Abstract
Spores of Bacillus species are said to be committed when they continue through nutrient germination even when germinants are removed or their binding to spores' nutrient germinant receptors (GRs) is both reversed and inhibited. Measurement of commitment and the subsequent release of dipicolinic acid (DPA) during nutrient germination of spores of Bacillus cereus and Bacillus subtilis showed that heat activation, increased nutrient germinant concentrations, and higher average levels of GRs/spore significantly decreased the times needed for commitment, as well as lag times between commitment and DPA release. These lag times were also decreased dramatically by the action of one of the spores' two redundant cortex lytic enzymes (CLEs), CwlJ, but not by the other CLE, SleB, and CwlJ action did not affect the timing of commitment. The timing of commitment and the lag time between commitment and DPA release were also dependent on the specific GR activated to cause spore germination. For spore populations, the lag times between commitment and DPA release were increased significantly in spores that germinated late compared to those that germinated early, and individual spores that germinated late may have had lower appropriate GR levels/spore than spores that germinated early. These findings together provide new insight into the commitment step in spore germination and suggest several factors that may contribute to the large heterogeneity among the timings of various events in the germination of individual spores in spore populations.
Spores of Bacillus species can remain dormant for long times and are extremely resistant to a variety of environmental stresses (26). However, under appropriate conditions, normally upon the binding of specific nutrients to spores' nutrient germinant receptors (GRs), spores can come back to active growth through a process called germination followed by outgrowth (19, 20, 25, 26). Germination of Bacillus subtilis spores can be triggered by l-alanine or l-valine or a combination of l-asparagine, d-glucose, d-fructose, and K+ (AGFK). These nutrient germinants trigger germination by binding to and interacting with GRs that have been localized to the spore's inner membrane (12, 20). l-Alanine and l-valine bind to the GerA GR, while the AGFK mixture triggers germination by interacting with both the GerB and GerK GRs (25). Normally, l-asparagine alone does not trigger B. subtilis spore germination. However, a mutant form of the GerB GR, termed GerB*, displays altered germinant specificity such that l-asparagine alone will trigger the germination of gerB* mutant spores (1, 18).
A number of events occur in a defined sequence during spore germination. Initially, exposure of spores to nutrient germinants causes a reaction that commits spores to germinate, even if the germinant is removed or displaced from its cognate GR (7, 10, 21, 27, 28). This commitment step is followed by release of monovalent cations, as well as the spore core's large pool of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) along with divalent cations, predominantly Ca2+, that are chelated with DPA (Ca-DPA). In Bacillus spores, the release of Ca-DPA triggers the hydrolysis of spores' peptidoglycan cortex by either of two cortex lytic enzymes (CLEs), CwlJ and SleB (11, 16, 23). CwlJ is activated during germination by Ca-DPA as it is being released from individual spores, while SleB activation requires that most Ca-DPA be released (14, 16, 17). Cortex hydrolysis, in turn, allows the spore core to expand and fully hydrate, which leads to activation of enzymes and initiation of metabolism in the spore core (21, 25).
As noted above, commitment is the first event that can be assessed during spore germination, although the precise mechanism of commitment is not known. Since much has been learned about proteins important in spore germination in the many years since commitment was last studied (25, 26), it seemed worth reexamining commitment, with the goal of determining those factors that influence this step in the germination process. Knowledge of factors important in determining kinetics of commitment could then lead to an understanding of what is involved in this reaction.
Kinetic analysis of spore germination, as well as commitment, has mostly been based on the decrease in optical density at 600 nm (OD600) of spore suspensions, which monitors a combination of events that occur well after commitment, including DPA release, cortex hydrolysis, and core swelling (25-27). In the current work, we have used a germination assay that measures DPA release, an early event in spore germination, and have automated this assay to allow routine measurement of commitment, as well as DPA release from large numbers of spore samples simultaneously. This assay has allowed comparison of the kinetics of DPA release and commitment during germination and study of the effects of heat activation, germinant concentration, GR levels, and CLEs on commitment.
MATERIALS AND METHODS
Bacterial strains and spore preparation and purification.
The B. subtilis strains used are isogenic derivatives of laboratory strain 168 and are listed in Table 1. B. subtilis strains were routinely grown in LB medium (17) with appropriate antibiotics. B. subtilis strains were sporulated at 37°C on 2× Schaeffer's glucose medium plates without antibiotics, and spores were harvested and purified as described previously (15). The Bacillus cereus strain used was strain T, originally obtained from H. O. Halvorson, and B. cereus spores were prepared at 30°C in a defined liquid medium and were purified as described previously (5, 9). All spore preparations were stored at 4°C protected from light and were 98% free of growing or sporulating cells, germinated spores, and cell debris as determined by observation in a phase-contrast microscope.
TABLE 1.
B. subtilis strains used in this study
| B. subtilis strain | Genotype and phenotypea | Reference |
|---|---|---|
| FB10 | gerB* wild-type level of the GerB* GR | 18 |
| FB111 | ΔcwlJ::tet Tcr | 16 |
| FB112 | ΔsleB::spc Spr | 16 |
| FB113 | ΔcwlJ::tet ΔsleB::spc Spr Tcr | 16 |
| PS533 | pUB110 Kmr wild type | 24 |
| PS3415 | PsspB:gerB* Spr 200-fold elevated level of the GerB* GR | 3 |
| PS3476 | PsspD::gerA MLSr ≥10-fold elevated level of the GerA GR | 3 |
| PS3502 | PsspD::gerB* Spr 20-fold elevated level of the GerB* GR | 3 |
Abbreviations: Kmr, kanamycin resistance (10 μg/ml); MLSr, macrolide-lincosamide-streptogramin B resistance (25 μg/ml lincomycin and 1 μg/ml erythromycin); Spr, spectinomycin resistance (100 μg/ml); Tcr, tetracycline resistance (10 μg/ml).
Spore germination and assay of DPA release.
Unless otherwise stated, B. subtilis spores at an OD600 of ∼10 were heat activated at 75°C for 30 min, B. cereus spores at an OD600 of ∼10 were heat activated at 70°C for 20 min, and the activated spores were cooled on ice before germination. Spores were germinated at 37°C (B. subtilis) or 30°C (B. cereus) at an OD600 of 0.5 in 200 μl of 25 mM HEPES buffer (pH 7.4) (B. subtilis) or 25 mM Tris-HCl buffer (pH 8.8) (B. cereus), with germinants added as noted in individual experiments. All germination incubations also contained 50 μM terbium chloride. Spore germination was initiated by addition of the germinant l-alanine, l-valine, or the AGFK mixture (equal concentrations of all four constituents, including KCl) for B. subtilis spores and a combination of 40 mM NH4Cl and various l-alanine concentrations for B. cereus spores.
Spore germination was carried out in a 96-well plate (Fisher Scientific) in a Gemini EM microplate fluorescence reader (Molecular Devices, Sunnyvale, CA). DPA release was monitored by real-time measurement of fluorescence emission at 545 nm with excitation at 270 nm, appropriate wavelengths for the Tb3+-DPA complex (29, 30). The background detected at zero time was used as a blank, and this blank was invariably ≤2% of the fluorescence seen when germination was complete. Control experiments also showed that under the assay conditions used, the number of relative fluorescence units (RFU) obtained in the assay was linear with DPA release. The maximum relative number of RFU reached upon the completion of germination experiments in 0.5 to 6 h was considered 100%, and the percentage of DPA release at time t was calculated as follows: (number of RFU at time t/maximum number of RFU reached in 0.5 to 6 h) × 100. Levels of actual DPA release in incubations at 0.5 to 6 h were determined with reference to the number of RFU obtained when all of the DPA was extracted from spore samples by boiling (15). The germination percentages of spore samples at the end of germination incubations were also routinely checked by phase-contrast microscopy, and these measurements invariably agreed with those determined from RFU counts (data not shown).
Measurement of germination commitment.
Two methods were used to measure the commitment step in spore germination. For l-alanine/l-valine germination, 2 μl of 1 M d-alanine was added to germination mixtures at various times after spores were mixed with germinant to give a final concentration of 10 mM d-alanine, and DPA release was measured as described above to quantify the degree of subsequent germination. Control incubations in which d-alanine was added at time zero together with l-alanine or l-valine exhibited no detectable DPA release over 2 to 4 h (see Results).
For AGFK or l-asparagine germination, 2 μl of 17.4 N acetic acid was added to germination incubations to lower the pH of the mixtures to 3.6. This acidification inhibited further commitment, but DPA release from committed spores was not blocked, as reported previously (2, 27) (see Results). Control incubations in which acetic acid was added together with AGFK or l-asparagine exhibited no detectable DPA release over 2 to 4 h (see Results). In all cases, percent commitment was calculated as the sum of the percent DPA released both at the time of and subsequent to the addition of d-alanine or acetic acid. Values of the percent DPA release before and after d-alanine or acetic acid addition were measured and calculated as described above.
All data reported are results from one experiment, although almost all experiments were repeated at least twice with the same spore preparation, while germination experiments with AGFK were repeated with two independent spore preparations. In all experiments, individual values of percent commitment or DPA release in replicates varied by less than 15%. In cases in which the commitment and DPA release of spores of isogenic B. subtilis strains were compared, the spores used were prepared and tested together.
RESULTS
Measurement of commitment of spores to germinate.
It is well known that nutrient germination of individual spores in populations is very heterogeneous, with some spores beginning germination immediately after the addition of germinants and others exhibiting no signs of germination for hours (4, 8, 9, 22, 23). Consequently, rates of events such as DPA release and almost certainly commitment determined with spore populations are generally a summation of the heterogeneous rates of these events for millions of individual spores.
l-Alanine or l-valine germination of B. subtilis spores via the GerA GR is very strongly inhibited by d-alanine, which blocks further commitment but does not inhibit subsequent germination events of committed spores (10, 27). Therefore, the time for a fraction of the spores in a population to become committed to germinate via the GerA GR can be determined by adding an excess of d-alanine at various times during l-alanine/l-valine germination and determining the ability of the spores to continue germination (27). Spores that have become committed to germinate at any time of d-alanine addition are those that (i) have already released their DPA and (ii) subsequently release their DPA. This approach has also been successful with B. cereus spores germinating with l-alanine (2). As expected, we found that continuous measurement of DPA release readily measured commitment during l-alanine/l-valine germination of B. cereus and B. subtilis spores using d-alanine to block further commitment (see Materials and Methods and below). However, while d-asparagine has been reported to block commitment in AGFK germination of B. subtilis spores (28), we did not observe this (data not shown). d-Alanine is also ineffective in blocking commitment during germination by a mixture of l-alanine and GFK via the GerB and GerK GRs (6). However, commitment is reported to be significantly more sensitive to low pH than are subsequent germination events, as a pH of ∼3.6 blocks further commitment of B. subtilis spores but allows committed spores to continue through later steps in germination, including DPA release (2, 27). Therefore, we measured commitment during the germination of B. subtilis spores with AGFK or l-asparagine by adding acetic acid to acidify germination incubations to pH 3.6. Control experiments showed that this acidification blocked further commitment but allowed DPA release from spores that were already committed (see below).
Distinguishing commitment and DPA release.
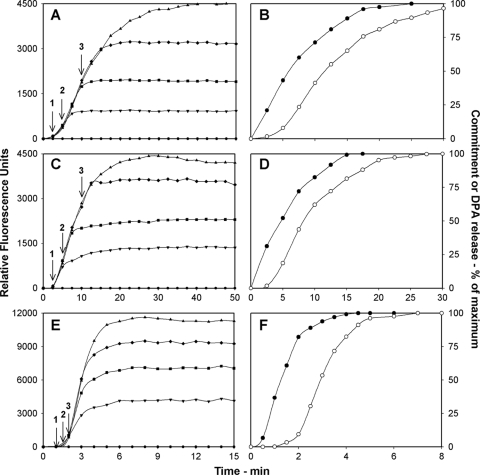
As expected, B. cereus and B. subtilis spores exposed to germinants for a short time continued to release DPA after the addition of d-alanine or acetic acid to stop further commitment in l-alanine or l-asparagine germination, respectively (Fig. 1 A, C, and E). Thus, wild-type B. subtilis spores incubated in 2 mM l-alanine for 2.5 min released only 1.7% of their DPA but released 21% of their DPA after the addition of d-alanine at 2.5 min and further incubation (Fig. 1A), B. subtilis spores with 200-fold elevated levels of the GerB* GR released ∼1.7% of their DPA when incubated for 2.5 min with 150 μM l-asparagine but released ∼31% of their DPA after acidification at 2.5 min and further incubation (Fig. 1C), and B. cereus spores incubated with NH4Cl and l-alanine released <0.1% of their DPA in 1 min but released 37% of their DPA upon the addition of d-alanine at 1 min and further incubation (Fig. 1E). Importantly, addition of d-alanine or acetic acid at the time of mixing of spores with germinants completely blocked all DPA release (Fig. 1A, C, and E). These results indicate that the addition of either d-alanine or acetic acid allows a distinction between commitment and DPA release in spore germination, thus allowing commitment alone to be assessed in spore populations as described above (Fig. 1B, D, and F).
FIG. 1.
(A to F) Commitment and DPA release during the germination of B. subtilis and B. cereus spores. Heat-activated spores of B. subtilis strain PS533 (wild type) were germinated in 2 mM l-alanine (A, B), heat-activated spores of B. subtilis strain PS3415 (PsspB::gerB*) were germinated in 150 μM l-asparagine (C, D), and heat-activated spores of B. cereus were germinated in 40 mM NH4Cl and 6 mM l-alanine (E, F). d-Alanine (A and E) or acetic acid (C) was added to germination incubations at zero time and other times, as indicated by arrows 1, 2, and 3, denoting 2.5, 5, and 10 min (A, C) or 1, 1.5, and 2 min (E), and commitment and DPA release during incubations with or without d-alanine or acetic acid addition were determined as described in Materials and Methods. Shown in panels B, D, and F are normalized commitment (•) and germination (○) curves derived from data in panels A, C, and E, respectively, as described in Materials and Methods. The maximum number of RFU that each germination assay reached upon germination for 2 h in panels A and C and for 30 min in panel E was set at 100%, but the actual germination percentages of spore samples at the end of germination incubations were 95, 90, and 95% in panels A, C, and E, respectively. Note that in the experiments in panels A, C, and E, d-alanine was added at many more times than shown and only data from three d-alanine addition times are shown for clarity. However, data for all d-alanine addition times are shown in panels B, D, and F.
Comparison of the kinetics of commitment and DPA release showed that, as expected, commitment precedes DPA release (Fig. 1B, D, F). For example, with wild-type B. subtilis spores germinating with 2 mM l-alanine (Fig. 1B), the times for spores to reach 25, 50, and 75% commitment (defined as C25,C50, and C75 values) were 2.9, 5.9, and 10.9 min, while the times to reach 25, 50, and 75% DPA release (defined as G25, G50, and G75 values) were 7.7, 11.5, and 17.3 min (Table 2). Subtraction of Ct values from the comparable Gt values gave lag times between commitment and DPA release (defined as Δ25, Δ50, and Δ75 values) of 4.8, 5.6, and 6.4 min at 25, 50, and 75% completion of the various steps, respectively (Table 2).
TABLE 2.
Effect of heat activation on timing of commitment and DPA release and lag times between commitment and DPA release during l-alanine germination of Bacillus spores
| Spores and heat activation | Time (min)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| C25 | G25 | Δ25 | C50 | G50 | Δ50 | C75 | G75 | Δ75 | |
| Wild-type B. subtilis | |||||||||
| Yes | 4 | 8 | 4 | 8 | 11.8 | 3.8 | 12.6 | 17.4 | 4.8 |
| No | 7 | 12 | 5 | 11 | 17.6 | 6.6 | 18 | 24.3 | 6.3 |
| B. cereus | |||||||||
| Yes | 1.1 | 2.8 | 1.7 | 1.7 | 3.6 | 1.9 | 2.5 | 4.8 | 2.3 |
| No | 2.1 | 3.7 | 1.6 | 2.7 | 5 | 2.3 | 3.2 | 6.7 | 3.5 |
Ct, Gt, and Δt values (times to reach 25%, 50%, and 75% commitment [C25, C50, and C75] and germination [G25, G50, and G75] and Gt − Ct times [Δ25, Δ50, and Δ75]) were determined from data in Fig. 2A and B.
As noted above, d-alanine blocks commitment but does not inhibit subsequent germination events of spores germinating in l-alanine or l-valine. Also as noted above, lowering the pH of germination incubations by acetic acid addition should be an alternative to the use of d-alanine to block commitment (2, 27). To determine if acetic acid addition is effective in blocking commitment but not subsequent DPA release, we compared the commitment levels seen during l-alanine germination of wild-type B. subtilis spores using either d-alanine or acetic acid addition to block further commitment. If acetic acid addition inhibited not only commitment but also subsequent DPA release, the relative kinetics of commitment and DPA release in l-alanine germination would be different when acetic acid or d-alanine was used to block further commitment. However, this was not the case, since the kinetics of commitment and DPA release were the same whether d-alanine or acetic acid addition was used (data not shown).
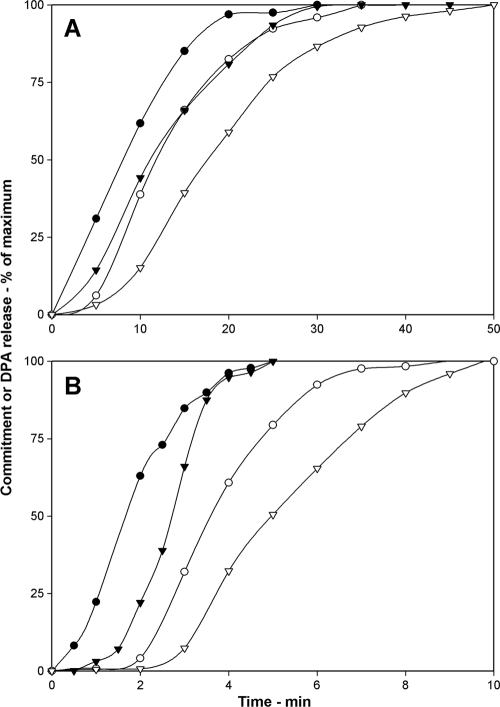
Effects of heat activation on commitment.
With confidence that we had methods to accurately measure commitment during spore germination with different nutrient germinants, we turned to analysis of the effects of various factors on commitment itself. The first factor we tested was heat activation, since heat activation accelerates the rate of spore germination, although the mechanism of this effect is not known (13). For B. subtilis spores germinating with 2 mM l-alanine, heat-activated spores reached 25, 50, or 75% commitment significantly faster than unactivated spores (Fig. 2 A; Table 2). As a result, the heat-activated spores completed 25, 50, or 75% DPA release faster than unactivated spores and the lag times between commitment and germination at various points were shorter with the heat-activated spores (Table 2). Previous work has shown that once DPA release has been initiated during GR-mediated spore germination, release of ≥85% of the total spore DPA takes only 1 to 2 min in individual wild-type spores (4, 22, 23). Consequently, values of 25, 50, and 75% DPA release in a spore population are essentially the times for 25, 50, and 75% of the spores in the population to release all of their DPA.
FIG. 2.
(A, B) Effect of heat activation on commitment and DPA release during spore germination. Commitment (•, ▾) and DPA release (○, ▿) curves of spores of B. subtilis PS533 (wild-type) germinating in 2 mM l-alanine (A) and B. cereus germinating with 40 mM NH4Cl plus 1 mM l-alanine (B) with (•, ○) or without (▾, ▿) prior heat activation were determined after d-alanine addition to germination incubations at various times as described in Materials and Methods. The maximum number of RFU that each germination assay reached upon germination for 2 h in panel A and for 30 min in panel B was set at 100%, but the actual germination percentages of spore samples at the end of germination incubations were 95 and 68% in panels A and B, respectively.
For B. cereus spores germinating with 40 mM NH4Cl and 6 mM l-alanine, heat activation also increased rates of commitment and DPA release and slightly shortened Δ50 and Δ75 values (Fig. 2B; Table 2). Since heat activation accelerated the commitment and germination of spores of both B. cereus and B. subtilis, this suggests that a major effect of heat activation is to decrease the time required to obtain commitment after mixing Bacillus spores with germinants (see Discussion).
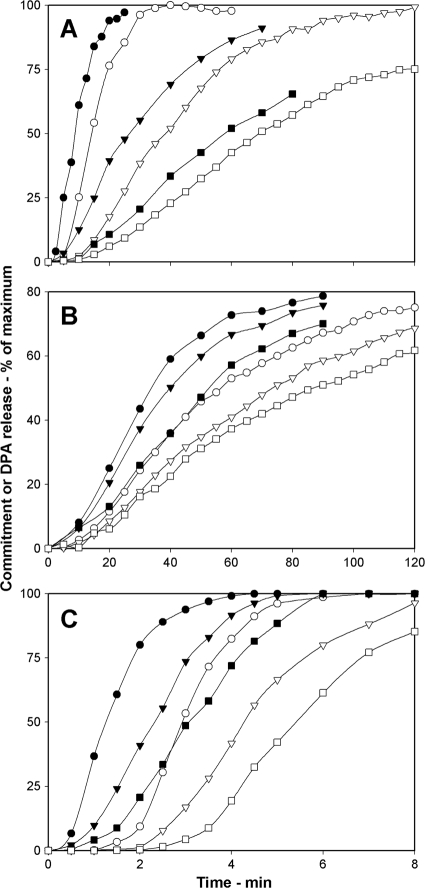
Effects of germinant concentrations on commitment.
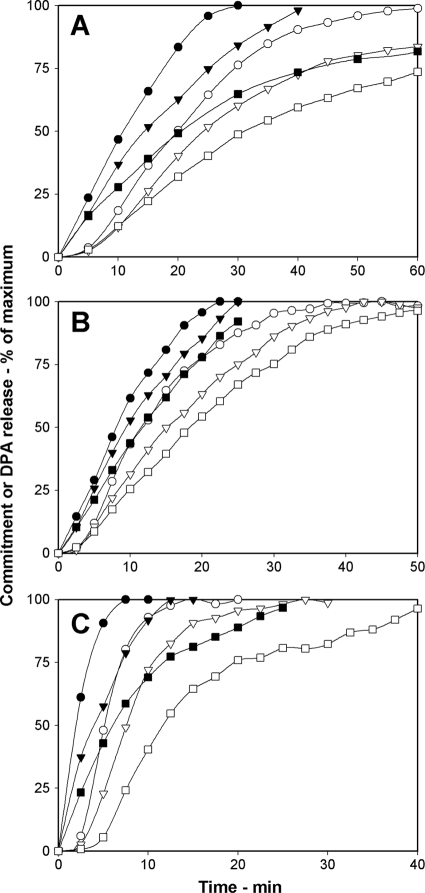
Another factor that affects rates of spore germination is the germinant concentration (1, 21, 25). Binding of nutrient germinants to GRs triggers commitment and subsequent germination events, including DPA release, although the entire signal transduction pathway in this process is not known. However, it seems likely that a low nutrient germinant concentration will generate only a low level of GR-bound germinants and thus give decreased rates of both commitment and DPA release. To test this prediction, we examined spore commitment and DPA release in both B. subtilis and B. cereus spores germinating with different concentrations of several nutrient germinants. For wild-type B. subtilis spores germinating with l-valine, times for spores to reach various levels of commitment and DPA release increased as the l-valine concentration decreased (Fig. 3 A; Table 3). In addition, the lag times between commitment and DPA release also increased as the germinant concentration decreased (Table 3). Similar behavior was seen with wild-type B. subtilis and B. cereus spores germinating with various concentrations of AGFK (Fig. 3B; Table 3) or l-alanine, respectively (Fig. 3C; Table 3). These results suggest that germinant concentrations not only affect the commitment step in spore germination but continue to play a role in postcommitment events that affect the lag time between commitment and DPA release.
FIG. 3.
(A to C) Effects of germinant concentrations on commitment and DPA release during spore germination. Shown are the commitment (•, ▾, ▪) and DPA release (○, ▿, □) of heat-activated wild-type B. subtilis (PS533) spores germinated with various concentrations of l-valine (A) and AGFK (B) and B. cereus spores germinated with 40 mM NH4Cl plus various concentrations of l-alanine (C) as described in Materials and Methods. Circles, triangles, and squares, respectively, denote l-valine concentrations of 10, 2, and 1 mM (A), concentrations of 10, 5, and 1.5 mM each component of AGFK (B), and l-alanine concentrations of 6, 1, and 0.3 mM (C). The maximum number of RFU that each germination assay reached upon germination for 6 h in panels A and B and for 30 min in panel C was set at 100%, but the actual germination percentages of spore samples at the end of germination incubations were 95, 82, and 54% in panel A, 85, 68, and 21% in panel B, and 95, 68, and 41% in panel C, going from the highest to the lowest germinant concentrations.
TABLE 3.
Effects of germinant concentrations on the timing of commitment and DPA release and lag times between commitment and DPA release during nutrient germination of Bacillus spores
| Spores, germinant (concn [mM]) | Time (min)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| C25 | G25 | Δ25 | C50 | G50 | Δ50 | C75 | G75 | Δ75 | |
| Wild-type B. subtilis, (l-Val) | |||||||||
| 10 | 5 | 10 | 5 | 8.6 | 14.2 | 5.6 | 13.2 | 19.6 | 6.4 |
| 2 | 15 | 23.8 | 8.8 | 26.5 | 38.2 | 11.7 | 45.6 | 56 | 10.4 |
| 1 | 33.8 | 42.3 | 8.5 | 57.5 | 69 | 11.5 | 93 | 116 | 23 |
| Wild-type B. subtilis, AGFK | |||||||||
| 10 | 20 | 30.5 | 10.5 | 34 | 56 | 22 | 70 | 112 | 42 |
| 5 | 22.5 | 37.5 | 15 | 40 | 73 | 33 | 85 | 152 | 67 |
| 1.5 | 29.2 | 42 | 12.8 | 52.5 | 86 | 33.5 | 102 | 165 | 63 |
| B. cereus, (l-Ala) | |||||||||
| 6 | 0.8 | 2.4 | 1.6 | 1.3 | 2.9 | 1.7 | 1.9 | 3.1 | 1.2 |
| 1 | 1.6 | 3.4 | 1.9 | 2.3 | 4.3 | 2.0 | 3.7 | 5.6 | 2.0 |
| 0.3 | 2.2 | 4.2 | 2.0 | 3.1 | 5.4 | 2.3 | 4.12 | 6.8 | 2.7 |
The rates of commitment and DPA release when wild-type B. subtilis spores germinated in AGFK were significantly slower than when these spores germinated in l-valine (Fig. 3; Table 3). For example, the C50 and G50 values with saturating AGFK levels (10 mM each component of AGFK) were 34 and 56 min, respectively, while the C50 and G50 values with saturating l-valine (10 mM) were 9 and 14 min, respectively; the lag times between commitment and DPA release with AGFK were also longer than those with l-valine (Table 3). These differences suggest first that the AGFK germination pathway that uses the GerB and GerK GRs is not as effective in triggering germination as the GerA pathway stimulated by l-alanine or l-valine and further that the levels and identity of activated GRs are important factors in determining not only the rate of commitment but also that of at least one postcommitment event, DPA release, as noted above.
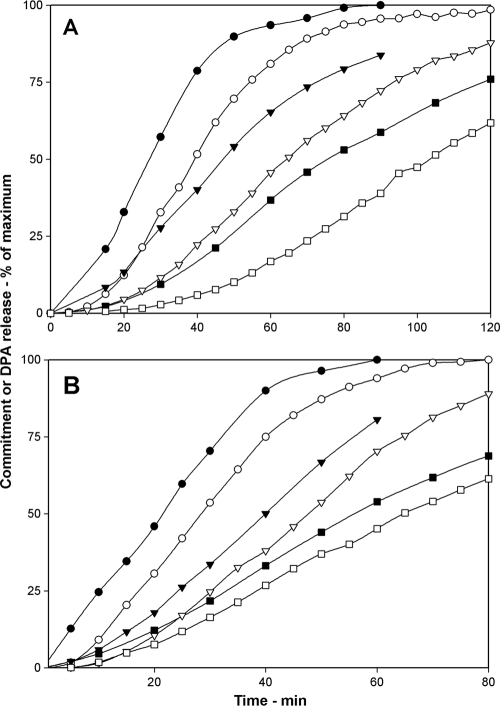
Effects of elevated GR levels on commitment.
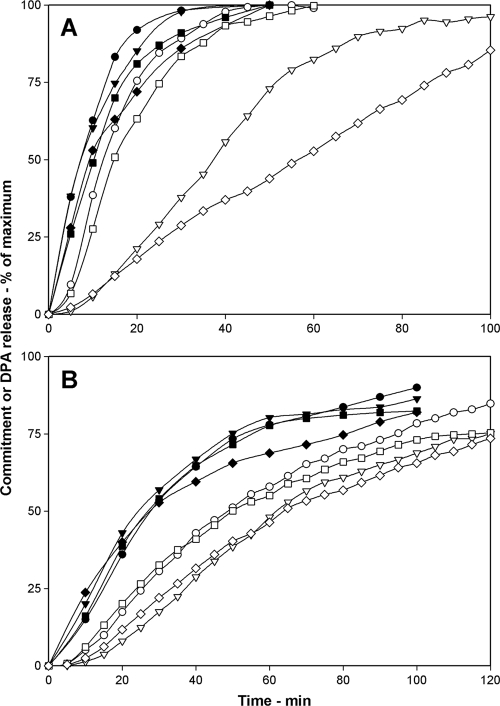
The above results are consistent with the idea that low germinant concentrations result in a lower level of activated GRs and thus trigger commitment and DPA release at a lower rate. If, as seems likely, the binding of nutrient germinants to GRs is what triggers the commitment step in spore germination, then a simple prediction is that raising the spores' levels of GRs should increase rates of both commitment and DPA release. To test this prediction, we compared commitment and DPA release during the germination of B. subtilis spores with wild-type levels of the GerA receptor and spores in which the gerA operon had been expressed under the control of the sspD promoter, a promoter that is stronger than the gerA promoter and generates levels of the GerA GR in spores that are likely ≥10-fold higher than those in wild-type spores (3). When germinating with comparable l-valine concentrations, the times to reach various levels of commitment and DPA release of spores with elevated GerA GR levels were consistently shorter than those of wild-type spores, as were the lag times between commitment and DPA release (Fig. 4; Table 4).
FIG. 4.
(A, B) Effects of elevated levels of the GerA GR on commitment and DPA release during B. subtilis spore germination. Commitment (•, ▾, ▪) and DPA release (○, ▿, □) of heat-activated spores of B. subtilis strains PS533 (wild type) (A) and PS3476 (PsspD::gerA) (B) germinating with various concentrations of l-valine were measured as described in Materials and Methods. Circles, triangles, and squares, respectively, denote l-valine concentrations of 10, 4, and 1 mM (A) or 10, 2, and 0.5 mM (B). The maximum number of RFU that each germination assay reached upon germination for 5 h in panel A and for 3 h in panel B was set at 100%, but the actual germination percentages of spore samples at the end of germination incubations were 95, 90, and 45% in panel A and 95, 86, and 74% in panel B, going from the highest to the lowest l-valine concentration. Note that the PS533 spore preparation used in the experiment shown is different from the one used in the experiment shown in Fig. 3. However, differences in rates of commitment and DPA release for l-valine germination between B. subtilis spore preparations did not affect the conclusions made in this work about factors important in determining the timing of commitment and DPA release for spore populations.
TABLE 4.
Effects of elevated levels of the GerA GR on the timing of commitment and DPA release and lag times between commitment and DPA release during nutrient germination of B. subtilis spores
| B. subtilis strain, germinant, concn (mM) | Time (min)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| C25 | G25 | Δ25 | C50 | G50 | Δ50 | C75 | G75 | Δ75 | |
| Wild type, l-Val | |||||||||
| 10 | 17.2 | 26.5 | 9.3 | 26.8 | 39.5 | 12.7 | 38 | 54.2 | 16.2 |
| 4 | 28 | 42.5 | 14.5 | 47.5 | 64.5 | 17 | 72.5 | 93.7 | 21.2 |
| 1 | 48.5 | 71.8 | 23.3 | 74.8 | 103.5 | 28.7 | 118 | 155 | 37.5 |
| PsspD::gerA, l-Val | |||||||||
| 10 | 10 | 17.2 | 7.2 | 21.5 | 28.3 | 6.8 | 32 | 40 | 8 |
| 2 | 24 | 30 | 6 | 40 | 47.8 | 7.8 | 55.8 | 64.5 | 8.7 |
| 0.5 | 33 | 38 | 5 | 56 | 65 | 9 | 93 | 104 | 11 |
A similar and more dramatic phenomenon was observed when B. subtilis spores with elevated levels of the GerB* GR (3) were germinated with l-asparagine (Fig. 5; Table 5). In this case, during germination with 1 mM l-asparagine, the times to reach 25, 50, and 75% commitment and DPA release decreased ≥50% with spores that had a 20-fold elevated GerB* GR level (strain PS3502) compared to spores in which the gerB operon was expressed from its normal promoter (strain FB10) and decreased even more for spores with 200-fold higher levels of the GerB* GR (strain PS3415). The lag times between commitment and DPA release also decreased significantly in spores with 20- and 200-fold GerB* GR levels and spores with elevated GerB* GR levels were much more responsive to low l-asparagine levels, as reported previously (3) (Table 5). These results show further that, at similar germinant concentrations, spores commit faster when levels of GRs are elevated and that increased average numbers of GRs/spore shorten the lag time between commitment and DPA release.
FIG. 5.
(A to C) Effects of elevated levels of the GerB* GR on commitment and DPA release during B. subtilis spore germination. Commitment (•, ▾, ▪) and DPA release (○, ▿, □) of heat-activated spores of B. subtilis strains FB10 (gerB*) (A), PS3502 (PsspD::gerB*) (B), and PS3415 (PsspB::gerB*) (C) germinating in various concentrations of l-asparagine were measured as described in Materials and Methods. Circles, triangles, and squares denote l-asparagine concentrations of 10, 1, and 0.25 mM (A), 10, 1, and 0.25 mM (B), and 1, 0.2, and 0.05 mM (C), respectively. The maximum number of RFU that each germination assay reached upon germination for 2 h in panels A and B and for 1 h in panel C was set at 100%, but the actual germination percentages of spore samples at the end of germination incubations were 95, 89, and 38% in panel A, 95, 88, and 48% in panel B, and 95, 95, and 46% in panel C, going from the highest to the lowest l-asparagine concentration.
TABLE 5.
Effects of elevated levels of the GerB* GR on the timing of commitment and DPA release and lag times between commitment and DPA release in B. subtilis spores
| B. subtilis strain, germinant, concn (mM) | Time (min)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| C25 | G25 | Δ25 | C50 | G50 | Δ50 | C75 | G75 | Δ75 | |
| FB10, l-Asn | |||||||||
| 10 | 5.3 | 11.8 | 6.5 | 11.8 | 20 | 8.2 | 17.6 | 24.4 | 6.8 |
| 1 | 8.8 | 14.6 | 5.8 | 14.2 | 24.1 | 9.9 | 25 | 42 | 17 |
| 0.25 | 8.8 | 16.3 | 7.5 | 20.3 | 31 | 10.7 | 42 | 62.5 | 20.5 |
| PS3502, l-Asn | |||||||||
| 10 | 4.4 | 7 | 2.6 | 8 | 11.8 | 3.8 | 13.4 | 18.6 | 5.2 |
| 1 | 4.8 | 8.1 | 3.3 | 9.4 | 15 | 5.6 | 16.2 | 25 | 8.8 |
| 0.25 | 5.8 | 9.8 | 4 | 11.5 | 18.1 | 6.6 | 19 | 30 | 11 |
| PS3415, l-Asn | |||||||||
| 1 | 1 | 3.8 | 2.8 | 2 | 5.2 | 3.2 | 3.4 | 7 | 3.6 |
| 0.2 | 1.5 | 5.3 | 3.8 | 4 | 7.6 | 3.6 | 7 | 10.5 | 3.5 |
| 0.05 | 2.7 | 7.6 | 4.9 | 6 | 11.6 | 5.6 | 11.7 | 19.6 | 7.9 |
Ct, Gt, and Δt values were determined from data in Fig. 5A and B.
Effect of CLEs on commitment.
The results described above indicate that DPA release during germination is accelerated as a result of faster commitment, with the latter due to either heat activation or increases in germinant concentration or average GR levels/spore. Previous reports have suggested that at least one CLE is also involved in modulating rates of DPA release during spore germination, presumably by degrading or modifying spores' peptidoglycan cortex (11, 16, 23). Spores of Bacillus species have two redundant CLEs, CwlJ and SleB, but only CwlJ appears to have a significant effect on rates of DPA release during germination (22, 23, 25). While rates of DPA release during germination of individual wild-type and sleB mutant spores are identical, these rates are much slower with individual cwlJ or cwlJ sleB mutant spores (22, 23). If the action of CLEs during germination occurs after the commitment step, then the absence of one or both CLEs should not affect rates of commitment. To test this prediction, we germinated cwlJ, sleB, and cwlJ sleB mutant B. subtilis spores in different nutrient germinants and compared the rates of commitment and DPA release of these spores with those of wild-type spores. For spores germinating with l-alanine, DPA release with cwlJ and cwlJ sleB mutant spores was 3- to 4-fold slower than with wild-type spores, while sleB mutant spores released DPA at a rate similar to that of wild-type spores, consistent with previous work (22) (Fig. 6 A; Table 6). Strikingly, despite differences in the rates of DPA release, the rates of commitment of all three mutant spores were essentially identical to those of wild-type spores, reaching 50% commitment within 7 to 10 min (Fig. 6A). Consequently, the cwlJ and cwlJ sleB mutant spores had much longer lag times between commitment and DPA release during l-alanine germination than did wild-type and sleB mutant spores (Table 6).
FIG. 6.
(A, B) Effects of CLEs on commitment and DPA release during B. subtilis spore germination. Commitment (•, ▾, ▪, ⧫) and DPA release (○, ▿, □, ⋄) of heat-activated spores of B. subtilis strains PS533 (wild type) (•,○), FB111 (cwlJ) (▾, ▿), FB112 (sleB) (▪, □), and FB113 (cwlJ sleB) (⧫, ⋄) germinating in 2 mM l-alanine (A) or 10 mM AGFK (B) were measured as described in Materials and Methods. The maximum number of RFU that each germination assay reached upon germination for 3 h in panel A and for 6 h in panel B was set at 100%, and saturating concentrations of l-alanine or AGFK were used in order to achieve maximum germination. Note that different preparations of PS533 spores were used in the experiments shown here and in Fig. 3 and 4.
TABLE 6.
Effects of CLEs on the timing of commitment and DPA release and lag times between commitment and DPA release during nutrient germination of B. subtilis spores
| Germinant (concn [mM]), strain | Time (min)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| C25 | G25 | Δ25 | C50 | G50 | Δ50 | C75 | G75 | Δ75 | |
| l-Ala (2) | |||||||||
| WTb | 3 | 7.9 | 4.9 | 7.2 | 12.3 | 5.1 | 13 | 19.8 | 6.8 |
| cwlJ | 3 | 22.2 | 19.2 | 7.2 | 37.3 | 30.1 | 15.2 | 52.5 | 37.3 |
| sleB | 4.8 | 9.5 | 4.7 | 10.3 | 14.8 | 4.5 | 16.8 | 25.2 | 8.4 |
| cwlJ sleB | 4.7 | 26 | 21.3 | 9.3 | 56 | 46.7 | 22 | 88 | 66 |
| AGFK (10) | |||||||||
| WT | 15 | 25.5 | 10.5 | 27.5 | 48.6 | 21.1 | 52.5 | 94.3 | 41.8 |
| cwlJ | 12 | 37 | 25 | 24 | 62 | 38 | 50 | 120 | 70 |
| sleB | 14 | 23.8 | 9.8 | 26.7 | 48.5 | 21.8 | 55.6 | 110 | 54.4 |
| cwlJ sleB | 10.5 | 33.3 | 22.8 | 27.5 | 64 | 36.5 | 80 | 124 | 44 |
AGFK was also used as a germinant to compare the commitment and DPA release of wild-type spores with those of spores lacking one or both CLEs. Similar to what was observed with l-alanine germination, during AGFK germination, both wild-type and CLE-deficient spores committed at similar rates, with C50 values ranging from 24 to 28 min (Fig. 6B; Table 6). During AGFK germination, DPA release with cwlJ and cwlJ sleB mutant spores was also slower than with wild-type and sleB mutant spores, but only slightly slower, perhaps because germination events are much slower during AGFK germination (Fig. 6B; Table 6). These results suggest that the absence of the CLE CwlJ does not alter the spore's ability to commit to germination but does slow postcommitment events, in particular, DPA release.
DISCUSSION
Three major conclusions from this work concern the factors that determine rates of commitment in the germination of spores of Bacillus species, (i) heat activation, (ii) nutrient germinant concentrations, and (iii) spores' levels of appropriate GRs, since commitment was enhanced by heat activation prior to germination, increased concentrations of nutrient germinants, and increased levels of GRs. Heat activation is known to potentiate spore germination (13) and has been reported to increase the rate of commitment of B. megaterium spores (27). However, the mechanism of heat activation is not clear, although the effect is reversible and may involve effects on the conformation of one or more proteins (13, 31). Since rates of commitment and DPA release were stimulated by heat activation and commitment precedes and is presumably necessary for DPA release, this suggests that a major effect of heat activation is to increase rates of commitment. However, the lag times between commitment and DPA release were also shortened by heat activation, similar to what was seen when average GR levels/spore were increased. These similar effects of heat activation and GR levels/spore are consistent with the idea that heat activation somehow increases GR availability or responsiveness, since such effects would, in effect, increase the number of functional GRs/spore. However, how heat activation might exert such effects is not known.
Nutrient germinant concentrations also affected rates of spore commitment markedly. Again, it seems likely that these effects are due to changes in levels of “activated” GRs, since changes in nutrient germinant concentrations will change the degree of saturation of GRs with their cognate nutrient germinant. It is, however, notable that lag times between commitment and DPA release were not constant at different nutrient germinant concentrations but increased significantly as times for commitment increased. This suggests that “activated” GR levels not only are important in determining commitment rates but also play a role in determining the timing of subsequent DPA release and perhaps even play some direct role in DPA release itself. The latter observation is also consistent with effects of average GR levels/spore on rates of commitment and DPA release, as increased average GR levels/spore resulted not only in more rapid commitment at all nutrient germinant concentrations but also shorter lag times between commitment and DPA release. Together, the three conclusions noted above strongly suggest that level of “activated” GRs/spore is the major factor determining rates of both commitment and DPA release during nutrient germination of spores of Bacillus species.
In contrast to the three factors noted above that play major roles in determining the timing of commitment during spore germination, CLEs appear to be relatively unimportant in commitment, even though action of the CLE CwlJ is clearly essential for rapid DPA release during spore germination. Consequently, even minimal levels of hydrolysis of the spores' peptidoglycan cortex are most likely not required for the commitment step. The results obtained with cwlJ mutant spores also indicate that commitment does not require the release of even minute levels of DPA from spores.
Another minor conclusion from the current work is that DPA release during spore germination takes place at similar rates at both pH 7.4 and pH 3.6, since committed spores released significant DPA following acidification at the same rate as spores maintained at pH 7.4. This was somewhat surprising in that CwlJ action is essential for fast DPA release, but perhaps CwlJ retains much activity at pH 3.6, has to cleave only a minimal number of bonds in peptidoglycan to allow rapid DPA release, or is present in large excess over what is needed to allow fast DPA release during germination.
Another minor but potentially important conclusion from the current work is that maximal rates of commitment and DPA release were different during germination triggered by nutrient germinants that target different GRs. This was seen most dramatically when comparing l-alanine and AGFK germination of B. subtilis spores, since the maximum rate of commitment was much lower in AGFK germination, and lag times between commitment and DPA release were also much longer for AGFK germination. This observation suggests that not only is the precise level of “activated” GR important in determining times for commitment and DPA release during spore germination but so is the nature of the GR or GRs involved in germination.
One other notable observation made in this work was that for both unactivated and heat-activated spores, as well as those with elevated GR levels, the lag times between 25% commitment and DPA release were almost always considerably shorter than between 75% commitment and DPA release. The reason for this effect is not clear. However, one possible explanation is that spores committing early in germination are those with the highest GR levels in the spore population, while spores that commit later in germination are those with lower GR levels. This idea is certainly consistent with the effects of average GR numbers/spore on rates of commitment and DPA release noted above. In addition, there is preliminary evidence that individual spores that germinate most rapidly in spore populations are those that have the highest levels of at least one GR (J. Zhang and J. Yu; personal communication). If this idea is indeed correct, it would also implicate variation in the numbers of GRs/spore as a major cause of the significant heterogeneity in the rates of germination of individual spores in spore populations, as has been suggested previously (4, 8, 9, 22).
While the current work produced a number of new observations and conclusions, we cannot yet begin to assess the mechanistic reasons for some of the major conclusions. In particular, we do not know (i) how binding of nutrient germinants to GRs causes the triggering of subsequent germination events, (ii) the nature of the event or events that result in the commitment of spores to germinate, and (iii) how commitment leads to DPA release during spore germination. The latter questions are some of the most crucial ones, the answers to which are essential for a thorough understanding of bacterial spore germination.
Acknowledgments
This work was supported by a Multi-University Research Initiative award from the U.S. Department of Defense to P.S.
Footnotes
Published ahead of print on 30 April 2010.
REFERENCES
- 1.Atluri, S., K. Ragkousi, D. E. Cortezzo, and P. Setlow. 2006. Co-operativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this co-operativity by alterations in the GerB receptor. J. Bacteriol. 188:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blocher, J. C., and F. F. Busta. 1985. Inhibition of germinant binding by bacterial spores in acidic environments. Appl. Environ. Microbiol. 50:274-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera-Martinez, R.-M., F. Tovar-Rojo, V. R. Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis J. Bacteriol. 185:2457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, D., S. S. Huang, and Y. Q. Li. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 78:6936-6941. [DOI] [PubMed] [Google Scholar]
- 5.Clements, M. O., and A. Moir. 1998. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 180:6729-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortezzo, D. E., B. Setlow, and P. Setlow. 2004. Analysis of the action of compounds that inhibit the germination of spores of Bacillus species. J. Appl. Microbiol. 96:725-741. [DOI] [PubMed] [Google Scholar]
- 7.Foster, S. J., and K. Johnstone. 1986. The use of inhibitors to identify early events during Bacillus megaterium KM spore germination. Biochem. J. 237:865-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh, S., and P. Setlow. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 191:1787-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh, S., and P. Setlow. 2010. The preparation, germination properties and stability of superdormant spores of Bacillus cereus J. Appl. Microbiol. 108:582-590. [DOI] [PubMed] [Google Scholar]
- 10.Halmann, M., and A. Keynan. 1962. Stages in germination of spores of Bacillus licheniformis J. Bacteriol. 84:1187-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heffron, J. D., E. A. Lambert, N. Sherry, and D. L. Popham. 2010. Contributions of four cortex lytic enzymes to germination of Bacillus anthracis spores. J. Bacteriol. 192:763-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson, K. D., B. M. Corfe, E. H. Kemp, P. J. Coote, and A. Moir. 2001. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J. Bacteriol. 183:4317-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keynan, A., and Z. Evenchick. 1969. Activation, p. 359-396. In G.W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, New York, NY.
- 14.Magge, A., A. C. Granger, P. G. Wahome, B. Setlow, V. R. Vepachedu, C. A. Loshon, L. Peng, D. Chen, Y.-Q. Li, and P. Setlow. 2008. Role of dipicolinic acid in the germination, stability, and viability of spores of Bacillus subtilis J. Bacteriol. 190:4798-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C.R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus John Wiley and Sons, Chichester, United Kingdom.
- 16.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paidhungat, M., and P. Setlow. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 181:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In A.L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its relatives: from genes to cells. American Society for Microbiology, Washington, DC.
- 22.Peng, L., D. Chen, P. Setlow, and Y.-Q. Li. 2009. Elastic and inelastic light scattering from single bacterial spores in an optical trap allows monitoring of spore germination dynamics. Anal. Chem. 81:4035-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setlow, B., L. Peng, C. A. Loshon, Y. Q. Li, G. Christie, and P. Setlow. 2009. Characterization of the germination of Bacillus megaterium spores lacking enzymes that degrade the spore cortex. J. Appl. Microbiol. 107:318-328. [DOI] [PubMed] [Google Scholar]
- 24.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 26.Setlow, P., and E. A. Johnson. 2007. Spores and their significance, p. 35-67. In M. P. Doyle and L. R. Beuchat (ed.), Food microbiology: fundamentals and frontiers, 3rd ed. ASM Press, Washington, DC.
- 27.Stewart, G. S. A. B., K. Johnstone, E. Hagelberg, and D. J. Ellar. 1981. Commitment of bacterial spores to germinate: a measure of the trigger reaction. Biochem. J. 198:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatasubramanian, P., and K. Johnstone. 1989. Biochemical analysis of the Bacillus subtilis 1604 spore germination response. J. Gen. Microbiol. 135:2723-2733. [DOI] [PubMed] [Google Scholar]
- 29.Yang, W. W., and A. Ponce. 2009. Rapid endospore viability assay of Clostridium sporogenes spores. Int. J. Food Microbiol. 133:213-216. [DOI] [PubMed] [Google Scholar]
- 30.Yung, P. T., and A. Ponce. 2008. Fast sterility assessment by germinable-endospore biodosimetry. Appl. Environ. Microbiol. 74:7669-7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, P., P. Setlow, and Y. Li. 2009. Characterization of single heat-activated Bacillus spores using laser tweezers Raman spectroscopy. Opt. Express. 17:16480-16491. [DOI] [PubMed] [Google Scholar]