Abstract
Influenza virus-like particles (VLPs) are a promising cell culture-based vaccine, and the skin is considered an attractive immunization site. In this study, we examined the immunogenicity and protective efficacy of influenza VLPs (H1N1 A/PR/8/34) after skin vaccination using vaccine dried on solid microneedle arrays. Coating of microneedles with influenza VLPs using an unstabilized formulation was found to decrease hemagglutinin (HA) activity, whereas inclusion of trehalose disaccharide preserved the HA activity of influenza VLP vaccines after microneedles were coated. Microneedle vaccination of mice in the skin with a single dose of stabilized influenza VLPs induced 100% protection against challenge infection with a high lethal dose. In contrast, unstabilized influenza VLPs, as well as intramuscularly injected vaccines, provided inferior immunity and only partial protection (≤40%). The stabilized microneedle vaccination group showed IgG2a levels that were 1 order of magnitude higher than those of other groups and had the lowest lung viral titers after challenge. Also, levels of recall immune responses, including hemagglutination inhibition titers, neutralizing antibodies, and antibody-secreting plasma cells, were significantly higher after skin vaccination with stabilized formulations. Therefore, our results indicate that HA stabilization, combined with vaccination via the skin using a vaccine formulated as a solid microneedle patch, confers protection superior to that with intramuscular injection and enables potential dose-sparing effects which are reflected by pronounced increases in rapid recall immune responses against influenza virus.
Influenza is a major health threat among infectious diseases, posing a significant burden for public health worldwide. Over 200,000 hospitalizations and approximately 36,000 deaths are estimated to occur annually in the United States alone (48, 49). Vaccination is the most cost-effective measure for controlling influenza. However, the influenza vaccine needs to be updated and manufactured every year due to changes in circulating viral strains. Current influenza vaccines rely on egg substrate-based production, a lengthy process with limited capacity that can cause shortages in available vaccine supplies. The recent 2009 outbreak of H1N1 influenza virus is a good example of the urgent need to develop a more effective vaccine platform and vaccination method (38).
Influenza virus-like particles (VLPs) have been suggested as a promising alternative candidate to current influenza vaccines. Influenza VLPs are noninfectious particles that mimic the virus in structure and morphology, can be produced using an egg-free cell culture system, and have been shown to be highly immunogenic, inducing protective immunity (9, 15, 19, 27, 35, 41, 42, 44). Most current vaccines are administered intramuscularly to humans in liquid formulations using hypodermic needles or syringes. Another strategy to meet the potential need for mass vaccination would be to develop an effective method for vaccine delivery to the skin (4, 8, 32, 50, 52). The skin is considered an important peripheral immune organ rich in potent immune-inducing cells, including Langerhans cells (LCs), dermal dendritic cells (DCs), and keratinocytes (5, 13, 14, 22). LCs and DCs residing in the epidermal and dermal layers of the skin have been shown to play an important role in antigen processing and presentation following skin immunization (1, 13, 14, 22). Intradermal (ID) vaccination delivering antigens to the dermal layer of the skin has been performed in many clinical studies and have demonstrated dose-sparing effects in some cases (4, 28, 29). Particularly, ID delivery of vaccines might be more effective in the elderly population (50), the highest risk group for influenza epidemics (49). However, ID delivery of vaccines using hypodermic needles is painful and needs highly trained medical personnel. In addition, more frequent local reactions at the injection site were observed after ID delivery. Therefore, a simple and effective approach for vaccination without using hypodermic needles would be highly desirable.
To overcome the skin barrier of the outer layer of stratum corneum, solid microneedles were previously coated with inactivated influenza viruses and used to successfully deliver vaccines to the skin, which provided protection comparable to that with conventional intramuscular immunizations (32, 52). Other vaccines have also been delivered using microneedles (17, 17a), but VLPs have never been used this way before. Delivery of a powdered form of inactivated influenza vaccines to the skin has also been demonstrated using a high-speed jet delivery device (10). These previous studies used high doses of vaccines, possibly due to the instability of vaccines in dry formulations.
Influenza hemagglutinin (HA) is responsible for attachment of the virus to sialic acid-containing receptors on target cells. However, it is not well understood how functional activity of HA affects the immunogenicity of influenza VLP vaccines. For the first time in this study, we investigated the effect of HA stability, immune responses, and protective efficacies of solid-microneedle VLP vaccines containing H1 HA as a major influenza viral component after delivery to the skin in comparison to results with intramuscular immunization. We found that the functional integrity of HA in influenza VLPs significantly influenced the immunological and protective outcomes for both microneedle and intramuscular vaccination. In addition, we have observed differential outcomes contributing to the protective immunity by the delivery of HA-stabilized VLPs to the skin in terms of the types of immune responses, recall antibody responses, and viral clearance at an early time point after challenge compared to those induced by intramuscular immunization.
MATERIALS AND METHODS
Virus and cells.
Influenza virus A/PR/8/1934 (H1N1; abbreviated as A/PR8) was grown in 10-day-old embryonated hen's eggs for 2.5 days at 36 to 37°C. Allantoic fluid was harvested from infected eggs after being stored overnight at 4°C and centrifuged to remove cell debris. The virus was purified from allantoic fluid by using a discontinuous sucrose gradient (15%, 30% and 60% layers) and ultracentrifugation (at 28,000 rpm for 60 min). The purified virus was inactivated by mixing the virus with formalin at a final concentration of 1:4,000 (vol/vol) as described previously (44). For use in challenge experiments, mouse-adapted A/PR8 was prepared as lung homogenates of infected mice. Spodoptera frugiperda Sf9 cells were maintained in suspension in serum-free SF900II medium (Gibco-BRL). MDCK cells were grown and maintained in Dulbecco's modified Eagle's medium (DMEM).
Preparation of influenza VLPs and microneedle vaccines.
Influenza VLPs containing HA and M1 proteins derived from A/PR8 were prepared as described previously (44). Briefly, Sf9 insect cells were coinfected with recombinant baculoviruses (BVs) expressing HA and M1 at multiplicities of infection of 2 and 1, respectively. Since insect cells rarely add sialic acids to N-glycans during posttranslational modification (33), VLPs containing HA without neuraminidase expression are effectively released from the insect cell surfaces, as demonstrated in previous studies (15, 44). The purified VLPs were characterized by Coomassie blue staining, Western blotting, and hemagglutination activity analysis, as shown in Fig. 1. The content of HA was approximately 10% of total proteins of influenza VLPs, which is similar to that found in a previous report (46). Microneedle preparations and coatings were performed as described previously (17, 32, 52). Influenza VLPs (0.35 μg of total protein) were coated onto a microneedle array with five needles in the presence or absence of a trehalose disaccharide stabilizer (15%, wt/vol; Sigma Aldrich) (30). Microneedles coated with VLP vaccines were either used directly to vaccinate animals or dissolved in phosphate-buffered saline (PBS) to reconstitute VLPs from microneedles for characterization of the VLP vaccines and to serve as controls for intramuscular immunization. Unprocessed influenza VLPs were also used intramuscularly as intact vaccine controls (IM-intact). Finally, mock vaccination was carried out with microneedles using a coating solution without VLP vaccine.
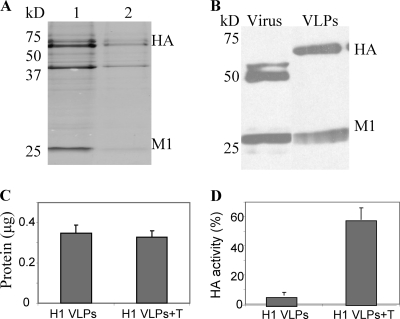
FIG. 1.
Characterization of influenza VLPs and coating onto microneedles. (A) Coomassie blue staining of influenza VLPs. Influenza VLP proteins (total protein of VLPs in lane 1, 10 μg; lane 2, 5 μg) were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and the gel was stained with Coomassie blue. The second major band between the molecular masses of 37 kDa and 50 kDa indicates unidentified insect cell or baculovirus-derived protein. (B) Influenza HA and M1 proteins on Western blot. The equivalent amount (5 μg of protein) of inactivated A/PR8/1934 virus (lane 1) and VLPs (lane 2) were run on an SDS-PAGE gel, and influenza proteins (HA and M1) were visualized by blotting with mouse anti-A/PR8/1934 virus serum. (C) Determination of total protein in influenza VLPs dissolved from coated microneedles. Equivalent amounts of influenza VLPs were coated onto microneedles in the presence of trehalose (H1 VLPs +T) or absence of trehalose (H1 VLPs). (D) Hemagglutination activities in influenza VLP vaccines dissolved from coated microneedles without trehalose (H1 VLPs) or with trehalose (H1 VLPs+T).
Immunization and challenge infection.
Female inbred BALB/c mice (Charles River) aged 6 to 8 weeks were used. Groups of mice (12 mice per group) were immunized with a microneedle array coated with VLP vaccine (0.35 μg of total VLP protein) for delivery to the skin or intramuscularly immunized with reconstituted vaccine (0.35 μg/100 μl) for intramuscular injection in the upper quadriceps muscles (each leg with 50 μl). The four groups of immunized mice were as follows: microneedle vaccine groups without trehalose (MN) and with trehalose (MN+T) and groups receiving intramuscular immunization of reconstituted vaccine in the absence of trehalose (IM) or presence of trehalose (IM+T). For microneedle delivery, mice were anesthetized with ketamine (110 mg/kg of body weight; Abbott Laboratories) mixed with xylaxine (11 mg/kg; Phoenix Scientific). Hair on the dorsal surface of mice was removed by hair-removing cream (Nair) with a moisturized cotton stick. After the skin was cleaned with a soaked cotton ball (70% ethanol) and dried with a hair dryer, an array of vaccine-coated microneedles was inserted into the skin and held for 10 min for release of the vaccine antigen from the coated microneedles.
For challenge infections, mice lightly anesthetized with isoflurane were intranasally infected with a lethal dose of A/PR8 virus (100 50% lethal doses [LD50]) in 50 μl of PBS at 7 weeks after a single VLP vaccine dose. Mice were observed daily to monitor changes in body weight and to record mortality. We followed an approved Emory IACUC protocol for this study, in which animals losing more than 25% of body weight were euthanized.
Antibody responses and HAI titer.
Influenza virus-specific antibodies of different isotypes (IgG, IgG1, IgG2a, and IgG2b) were determined by enzyme-linked immunosorbent assay (ELISA) plates coated with A/PR8 viral antigen and by using anti-mouse IgG isotype-specific secondary antibodies, as described previously (44). For determination of hemagglutination inhibition (HAI) titers, serum samples were first treated with receptor-destroying enzyme (Denka Seiken) by incubation overnight at 37°C and then incubated for 30 min at 56°C. Serum was serially diluted, mixed with 4 HA units (HAU) of influenza A/PR8 virus, and incubated for 30 min at room temperature prior to the addition of 0.5% chicken red blood cells. The reciprocal of the highest serum dilution preventing hemagglutination was scored as the HAI titer.
Neutralization, lung viral titer, and lung inflammatory cytokine assays.
A virus neutralization assay was performed using MDCK cells (American Type Culture Collection) following a previously described procedure (44). Lung viral titers at day 4 postchallenge were determined by counting plaques formed on the MDCK cells as described previously (44, 46). Inflammatory cytokines (interleukin-6 [IL-6]) in lungs collected at day 4 postchallenge were analyzed by Ready-Set-Go cytokine kits (eBioscience) following the manufacturer's procedure, as previously described (44).
Analysis of antibody-secreting B-cell and gamma interferon (IFN-γ)-producing T-cell responses.
Recall immune responses were determined from serum, lung, bone marrow, and spleen at day 4 postchallenge. Viral antigen-specific serum and lung IgG responses were determined using an ELISA as described above (44, 46). To determine antibody-producing cell spots in vitro, bone marrow and spleen cells were cultured in multiscreen 96-well filtration plates (Millipore) coated with inactivated A/PR8 viral antigen in RPMI medium for 2 days (5 × 105 cells per well), and cell spots were counted by an enzyme-linked immunospot (ELISPOT) reader as described previously (44). To determine T-cell responses, spleen cells were harvested from immunized and mock control mice at day 4 postchallenge and used to determine IFN-γ producing T-cell responses by ELISA. Briefly, spleen cells (106 cells per well of 96-well plates) were stimulated in vitro with A/PR8 hemagglutinin-specific peptides, a mixture of two major histocompatibility complex class I (MHC-I) peptides (IYSTVASSL and LYEKVKSQL) or a pool of five MHC-II peptides (SFERFEIFPKE, HNTNGVTAACSH, CPKYVRSAKLRM, KLKNSYVNKKGK, and NAYVSVVTSNYNRRF). Anti-IFN-γ antibodies for cytokine ELISA were purchased from BD/Pharmingen (San Diego, CA).
Statistics.
All parameters were recorded for individuals within all groups. A two-tailed Student's t test was performed when two different conditions were compared. Statistical comparisons of three or more conditions were carried out using the correlation and regression test of the PC-SAS system (SAS Institute). A P value of less than 0.05 was considered to be significant.
RESULTS
Microneedles coated with influenza VLP vaccines.
VLPs are an attractive format of vaccine that has been demonstrated to be effective in inducing protective immunity against influenza virus infection (9, 15, 19, 27, 35, 41, 42, 44). However, no previous studies have reported vaccination using influenza VLPs in the skin, which is an attractive alternative route of vaccination (5, 13, 14, 22). Delivery of inactivated influenza virus vaccines to the skin using microneedles was recently shown to be a promising approach for needle-free vaccination (32, 52). In this study, we used VLPs containing M1 and HA of A/PR8 virus as a model system for microneedle delivery of influenza VLP vaccine to the skin. The length of the microneedles used was approximately 700 μm, a size that penetrates epidermal and dermal layers of skin, as described in previous studies (17, 30, 32, 52).
The Coomassie blue-stained gel indicated that influenza VLPs contain HA as a major component in VLPs (Fig. 1A). The content of HA in VLPs was found to be similar to that in influenza virus, and its estimated molecular weight was higher than that in the virus, as shown in Western blot analysis (Fig. 1B), indicating the uncleaved precursor form of HA. Influenza VLPs containing HA without neuraminidase have been shown previously to confer protection (44).
To prepare microneedles coated with influenza VLP vaccines, microneedles were coated with influenza VLPs in suspension and air dried. Trehalose was previously shown to stabilize whole inactivated influenza vaccines and was tested as a stabilizer in the coating process (2, 23). To determine the total protein present on the needle (Fig. 1C) and associated hemagglutination activities (Fig. 1D), we dissolved the VLP coating from microneedles in PBS. The amount of VLP protein coated on one array of five microneedles was approximately 0.35 μg (Fig. 1C). Hemagglutinin activities of VLP vaccines dissolved from coated microneedles with and without trehalose were approximately 58% and 6%, respectively, of the original unprocessed VLP vaccines (Fig. 1D). Therefore, these results indicate that microneedle coating of VLP vaccines caused a significant decrease in HA activity and that the addition of trehalose preserved most of the HA activity after microneedle coating.
Microneedle VLP vaccination in the skin induces high levels of virus-specific antibodies.
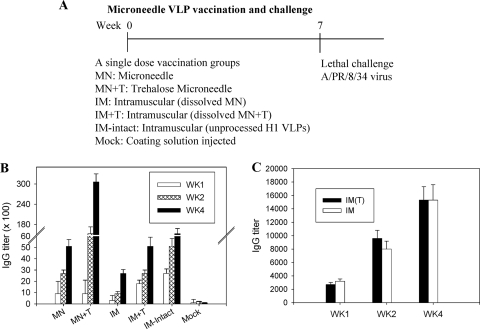
Groups of mice received VLP vaccination, and challenge experiments were then carried out as shown in Fig. 2 A. Virus-specific antibody responses were determined in serum samples collected at weeks 1, 2, and 4 after a single vaccination. At an early time point (week 1), microneedle groups (MN+T and MN) showed lower levels of antibody responses than groups intramuscularly immunized with stabilized VLP vaccines (IM+T and IM-intact) (Fig. 2B). However, at week 4, the trehalose-stabilized microneedle group (MN+T) showed the highest levels of virus-specific antibodies (Fig. 2B), which were 5-fold higher than those induced by the stabilized or intact intramuscular immunization group (IM+T and IM-intact). Notably, the intramuscular immunization with a redissolved unstabilized microneedle vaccine showed the lowest levels of antibodies, which were 2- to 3-fold lower than those induced by stabilized intramuscular (IM+T), intact IM, or the corresponding unstabilized microneedle (MN) immunization (Fig. 2B). To determine if there is a potential adjuvant effect of trehalose, additional groups of mice were intramuscularly immunized with intact VLPs (0.35 μg) with or without 15% trehalose, and virus-specific antibody responses were determined (Fig. 2C). No significant differences in antibody levels were observed between the groups with and without trehalose, indicating that trehalose does not have an adjuvant role.
FIG. 2.
Influenza A/PR8 virus-specific IgG responses. (A) Groups of mice (n = 12) immunized with a single dose of VLPs using different formulations and different routes of administration. Mice (n = 12 per group) were immunized with microneedles coated with 0.35 μg of influenza VLPs or by intramuscular injection with 0.35 μg of influenza VLPs dissolved from coated microneedles or by intramuscular injection with 0.35 μg of unprocessed intact inactivated influenza vaccine. At 7 weeks after vaccination, mice were challenged with a high lethal dose of A/PR8 virus (100 LD50). Groups were divided as follows: MN, microneedle vaccine without trehalose; MN+T, microneedle vaccine with trehalose; IM, intramuscular injection of reconstituted vaccine without trehalose; IM+T, intramuscular injection of reconstituted vaccine from trehalose-formulated microneedles; Mock, microneedles with trehalose coating buffer only (without vaccine). (B) Virus-specific total IgG antibody responses. Blood samples were collected at weeks 1, 2, and 4 after vaccination. Virus-specific antibody responses measured by ELISA are expressed as the reciprocals of serum dilutions that give antibody titers 2-fold higher than the standard deviations of naïve serum samples. Statistical significance among groups compared are as follows: at week 1, for IM+T and IM-intact versus MN+T, MN, and IM, P < 0.05; at week 2, for MN+T versus MN, IM, and IM+T, P < 0.01; for IM-intact versus IM, P < 0.05; at week 4, for MN+T versus MN, IM+T, and IM-intact, P < 0.05; for MN+T versus IM, P < 0.01. (C) Lack of trehalose effect on inducing antibody responses. Virus-specific total IgG antibodies were determined by ELISA from blood samples collected at weeks 1, 2, and 4 after a single intramuscular VLP immunization (n = 6 BALB/c mice per group) with or without 15% trehalose. IM(T), 0.35 μg of VLPs plus trehalose; IM, 0.35 μg of VLPs.
Taken together, the results suggest that microneedle immunizations in the skin with VLP vaccines (MN and MN+T) were more effective in inducing virus-specific antibodies than the corresponding intramuscular immunizations (IM and IM+T). Also, the trehalose stabilization of influenza VLPs was found to be important for inducing higher levels of virus-specific antibodies than those obtained with intramuscular immunizations, resulting in potential dose-sparing effects.
Microneedle vaccination of stabilized VLP vaccine induces superior protection.
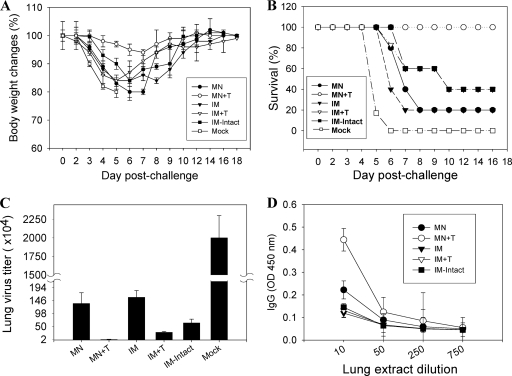
To determine the efficacies of VLP vaccines, groups of mice including a mock control (microneedle-treated without VLPs) were challenged with a high lethal dose of A/PR8 virus (100 LD50) at 7 weeks after a single microneedle vaccination dose in the skin or intramuscular immunization (Fig. 3). All mice in the mock control rapidly lost body weight and were euthanized by day 6 postchallenge. Intramuscular immunization groups with stabilized (IM+T) or intact VLP vaccines exhibited 40 to 60% survival rates accompanied by significant body weight loss of 15 to 17%. Remarkably, the group immunized by microneedle vaccination in the skin with stabilized VLPs was 100% protected against a high lethal challenge dose and exhibited only a transient body weight loss of 7%. Vaccination with unstabilized influenza VLPs at a similar dose (0.35 μg) in both the microneedle and intramuscularly immunized groups showed only 20% survival rates, with severe body weight loss in survivors. Microneedle vaccination with a 3-fold higher dose of influenza VLPs (1 μg) induced 100% protection even in the absence of trehalose stabilization (Table 1), which is consistent with results reported in previous studies using inactivated virus vaccines showing protection with unstabilized antigen at higher doses (32, 52).
FIG. 3.
Body weight changes, survival, lung virus titers, and lung IgG response after lethal challenge. (A) Body weight changes. (B) Survival rates. At week 7 after a single vaccination, mice (n = 12) were challenged with a lethal dose (A/PR8 virus, 100 LD50) and were monitored daily to record body weight changes and survival rates. (C) Lung virus titers. Lungs from individual mice in each group (n = 6 out of 12 mice per group) were collected on day 4 postchallenge, and lung virus titers (number of PFU) were determined. (D) Lung IgG antibody responses. Lung IgG responses were determined from the lung extracts collected at day 4 postchallenge. OD, optical density.
TABLE 1.
Protection by microneedle vaccination with high versus low doses of influenza VLPs in the absence of trehalose stabilizationa
| Treatment group | Body wt (%) at the indicated day postchallenge |
Survival (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 9 | Day 13 | ||
| VLP vaccine | |||||||||
| 1 μg | 100 | 99.5 | 99 | 98.7 | 98.5 | 98.7 | 99 | 99.8 | 100 |
| 0.35 μg | 100 | 96 | 89 | 83 | 80b | 94 | 97 | 100 | 20 |
| Naïve | 100 | 90 | 82 | 80 | 0 | ||||
Groups of mice (n = 5) were vaccinated with influenza VLPs (1 μg or 0.35 μg of VLPs) via microneedles coated without trehalose. After 7 weeks, mice were challenged with a lethal dose of A/PR8/1934 (100 LD50) and observed daily to monitor changes in body weight and to record mortality (animals were euthanized after losing 25% in body weight to reduce suffering).
Four out of 5 were eutharized on day 6.
To better assess the efficacy of vaccines, we determined the viral titers in lungs at day 4 postchallenge infection. The mock control group showed the highest viral titers, 2 × 107, which reflects the high dose of challenge virus. The microneedle vaccination with stabilized VLPs (MN+T) significantly reduced lung viral titers by 1,000-fold compared to those in the mock control (Fig. 3C), whereas lung viral titers in the conventional intramuscular immunization groups with the stabilized (IM+T) or intact (IM-intact) VLPs were only 30- to 60-fold lower than those in the mock control group. Importantly, lung viral titers in the unstabilized microneedle vaccination group (MN) were more than 50-fold higher than those in the stabilized microneedle group (MN+T) although lung viral titers of the MN group were still 16-fold lower than those of the mock control group. In addition, significant levels of virus-specific lung IgG antibodies were observed at day 4 after challenge in the stabilized microneedle group but not in other groups (Fig. 3D) (P < 0.005).
These results indicate that a single dose of VLP vaccination using microneedles in the skin was superior to conventional intramuscular immunization in inducing protection and in controlling lung viral replication. Thus, trehalose-mediated stabilization of influenza VLP vaccines during microneedle coating significantly contributed to providing effective protection.
Microneedle vaccination with influenza VLPs induces rapid recall antibody responses.
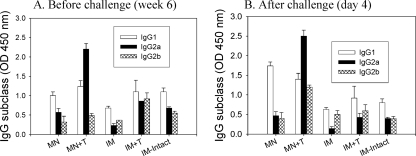
To better understand the superior protective immunity induced by microneedle vaccination, isotypes of serum antibodies were determined at week 6 postvaccination and at day 4 after lethal challenge (Fig. 4). Microneedle vaccination with the stabilized VLP vaccine (MN+T) induced IgG2a antibody as a dominant isotype and showed that levels of IgG1 and IgG2b isotypes increased by 1 order of magnitude after challenge (Table 2) (Fig. 4). However, in groups without trehalose stabilization of VLP vaccines (IM and MN), IgG1 antibody was found to be the dominant isotype, with a trend toward higher levels of IgG1 in the microneedle immunization at an early time postchallenge (Fig. 4B). Although intramuscular immunizations with either intact (IM-intact) or stabilized (IM+T) VLP vaccine induced all isotypes (IgG1, IgG2a, and IgG2b) at similar levels, there was a slightly decreased level of IgG2a antibody at day 4 postchallenge (Fig. 4B and Table 2).
FIG. 4.
Virus-specific isotype antibody responses. (A) Antibody isotypes before challenge. (B) Antibody isotypes after challenge. A/PR8 virus-specific isotype antibody responses (IgG1, IgG2a, and IgG2b) were determined before and after challenge. Results are expressed as averages of optical density readings at 450 nm (OD450) with 100-fold diluted serum samples in each group of mice (n = 12). Groups of mice are described in the legend of Fig. 2. Antibody titers are provided in Table 2.
TABLE 2.
Antibody titers in serum before and after challenge
| Groupb | Antibody titer (102)a |
|||||
|---|---|---|---|---|---|---|
| IgG1 |
IgG2a |
IgG2b |
||||
| Before | After | Before | After | Before | After | |
| MN | 44 ± 6.8 | 140 ± 22 | 20 ± 3.6 | 20 ± 3.0 | 8 ± 2.4 | 10 ± 2.1 |
| MN+T | 60 ± 10.5 | 186 ± 34 | 110 ± 24 | 280 ± 42 | 10 ± 2.8 | 36 ± 8.0 |
| IM | 24 ± 6.2 | 22 ± 3.9 | 6 ± 1.8 | 2.0 ± 0.6 | 11 ± 3.3 | 10 ± 2.0 |
| IM+T | 52 ± 12.5 | 40 ± 7.2 | 24 ± 7.2 | 16 ± 4.8 | 40 ± 5.0 | 24 ± 4.8 |
| IM-intact | 50 ± 6.5 | 36 ± 5.1 | 20 ± 5.8 | 16 ± 3.4 | 24 ± 6.0 | 16 ± 2.6 |
Virus-specific isotype antibody responses were determined and compared among groups before challenge. Titers are expressed as the highest dilution having a mean optical density at 450 nm greater than the mean value plus 3 standard deviations of naïve or mock-treated serum samples. Before, 6 weeks after a single-dose microneedle or intramuscular immunization (n = 12); after, day 4 postchallenge (n = 4).
MN, microneedle H1 VLP vaccine without trehalose; MN+T, microneedle H1 VLP vaccine with trehalose; IM, reconstituted H1 VLP vaccine from microneedles without trehalose; IM+T, reconstituted H1 VLP vaccine from trehalose-formulated microneedles; IM-intact, intramuscular immunization with unprocessed H1 VLPs.
Therefore, our observations suggest that an effective IgG2a recall immune response and lung IgG antibody responses induced by microneedle vaccination of stabilized VLP vaccine are important for enhancing protection. Also, stabilization of influenza VLP vaccines as well as the route of vaccine delivery significantly affects the pattern of antibody isotypes induced.
Microneedle vaccination with VLPs induces rapid increases in protective immune responses after lethal challenge.
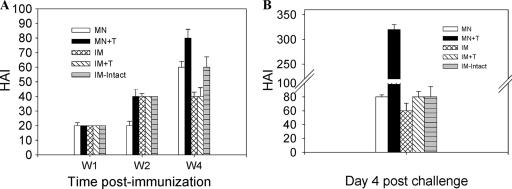
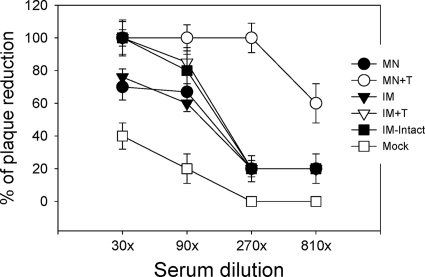
Functional antibodies such as hemagglutination inhibition (HAI) and virus-neutralizing antibodies can provide better immune correlates for predicting protection than binding antibodies. HAI titers were determined from immune serum collected at weeks 1, 2, and 4 postvaccination and at day 4 postchallenge. HAI titers were low in all groups at week 1 or 2 postvaccination (Fig. 5 A). At week 4, HAI titers of 80 were observed in the microneedle vaccination group with stabilized VLPs (MN+T) but not in other groups. The same microneedle group (MN+T) showed rapidly increased HAI titers to 320 at day 4 postchallenge, which were approximately 4-fold higher than titers of other groups (Fig. 5B). However, the group intramuscularly immunized with intact VLPs (IM-intact) and other groups (IM and MN) did not show such an increase in HAI titers at day 4 postchallenge. Consistent with HAI titers, at day 4 postchallenge infection, the microneedle vaccination with stabilized VLPs (MN+T) showed a rapid increas in neutralizing titers to 270, with approximately 100% plaque reduction (Fig. 6). Other groups immunized with unstabilized VLPs by microneedle (MN) or intramuscularly immunized with stabilized (IM+T) or intact (IM-intact) VLPs showed only moderate increases in neutralizing titers of 90, with approximately 80% plaque reduction.
FIG. 5.
Hemagglutination inhibition titers. (A) HAI titers before challenge. (B) HAI titers at day 4 postchallenge. HAI titers were determined at weeks 1, 2, and 4 (W1, W2, and W4, respectively) postimmunization and at day 4 postchallenge. Significant differences were found among groups of mice: at week 4, for MN+T versus IM-intact, P < 0.05; for MN+T versus MN, and IM+T, P < 0.01; At postchallenge, for MN+T versus MN, IM, IM+T, and IM-intact, P < 0.001.
FIG. 6.
Neutralizing activities determined at day 4 postchallenge. Serial dilutions of serum samples were incubated with infectious influenza viruses and percentages of PFU were determined. Neutralizing activities were expressed as the percentage of plaque reduction compared to the medium control. Significant differences were found among groups of mice: for neutralizing titers at both 270 and 810 dilutions for plaque reduction, P was <0.001 between MN+T and other groups.
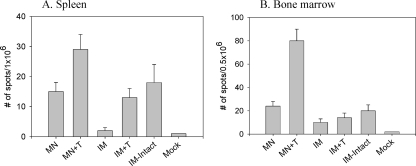
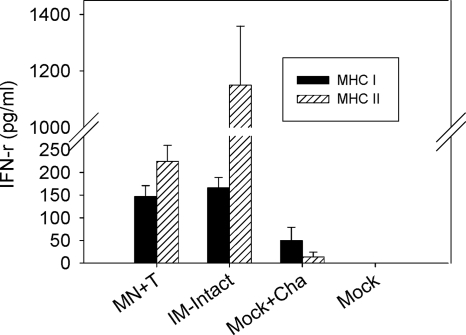
Since rapid increases in levels of serum functional antibodies were observed in the stabilized microneedle vaccination group (MN+T) upon viral challenge, we wanted to determine the levels of antibody-secreting plasma cells. The stabilized influenza vaccines and microneedle vaccinations elicited higher levels of recall antibody-secreting spleen cells at day 4 postchallenge than those found by corresponding intramuscular immunizations (Fig. 7 A). Most notably, microneedle vaccination with stabilized VLPs (MN+T) induced the highest levels of antibody-secreting cell spots in bone marrow, levels that were higher even than those induced by intramuscular immunization with stabilized (IM+T) or intact influenza vaccine (IM-intact) (Fig. 7B). Levels of IFN-γ in culture supernatants of spleen cells were observed to be similar in both stabilized microneedle and intramuscular groups in response to the CD8 T-cell-stimulating MHC-I peptides, whereas the intramuscular group showed higher levels of IFN-γ secretion upon stimulation with a pool of CD4 T-cell-activating MHC-II peptides (Fig. 8). Therefore, these cytokine results indicate that no correlation was found between IFN-γ-producing T-cell responses and protection.
FIG. 7.
Antibody-secreting cells (ASC) induced by microneedle VLP vaccine. Spleen and bone marrow samples were collected from individual mice in each group (n = 6) at day 4 postchallenge. Spots representing antibody-secreting cells were determined by ELISPOT assay. Significant differences were found among the groups: in spleen (A) for MN+T versus MN, and IM+T, IM-intact, P < 0.05; for MN versus IM, P < 0.05; in bone marrow (B), for MN+T versus MN, IM+T, and IM-intact, P < 0.01; for MN versus IM, P < 0.05.
FIG. 8.
IFN-γ production in spleen cells. Groups of mice (n = 6) were immunized with stabilized microneedle VLP vaccines (MN+T) or with unprocessed influenza VLPs (IM-intact) and challenged with A/PR8 virus at week 7 after vaccination. To collect spleen cells, mice were sacrificed at day 4 postchallenge. Levels of IFN-γ secreted into splenocyte culture supernatants were determined using a cytokine ELISA. Cells (1 × 106/well) from groups of mice (see legend of Fig. 2 for descriptions) were stimulated with A/PR8 HA-specific MHC-I and MHC-II peptides. Mock+Cha, group administered microneedles with trehalose coating buffer only (without vaccine) after challenge.
Overall, these results indicate that microneedle vaccination in the skin with stabilized VLPs is more effective in inducing rapid recall HAI and neutralizing antibody responses as well as antibody-secreting cells in spleen and bone marrow upon lethal challenge infection than conventional intramuscular immunization. The trehalose-mediated stabilization of influenza vaccines retaining HA functional activity during the microneedle coating process is an important factor for potential dose-sparing effects in inducing protective immunity.
DISCUSSION
The immunologic response to influenza VLPs after delivery to the skin has not previously been studied in a parallel comparison with intramuscular immunization. Influenza VLPs have a particulate structure which is too large to penetrate the intact skin (18). An objective of this study was to overcome the barrier of the stratum corneum layer for vaccine delivery by using microneedles coated with influenza VLPs. Microneedle coating with influenza VLPs involves a drying process that requires a change of vaccine formulation from liquid to solid. The stability of influenza VLPs after microneedle coating was found to decrease significantly, as evidenced by a loss of hemagglutinin (HA) activity. The addition of trehalose to the coating buffer significantly improved the stability of influenza VLPs after microneedle coating. A single vaccination with microneedles coated with stabilized influenza VLPs provided protection superior to that provided by conventional intramuscular immunization or MN vaccination with the unstabilized VLP vaccines. Microneedle vaccination in the skin with influenza VLPs was more effective than conventional intramuscular immunization in inducing humoral responses as well as enhanced recall immune responses, including functional antibodies and antibody-secreting cell responses. Overall, the results suggest that both HA stability and microneedle skin vaccination play important roles in providing protective immunity.
The data in this study provide evidence that the maintenance of the functional integrity of HA plays a significant role in inducing protective immunity against influenza virus infection. Previous studies have reported the effects of influenza virus HA on host pathological and immunological responses. The HA of the 1918 pandemic strain of influenza virus was reported to contribute to its high virulence, as evidenced by induction of high levels of chemokines and cytokines and infiltration of inflammatory cells (31). Inactivated whole influenza virus was shown to activate dendritic cells through the viral single-stranded RNA-mediated Toll-like receptor 7 (TLR7) pathway, and the fusion activity of HA was considered to be required due to its role in enhancing delivery of viral RNA (16). With regard to the role of HA in activating B cells, inactivated influenza virus with an H2 subtype HA was able to induce B-cell proliferation, which is involved in signaling of the innate immune system (36). The expression of class II cell surface I-E molecules on B cells was needed for the induction of B-cell proliferation by an influenza virus (3, 47). In line with these previous studies with whole influenza virus, the receptor-binding activity of HA in inactivated influenza virus was found to be important for inducing effective T helper type 1 (Th1) immune responses (45).
Because inactivated whole-virus or subunit vaccines contain many viral components including neuraminidase, single-stranded RNAs, and other viral molecules, it is difficult to define the effects of HA functional integrity on inducing protective immunity. Here, we found that maintaining the stability of influenza VLPs as determined by HA activity was more effective in inducing IgG2a isotype antibodies as a representative of Th1 immune responses, regardless of the vaccine delivery route. Activation of the innate immune system, such as MyD88-dependent or -independent TLRs or other signaling pathways, was shown to be important for inducing protective immune responses, including the induction of IgG2a antibody (20, 34, 39). Therefore, this study suggests that the functional integrity of HA might be required for effective interactions with B cells, dendritic cells, and/or other cells to stimulate the innate immune system and to induce IgG2a-dominant-type immune responses.
Conventional intradermal (ID) immunization involves the delivery of vaccine antigens in liquid form and the initiation of an immune response just below the skin surface. However, despite many clinical studies (6, 11, 28, 50), the detailed immunological advantages of influenza vaccination in the skin are not clear. Some studies reported vaccine dose-sparing effects by ID delivery compared to intramuscular immunization although low-dose intramuscular control groups were not included (6, 11, 28, 50). A well-controlled clinical study suggested that ID immunization induced HAI titers similar to those induced by intramuscular injection with equivalent low-dose vaccines (7) although a study in the elderly showed higher HAI titers after ID vaccination (21).
Vaccination of mice in the skin using solid microneedles coated with whole inactivated viruses at high doses (10 μg) has shown protection comparable to that with intramuscular immunization (32, 52), with IgG1 antibody as a dominant isotype (32). However, these studies did not investigate the stability issues associated with the dried formulation of microneedle vaccines (32, 52). A recent study confirmed that microneedle vaccination with inactivated virus in the absence of trehalose stabilization induced IgG1 as a dominant isotype antibody but there was a switch to IgG2a dominance using trehalose-stabilized antigen (45). Similarly, the present results show that influenza VLPs delivered to the mouse skin using microneedles without trehalose stabilization elicited high levels of IgG1, whereas trehalose-stabilized formulations induce high levels of IgG2a. Therefore, we suggest that the maintenance of HA receptor binding activity might be important for inducing IgG2a isotype antibody responses, contributing to superior protection with a lower vaccine dose. Interestingly, a 3-fold higher dose of influenza VLPs delivered via microneedles could provide 100% protection even in the absence of trehalose stabilization, indicating that trehalose stabilization results in dose-sparing effects for microneedle vaccination.
We suggest that the superior protection by skin vaccination with stabilized VLPs is probably due to higher levels of IgG2a antibodies as well as to rapid recall immune responses, including HAI titers, neutralizing activities, lung antibodies, and antibody-secreting cells. The induction of IgG2a antibodies has been demonstrated to be more effective than induction of other isotypes in clearing virus infection through multiple mechanisms, including activation of the complement system, stimulation of antibody-dependent cellular cytotoxicity, and clearance of opsonized virus by macrophages (24, 26, 37). Therefore, the results from the present study suggest important roles of functional and probably structural integrity of HA in inducing superior protective immunity by microneedle vaccination with influenza VLPs.
The mechanisms by which microneedle vaccination induces superior immunity to that with IM immunization are unknown. It has been suggested that vaccine antigens delivered to the skin are more likely to be captured by antigen-presenting cells, such as Langerhans cells and dermal DCs, and transported to the draining lymph nodes for B cells to respond (12, 43, 51). In contrast, antigens delivered into muscular tissue, which is relatively inefficient in capturing antigens by immune cells, are thought to be passively drained to the regional lymph nodes via the lymphatic conduits or bloodstream (28, 40). Direct migration of antigens to the lymph nodes was demonstrated to be faster than transport via antigen-presenting cells capturing or loading the antigens (25, 40). In support of this hypothesis, we found that higher antibody responses were detected at an early time point (week 1) postvaccination in the intramuscularly immunized groups (IM+T and IM-intact) than in the group receiving microneedle vaccination (MN+T). However, the kinetics of antibody production appear to be slower in the microneedle groups. Nevertheless, vaccines delivered to the skin were more effective than those administered by intramuscular injection in enhancing antibody levels after 2 weeks, resulting in the highest levels in the stabilized VLP microneedle vaccination group. Thus, it is likely that vaccine antigens delivered to the skin are effectively captured by cells which present antigens and effectively stimulate B cells.
In conclusion, we demonstrate that a single microneedle vaccination in the skin with stabilized influenza VLPs provides protection superior to that of conventional intramuscular immunization. The stabilization of influenza vaccines as measured by retention of the HA functional activity is an important parameter in predicting vaccine efficacy. HA stabilization, combined with vaccination via the skin using vaccine formulated as a solid-microneedle patch (MN+T), exhibited some advantages in the primary antibody response following an equivalent single-dose vaccination and showed more pronounced differences after lethal virus challenge: (i) 100% survival, when all other groups had ≤ 40% survival; (ii) at least 26-fold lower lung viral titers than all other groups; (iii) at least 14-fold higher IgG2a levels than all other groups and 4-fold higher IgG1 levels than all non-microneedle groups; and (iv) at least 4-fold higher HAI titers, 3-fold higher neutralizing activities, and substantially higher levels of antibody-secreting plasma cells than all other groups. Altogether, this study shows that powerful rapid recall immune responses are enabled by microneedle vaccination in the skin. Combining the immunologic and logistic advantages associated with avoidance of hypodermic needles and syringes, potential for rapid distribution and administration of microneedle patches, and the versatility of VLPs as a vaccination modality, this study indicates that microneedle-based immunization using influenza VLPs could have a great impact on improving vaccination efficacy, coverage, and public health.
Acknowledgments
The project described in this study was supported in part by NIH grants EB006369 (M.R.P.), AI068003 (R.W.C.), AI074579 (R.W.C.), and SERCEB AI057157 (R.W.C.) and the Georgia Research Alliance (S.M.K).
We thank Huan Nguyen for the mouse-adapted influenza virus A/PR8/34 strain and Mark Allen for use of laser cutting microfabrication facilities.
R.W.C. and S.M.K. have equity in Zetra Biologicals which is developing VLP technology under license from Emory University. M.R.P. serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. These possible conflicts of interest have been disclosed and are being managed by Georgia Tech and Emory University.
Footnotes
Published ahead of print on 19 May 2010.
REFERENCES
- 1.Akbari, O., N. Panjwani, S. Garcia, R. Tascon, D. Lowrie, and B. Stockinger. 1999. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J. Exp. Med. 189:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amorij, J. P., J. Meulenaar, W. L. Hinrichs, T. Stegmann, A. Huckriede, F. Coenen, and H. W. Frijlink. 2007. Rational design of an influenza subunit vaccine powder with sugar glass technology: preventing conformational changes of haemagglutinin during freezing and freeze-drying. Vaccine 25:6447-6457. [DOI] [PubMed] [Google Scholar]
- 3.Anders, E. M., A. A. Scalzo, and D. O. White. 1985. Mitogenic activity of influenza virus and haemagglutinin. Vaccine 3:241-244. [DOI] [PubMed] [Google Scholar]
- 4.Auewarakul, P., U. Kositanont, P. Sornsathapornkul, P. Tothong, R. Kanyok, and P. Thongcharoen. 2007. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine 25:659-663. [DOI] [PubMed] [Google Scholar]
- 5.Barker, J. N., R. S. Mitra, C. E. Griffiths, V. M. Dixit, and B. J. Nickoloff. 1991. Keratinocytes as initiators of inflammation. Lancet 337:211-214. [DOI] [PubMed] [Google Scholar]
- 6.Belshe, R. B., F. K. Newman, J. Cannon, C. Duane, J. Treanor, C. Van Hoecke, B. J. Howe, and G. Dubin. 2004. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 351:2286-2294. [DOI] [PubMed] [Google Scholar]
- 7.Belshe, R. B., F. K. Newman, K. Wilkins, I. L. Graham, E. Babusis, M. Ewell, and S. E. Frey. 2007. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine 25:6755-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beran, J., A. Ambrozaitis, A. Laiskonis, N. Mickuviene, P. Bacart, Y. Calozet, E. Demanet, S. Heijmans, P. Van Belle, F. Weber, and C. Salamand. 2009. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bright, R. A., D. M. Carter, C. J. Crevar, F. R. Toapanta, J. D. Steckbeck, K. S. Cole, N. M. Kumar, P. Pushko, G. Smith, T. M. Tumpey, and T. M. Ross. 2008. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an Influenza virus-like particle. PLoS One 3:e1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, D., R. L. Endres, C. A. Erickson, K. F. Weis, M. W. McGregor, Y. Kawaoka, and L. G. Payne. 2000. Epidermal immunization by a needle-free powder delivery technology: immunogenicity of influenza vaccine and protection in mice. Nat. Med. 6:1187-1190. [DOI] [PubMed] [Google Scholar]
- 11.Chiu, S. S., J. S. Peiris, K. H. Chan, W. H. Wong, and Y. L. Lau. 2007. Immunogenicity and safety of intradermal influenza immunization at a reduced dose in healthy children. Pediatrics 119:1076-1082. [DOI] [PubMed] [Google Scholar]
- 12.Dubois, B., C. Massacrier, and C. Caux. 2001. Selective attraction of naive and memory B cells by dendritic cells. J. Leukoc. Biol. 70:633-641. [PubMed] [Google Scholar]
- 13.Enioutina, E. Y., D. Visic, and R. A. Daynes. 2000. The induction of systemic and mucosal immune responses to antigen-adjuvant compositions administered into the skin: alterations in the migratory properties of dendritic cells appears to be important for stimulating mucosal immunity. Vaccine 18:2753-2767. [DOI] [PubMed] [Google Scholar]
- 14.Flacher, V., M. Bouschbacher, E. Verronese, C. Massacrier, V. Sisirak, O. Berthier-Vergnes, B. de Saint-Vis, C. Caux, C. Dezutter-Dambuyant, S. Lebecque, and J. Valladeau. 2006. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J. Immunol. 177:7959-7967. [DOI] [PubMed] [Google Scholar]
- 15.Galarza, J. M., T. Latham, and A. Cupo. 2005. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 18:244-251. [DOI] [PubMed] [Google Scholar]
- 16.Geeraedts, F., N. Goutagny, V. Hornung, M. Severa, A. de Haan, J. Pool, J. Wilschut, K. A. Fitzgerald, and A. Huckriede. 2008. Superior immunogenicity of inactivated whole virus H5N1 influenza vaccine is primarily controlled by Toll-like receptor signalling. PLoS Pathog. 4:e1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill, H. S., and M. R. Prausnitz. 2007. Coated microneedles for transdermal delivery. J. Control Release 117:227-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Gill, H. S., J. Söderholm, M. R. Prausnitz, and M. Sällbeng. 2010. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 17:811-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenn, G. M., R. T. Kenney, L. R. Ellingsworth, S. A. Frech, S. A. Hammond, and J. P. Zoeteweij. 2003. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev. Vaccines 2:253-267. [DOI] [PubMed] [Google Scholar]
- 19.Haynes, J. R., L. Dokken, J. A. Wiley, A. G. Cawthon, J. Bigger, A. G. Harmsen, and C. Richardson. 2009. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine 27:530-541. [DOI] [PubMed] [Google Scholar]
- 20.Heer, A. K., A. Shamshiev, A. Donda, S. Uematsu, S. Akira, M. Kopf, and B. J. Marsland. 2007. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J. Immunol. 178:2182-2191. [DOI] [PubMed] [Google Scholar]
- 21.Holland, D., R. Booy, F. De Looze, P. Eizenberg, J. McDonald, J. Karrasch, M. McKeirnan, H. Salem, G. Mills, J. Reid, F. Weber, and M. Saville. 2008. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J. Infect. Dis. 198:650-658. [DOI] [PubMed] [Google Scholar]
- 22.Hon, H., and J. Jacob. 2004. Tracking dendritic cells in vivo: insights into DC biology and function. Immunol. Res. 29:69-80. [DOI] [PubMed] [Google Scholar]
- 23.Huang, J., R. J. Garmise, T. M. Crowder, K. Mar, C. R. Hwang, A. J. Hickey, J. A. Mikszta, and V. J. Sullivan. 2004. A novel dry powder influenza vaccine and intranasal delivery technology: induction of systemic and mucosal immune responses in rats. Vaccine 23:794-801. [DOI] [PubMed] [Google Scholar]
- 24.Huber, V. C., J. M. Lynch, D. J. Bucher, J. Le, and D. W. Metzger. 2001. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 166:7381-7388. [DOI] [PubMed] [Google Scholar]
- 25.Itano, A. A., S. J. McSorley, R. L. Reinhardt, B. D. Ehst, E. Ingulli, A. Y. Rudensky, and M. K. Jenkins. 2003. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19:47-57. [DOI] [PubMed] [Google Scholar]
- 26.Jayasekera, J. P., E. A. Moseman, and M. C. Carroll. 2007. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 81:3487-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang, S. M., D. G. Yoo, A. S. Lipatov, J. M. Song, C. T. Davis, F. S. Quan, L. M. Chen, R. O. Donis, and R. W. Compans. 2009. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS One 4:e4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenney, R. T., S. A. Frech, L. R. Muenz, C. P. Villar, and G. M. Glenn. 2004. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 351:2295-2301. [DOI] [PubMed] [Google Scholar]
- 29.Khanlou, H., S. Sanchez, M. Babaie, C. Chien, G. Hamwi, J. C. Ricaurte, T. Stein, L. Bhatti, P. Denouden, and C. Farthing. 2006. The safety and efficacy of dose-sparing intradermal administration of influenza vaccine in human immunodeficiency virus-positive patients. Arch. Intern. Med. 166:1417. [DOI] [PubMed] [Google Scholar]
- 30.Kim, Y. C., F. S. Quan, R. W. Compans, S. M. Kang, and M. R. Prausnitz. 2009. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control Release 42:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobasa, D., A. Takada, K. Shinya, M. Hatta, P. Halfmann, S. Theriault, H. Suzuki, H. Nishimura, K. Mitamura, N. Sugaya, T. Usui, T. Murata, Y. Maeda, S. Watanabe, M. Suresh, T. Suzuki, Y. Suzuki, H. Feldmann, and Y. Kawaoka. 2004. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 431:703-707. [DOI] [PubMed] [Google Scholar]
- 32.Koutsonanos, D. G., M. del Pilar Martin, V. G. Zarnitsyn, S. P. Sullivan, R. W. Compans, M. R. Prausnitz, and I. Skountzou. 2009. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One 4:e4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanford, R. E., V. Luckow, R. C. Kennedy, G. R. Dreesman, L. Notvall, and M. D. Summers. 1989. Expression and characterization of hepatitis B virus surface antigen polypeptides in insect cells with a baculovirus expression system. J. Virol. 63:1549-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, L., A. J. Gerth, and S. L. Peng. 2004. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur. J. Immunol. 34:1483-1487. [DOI] [PubMed] [Google Scholar]
- 35.Mahmood, K., R. A. Bright, N. Mytle, D. M. Carter, C. J. Crevar, J. E. Achenbach, P. M. Heaton, T. M. Tumpey, and T. M. Ross. 2008. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine 26:5393-5399. [DOI] [PubMed] [Google Scholar]
- 36.Marshall-Clarke, S., L. Tasker, O. Buchatska, J. Downes, J. Pennock, S. Wharton, P. Borrow, and D. Z. Wiseman. 2006. Influenza H2 haemagglutinin activates B cells via a MyD88-dependent pathway. Eur. J. Immunol. 36:95-106. [DOI] [PubMed] [Google Scholar]
- 37.Mozdzanowska, K., J. Feng, M. Eid, D. Zharikova, and W. Gerhard. 2006. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology 352:418-426. [DOI] [PubMed] [Google Scholar]
- 38.Nava, G. M., M. S. Attene-Ramos, J. K. Ang, and M. Escorcia. 2009. Origins of the new influenza A (H1N1) virus: time to take action. Euro Surveill. 14:pii19228. [DOI] [PubMed] [Google Scholar]
- 39.Nemazee, D., A. Gavin, K. Hoebe, and B. Beutler. 2006. Immunology: Toll-like receptors and antibody responses. Nature 441:E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pape, K. A., D. M. Catron, A. A. Itano, and M. K. Jenkins. 2007. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 26:491-502. [DOI] [PubMed] [Google Scholar]
- 41.Perrone, L. A., A. Ahmad, V. Veguilla, X. Lu, G. Smith, J. M. Katz, P. Pushko, and T. M. Tumpey. 2009. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J. Virol. 83:5726-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pushko, P., T. M. Tumpey, F. Bu, J. Knell, R. Robinson, and G. Smith. 2005. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 23:5751-5759. [DOI] [PubMed] [Google Scholar]
- 43.Qi, H., J. G. Egen, A. Y. Huang, and R. N. Germain. 2006. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312:1672-1676. [DOI] [PubMed] [Google Scholar]
- 44.Quan, F. S., C. Huang, R. W. Compans, and S. M. Kang. 2007. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 81:3514-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quan, F. S., Y. C. Kim, D. G. Yoo, R. W. Compans, M. R. Prausnitz, and S. M. Kang. 2009. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One 4:e7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quan, F. S., D. G. Yoo, J. M. Song, J. D. Clements, R. W. Compans, and S. M. Kang. 2009. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J. Virol. 83:4489-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scalzo, A. A., and E. M. Anders. 1985. Influenza viruses as lymphocyte mitogens. II. Role of I-E molecules in B cell mitogenesis by influenza A viruses of the H2 and H6 subtypes. J. Immunol. 135:3524-3529. [PubMed] [Google Scholar]
- 48.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, C. B. Bridges, N. J. Cox, and K. Fukuda. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333-1340. [DOI] [PubMed] [Google Scholar]
- 49.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, N. Cox, L. J. Anderson, and K. Fukuda. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179-186. [DOI] [PubMed] [Google Scholar]
- 50.Van Damme, P., F. Oosterhuis-Kafeja, M. Van der Wielen, Y. Almagor, O. Sharon, and Y. Levin. 2009. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine 27:454-459. [DOI] [PubMed] [Google Scholar]
- 51.Wykes, M., A. Pombo, C. Jenkins, and G. G. MacPherson. 1998. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161:1313-1319. [PubMed] [Google Scholar]
- 52.Zhu, Q., V. G. Zarnitsyn, L. Ye, Z. Wen, Y. Gao, L. Pan, I. Skountzou, H. S. Gill, M. R. Prausnitz, C. Yang, and R. W. Compans. 2009. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. U. S. A. 106:7968-7973. [DOI] [PMC free article] [PubMed] [Google Scholar]