciliated cells in CF HAE were transduced with rgRSV-CFTR12 than with rgRSV-CFTR10, correlating roughly with the viral inoculum (Fig. 4) and with the amounts of RSV N protein mRNA produced (Fig. 5B). However, cultures infected with rgRSV-CFTR10 produced ∼5.3 times more CFTR mRNA than cultures infected with rgRSV-CFTR12 (Fig. 5A). From the N and CFTR mRNA measurements, we can extrapolate that the CFTR gene inserted into the 10th position of the RSV genome is transcribed ∼4 times more efficiently than from the 12th position. This result illustrates the ability to control the amount of transgene expression simply by changing the gene position in the RSV genome. Incidentally, the observation that there was only an ∼4-fold decrease in CFTR mRNA expression between the 10th and the 12th positions suggests that the presence of the very large 6.5-kb L gene before the CFTR gene in rgRSV-CFTR12 did not result in a high level of polymerase fall-off. During RSV infection, the relative molar amount of L mRNA that accumulates in infected cells is much smaller than that of the other RSV mRNAs, a difference that cannot be accounted for solely by the transcriptional gradient (19). One possibility was that this difference in mRNA concentration was due to intragenic polymerase fall-off during transcription of the long L gene. However, the present finding of only an ∼4-fold reduction in mRNA transcribed from a foreign gene inserted before the M2 gene and after the L gene indicates that this is not the case. Some other factor, possibly mRNA instability, may account for the unexpectedly low accumulation of L mRNA.
Both rgRSV-CFTR10 and rgRSV-CFTR12 produced functional CFTR, as measured by Cl− transport in Ussing chambers, but the magnitude of forskolin-mediated CFTR activation correlated closely with the number of infected cells rather than with the levels of total CFTR mRNA being produced. This result suggests that there is a limit to the CFTR activity per cell and that this limit had been reached by both vectors. One possible explanation for this limit on CFTR function in the presence of CFTR overexpression is that there are a limited number of CFTR membrane sites and, once these are occupied, no additional CFTR molecules are allowed in the membrane and no further effects on intracellular ion balance are possible. Thus, even though rgRSV-CFTR10 produced more CFTR per cell (Fig. 2A and 5A), rgRSV-CFTR12 caused greater overall CFTR function (Fig. 7C) because it was delivered to more cells in these particular experiments (Fig. 4). These results are consistent with previous findings indicating that, as long as CFTR is expressed above endogenous levels, it is more effective to deliver CFTR to more cells than to express a larger amount of CFTR in fewer cells (25, 30, 93).
At the MOI used in these experiments, rgRSV-CFTR10 infected 10% of the apical CF HAE (Fig. 4) and exhibited a cAMP-activated CFTR activity that was 37% of that in non-CF HAE (Fig. 7C). Similarly, rgRSV-CFTR12 infecting 35% of CF HAE generated a CFTR response that was statistically equivalent to that of non-CF cells, though numerically higher. In both cases, overexpression of CFTR in individual infected cells led to greater CFTR function per cell than in non-CF cells. With rgRSV-CFTR12, given its higher input MOI, we were able to target more than the suggested number of cells needed to reverse the chloride ion transport defect in the CF lung (2, 42, 60). Also, the degree of CFTR function provided by rgRSV-CFTR12 exceeds the amount that is thought to be needed to reverse many of the other defects in the CF lung, such as sodium hyperabsorption and mucus thickening (25, 93). Indeed, our results suggest that RSV expression of CFTR in CF HAE has some effect on ENaC function (Fig. 7B) and confirm that the ASL is maintained at a higher volume in cultures infected with rgRSV-CFTR12 (Fig. 8). In addition to correcting the ASL dehydration phenotype of CF HAE, rgRSV-CFTR12 also infected a percentage of cells in the cultures that exceeded the amount (35% versus 25%) speculated as being required for the correction of other defects (i.e., ciliary beat frequency and mucociliary transport efficiency), as shown in the PIVCFTR vector study (93).
FIG. 8.
ASL measurements of CF HAE 48 h after infection with rgRSV, rgRSV-CFTR10, or rgRSV-CFTR12 (2 × 105 PFU, 2 × 105 PFU, and 1 × 106 PFU, respectively) or mock infection. Each bar represents three measurements on each of four cultures. All results are shown as means and standard deviations. “ns” indicates a difference that is not statistically significant (P value > 0.05). The asterisk indicates a statistically significant difference (P value < 0.05), as determined by an unpaired Student t test.
Recently, Sendai virus, a paramyxovirus that naturally infects the airways of mice, was shown to express functional CFTR in vitro and in vivo in CFTR-deficient mice and was proposed as a gene therapy vector (26). Generally, when a virus that naturally infects one species, in this case mice, is used to infect another, in this case humans, it induces a powerful innate immune response because viral mechanisms of immune evasion often do not operate efficiently in a foreign host (10). In addition to inhibiting virus replication, stimulation of innate immunity also enhances the adaptive immune response. It is likely that Sendai virus would not be able to control the innate response in human cells, resulting in inhibition of virus replication and stimulation of adaptive immunity. On the other hand, such a response could be an advantage for a vaccine vector, and Sendai virus is currently being developed as a vaccine vector for multiple respiratory viruses (43, 73, 74, 80, 91). RSV suppresses the innate immune response in human cells via its two unique nonstructural proteins, NS1 and NS2. These proteins attenuate interferon induction, as well as the antiviral response caused by interferon (23, 55, 64, 75). The reduced interferon response to RSV infection may also play a role in dampening the adaptive immune response to RSV (22, 50).
RSV infection does elicit a muted adaptive host immune response that allows repeated infections throughout life without the need for significant antigenic change (8, 37, 71), suggesting that an RSV-based gene therapy vector might be successfully readministered. In one study, 7 of 15 individuals could be infected three or more times over a 26-month period, as detected by shed virus or a rise in RSV antibody titer. Furthermore, infection can occur without virus shedding, as shown recently for another paramyxovirus, PIV3, in nonhuman primates (13). In this case, shed virus may have been neutralized by antibody, perhaps as the result of a memory response. Repeat infections with RSV are usually limited to the upper respiratory tract, suggesting that the level of preexisting antibody is not high enough to be protective but that the memory immune response is successful at preventing the spread of RSV from the upper to the lower respiratory tract. However, a vector delivered by aerosol could be distributed throughout the lung simultaneously (6, 51), enabling vector infection before a memory response could be mounted. Furthermore, recent in vivo data have shown that while previous exposure to RSV does not prevent reinfection, it instead causes the challenge infection to become abortive: RSV enters cells and replicates but fails to generate infectious progeny virions (9), perhaps because they are neutralized by an antibody recall response. This situation might be ideal for a gene transfer vector, reducing the likelihood of cell-to-cell spread and disease symptoms.
In addition, through efforts to develop RSV vaccines, mutations and gene deletions that attenuate the virus have been identified (21, 44, 45), some of which could be incorporated into RSV-CFTR vectors to reduce or eliminate pathogenesis. The observation that CFTR expression from RSV is robust, and possibly too robust from the second and sixth positions, indicates that substantial reductions in overall RSV gene expression could be accommodated while maintaining adequate levels of CFTR expression to correct ciliated-cell defects. This suggests that an RSV vector could be attenuated without the loss of sufficient CFTR expression. Attenuation could also reduce the immunogenicity of the infecting virus, as has been found in some clinical trials with live attenuated vaccine candidates, and in turn may facilitate reinfection. Further studies in applicable animal models will need to be performed to determine the immune response to this vector, as well as its persistence and its ability to infect upon readministration. Newly developed models of CF lung disease in the pig and ferret may provide animal models for testing an RSV-based vector due to their ability to be infected with RSV and their similarities in lung physiology to humans (15, 17, 27, 65, 66, 79).
Collectively, our data support further investigation of RSV as a vector for CF gene therapy. RSV infects the ciliated cells of both non-CF and CF HAE cultures, thus delivering CFTR to the appropriate target. rgRSV-delivered CFTR is expressed correctly at the apical surface and is functional at a level that recapitulates the Cl− channel activity observed in normal HAE cultures. rgRSV-expressed CFTR also improves ASL dehydration in CF HAE cultures, thus correcting a hallmark defect of CF lung disease. The ability to incorporate transgenes into RSV at various positions to control their expression level and the immune evasion properties of RSV, as well as the potential for generating less-cytopathic RSV vectors, further strengthen the potential of RSV as a gene therapy vector for CF and for other diseases affecting the airways.
Acknowledgments
This work was funded by National Institutes of Health PO1 HL051818 (M.E.P. and R.J.P.) and NIH Molecular Therapy Core Center P30 DK065988-01 (R.J.P.). A.R.K. was supported by a graduate fellowship from the General Mason Scholarship Fund of The Research Institute at Nationwide Children's Hospital. P.L.C. was supported by the Intramural Program of NIAID, NIH.
We thank Rachel Fearns for the D53-BsiWI construct; Arnold Hampel for the 5′ ribozyme; Naina Barretto for some of the construction of RW30; Apath, LLC, for support in making other components of RW30; John Riordan at UNC for the MAb to CFTR; Barb Newton, Steve Kwilas, and Arife Unal for their help in vector construction; and the directors and teams of the UNC Cystic Fibrosis Center Tissue Culture Core, the Morphology and Morphometry Core, and the Correction Core-EPA Clinical Proteomics Core for supplying reagents and technical expertise.
Footnotes
Published ahead of print on 26 May 2010.
REFERENCES
- 1.Altschuler, M., R. Tritz, and A. Hampel. 1992. A method for generating transcripts with defined 5′ and 3′ termini by autolytic processing. Gene 122:85-90. [DOI] [PubMed] [Google Scholar]
- 2.Amaral, M. D. 2005. Processing of CFTR: traversing the cellular maze—how much CFTR needs to go through to avoid cystic fibrosis? Pediatr. Pulmonol. 39:479-491. [DOI] [PubMed] [Google Scholar]
- 3.Apolonia, L., S. N. Waddington, C. Fernandes, N. J. Ward, G. Bouma, M. P. Blundell, A. J. Thrasher, M. K. Collins, and N. J. Philpott. 2007. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol. Ther. 15:1947-1954. [DOI] [PubMed] [Google Scholar]
- 4.Baatz, J. E., Y. Zou, and T. R. Korfhagen. 2001. Inhibitory effects of tumor necrosis factor-alpha on cationic lipid-mediated gene delivery to airway cells in vitro. Biochim. Biophys. Acta 1535:100-109. [DOI] [PubMed] [Google Scholar]
- 5.Banasik, M. B., and P. B. McCray, Jr. 2010. Integrase-defective lentiviral vectors: progress and applications. Gene Ther. 17:150-157. [DOI] [PubMed] [Google Scholar]
- 6.Beck, S. E., B. L. Laube, C. I. Barberena, A. C. Fischer, R. J. Adams, K. Chesnut, T. R. Flotte, and W. B. Guggino. 2002. Deposition and expression of aerosolized rAAV vectors in the lungs of Rhesus macaques. Mol. Ther. 6:546-554. [DOI] [PubMed] [Google Scholar]
- 7.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 8.Bont, L., J. Versteegh, W. T. Swelsen, C. J. Heijnen, A. Kavelaars, F. Brus, J. M. Draaisma, M. Pekelharing-Berghuis, R. A. van Diemen-Steenvoorde, and J. L. Kimpen. 2002. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr. Res. 52:363-367. [DOI] [PubMed] [Google Scholar]
- 9.Boukhvalova, M. S., G. A. Prince, and J. C. Blanco. 2007. Respiratory syncytial virus infects and abortively replicates in the lungs in spite of preexisting immunity. J. Virol. 81:9443-9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bousse, T., R. L. Chambers, R. A. Scroggs, A. Portner, and T. Takimoto. 2006. Human parainfluenza virus type 1 but not Sendai virus replicates in human respiratory cells despite IFN treatment. Virus Res. 121:23-32. [DOI] [PubMed] [Google Scholar]
- 11.Bragonzi, A., and M. Conese. 2002. Non-viral approach toward gene therapy of cystic fibrosis lung disease. Curr. Gene Ther. 2:295-305. [DOI] [PubMed] [Google Scholar]
- 12.Buckley, S. M., S. J. Howe, V. Sheard, N. J. Ward, C. Coutelle, A. J. Thrasher, S. N. Waddington, and T. R. McKay. 2008. Lentiviral transduction of the murine lung provides efficient pseudotype and developmental stage-dependent cell-specific transgene expression. Gene Ther. 15:1167-1175. [DOI] [PubMed] [Google Scholar]
- 13.Bukreyev, A. A., J. M. Dinapoli, L. Yang, B. R. Murphy, and P. L. Collins. 2010. Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology 399:290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrappa, S., D. K. Gavin, and K. C. Gupta. 1995. A highly efficient procedure for site-specific mutagenesis of full-length plasmids using Vent DNA polymerase. Genome Res. 5:404-407. [DOI] [PubMed] [Google Scholar]
- 15.Byrd, L. G., and G. A. Prince. 1997. Animal models of respiratory syncytial virus infection. Clin. Infect. Dis. 25:1363-1368. [DOI] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.Clyde, W. A., Jr. 1980. Experimental models for study of common respiratory viruses. Environ. Health Perspect. 35:107-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins, P. L., M. G. Hill, E. Camargo, H. Grosfeld, R. M. Chanock, and B. R. Murphy. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. U. S. A. 92:11563-11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins, P. L., and G. W. Wertz. 1983. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc. Natl. Acad. Sci. U. S. A. 80:3208-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford, I., P. C. Maloney, P. L. Zeitlin, W. B. Guggino, S. C. Hyde, H. Turley, K. C. Gatter, A. Harris, and C. F. Higgins. 1991. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc. Natl. Acad. Sci. U. S. A. 88:9262-9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowe, J. E., Jr. 2001. Respiratory syncytial virus vaccine development. Vaccine 20(Suppl. 1):S32-S237. [DOI] [PubMed] [Google Scholar]
- 22.Durbin, J. E., and R. K. Durbin. 2004. Respiratory syncytial virus-induced immunoprotection and immunopathology. Viral Immunol. 17:370-380. [DOI] [PubMed] [Google Scholar]
- 23.Elliott, J., O. T. Lynch, Y. Suessmuth, P. Qian, C. R. Boyd, J. F. Burrows, R. Buick, N. J. Stevenson, O. Touzelet, M. Gadina, U. F. Power, and J. A. Johnston. 2007. Respiratory syncytial virus NS1 protein degrades STAT2 by using the Elongin-Cullin E3 ligase. J. Virol. 81:3428-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Excoffon, K. J., J. T. Koerber, D. D. Dickey, M. Murtha, S. Keshavjee, B. K. Kaspar, J. Zabner, and D. V. Schaffer. 2009. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc. Natl. Acad. Sci. U. S. A. 106:3865-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farmen, S. L., P. H. Karp, P. Ng, D. J. Palmer, D. R. Koehler, J. Hu, A. L. Beaudet, J. Zabner, and M. J. Welsh. 2005. Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl- transport and overexpression can generate basolateral CFTR. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L1123-L1130. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari, S., U. Griesenbach, A. Iida, R. Farley, A. M. Wright, J. Zhu, F. M. Munkonge, S. N. Smith, J. You, H. Ban, M. Inoue, M. Chan, C. Singh, B. Verdon, B. E. Argent, B. Wainwright, P. K. Jeffery, D. M. Geddes, D. J. Porteous, S. C. Hyde, M. A. Gray, M. Hasegawa, and E. W. Alton. 2007. Sendai virus-mediated CFTR gene transfer to the airway epithelium. Gene Ther. 14:1371-1379. [DOI] [PubMed] [Google Scholar]
- 27.Fishaut, M., J. D. Schwartzman, K. McIntosh, and S. R. Mostow. 1978. Behavior of respiratory syncytial virus in piglet tracheal organ culture. J. Infect. Dis. 138:644-649. [DOI] [PubMed] [Google Scholar]
- 28.Flotte, T. R. 2007. Gene therapy: the first two decades and the current state-of-the-art. J. Cell. Physiol. 213:301-305. [DOI] [PubMed] [Google Scholar]
- 29.Flotte, T. R., S. A. Afione, C. Conrad, S. A. McGrath, R. Solow, H. Oka, P. L. Zeitlin, W. B. Guggino, and B. J. Carter. 1993. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. U. S. A. 90:10613-10617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flotte, T. R., E. M. Schwiebert, P. L. Zeitlin, B. J. Carter, and W. B. Guggino. 2005. Correlation between DNA transfer and cystic fibrosis airway epithelial cell correction after recombinant adeno-associated virus serotype 2 gene therapy. Hum. Gene Ther. 16:921-928. [DOI] [PubMed] [Google Scholar]
- 31.Fulcher, M. L., S. Gabriel, K. A. Burns, J. R. Yankaskas, and S. H. Randell. 2005. Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 107:183-206. [DOI] [PubMed] [Google Scholar]
- 32.Garcia, D. F., P. W. Hiatt, A. Jewell, S. L. Schoonover, S. G. Cron, M. Riggs, S. Grace, C. M. Oermann, and P. A. Piedra. 2007. Human metapneumovirus and respiratory syncytial virus infections in older children with cystic fibrosis. Pediatr. Pulmonol. 42:66-74. [DOI] [PubMed] [Google Scholar]
- 33.Goldman, M. J., P. S. Lee, J. S. Yang, and J. M. Wilson. 1997. Lentiviral vectors for gene therapy of cystic fibrosis. Hum. Gene Ther. 8:2261-2268. [DOI] [PubMed] [Google Scholar]
- 34.Grubb, B. R., R. J. Pickles, H. Ye, J. R. Yankaskas, R. N. Vick, J. F. Engelhardt, J. M. Wilson, L. G. Johnson, and R. C. Boucher. 1994. Inefficient gene transfer by adenovirus vector to cystic fibrosis airway epithelia of mice and humans. Nature 371:802-806. [DOI] [PubMed] [Google Scholar]
- 35.Grubb, B. R., T. D. Rogers, P. C. Diggs, R. C. Boucher, and L. E. Ostrowski. 2006. Culture of murine nasal epithelia: model for cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L270-7. [DOI] [PubMed] [Google Scholar]
- 36.Halbert, C. L., J. M. Allen, and A. D. Miller. 2001. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J. Virol. 75:6615-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall, C. B., E. E. Walsh, C. E. Long, and K. C. Schnabel. 1991. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 163:693-698. [DOI] [PubMed] [Google Scholar]
- 38.Hallak, L., D. Spillman, P. L. Collins, and M. E. Peeples. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 74:10508-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvey, B. G., P. L. Leopold, N. R. Hackett, T. M. Grasso, P. M. Williams, A. L. Tucker, R. J. Kaner, B. Ferris, I. Gonda, T. D. Sweeney, R. Ramalingam, I. Kovesdi, S. Shak, and R. G. Crystal. 1999. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J. Clin. Invest. 104:1245-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang, Q., and J. F. Engelhardt. 1998. Cellular heterogeneity of CFTR expression and function in the lung: implications for gene therapy of cystic fibrosis. Eur. J. Hum. Genet. 6:12-31. [DOI] [PubMed] [Google Scholar]
- 41.Johnson, L. G., J. C. Olsen, L. Naldini, and R. C. Boucher. 2000. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 7:568-574. [DOI] [PubMed] [Google Scholar]
- 42.Johnson, L. G., J. C. Olsen, B. Sarkadi, K. L. Moore, R. Swanstrom, and R. C. Boucher. 1992. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 2:21-25. [DOI] [PubMed] [Google Scholar]
- 43.Jones, B., X. Zhan, V. Mishin, K. S. Slobod, S. Surman, C. J. Russell, A. Portner, and J. L. Hurwitz. 2009. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine 27:1848-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karron, R. A., P. F. Wright, R. B. Belshe, B. Thumar, R. Casey, F. Newman, F. P. Polack, V. B. Randolph, A. Deatly, J. Hackell, W. Gruber, B. R. Murphy, and P. L. Collins. 2005. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J. Infect. Dis. 191:1093-1104. [DOI] [PubMed] [Google Scholar]
- 45.Karron, R. A., P. F. Wright, J. E. Crowe, Jr., M. L. Clements-Mann, J. Thompson, M. Makhene, R. Casey, and B. R. Murphy. 1997. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. J. Infect. Dis. 176:1428-1436. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi, M., A. Iida, Y. Ueda, and M. Hasegawa. 2003. Pseudotyped lentivirus vectors derived from simian immunodeficiency virus SIVagm with envelope glycoproteins from paramyxovirus. J. Virol. 77:2607-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kosorok, M. R., W. H. Wei, and P. M. Farrell. 1996. The incidence of cystic fibrosis. Stat. Med. 15:449-462. [DOI] [PubMed] [Google Scholar]
- 48.Kreda, S. M., M. Mall, A. Mengos, L. Rochelle, J. Yankaskas, J. R. Riordan, and R. C. Boucher. 2005. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol. Biol. Cell 16:2154-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 50.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461-470. [DOI] [PubMed] [Google Scholar]
- 51.Leung, K., E. Louca, K. Munson, B. Dutzar, P. Anklesaria, and A. L. Coates. 2007. Calculating expected lung deposition of aerosolized administration of AAV vector in human clinical studies. J. Gene Med. 9:10-21. [DOI] [PubMed] [Google Scholar]
- 52.Li, W., L. Zhang, J. S. Johnson, W. Zhijian, J. C. Grieger, X. Ping-Jie, L. M. Drouin, M. Agbandje-McKenna, R. J. Pickles, and R. J. Samulski. 2009. Generation of novel AAV variants by directed evolution for improved CFTR delivery to human ciliated airway epithelium. Mol. Ther. 17:2067-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Limberis, M. P., L. H. Vandenberghe, L. Zhang, R. J. Pickles, and J. M. Wilson. 2009. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol. Ther. 17:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Limberis, M. P., and J. M. Wilson. 2006. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. U. S. A. 103:12993-12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo, M. S., R. M. Brazas, and M. J. Holtzman. 2005. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and alpha/beta interferon responsiveness. J. Virol. 79:9315-9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma, T., J. R. Thiagarajah, H. Yang, N. D. Sonawane, C. Folli, L. J. Galietta, and A. S. Verkman. 2002. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 110:1651-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pauwels, K., R. Gijsbers, J. Toelen, A. Schambach, K. Willard-Gallo, C. Verheust, Z. Debyser, and P. Herman. 2009. State-of-the-art lentiviral vectors for research use: risk assessment and biosafety recommendations. Curr. Gene Ther. 9:459-474. [DOI] [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.Perrotta, A. T., and M. D. Been. 1991. A pseudoknot-like structure required for efficient self-cleavage of hepatitis delta virus RNA. Nature 350:434-436. [DOI] [PubMed] [Google Scholar]
- 60.Pickles, R. J. 2004. Physical and biological barriers to viral vector-mediated delivery of genes to the airway epithelium. Proc. Am. Thorac. Soc. 1:302-308. [DOI] [PubMed] [Google Scholar]
- 61.Pickles, R. J. 1997. Towards a gene therapy for cystic fibrosis lung disease. Exp. Opin. Invest. Drugs 6:1459-1463. [DOI] [PubMed] [Google Scholar]
- 62.Pickles, R. J., D. McCarty, H. Matsui, P. J. Hart, S. H. Randell, and R. C. Boucher. 1998. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 72:6014-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porteous, D. J., J. R. Dorin, G. McLachlan, H. Davidson-Smith, H. Davidson, B. J. Stevenson, A. D. Carothers, W. A. Wallace, S. Moralee, C. Hoenes, G. Kallmeyer, U. Michaelis, K. Naujoks, L. P. Ho, J. M. Samways, M. Imrie, A. P. Greening, and J. A. Innes. 1997. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene Ther. 4:210-218. [DOI] [PubMed] [Google Scholar]
- 64.Ramaswamy, M., L. Shi, S. M. Varga, S. Barik, M. A. Behlke, and D. C. Look. 2006. Respiratory syncytial virus nonstructural protein 2 specifically inhibits type I interferon signal transduction. Virology 344:328-339. [DOI] [PubMed] [Google Scholar]
- 65.Rogers, C. S., Y. Hao, T. Rokhlina, M. Samuel, D. A. Stoltz, Y. Li, E. Petroff, D. W. Vermeer, A. C. Kabel, Z. Yan, L. Spate, D. Wax, C. N. Murphy, A. Rieke, K. Whitworth, M. L. Linville, S. W. Korte, J. F. Engelhardt, M. J. Welsh, and R. S. Prather. 2008. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J. Clin. Invest. 118:1571-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogers, C. S., D. A. Stoltz, D. K. Meyerholz, L. S. Ostedgaard, T. Rokhlina, P. J. Taft, M. P. Rogan, A. A. Pezzulo, P. H. Karp, O. A. Itani, A. C. Kabel, C. L. Wohlford-Lenane, G. J. Davis, R. A. Hanfland, T. L. Smith, M. Samuel, D. Wax, C. N. Murphy, A. Rieke, K. Whitworth, A. Uc, T. D. Starner, K. A. Brogden, J. Shilyansky, P. B. McCray, Jr., J. Zabner, R. S. Prather, and M. J. Welsh. 2008. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321:1837-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenfeld, M. A., K. Yoshimura, B. C. Trapnell, K. Yoneyama, E. R. Rosenthal, W. Dalemans, M. Fukayama, J. Bargon, L. E. Stier, L. Stratford-Perricaudet, et al. 1992. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell 68:143-155. [DOI] [PubMed] [Google Scholar]
- 68.Rowe, S. M., S. Miller, and E. J. Sorscher. 2005. Cystic fibrosis. N. Engl. J. Med. 352:1992-2001. [DOI] [PubMed] [Google Scholar]
- 69.Ruiz, F. E., J. P. Clancy, M. A. Perricone, Z. Bebok, J. S. Hong, S. H. Cheng, D. P. Meeker, K. R. Young, R. A. Schoumacher, M. R. Weatherly, L. Wing, J. E. Morris, L. Sindel, M. Rosenberg, F. W. van Ginkel, J. R. McGhee, D. Kelly, R. K. Lyrene, and E. J. Sorscher. 2001. A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis. Hum. Gene Ther. 12:751-761. [DOI] [PubMed] [Google Scholar]
- 70.Sarkis, C., S. Philippe, J. Mallet, and C. Serguera. 2008. Non-integrating lentiviral vectors. Curr. Gene Ther. 8:430-437. [DOI] [PubMed] [Google Scholar]
- 71.Singleton, R., N. Etchart, S. Hou, and L. Hyland. 2003. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J. Virol. 77:11303-11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinn, P. L., E. R. Burnight, M. A. Hickey, G. W. Blissard, and P. B. McCray, Jr. 2005. Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer. J. Virol. 79:12818-12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Skiadopoulos, M. H., S. R. Surman, J. M. Riggs, W. R. Elkins, M. St Claire, M. Nishio, D. Garcin, D. Kolakofsky, P. L. Collins, and B. R. Murphy. 2002. Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology 297:153-160. [DOI] [PubMed] [Google Scholar]
- 74.Slobod, K. S., J. L. Shenep, J. Lujan-Zilbermann, K. Allison, B. Brown, R. A. Scroggs, A. Portner, C. Coleclough, and J. L. Hurwitz. 2004. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 22:3182-3186. [DOI] [PubMed] [Google Scholar]
- 75.Spann, K. M., K. C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stocker, A. G., K. L. Kremer, R. Koldej, D. S. Miller, D. S. Anson, and D. W. Parsons. 2009. Single-dose lentiviral gene transfer for lifetime airway gene expression. J. Gene Med. 11:861-867. [DOI] [PubMed] [Google Scholar]
- 77.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sumner-Jones, S. G., L. A. Davies, A. Varathalingam, D. R. Gill, and S. C. Hyde. 2006. Long-term persistence of gene expression from adeno-associated virus serotype 5 in the mouse airways. Gene Ther. 13:1703-1713. [DOI] [PubMed] [Google Scholar]
- 79.Sun, X., Z. Yan, Y. Yi, Z. Li, D. Lei, C. S. Rogers, J. Chen, Y. Zhang, M. J. Welsh, G. H. Leno, and J. F. Engelhardt. 2008. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J. Clin. Invest. 118:1578-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takimoto, T., J. L. Hurwitz, C. Coleclough, C. Prouser, S. Krishnamurthy, X. Zhan, K. Boyd, R. A. Scroggs, B. Brown, Y. Nagai, A. Portner, and K. S. Slobod. 2004. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J. Virol. 78:6043-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reference deleted.
- 82.Tarran, R., and R. C. Boucher. 2002. Thin-film measurements of airway surface liquid volume/composition and mucus transport rates in vitro. Methods Mol. Med. 70:479-492. [DOI] [PubMed] [Google Scholar]
- 83.Techaarpornkul, S., N. Barretto, and M. E. Peeples. 2001. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J. Virol. 75:6825-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teramoto, S., J. S. Bartlett, D. McCarty, X. Xiao, R. J. Samulski, and R. C. Boucher. 1998. Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors. J. Virol. 72:8904-8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang, G., V. Slepushkin, J. Zabner, S. Keshavjee, J. C. Johnston, S. L. Sauter, D. J. Jolly, T. W. Dubensky, Jr., B. L. Davidson, and P. B. McCray, Jr. 1999. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J. Clin. Invest. 104:R55-R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wanisch, K., and R. J. Yanez-Munoz. 2009. Integration-deficient lentiviral vectors: a slow coming of age. Mol. Ther. 17:1316-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wat, D., and I. Doull. 2003. Respiratory virus infections in cystic fibrosis. Paediatr. Respir. Rev. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 88.Wei, L. Y., M. J. Stutts, M. M. Hoffman, and P. D. Roepe. 1995. Overexpression of the cystic fibrosis transmembrane conductance regulator in NIH 3T3 cells lowers membrane potential and intracellular pH and confers a multidrug resistance phenotype. Biophys. J. 69:883-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zabner, J., M. Seiler, R. Walters, R. M. Kotin, W. Fulgeras, B. L. Davidson, and J. A. Chiorini. 2000. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 74:3852-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zaiss, A. K., and D. A. Muruve. 2005. Immune responses to adeno-associated virus vectors. Curr. Gene Ther. 5:323-331. [DOI] [PubMed] [Google Scholar]
- 91.Zhan, X., K. S. Slobod, S. Krishnamurthy, L. E. Luque, T. Takimoto, B. Jones, S. Surman, C. J. Russell, A. Portner, and J. L. Hurwitz. 2008. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine 26:3480-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang, L., A. Bukreyev, C. I. Thompson, B. Watson, M. E. Peeples, P. L. Collins, and R. J. Pickles. 2005. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 79:1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang, L., B. Button, S. E. Gabriel, S. Burkett, Y. Yan, M. H. Skiadopoulos, Y. L. Dang, L. N. Vogel, T. McKay, A. Mengos, R. C. Boucher, P. L. Collins, and R. J. Pickles. 2009. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 7:e1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang, L., M. E. Peeples, R. C. Boucher, P. L. Collins, and R. J. Pickles. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 76:5654-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]