Abstract
We report that the N terminus of the type III secretion system translocator proteins EspB, EspD, and EspA mediate protein secretion and translocation from wild-type enteropathogenic Escherichia coli and hypersecretion from sepL and sepD mutants. EspA containing the translocation signal of Map and Tir containing the secretion signal of EspA are biologically active.
Deployment of a type III secretion system (T3SS) is an infection strategy used by many human pathogens (reviewed in references 15 and 24). The T3SS is composed of a cytosolic ATPase, inner and outer membrane rings, and an extracellular needle. In Yersinia, LcrV forms a distal needle tip structure (25), while in enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC), the needle is extended by long EspA filaments (9, 19). The substrates of the T3SS are translocator and effector proteins. Translocator proteins of the Yersinia YopB and YopD family form a translocation pore in the host cell plasma membrane that enables effector protein translocation (reviewed in reference 24). Once translocated, the effectors affect host cell functions, interfering with signaling pathways and a broad range of physiological processes (reviewed in references 14 and 16).
In EPEC and EHEC, effector protein translocation into cultured cells leads to the formation of actin-rich pedestal-like structures at the bacterial attachment sites, which can be visu-alized by the fluorescent actin staining test (reviewed in reference 13). The T3SS components in EPEC and EHEC are encoded within the locus of enterocyte effacement (LEE) (23). The LEE also encodes gene regulators, chaperones, the translocators EspB and EspD, the EspA filament subunit EspA, and the effectors Map, EspF, EspG, EspH, EspZ, and Tir (reviewed in references 13 and 16).
The fact that the translocators are required for injection of the effectors suggests the existence of a secretion hierarchy whereby the former are guided to the secretion apparatus prior to the latter. A key unanswered question is how this specificity is achieved (7, 11). An invE mutant of Salmonella (20), a mixC mutant of Shigella (3), and sepD and sepL mutants of EPEC (12, 26) are defective for translocator secretion yet oversecrete effectors. SepD and SepL are LEE-encoded proteins that interact with each other at the bacterial membrane (8, 26). It is not known how this complex functions, although it might sense certain environmental signals to switch from the secretion of translocators to that of effectors (12).
Despite the importance of the T3SS in bacterial pathogenesis, the mechanism involved in the recognition of type III substrates remains poorly understood. Studies conducted over many years focused on the N-terminal ca. 20-amino-acid secretion signal of the effectors. Indeed, recent studies using machine-learning approaches have shown that it is possible to identify T3SS effectors across multiple microorganisms based on the N-terminal secretion signals (1, 27). Although not able to provide a consensus secretion signal, these studies identified sequence biases, conserved structural features, and shared structural elements. In contrast, very little is currently known about the N-terminal secretion signals of translocators. In Yersinia, a mutation in the autocleavable protein YscU, YcsUN236A, did not affect the secretion of effectors but abolished the secretion of YopB, YopD, and LcrV (29). Replacing the N-terminal secretion signal of LcrV with the first 15 amino acids of the effector YopE restored secretion and function, which led the authors to suggest that translocators have a specific N-terminal secretion signal that is distinct from that of effectors (29). The aim of this study was to analyze the role of the N-terminal 20 amino acids of translocators in secretion, translocation, and function using EPEC EspA, EspB, and EspD as model proteins.
We investigated the ability of the N-terminal 20 amino acids of the translocators EspB (20EspB), EspD (20EspD), and EspA (20EspA) to mediate the secretion and translocation of the β-lactamase TEM-1 reporter (4). As positive and negative controls we used TEM fusions with the first 20 residues of the effector EspF (20EspF) (4) and a random sequence (20R) derived from the C terminus of maltose binding protein, respectively. In order to construct 20EspA-TEM, 20EspB-TEM, 20EspD-TEM, 20EspF-TEM, and 20R-TEM (the plasmids used are listed in Table 1), the respective primer pairs (Table 2) were annealed and ligated into pCX340 digested with NdeI/EcoRI. All plasmids were checked by sequencing before they were transformed into the wild-type and mutant EPEC E2348/69 strains listed in Table 1.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Description | Reference | Primers used |
|---|---|---|---|
| Strains | |||
| E2348/69 | Wild-type EPEC O127:H6 | 22 | |
| ICC192 | E2348/69 ΔescN | 17 | |
| ICC305 | E2348/69 ΔsepD | This study | |
| ICC306 | E2348/69 ΔsepL | This study | |
| ICC225 | E2348/69 Δtir | 2 | |
| UMD870 | E2348/69ΔespD::aphA3 | 21 | |
| UMD872 | E2348/69ΔespA::aphA3 | 18 | |
| Plasmids | |||
| pCX340 | pBR322 derivative, cloning vector used to fuse genes to blaM, which encodes the mature form of TEM-1 β-lactamase 3 | 4 | |
| pSA10 | pKK177-3 derivative containing lacIq gene | 28 | |
| pICC460 | Residues 1 to 20 of EspA fused to TEM-1 | This study | For-20EspA/Rev-20EspA |
| pICC492 | Residues 1 to 20 of EspB fused to TEM-1 | This study | For-20EspB/Rev-20EspB |
| pICC493 | Residues 1 to 20 of EspD fused to TEM-1 | This study | For-20EspD/Rev-20EspD |
| pICC461 | 20 random residues fused to TEM-1 | This study | For-20R/Rev-20R |
| pICC459 | Residues 1 to 20 of EspF fused to TEM-1 | This study | For-20EspF/Rev-20EspF |
| pICC285 | pSA10 derivative expressing EspA | 9 | |
| pICC487 | pSA10 derivative expressing residues 1 to 20 of Map fused to Δ20EspA | This study | Eco-Map-EspA/EspA-PstI |
| pICC462 | Fusion of Map to TEM-1 | This study | NdeI-MAP-b/MAP-EcoRI |
| pICC464 | Residues 1 to 20 of EspA fused to Δ20Map-TEM-1 | This study | NdeI-EspA20MAP/MAP-EcoRI |
| pICC476 | Fusion of Tir to TEM-1 | This study | F-KpnI-Tir/R-Tir-EcoRI |
| pICC478 | Residues 1 to 20 of EspA fused to Δ20Tir-TEM-1 | This study | F-KpnI-EspA20Tir/R-Tir-EcoRI |
| pICC481 | pSA10 derivative expressing Tir, HAa tagged | This study | Tir-E69-1/Tir-E69-HA |
| pICC483 | pSA10 derivative expressing residues 1 to 20 of EspA fused to Δ20Tir, HA tagged | This study | EspA20Tir-EcoRI/Tir-E69-HA |
HA, hemagglutinin.
TABLE 2.
Primers used in this study
| Primer | Nucleotide sequence |
|---|---|
| ΔsepL-Kan-fw | 5′-GGTATTGAATTTAATCAAAACCCCGCATCTGTTTTTAATTCTAATTCATTATGTGTAGGCTGGAGCTGCTTCG-3′ |
| ΔsepL-Kan-rv | 5′-AACATCCTCCTTATAATCTATCACTTTACCAATCATTAATAATGTATTACTCATATGAATATCCTCCTTAGTTCC-3′ |
| ΔsepD-Cm-fw | 5′-ATGAACAATAATAATGGCATAGCAAAGAATGATTGTGACTGGCTAACGGCCCATATGAATATCCTCCTTAGTTCC-3′ |
| ΔsepD-Cm-rv | 5′-TTACAACAATTCGTCCTATATCAGAAAACAAGTGTGTTGGCGGCATCATTGTTGTGTAGGCTGGAGCTGCTTCG-3′ |
| For-20EspA | 5′-TATGGATACATCAACTACAGCATCAGTTGCTAGTGCGAATGCGAGTACTTCGACATCAATGGAG-3′ |
| Rev-20EspA | 5′-AATTCTCCATTGATGTCGAAGTACTCGCATTCGCACTAGCAACTGATGCTGTAGTTGATGTATCCA-3′ |
| For-20EspB | 5′-TATGAATACTATCGATAATAACAATGCGGCAATCGCAGTTAATTCTGTTTTGAGCAGCACGGAG-3′ |
| Rev-20EspB | 5′-AATTCTCCGTGCTGCTCAAAACAGAATTAACTGCGATTGCCGCATTGTTATTATCGATAGTATTCA-3′ |
| For-20EspD | 5′-TATGCTTAATGTAAATAACGATATCCAGTCTGTGAGGTCTGGAGCCAGTGCTGCTACGGCTGAG-3′ |
| Rev-20EspD | 5′-AATTCTCAGCCGTAGCAGCACTGGCTCCAGACCTCACAGACTGGATATCGTTATTTACATTAAGCA-3′ |
| For-20R | 5′-TATGAGCGGTCGTCAGACTGTCGATGAAGCCCTGAAAGACGCGCAGACTCGTATCACCAAGGAG-3′ |
| Rev-20R | 5′-AATTCTCCTTGGTGATACGAGTCTGCGCGTCTTTCAGGGCTTCATCGACAGTCTGACGACCGCTCA-3′ |
| For-20EspF | 5′-TATGCTTAATGGAATTAGTAACGCTGCTTCTACACTAGGGCGGCAGCTTGTAGGTATCGCAGAG-3′ |
| Rev-20EspF | 5′-AATTCTCTGCGATACCTACAAGCTGCCGCCCTAGTGTAGAAGCAGCGTTACTAATTCCATTAAGCA-3′ |
| Eco-Map-EspA | 5′-CAGAATTCATGTTTAGTCCAACGGCAATGGTAGGTAGAGCGTTAGCTCAGGCGGTTACACAAACTCTTGCCTATGATTTAGGGAGCATGT-3′ |
| EspA-PstI | 5′-ATTGTCGAAGCTTGGCTGCAGTTATTTACCAAGGGATATTCCTG-3′ |
| NdeI-MAP-b | 5′-GGAATAACATATGTTTAGTCCAACGGCAAT-3′ |
| MAP-EcoRI | 5′-GTGCGAATTCAGCCGAGTATCCTGCACA-3′ |
| NdeI-EspA20MAP | 5′-GGAATAACATATGGATACATCAACTACAGCATCAGTTGCTAGTGCGAATGCGAGTACTTCGACATCAATGAGACCCGCTGTAACCAAGGCTGC-3′ |
| F-KpnI-Tir | 5′-TGGGTACCAAGAAGGAGATATACCATGCCTATTGGTAACCTTGGT-3′ |
| R-Tir-EcoRI | 5′-GTGCGAATTCTCAACGAAACGTACTGGTCCCG-3′ |
| F-KpnI-EspA20Tir | 5′-TGGGTACCAAGAAGGAGATATACCATGGATACATCAACTACAGCATCAGTTGCTAGTGCGAATGCGAGTACTTCGACATCAATGCCACTACCTTCACAAACAGA-3′ |
| Tir-E69-1 | 5′-TGTGAATTCATGCCTATTGGTAACCTTGG-3′ |
| Tir-E69-HA | 5′-AAGCGTCGACAGTCGACTTAAGCGTAATCTGGAACATCGTATGGGTATGCACCAACGAAACGTACTGGTCCCGGCGT-3′ |
| EspA20Tir-EcoRI | 5′-ACAGAATTCATGGATACATCAACTACAGCATCAGTTGCTAGTGCGAATGCGAGTACTTCGACATCAATGCCACTACCTTCACAAACAGACGG-3′ |
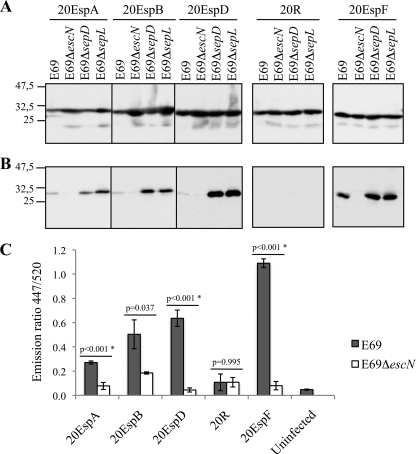
We expressed the 20EspA-TEM, 20EspB-TEM, and 20EspD-TEM fusions along with control 20R-TEM and 20EspF-TEM fusions in wild-type EPEC strain E2348/69, a ΔescN (encoding the ATPase) mutant, and sepD and sepL EPEC mutants (Table 1), which were constructed using the one-step λ red recombination system (10). Briefly, pKD3 and pKD4 were used as PCR templates to amplify the chloramphenicol (sepD) or kanamycin (sepL) resistance gene using primers containing 50-bp sepD and sepL sequences (Table 2). The PCR products were electroporated into wild-type EPEC carrying pKD46, and the mutants were selected on LB plates with chloramphenicol at 2.5 μg/ml or kanamycin at 20 μg/ml. Analysis of proteins secreted into the culture supernatants was done once the bacterial strains were grown overnight in LB containing antibiotics at 37°C with shaking. The cultures were diluted 1/50 in 15 ml Dulbecco's modified Eagle's medium (DMEM; serum free, low glucose [1 g/liter]) and grown at 37°C at 100 rpm to an optical density at 600 nm (OD600) of 0.4 to 0.5. A 1 mM concentration of isopropyl β-d-thiogalactopyranoside (IPTG) was added, and the incubation was continued for an additional 3 h under the same conditions. Five OD600 units of each culture was centrifuged at 4,000 rpm for 10 min, and the pellets were resuspended in 1 ml sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. The supernatant was filtered through 0.2-μm filters into Amicon Ultra-15 centrifugal filter devices (UFC 900524; Millipore) and concentrated to 1 ml. Trichloroacetic acid was added to a final concentration of 20%, and the samples were incubated on ice overnight. The samples were then centrifuged, washed with cold acetone, and resuspended in 35 μl SDS-PAGE loading buffer. Following gel electrophoresis, the samples were analyzed by Western blotting using anti-TEM-1 antibody as described previously (4). This showed the comparable expression of all of the TEM fusion proteins in the different EPEC strains (Fig. 1 A). Analyzing the culture supernatants revealed that 20EspA-TEM, 20EspB-TEM, and 20EspD-TEM were secreted at low levels from wild-type EPEC compared with the 20EspF-TEM control. However, secretion of the translocator N-terminal TEM fusions from either the sepD or the sepL mutant was greatly enhanced compared to secretion from wild-type EPEC (Fig. 1B). The control 20R-TEM was not secreted from any of the strains. Secretion of all of the TEM fusions from the EPECΔescN control was undetectable.
FIG. 1.
Secretion and translocation of translocator N-terminal sequences fused to TEM. (A) Analysis of whole-cell lysates reveals comparable expression levels of the TEM fusions in the different EPEC E2348/69 (E69) strains. (B) Secretion profiling reveal that 20EspA, 20EspB, and 20EspD, but not 20R, can mediate TEM secretion from wild-type EPEC, which was enhanced when the fusions were expressed in the sepD and sepL mutants. 20EspF was included as a positive control. Neither protein was secreted from the escN mutant. Molecular mass markers (in kilodaltons) are indicated to the left. (C) 20EspA, 20EspB, and 20EspD, but not 20R, mediate TEM protein translocation from wild-type EPEC. 20EspF was included as a positive control. The data presented are averages of triplicate values obtained from two independent experiments. The statistical significance (*) of the difference between E69 and E69ΔescN expressing the indicated TEM fusion (horizontal lines) was determined by Student's t test.
These results show, for the first time, that the N-terminal sequences of translocators and EspA also contain a signal that can mediate the secretion of a bystander reporter. Secretion of TEM by the EspA, EspB, and EspD signals is specific, as a random sequence did not allow protein secretion. These results are different from those reported by Chiu et al. (5), who showed that the first 21 amino acids of EspB from EHEC do not mediate the secretion of a reporter. The apparent disagreement could be due to the different reporter protein employed, the detection sensitivity, or the fact that the sequences of the first 20 amino acids of the EPEC and EHEC EspB proteins are only 55% identical. Nonetheless, our results suggest that the SepL/D complex functions as a checkpoint assessing N-terminal secretion signals and/or recognizes downstream translocator sequences.
Next we investigated if the 20EspA-TEM, 20EspB-TEM, and 20EspD-TEM fusions are translocated into HeLa cells. 20R-TEM and 20EspF-TEM fusions were used as negative and positive controls, respectively. Cultures of wild-type EPEC, the ΔescN mutant, or ΔespD mutant strain UMD870 (Table 1), which served as an additional negative control (sepD and sepL mutants were not analyzed, as they are deficient in protein translocation), expressing each of the TEM fusions were primed in DMEM for 3 h, conditions that are conducive to LEE gene expression (6), prior to the infection of HeLa cells. After 90 min, the cells were washed, the β-lactamase substrate CCF2/AM was added, and the cultures were incubated for an additional 90 min as previously described (4). Translocation was expressed as the emission ratio at 447/520 nm to normalize the β-lactamase activity.
20EspA-TEM, 20EspB-TEM, and 20EspD-TEM were translocated into HeLa cells from wild-type EPEC, albeit at lower efficiency than 20EspF-TEM. The translocation efficiency order of the different TEM fusions was 20EspA < 20EspB < 20EspD < 20EspF. The control 20R-TEM was not translocated. Neither protein was translocated from the ΔescN (Fig. 1C) or ΔespD (data not shown) negative control. These results show, also for the first time, that the N-terminal secretion signal of translocators, when expressed in the context of a reporter, can mediate protein translocation.
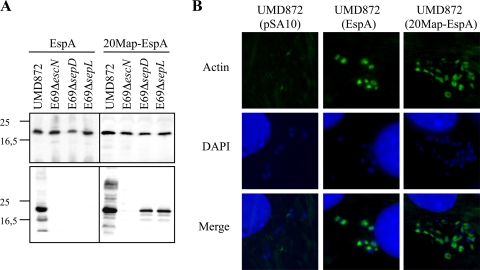
We next investigated the specificity of the N-terminal secretion signal of EspA. Toward this end, we generated a version of pSA10 expressing native EspA (9) in which 20EspA was replaced with the first 20 residues of Map (20Map). The gene encoding EspA was amplified by PCR (the primers used are listed in Table 2) using EPEC strain E2348/69 genomic DNA as a template with Pfu polymerase. The resulting PCR product was digested with EcoRI/PstI, cloned into pSA10, and transformed into host strains. We first analyzed protein secretion from ΔespA mutant EPEC strain UMD872 (Table 1) complemented with a plasmid encoding wild-type EspA and 20Map-EspA. As SepD and SepL are essential for the secretion of translocators but not effectors (12, 26), we also analyzed the secretion profile from the sepD and sepL mutants. EPECΔescN was used as a negative control. EspA was detected by Western blotting with rabbit polyclonal EPEC EspA antiserum (19), followed by anti-rabbit peroxidase conjugate (Sigma) as a secondary antibody. Western blot analysis confirmed that the EspA variants are expressed in these host strains (Fig. 2 A, top panel). Native EspA and 20Map-EspA were secreted from UMD872 but not from the control ΔescN mutant strain (Fig. 2A, bottom panel). Importantly, while native EspA was not secreted from the sepD or sepL mutant, 20Map-EspA was secreted by both mutant strains (Fig. 2A, bottom panel). These results show that when the secretion signal of EspA is replaced with 20Map it gains partial effector characteristics.
FIG. 2.
20Map can replace the secretion signal of EspA. (A, top panel) Shown are comparable protein expression levels in whole-cell lysates of the different EPEC E2348/69 (E69) strains. (A, bottom panel) Native EspA is secreted from EPECΔespA (UMD872) but not from the escN, sepD, and sepL mutants, while 20Map-EspA is secreted from UMD872 and sepD and sepL mutants. Molecular mass markers (in kilodaltons) are indicated to the left. (B) 20Map-EspA is biologically active, as judged by its ability to complement an espA mutant in terms of actin polymerization at the bacterial attachment sites. Actin was labeled using Oregon Green-conjugated phalloidin.
We investigated if 20Map-EspA is biologically active. Toward this end, Swiss mouse 3T3 cells were infected with primed EPEC UMD872 expressing EspA, 20Map-EspA, or the empty vector pSA10. After 3 h of infection, cells were washed, fixed with paraformaldehyde, and stained for immunofluorescence microscopy with Oregon Green-conjugated phalloidin (Invitrogen) and 0.1% 4′,6-diamidino-2-phenylindole (DAPI) diluted in phosphate-buffered saline (PBS)-donkey serum (Jackson ImmunoResearch) for 45 min. Cells were washed three times with PBS, mounted in Dako mountain medium, and examined by conventional epifluorescence microscopy using a Zeiss Axio Imager microscope. 20Map-EspA restored the ability of UMD872 to trigger actin polymerization as a marker of protein translocation to the same extent as native EspA (Fig. 2B). These results are consistent with the data of Sorg et al. (29) that showed that the 15 amino acids of YopE are functionally exchangeable with the N-terminal amino acids of LcrV.
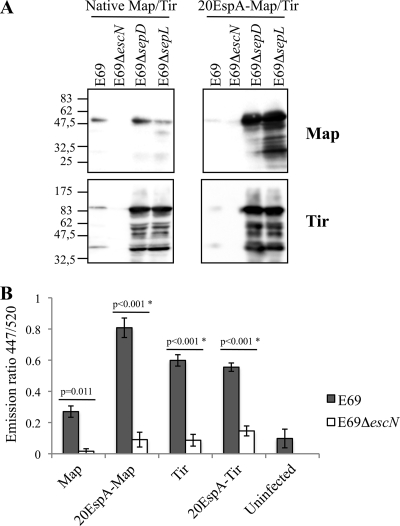
We investigated if the secretion signal of an effector is exchangeable with that of EspA. Toward this end, we replaced the N-terminal secretion signals of Tir and Map with 20EspA. The genes encoding Tir and Map were amplified by PCR (the primers used are listed in Table 2) using E2348/69 genomic DNA as the template. The resulting PCR products were digested with KpnI/EcoRI or NdeI/EcoRI (for tir and map, respectively), cloned into the same restriction sites in pCX340, and transformed into the wild-type and mutant E2348/69 strains. Analysis of whole-cell extracts revealed that replacing the native secretion signal sequences with 20EspA did not affect protein expression levels or stability (data not shown). Analyzing the secretion profiles has shown that the native effector-TEM fusions were secreted from wild-type EPEC but not from the ΔescN mutant (Fig. 3 A). Secretion of native Tir-TEM, but importantly not of Map-TEM, from the sepD and sepL mutants was dramatically enhanced (Fig. 3A). 20EspA-Map and 20EspA-Tir fusions were secreted at low levels from wild-type EPEC, but enhanced secretion from the sepD and sepL mutants was observed. For reasons that are currently not known, secretion of 20EspA-Map was particularly enhanced when it was expressed in the sepD and sepL mutants (Fig. 3A).
FIG. 3.
Secretion and translocation of native and 20EspA-Map/Tir-TEM fusions. (A) Native Map and Tir and 20EspA-Map and 20EspA-Tir fused to TEM were secreted from wild-type EPEC E2348/69 (E69); enhanced secretion of native Tir, 20EspA-Map, and 20EspA-Tir from the sepD and sepL mutants was detected. Neither protein was secreted from the escN mutant. Molecular mass markers (in kilodaltons) are indicated to the left. (B) A translocation assay using wild-type EPEC revealed that 20EspA enhanced the translocation of Map, while the translocation of 20EspA-Tir remained at the same levels as that of native Tir. Neither effector was translocated from the escN mutant. The data presented are averages of triplicate values obtained from two independent experiments. The statistical significance (*) of the difference between E69 and E69ΔescN expressing the indicated TEM fusion (horizontal lines) was determined by Student's t test.
In order to determine if 20EspA-Map and 20EspA-Tir are translocated into HeLa cells, they were expressed in wild-type EPEC; the ΔescN and ΔespD mutants were used as negative controls. 20EspA-Map and 20EspA-Tir were translocated from wild-type EPEC, while no translocation from EPECΔescN (Fig. 3B) or EPECΔespD (data not shown) was detected. Interestingly, compared with the native secretion signal, 20EspA enhanced the translocation efficiency of Map (Fig. 3B) while translocation of 20EspA-Tir remained at the same levels as that of native Tir.
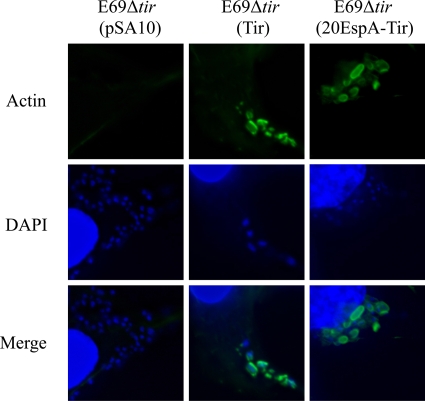
Finally, we tested if 20EspA fused to an effector can mediate protein translocation by expressing 20EspA-Tir in EPECΔtir. However, as the Tir-TEM fusion did not complement EPECΔtir (data not shown), we generated a pSA10 derivative encoding 20EspA-Tir; native Tir and the empty vector were used as controls (the primers used are listed in Table 2). Infection of Swiss mouse 3T3 cells with primed bacteria has shown that while the empty pSA10 vector was unable to complement EPECΔtir, both native Tir and 20EspA-Tir complemented the mutant, as measured by the ability of the strains to trigger actin polymerization at the bacterial attachment sites (Fig. 4).
FIG. 4.
20EspA-Tir is biologically active. EPECΔtir containing pSA10 encoding native Tir or 20EspA-Tir, but not the empty vector, triggers actin polymerization in infected Swiss mouse 3T3 cells. Actin was labeled using Oregon Green-conjugated phalloidin.
In conclusion, our data show that the N termini of translocators (EspB and EspD) and a structural needle tip protein (EspA) contain an N-terminal secretion signal that, when expressed in the context of a reporter or an effector, can mediate protein translocation.
Acknowledgments
We thank Mona Singh and Navjyot Sangha for preliminary data.
This project was supported by the Wellcome Trust.
Footnotes
Published ahead of print on 16 April 2010.
REFERENCES
- 1.Arnold, R., S. Brandmaier, F. Kleine, P. Tischler, E. Heinz, S. Behrens, A. Niinikoski, H. W. Mewes, M. Horn, and T. Rattei. 2009. Sequence-based prediction of type III secreted proteins. PLoS Pathog. 5:e1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, C. N., V. F. Crepin, M. A. Jepson, A. Arbeloa, and G. Frankel. 2009. The mechanisms used by enteropathogenic Escherichia coli to control filopodia dynamics. Cell. Microbiol. 11:309-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botteaux, A., M. P. Sory, L. Biskri, C. Parsot, and A. Allaoui. 2009. MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 71:449-460. [DOI] [PubMed] [Google Scholar]
- 4.Charpentier, X., and E. Oswald. 2004. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J. Bacteriol. 186:5486-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu, H. J., W. S. Lin, and W. J. Syu. 2003. Type III secretion of EspB in enterohemorrhagic Escherichia coli O157:H7. Arch. Microbiol. 180:218-226. [DOI] [PubMed] [Google Scholar]
- 6.Collington, G. K., I. W. Booth, and S. Knutton. 1998. Rapid modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut 42:200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creasey, E. A., R. M. Delahay, A. A. Bishop, R. K. Shaw, B. Kenny, S. Knutton, and G. Frankel. 2003. CesT is a bivalent enteropathogenic Escherichia coli chaperone required for translocation of both Tir and Map. Mol. Microbiol. 47:209-221. [DOI] [PubMed] [Google Scholar]
- 9.Crepin, V. F., R. Shaw, C. M. Abe, S. Knutton, and G. Frankel. 2005. Polarity of enteropathogenic Escherichia coli EspA filament assembly and protein secretion. J. Bacteriol. 187:2881-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deane, J. E., P. Abrusci, S. Johnson, and S. M. Lea. 2010. Timing is everything: the regulation of type III secretion. Cell. Mol. Life Sci. 67:1065-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng, W., Y. Li, P. R. Hardwidge, E. A. Frey, R. A. Pfuetzner, S. Lee, S. Gruenheid, N. C. Strynakda, J. L. Puente, and B. B. Finlay. 2005. Regulation of type III secretion hierarchy of translocators and effectors in attaching and effacing bacterial pathogens. Infect. Immun. 73:2135-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 14.Galán, J. E. 2009. Common themes in the design and function of bacterial effectors. Cell Host Microbe 5:571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galán, J. E., and H. Wolf-Watz. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567-573. [DOI] [PubMed] [Google Scholar]
- 16.Garmendia, J., G. Frankel, and V. F. Crepin. 2005. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 73:2573-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garmendia, J., A. D. Phillips, M. F. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. K. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 6:1167-1183. [DOI] [PubMed] [Google Scholar]
- 18.Kenny, B., L.-C. Lai, B. B. Finlay, and M. S. Donnenberg. 1996. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol. Microbiol. 20:313-323. [DOI] [PubMed] [Google Scholar]
- 19.Knutton, S., I. Rosenshine, J. M. Pallen, I. Nisan, C. B. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubori, T., and J. E. Galán. 2002. Salmonella type III secretion-associated protein InvE controls translocation of effector proteins into host cells. J. Bacteriol. 184:4699-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai, L. C., L. A. Wainwright, K. D. Stone, and M. S. Donnenberg. 1997. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect. Immun. 65:2211-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet 1(8074):1119-1122. [DOI] [PubMed] [Google Scholar]
- 23.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. U. S. A. 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mota, L. J., and G. R. Cornelis. 2005. The bacterial injection kit: type III secretion systems. Ann. Med. 37:234-249. [DOI] [PubMed] [Google Scholar]
- 25.Mueller, C. A., P. Broz, S. A. Muller, P. Ringler, F. Erne-Brand, I. Sorg, M. Kuhn, A. Engel, and G. R. Cornelis. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674-676. [DOI] [PubMed] [Google Scholar]
- 26.O'Connell, C. B., E. A. Creasey, S. Knutton, S. Elliott, L. J. Crowther, W. Luo, M. J. Albert, J. B. Kaper, G. Frankel, and M. S. Donnenberg. 2004. SepL, a protein required for enteropathogenic Escherichia coli type III translocation, interacts with secretion component SepD. Mol. Microbiol. 52:1613-1625. [DOI] [PubMed] [Google Scholar]
- 27.Samudrala, R., F. Heffron, and J. E. McDermott. 2009. Accurate prediction of secreted substrates and identification of a conserved putative secretion signal for type III secretion systems. PLoS Pathog. 5:e1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlosser-Silverman, E., M. Elgrably-Weiss, I. Rosenshine, R. Kohen, and S. Altuvia. 2000. Characterization of Escherichia coli DNA lesions generated within J774 macrophages. J. Bacteriol. 182:5225-5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorg, I., S. Wagner, M. Amstutz, S. A. Muller, P. Broz, Y. Lussi, A. Engel, and G. R. Cornelis. 2007. YscU recognizes translocators as export substrates of the Yersinia injectisome. EMBO J. 26:3015-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]