Abstract
Long-term use of supplemental oxygen improves survival in patients with COPD and severe resting hypoxemia. However, the role of oxygen in symptomatic patients with COPD and more moderate hypoxemia at rest and desaturation with activity is unclear. The few long-term reports of supplemental oxygen in this group have been of small size and insufficient to demonstrate a survival benefit. Short-term trials have suggested beneficial effects other than survival in patients with COPD and moderate hypoxemia at rest. In addition, supplemental oxygen appeared to improve exercise performance in small short-term investigations of patients with COPD and moderate hypoxemia at rest and desaturation with exercise, but long-term trials evaluating patient-reported outcomes are lacking. This article reviews the evidence for long-term use of supplemental oxygen therapy and provides a rationale for the National Heart, Lung, and Blood Institute Long-term Oxygen Treatment Trial. The trial plans to enroll subjects with COPD with moderate hypoxemia at rest or desaturation with exercise and compare tailored oxygen therapy to no oxygen therapy.
Use of supplemental long-term oxygen therapy (LTOT) by patients with COPD is common, with more than 1 million Medicare recipients using oxygen at an annual cost of more than $2 billion.1,2 Although current indications for LTOT are based on the results of older randomized trials,3,4 a recent conference identified uncertainties regarding LTOT in COPD, including its efficacy in patients with more moderate hypoxemia.1
This article reviews the available evidence regarding the efficacy of LTOT for individuals with COPD and frames current clinical research needs. We review the evidence regarding use of LTOT for patients with severe hypoxemia at rest, moderate hypoxemia at rest, hypoxemia only during activity, and desaturation only at night. We analyzed articles from a Medline search of published literature in the English language on oxygen therapy in patients with COPD. Because the number of randomized controlled trials are limited for subjects with less severe hypoxemia, we also reviewed nonrandomized trials. Finally, we introduce a recently launched multicenter National Heart, Lung, and Blood Institute trial (the Long-term Oxygen Treatment Trial [LOTT]) of supplemental oxygen for patients with COPD and moderate hypoxemia at rest or with desaturation only with exercise.
The Role of LTOT in Patients With COPD and Severe Hypoxemia at Rest
Survival
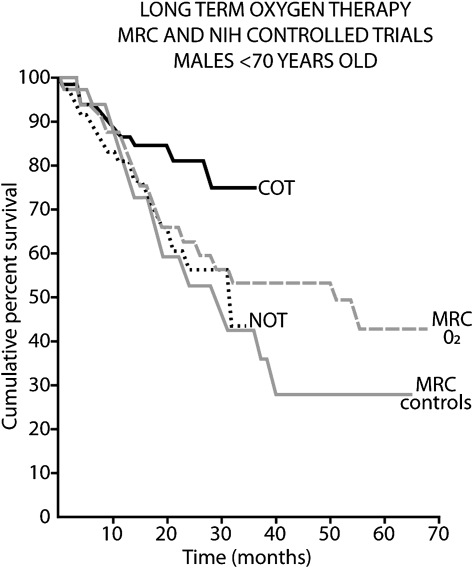
Supplemental oxygen is a well-established therapy with clear evidence for benefit in patients with COPD and severe resting hypoxemia, which is defined as a room air Pao2 ≤ 55 mm Hg or ≤ 59 mm Hg with signs of right-sided heart strain or polycythemia. Oxygen was the first treatment shown to prolong life in people with COPD.3,4 Current recommendations for prescribing LTOT (Table 1) are based on results from two randomized trials in patients with COPD published almost 30 years ago: the Nocturnal Oxygen Therapy Trial (NOTT) and the Medical Research Council (MRC) study (Fig 1).3,4
Table 1.
—Current Indications for Continuous Oxygen Use in COPD
| Based on Randomized Controlled Clinical Trials3,4 | Based on Less Evidencea |
| Continuous oxygen use | Intermittent oxygen use |
| Resting Pao2 ≤ 55 mm Hg | Desaturation (Spo2 ≤ 88%) with activity |
| Resting Pao2 of 56-59 mm Hg with any one of the following: | Desaturation (Spo2 ≤ 88%) at night |
| Dependent edema | |
| P pulmonale on the electrocardiogram (P wave exceeding 3 mm in standard lead II, III, or aVF) | |
| Polycythemia (hematocrit, > 56%) |
Spo2 = oxygen saturation by pulse oximetry.
Reimbursed by the Centers for Medicare & Medicaid Services.
Figure 1.
Long-term oxygen therapy in Medical Research Council and National Institutes of Health controlled trials in men aged < 70 years, examining the fraction of subjects surviving compared to the time from randomization or duration of treatment. COT = continuous oxygen therapy3; MRC = Medical Research Council4; NOT = nocturnal oxygen therapy.3
The MRC study was a randomized controlled oxygen therapy trial designed to assess whether use of supplemental oxygen for 15 h/d (including overnight) compared with no supplemental oxygen conferred a survival advantage over ≥ 3 years.3 Eighty-seven subjects with severe airflow limitation (mean FEV1, 0.58 L), marked hypoxemia (Pao2, 49-52 mm Hg), hypercapnia (Pco2, 56-59 mm Hg), and mild pulmonary hypertension were enrolled. Oxygen was delivered at 2 L/min (or higher if needed to achieve a Pao2 > 60 mm Hg). Results in the 87 subjects showed that supplemental oxygen use improved survival (55% vs 33% in controls; P < .05). Secondary outcome measures showed no significant benefit of supplemental oxygen (ie, days spent working, days spent in the hospital for COPD exacerbations, RBC mass, pulmonary hemodynamics). The results of this trial established the survival advantage of nocturnal supplemental oxygen in subjects with COPD and severe resting hypoxemia.
Further evidence of enhanced survival benefit with supplemental oxygen is provided by the National Institutes of Health NOTT.4 The study assessed whether continuous supplemental oxygen improved survival compared with nocturnal oxygen. Eligible subjects had COPD and severe resting hypoxemia (Pao2 ≤ 55 mm Hg or Pao2 ≤ 59 mm Hg with either edema, polycythemia [hematocrit, ≥ 55%], or P pulmonale on electrocardiogram). The study enrolled 203 subjects. Over a mean follow-up period of 19.3 months, use of continuous oxygen conferred a significant survival benefit (P = .01), with a relative risk of death of 1.94 (95% CI, 1.17-3.24) with use of nocturnal oxygen compared with continuous oxygen. Among secondary outcome measures, hematocrit fell more in continuous than in nocturnal oxygen users (P = .008 at 18 months), and pulmonary vascular resistance fell 11.1% in continuous oxygen users but rose 6.5% in nocturnal oxygen users (P = .04 at 6 months). Subjects receiving continuous oxygen therapy averaged (mean ± SD) 17.7 ± 4.8 h/d, and subjects receiving nocturnal oxygen therapy averaged 12.0 ± 2.5 h/d.
Results of the NOTT demonstrated that use of continuous supplemental oxygen enhanced survival compared with use of nocturnal supplemental oxygen. Taken together with the results of the MRC study, the findings suggest that in patients with COPD and resting hypoxemia, some oxygen is better than none, and continuous oxygen is better than nocturnal oxygen.
Pulmonary Hemodynamics
The effects of supplemental oxygen on pulmonary hemodynamics in patients with COPD using LTOT for > 13 h/d also have been investigated.5,6 Weitzenblum et al5 evaluated 16 patients with severe COPD (mean FEV1, 0.89 L) and hypoxemia (mean Pao2, 59.3 mm Hg) over a mean of 78 months. Pulmonary artery (PA) pressure was measured by right-sided heart catheterization before and twice after initiating supplemental oxygen for > 15 h/d to achieve a Pao2 ≥ 65 mm Hg. Supplemental oxygen reversed a pretreatment trend toward worsening pulmonary hypertension. Between the baseline measurement and the first post-oxygen catheterization, mean PA pressure rose by 1.47 mm Hg, whereas over the ensuing 31 months, mean PA pressure fell by 2.15 mm Hg.
In the longest hemodynamic study of patients with COPD and hypoxemia (mean PaO2, 55 mm Hg) receiving supplemental oxygen for a mean of 14.7 h/d, Zieliński et al6 measured PA pressures serially for up to 6 years. In a subset (39 of 73) of subjects who underwent follow-up right-sided heart catherization at 2 years, mean PA pressure fell slightly from 25 mm Hg to 23 mm Hg. In those who continued supplemental oxygen for 4 years, mean PA pressure remained stable. In 12 subjects who completed 6 years of supplemental oxygen use, mean PA pressure fell from baseline to 2-year follow-up (from 25 mm Hg to 21 mm Hg) but then returned to baseline values thereafter (mean PA pressure, 26 mm Hg at 4- and 6-year follow-up). The authors concluded that supplemental oxygen in patients with COPD and hypoxemia caused a short-term decline in PA pressure followed by subsequent return and stabilization of PA pressures to baseline levels.
The Role of LTOT in Patients With COPD and Moderate Hypoxemia
Mortality
In contrast with the results of the MRC study and NOTT, supplemental oxygen has not been shown to improve survival in patients with COPD and moderate hypoxemia.7,8 Górecka et al7 randomly assigned 135 patients with a resting room air Pao2 of 56 to 65 mm Hg to receive supplemental oxygen for > 17 h/d (to raise Pao2 to ≥ 65 mm Hg) or no supplemental oxygen. Over a mean observation of 40.9 months, cumulative survival in the treatment and control groups did not differ significantly. Furthermore, no survival difference was observed for patients using supplemental oxygen for more than 15 h/day vs those using it for shorter periods.
Haidl et al8 randomly assigned 28 patients with COPD (mean FEV1, 40.8 ± 10.2% predicted) and moderate hypoxemia (mean Pao2, 66.5 ± 6.3 mm Hg) to supplemental oxygen (2 L/min for > 15 h/d) or no supplemental oxygen for 3 years. At 1 year, cycle ergometry endurance time and end-exercise dyspnea were better in patients who received LTOT. The mortality rate was similar in both groups. Because of the small study population and large number of dropouts, later outcomes could not be assessed.
Analysis of the survival in these two studies in patients with COPD and moderate hypoxemia demonstrates no survival benefit for LTOT (odds ratio, 1.39; 95% CI, 0.74-2.59).9 However, these studies enrolled small numbers of subjects. As noted later in this article, the LOTT plans to enroll 1,134 subjects and follow them for up to 4½ years to assess a potential benefit in survival or hospitalization rate.
The Role of Supplemental Oxygen in Patients With Hypoxemia During Activity
Definition
Important challenges in ascertaining the effectiveness of supplemental oxygen during activity in patients with COPD are the lack of uniform criteria for defining exertional desaturation and standardized exercise protocols. Threshold values for oxygen desaturation range from 88% to 90%, and relative declines vary from 2% to 5% in published investigations. Some studies require maintenance of the oxygen saturation by pulse oximetry (Spo2) below a threshold value for a specified interval (usually between 0.5 min and 5 min) (Table 2). The techniques for inducing exertion vary from activities of daily living to incremental maximal cycle ergometry.
Table 2.
—Studies of Oxygen Therapy in Subjects With COPD and Exertional Desaturation
| Authors | No. | Subject Characteristicsa | Design | Benefits of Oxygen |
| Drummond et al10 | 471 | Severe COPD (moderate-severe emphysema; mean FEV1, 0.73 L [continuous oxygen], 0.77 L [intermittent oxygen], 0.86 L [no oxygen group]) randomized to medical therapy in NETT; Pao2 > 60 mm Hg; desaturation to < 90% during treadmill walk | Retrospective review of patients treated with continuous oxygen, intermittent supplemental oxygen, and no supplemental oxygen | No difference in survival |
| Stein et al11 | 9 | Severe COPD (mean FEV1, 0.87 L; mean Pao2, 63 mm Hg; range, 50-78 mm Hg) | Treadmill exercise while breathing 30% oxygen or compressed air | Increased duration of exercise; reduced minute ventilation; reduced tidal volume |
| McDonald et al12 | 26 | Severe COPD (mean FEV1, 0.9 L; mean Pao2, 69 mm Hg; mean Sao2, 94%) | Double-blind randomized crossover study comparing supplemental air or oxygen during 6MW and step tests after using either supplemental air or oxygen with activity for 6 weeks | Increased 6MW distance and steps climbed; no change in Borg dyspnea score |
| Jolly et al13 | 11 | Severe COPD (mean FEV1, 0.9 L; mean Pao2, 74 mm Hg; Spo2 reduction, ≥ 5% and absolute value < 90% during 6MW) | Double-blind, randomized, placebo-controlled trial comparing supplemental oxygen and compressed air during 6MW | Increased 6MW distance; reduced dyspnea |
| Somfay et al14 | 10 | Severe COPD (mean FEV1, 0.92 L; mean Sao2 at rest, 95.7%; mean Sao2 with exercise, 92%) | Single-blind randomized controlled study comparing compressed air and 30%, 50%, 75%, and 100% oxygen during cycle exercise testing at 75% of maximal work rate | Increased exercise duration; reduced dyspnea in dose-dependent manner due to decreased dynamic hyperinflation and respiratory rate |
| Rooyackers et al15 | 24 | Moderate to severe COPD (mean FEV1, 1.2 L; mean Pao2, 78.9 mm Hg; exercise Sao2, < 90%) | Ten-week inpatient pulmonary rehabilitation program with general exercise training breathing either supplemental oxygen or room air | No difference in training effect |
| Garrod et al16 | 25 | Severe COPD (mean FEV1, 0.76 L; mean Pao2, 63.6 mm Hg; exercise Sao2, 82%) | Six-week pulmonary rehabilitation training program using either compressed air or supplemental oxygen | Reduced breathlessness; no effect on exercise tolerance, health status, mood state, or performance of daily activities |
| Wadell et al17 | 20 | COPD (median FEV1 51.6% predicted [air group], 39.3% predicted [oxygen group]; median Pao2 69.9 mm Hg [air group], 71.4 mm Hg [oxygen group] exercise Sao2, ≤ 92%) | Single-blind randomized controlled study comparing supplemental oxygen or air during 8 weeks of exercise training three times/week for 30 min | No difference in training effect; dyspnea less in subjects receiving air |
| Emtner et al18 | 29 | COPD (mean FEV1, 36% predicted; Pao2, > 55 mm Hg; exercise Sao2, ≥ 88%) | Double-blind trial comparing supplemental oxygen and compressed air during 7 weeks of three times/week high-intensity cycle ergometry training | Improved maximal work rate; increased exercise endurance; increased training work rate more rapidly; reduced exercise breathing rate |
| Eaton et al19 | 41 | COPD (mean FEV1, 25.9% predicted; mean Pao2, 69 mm Hg; mean exercise Sao2, 82%) | Twelve-week double-blind randomized crossover trial comparing compressed supplemental air and oxygen during dyspnea-inducing exertion | Improved health-related quality of life |
6MW = 6-min walk test; NETT = National Emphysema Treatment Trial; Sao2 = arterial oxygen saturation. See Table 1 for expansion of other abbreviation.
Pao2 assessed at rest while breathing room air, unless otherwise noted.
Mortality
Several studies suggested that exertional desaturation may portend a poor prognosis for patients with COPD.10,20‐23 In a retrospective review of 144 patients, Takigawa and coworkers21 showed that a fall in Spo2 ≥ 6% during a 6-min walk predicted mortality. Similarly, in a prospective study of 576 patients with stable COPD, Casanova and colleagues22 demonstrated that desaturation (a decrease in the Spo2 ≥ 4% or Spo2 < 90% on 6-min walk) predicted mortality with a relative risk of 2.63. The Pao2 slope (rate of change of Pao2 and oxygen consumption) during incremental cardiopulmonary exercise testing and age were the most significant independent prognostic factors associated with survival in 120 patients with COPD.24 In a cohort of 64 patients with hypercapnia followed for ≤ 15 years, the decline in arterial oxygen saturation (Sao2) and increase in Paco2 during exercise were significantly greater in those who died.23 A retrospective review of 471 subjects with emphysema, resting normoxemia, and exertional desaturation randomized to medical treatment in the National Emphysema Treatment Trial10 demonstrated no differences in survival among subjects treated with continuous oxygen, intermittent oxygen, or no supplemental oxygen. Although exertional desaturation in patients with COPD and resting normoxemia appears to predict a poor prognosis, the effect of continuous supplemental oxygen on survival in this group has not been prospectively assessed in a large population.
Dyspnea, Exercise Performance, and Health-Related Quality of Life
In some studies, supplemental oxygen has been shown to enhance exercise performance in patients with COPD who are normoxemic at rest but desaturate with exertion.11‐13 However, these studies only examined the effects of relatively short-term oxygen use.
In 11 patients with COPD and resting normoxemia, the distance walked during 6 min increased from 391 ± 36 m to 450 ± 29 m, and the level of dyspnea decreased with supplemental oxygen but not with room air.13 Somfay et al14 examined the mechanism for this improvement and showed that supplemental oxygen increases exercise endurance time and reduces respiratory rate and dynamic hyperinflation during exercise in patients with COPD and mild hypoxemia. In a 12-week double-blind, randomized, crossover study that compared use of supplemental oxygen and air in 26 patients with resting near-normal Sao2 (94% ± 2.1%), McDonald and colleagues12 found that supplemental oxygen acutely increased 6-min walk distance and step test duration but did not have any long-term benefit in exercise performance, dyspnea, or quality of life.
Small studies of the short-term effects of supplemental oxygen in patients undergoing pulmonary rehabilitation suggested improvement in exercise performance but inconsistent outcomes on exercise training, an effect possibly related to methodologic variation.15‐18,25 During 7 weeks of high-intensity cycle ergometer exercise in patients with COPD and resting normoxemia, a higher maximal workload and greater endurance were demonstrated in the group treated with supplemental oxygen than in the group treated with compressed air.17 In 24 patients with exertional desaturation to < 90%, supplemental oxygen did not add to exercise performance or quality of life.15 During pulmonary rehabilitation in 25 patients with exertional desaturation, supplemental oxygen reduced dyspnea but did not affect exercise tolerance, health status, mood state, or performance of daily activities compared with room air.16 A meta-analysis of oxygen supplementation during training in patients with COPD concluded that oxygen augmented the benefits of exercise but emphasized the limited numbers of enrolled subjects and varied study design.26
An additional study examined health-related quality of life in patients with COPD without concomitant pulmonary rehabilitation and exertional desaturation treated with supplemental oxygen. In 50 patients with COPD (Sao2, 82% ± 5.4% after 6-min walk), Eaton and coworkers19 evaluated the effect of 12 weeks of supplemental oxygen on health-related quality of life in a double-blind, randomized, crossover trial. Supplemental oxygen significantly improved respiratory and general health-related quality of life, anxiety, and depression. However, the effect of supplemental oxygen on the 6-min walk distance and the Borg dyspnea scale did not correlate with the patient-reported outcomes, and 41% of the responders elected not to continue supplemental oxygen after the trial.
Other Outcomes
Cerebral Sao2 decreases during exercise in COPD. Supplemental oxygen reduces deoxyhemoglobin and improves cerebral oxygenation, thereby possibly helping to sustain cerebral function during exertion.27
As-Needed or Short-Burst Oxygen Therapy
Short duration, intermittent supplemental oxygen has been used to relieve breathlessness with exercise.28‐30 There is no uniform definition of the amount or duration of oxygen therapy used for short periods of time. Published reports have used oxygen to relieve breathlessness as needed, before exercise, during exercise, or after exercise.
Several early studies suggested that short-burst oxygen immediately before or just after exertion reduces dyspnea and increases 6-min walk distance.31‐33 Subsequent studies failed to demonstrate such benefits.34,35 Short-burst oxygen either before or after a 6-min walk does not improve the distance walked or the Borg dyspnea scale in patients with COPD and normoxemia at rest and desaturation with exertion.36 Compared with air, supplemental oxygen after the completion of exercise decreases the time to recovery from dynamic hyperinflation but does not affect the time to return to baseline breathlessness or maximal perception of dyspnea during recovery.37 Thus, there are few randomized controlled studies of short-term supplemental oxygen use. A metaanalysis of short-burst oxygen therapy concluded that there is no reduction in breathlessness and inconsistent effects on exercise capacity.38
It is not clear whether patients with COPD who desaturate during the day with exercise also desaturate at night. Because the mechanisms of desaturation during exercise and during sleep differ, patients who desaturate with activity may not desaturate at night.
The Role of LTOT in Patients With COPD and Nocturnal Desaturation
Definition
Nocturnal oxygen desaturation (NOD) has been reported in patients with COPD with an awake Pao2 > 60 mm Hg.39‐45 The most significant episodes of NOD occur during rapid eye movement sleep, with a reported prevalence of 27%.46 However, there are no accepted standards for the level or duration of desaturation that define NOD in patients with COPD45‐47 (Table 3).
Table 3.
—Studies of Oxygen Therapy in Subjects With COPD and Nocturnal Desaturation
| Authors | No. | Subject Characteristicsa | Design | Benefits of Oxygen |
| Górecka et al7 | 135 | COPD (mean FEV1, 0.83 L; mean Pao2, 60 mm Hg; range, 56-69 mm Hg) | Randomized study of conventional therapy vs conventional therapy plus continuous oxygen therapy (mean oxygen use, 14 h/d); followed over 3 years | No difference in survival, even in those using oxygen > 15 h/d; survival better with younger age, higher FEV1, and higher BMI |
| Chaouat et al48 | 76 | COPD (mean FEV1, 1.08 L in nocturnal oxygen group and 0.98 L in controls; mean Pao2, 62.7 mm Hg; range 56-69 mm Hg; mean nocturnal Spo2, 88%) | Randomized study of conventional therapy vs conventional therapy plus supplemental nocturnal oxygen therapy; followed over 2 years | No difference in survival and pulmonary hemodynamics; no delay in need for supplemental oxygen (defined as Pao2 < 55 mm Hg during the day) |
| Fletcher et al49 | 16 | COPD (mean FEV1, 1.42 L; mean Pao2, 76 mm Hg; isolated NOD Spo2 < 90% for ≥ 5 min; nadir, ≤ 85%) | Randomized to supplemental nocturnal oxygen therapy vs a sham control | No difference in survival at the end of 3 years; small improvement in pulmonary hemodynamics |
| Calverly et al40 | 6 | Severe COPD (mean FEV1, 0.6 L [all with FEV1 < 1 L]; mean Pao2, 48 mm Hg) | Sleep studies off and on supplemental oxygen | Improved sleep quality compared with control night |
| Fleetham et al44 | 15 | Severe COPD (mean FEV1, 24% predicted; mean Pao2, 52 mm Hg) | Sleep studies off and on supplemental oxygen | No improvement in sleep quality |
| Flick and Block50 | 10 | Severe COPD (mean FEV1, 0.76 L; mean Sao2, 86%) | Continuous 24-h electrocardiogram monitoring with and without supplemental oxygen (2 L/min) | Decrease in the number of nocturnal PVCs |
Mortality
Although retrospective data suggest a decreased survival in patients with NOD,49 only a few studies examined the impact of nocturnal supplemental oxygen therapy on mortality in patients with COPD and NOD.7,48,51 In patients with mild to moderate daytime hypoxemia (Pao2, 56-69 mm Hg) and associated NOD, no improvement in survival was noted with nocturnal supplemental oxygen therapy at the end of 2 years.48 A similar lack of improvement in survival was seen in patients with COPD and isolated NOD who were randomized to nocturnal oxygen therapy for 3 years.51 Therefore, based on limited available data in small numbers of subjects, it is unknown whether continuous supplemental oxygen therapy affects survival in patients with COPD and isolated NOD.
Sleep Quality
Sleep quality is poor in patients with COPD.39‐42,52‐54 Subjective complaints include difficulty falling and staying asleep, morning tiredness, early awakenings, and excessive daytime sleepiness.41,52‐54 Objective assessment of sleep quality demonstrates increased sleep latency, decreased total sleep time, increased number of arousals, and a decrease in rapid eye movement sleep.40,44
The results of studies investigating the effects of oxygen therapy on sleep quality are limited and conflicting, with one study demonstrating improved sleep quality40 and another noting no change.44 Therefore, although sleep quality is known to be poor in patients with COPD, the effects of nocturnal supplemental oxygen therapy are unknown.
Other Outcomes
Premature ventricular contractions (PVCs) occur during the night in 64% of patients with COPD,55 with a frequency more than twice that during the day.50 In patients with more significant nocturnal hypoxemia, there is a > 150% increase in the frequency of PVCs.55 In one study, supplemental oxygen did not decrease the mean number of PVCs in all subjects; however, four of the 10 subjects experienced a 50% decrease in PVC frequency.56 Although some investigators have reported elevations in PA pressure and pulmonary vascular resistance in patients with NOD,56 others have not.47 Similarly, studies of nocturnal supplemental oxygen therapy on pulmonary hemodynamics have shown conflicting results.48,56 In patients with isolated NOD, Fletcher et al56 showed a downward trend in PA pressure (−4 mm Hg) with supplemental oxygen therapy compared with an increase (+4 mm Hg) in the control group. Another study found no change in PA pressures in patients with NOD.48 Overall, whether supplemental oxygen therapy in patients with NOD affects the prevalence of cardiac dysrhythmias and the development of pulmonary hypertension has not been determined.
The LOTT
Based on the evidence and unanswered questions summarized herein and the research needs identified by the Sixth Oxygen Consensus Conference2 and a workshop report on LTOT,1 the National Heart, Lung, and Blood Institute and Centers for Medicare & Medicaid Services are sponsoring the LOTT. The LOTT is a multicenter, randomized clinical trial of tailored oxygen therapy (ie, continuous oxygen therapy [24 h/d] for subjects with moderate resting hypoxemia and supplemental oxygen therapy with sleep and activity for subjects with exercise desaturation) vs no supplemental oxygen therapy. The goal is to randomize 1,134 patients over 2½ years, with a maximal follow-up period of 4.5 years (minimum follow-up, 1 year). The primary objective of LOTT is to determine whether continuous supplemental oxygen therapy increases time to the composite outcome of all-cause mortality or all-cause hospitalization as well as deterioration of disease-specific quality of life (St. George Respiratory Questionnaire) and preference-weighted quality of life (Quality of Well-Being Scale).
Eligible subjects are aged ≥ 40 years, have COPD (postbronchodilator FEV1, ≤ 65% predicted; FEV1/FVC, < 0.70), and have a dyspnea rating ≥ 1 by the modified MRC scale. Subjects will use oxygen delivery devices designed to be wearable and convenient and to deliver adequate, tailored supplemental oxygen according to the protocols (either continuously in those with moderate resting hypoxemia or during activity and sleep in those with exercise desaturation).
It is hoped that LOTT will offer generalizable conclusions with regard to the safety, efficacy, and cost-effectiveness of LTOT in patients with COPD and moderate hypoxemia at rest or desaturation during exercise. Health-care practitioners are encouraged to refer candidates for the LOTT to one of the clinical centers listed at www.jhucct.com/lott/open/centers/centers.htm.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to the CHEST the following conflicts of interest: Dr Panos has contracted research with Novartis, Boehringer-Ingelheim, and Pearl Therapeutics. Dr Make has participated in two advisory boards for Respironics over the past 3 years. Dr Stoller has served as a consultant to Respironics, Talecris, CSL-Behring, Boehringer-Ingelheim, and Asthmatx and has presented lectures supported by Grifols, Baxter Healthcare, Talecris, Respironics, and CSL-Behring. Drs Krachman and Doherty have reported that no conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- LOTT
Long-term Oxygen Treatment Trial
- LTOT
long-term oxygen therapy
- MRC
Medical Research Council
- NOD
nocturnal oxygen desaturation
- NOTT
Nocturnal Oxygen Therapy Trial
- PA
pulmonary artery
- PVC
premature ventricular contraction
- Sao2
arterial oxygen saturation
- Spo2
oxygen saturation by pulse oximetry
Funding/Support: This project is supported through federal funding from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services [contracts HHSN268200736183C, HHSN268200736184C, HSN268200736185C, HHSN268200736186C, HHSN268200736187C, HHSN268200736188C, HHSN268200736189C, HHSN268200736190C, HHSN268200736191C, HHSN268200736192C, HHSN268200736193C, HHSN268200736194C, HHSN268200736195C, HHSN268200736196C, HHSN268200736197C, Y1-HR-7019-01, and Y1-HR-8076-01] in cooperation with the Centers for Medicare and Medicaid Services, Department of Health and Human Services.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373–378. doi: 10.1164/rccm.200507-1161WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doherty DE, Petty TL, Bailey W, et al. Recommendations of the 6th long-term oxygen therapy consensus conference. Respir Care. 2006;51(5):519–525. [PubMed] [Google Scholar]
- 3.Report of the Medical Research Council Working Party Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1(8222):681–686. [PubMed] [Google Scholar]
- 4.Nocturnal Oxygen Therapy Trial Group Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93(3):391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 5.Weitzenblum E, Sautegeau A, Ehrhart M, Mammosser M, Pelletier A. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;131(4):493–498. doi: 10.1164/arrd.1985.131.4.493. [DOI] [PubMed] [Google Scholar]
- 6.Zieliński J, Tobiasz M, Hawryłkiewicz I, Sliwiński P, Pałasiewicz G. Effects of long-term oxygen therapy on pulmonary hemodynamics in COPD patients: a 6-year prospective study. Chest. 1998;113(1):65–70. doi: 10.1378/chest.113.1.65. [DOI] [PubMed] [Google Scholar]
- 7.Górecka D, Gorzelak K, Sliwiński P, Tobiasz M, Zieliński J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax. 1997;52(8):674–679. doi: 10.1136/thx.52.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haidl P, Clement C, Wiese C, Dellweg D, Köhler D. Long-term oxygen therapy stops the natural decline of endurance in COPD patients with reversible hypercapnia. Respiration. 2004;71(4):342–347. doi: 10.1159/000079637. [DOI] [PubMed] [Google Scholar]
- 9.Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;(4):CD001744. doi: 10.1002/14651858.CD001744.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MB, Blackford AL, Benditt JO, et al. NETT Investigators Continuous oxygen use in nonhypoxemic emphysema patients identifies a high-risk subset of patients: retrospective analysis of the National Emphysema Treatment Trial. Chest. 2008;134(3):497–506. doi: 10.1378/chest.08-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein DA, Bradley BL, Miller WC. Mechanisms of oxygen effects on exercise in patients with chronic obstructive pulmonary disease. Chest. 1982;81(1):6–10. doi: 10.1378/chest.81.1.6. [DOI] [PubMed] [Google Scholar]
- 12.McDonald CF, Blyth CM, Lazarus MD, Marschner I, Barter CE. Exertional oxygen of limited benefit in patients with chronic obstructive pulmonary disease and mild hypoxemia. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1616–1619. doi: 10.1164/ajrccm.152.5.7582304. [DOI] [PubMed] [Google Scholar]
- 13.Jolly EC, Di Boscio V, Aguirre L, Luna CM, Berensztein S, Gené RJ. Effects of supplemental oxygen during activity in patients with advanced COPD without severe resting hypoxemia. Chest. 2001;120(2):437–443. doi: 10.1378/chest.120.2.437. [DOI] [PubMed] [Google Scholar]
- 14.Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J. 2001;18(1):77–84. doi: 10.1183/09031936.01.00082201. [DOI] [PubMed] [Google Scholar]
- 15.Rooyackers JM, Dekhuijzen PN, Van Herwaarden CL, Folgering HT. Training with supplemental oxygen in patients with COPD and hypoxaemia at peak exercise. Eur Respir J. 1997;10(6):1278–1284. doi: 10.1183/09031936.97.10061278. [DOI] [PubMed] [Google Scholar]
- 16.Garrod R, Mikelsons C, Paul EA, Wedzicha JA. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1335–1341. doi: 10.1164/ajrccm.162.4.9912029. [DOI] [PubMed] [Google Scholar]
- 17.Wadell K, Henriksson-Larsén K, Lundgren R. Physical training with and without oxygen in patients with chronic obstructive pulmonary disease and exercise-induced hypoxaemia. J Rehabil Med. 2001;33(5):200–205. doi: 10.1080/165019701750419581. [DOI] [PubMed] [Google Scholar]
- 18.Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med. 2003;168(9):1034–1042. doi: 10.1164/rccm.200212-1525OC. [DOI] [PubMed] [Google Scholar]
- 19.Eaton T, Garrett JE, Young P, et al. Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled study. Eur Respir J. 2002;20(2):306–312. doi: 10.1183/09031936.02.00301002. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami Y, Terai T, Yamamoto H, Murao M. Exercise and oxygen inhalation in relation to prognosis of chronic obstructive pulmonary disease. Chest. 1982;81(2):182–188. doi: 10.1378/chest.81.2.182. [DOI] [PubMed] [Google Scholar]
- 21.Takigawa N, Tada A, Soda R, et al. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir Med. 2007;101(3):561–567. doi: 10.1016/j.rmed.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134(4):746–752. doi: 10.1378/chest.08-0520. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbergh E, Clement J, Van de Woestijne KP. Course and prognosis of patients with advanced chronic obstructive pulmonary disease. Evaluation by means of functional indices. Am J Med. 1973;55(6):736–746. doi: 10.1016/0002-9343(73)90254-4. [DOI] [PubMed] [Google Scholar]
- 24.Hiraga T, Maekura R, Okuda Y, et al. Prognostic predictors for survival in patients with COPD using cardiopulmonary exercise testing. Clin Physiol Funct Imaging. 2003;23(6):324–331. doi: 10.1046/j.1475-0961.2003.00514.x. [DOI] [PubMed] [Google Scholar]
- 25.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131(5) suppl:4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 26.Nonoyama ML, Brooks D, Lacasse Y, Guyatt GH, Goldstein RS. Oxygen therapy during exercise training in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;(2):CD005372. doi: 10.1002/14651858.CD005372.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen G, Nielsen HB, Ide K, et al. Cerebral oxygenation during exercise in patients with terminal lung disease. Chest. 2002;122(2):445–450. doi: 10.1378/chest.122.2.445. [DOI] [PubMed] [Google Scholar]
- 28.Okubadejo AA, Paul EA, Wedzicha JA. Domiciliary oxygen cylinders: indications, prescription and usage. Respir Med. 1994;88(10):777–785. doi: 10.1016/s0954-6111(05)80201-x. [DOI] [PubMed] [Google Scholar]
- 29.Roberts CM. Short burst oxygen therapy for relief of breathlessness in COPD. Thorax. 2004;59(8):638–640. doi: 10.1136/thx.2003.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Neill B, Bradley JM, Heaney L, O’Neill C, MacMahon J. Short burst oxygen therapy in chronic obstructive pulmonary disease: a patient survey and cost analysis. Int J Clin Pract. 2005;59(7):751–753. doi: 10.1111/j.1368-5031.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- 31.Woodcock AA, Gross ER, Geddes DM. Oxygen relieves breathlessness in “pink puffers”. Lancet. 1981;1(8226):907–909. doi: 10.1016/s0140-6736(81)91612-3. [DOI] [PubMed] [Google Scholar]
- 32.Evans TW, Waterhouse JC, Carter A, Nicholl JF, Howard P. Short burst oxygen treatment for breathlessness in chronic obstructive airways disease. Thorax. 1986;41(8):611–615. doi: 10.1136/thx.41.8.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Killen JW, Corris PA. A pragmatic assessment of the placement of oxygen when given for exercise induced dyspnoea. Thorax. 2000;55(7):544–546. doi: 10.1136/thorax.55.7.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKeon JL, Murree-Allen K, Saunders NA. Effects of breathing supplemental oxygen before progressive exercise in patients with chronic obstructive lung disease. Thorax. 1988;43(1):53–56. doi: 10.1136/thx.43.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nandi K, Smith AA, Crawford A, et al. Oxygen supplementation before or after submaximal exercise in patients with chronic obstructive pulmonary disease. Thorax. 2003;58(8):670–673. doi: 10.1136/thorax.58.8.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis CA, Eaton TE, Young P, Kolbe J. Short-burst oxygen immediately before and after exercise is ineffective in nonhypoxic COPD patients. Eur Respir J. 2003;22(4):584–588. doi: 10.1183/09031936.03.00027603a. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson NJ, Calverley PM. Effect of oxygen on recovery from maximal exercise in patients with chronic obstructive pulmonary disease. Thorax. 2004;59(8):668–672. doi: 10.1136/thx.2003.014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Neill B, Mahon JM, Bradley J. Short-burst oxygen therapy in chronic obstructive pulmonary disease. Respir Med. 2006;100(7):1129–1138. doi: 10.1016/j.rmed.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 39.Wynne JW, Block AJ, Hemenway J, Hunt LA, Flick MR. Disordered breathing and oxygen desaturation during sleep in patients with chronic obstructive lung disease (COLD) Am J Med. 1979;66(4):573–579. doi: 10.1016/0002-9343(79)91166-5. [DOI] [PubMed] [Google Scholar]
- 40.Calverley PM, Brezinova V, Douglas NJ, Catterall JR, Flenley DC. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis. 1982;126(2):206–210. doi: 10.1164/arrd.1982.126.2.206. [DOI] [PubMed] [Google Scholar]
- 41.Cormick W, Olson LG, Hensley MJ, Saunders NA. Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung disease. Thorax. 1986;41(11):846–854. doi: 10.1136/thx.41.11.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krachman SL, Chatila W, Martin UJ, et al. National Emphysema Treatment Trial Research Group Effects of lung volume reduction surgery on sleep quality and nocturnal gas exchange in patients with severe emphysema. Chest. 2005;128(5):3221–3228. doi: 10.1378/chest.128.5.3221. [DOI] [PubMed] [Google Scholar]
- 43.Koo KW, Sax DS, Snider GL. Arterial blood gases and pH during sleep in chronic obstructive pulmonary disease. Am J Med. 1975;58(5):663–670. doi: 10.1016/0002-9343(75)90502-1. [DOI] [PubMed] [Google Scholar]
- 44.Fleetham J, West P, Mezon B, Conway W, Roth T, Kryger M. Sleep, arousals, and oxygen desaturation in chronic obstructive pulmonary disease. The effect of oxygen therapy. Am Rev Respir Dis. 1982;126(3):429–433. doi: 10.1164/arrd.1982.126.3.429. [DOI] [PubMed] [Google Scholar]
- 45.Bradley TD, Mateika J, Li D, Avendano M, Goldstein RS. Daytime hypercapnia in the development of nocturnal hypoxemia in COPD. Chest. 1990;97(2):308–312. doi: 10.1378/chest.97.2.308. [DOI] [PubMed] [Google Scholar]
- 46.Fletcher EC, Miller J, Divine GW, Fletcher JG, Miller T. Nocturnal oxyhemoglobin desaturation in COPD patients with arterial oxygen tensions above 60 mm Hg. Chest. 1987;92(4):604–608. doi: 10.1378/chest.92.4.604. [DOI] [PubMed] [Google Scholar]
- 47.Chaouat A, Weitzenblum E, Kessler R, et al. Sleep-related O2 desaturation and daytime pulmonary haemodynamics in COPD patients with mild hypoxaemia. Eur Respir J. 1997;10(8):1730–1735. doi: 10.1183/09031936.97.10081730. [DOI] [PubMed] [Google Scholar]
- 48.Chaouat A, Weitzenblum E, Kessler R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J. 1999;14(5):1002–1008. doi: 10.1183/09031936.99.14510029. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher EC, Luckett RA, Goodnight-White S, Miller CC, Qian W, Costarangos-Galarza C. A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mm Hg. Am Rev Respir Dis. 1992;145:1070–1076. doi: 10.1164/ajrccm/145.5.1070. [DOI] [PubMed] [Google Scholar]
- 50.Flick MR, Block AJ. Nocturnal vs diurnal cardiac arrhythmias in patients with chronic obstructive pulmonary disease. Chest. 1979;75(1):8–11. doi: 10.1378/chest.75.1.8. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher EC, Donner CF, Midgren B, et al. Survival in COPD patients with a daytime PaO2 greater than 60 mm Hg with and without nocturnal oxyhemoglobin desaturation. Chest. 1992;101(3):649–655. doi: 10.1378/chest.101.3.649. [DOI] [PubMed] [Google Scholar]
- 52.Bellia V, Catalano F, Scichilone N, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep. 2003;26(3):318–323. doi: 10.1093/sleep/26.3.318. [DOI] [PubMed] [Google Scholar]
- 53.Klink ME, Dodge R, Quan SF. The relation of sleep complaints to respiratory symptoms in a general population. Chest. 1994;105(1):151–154. doi: 10.1378/chest.105.1.151. [DOI] [PubMed] [Google Scholar]
- 54.Dodge R, Cline MG, Quan SF. The natural history of insomnia and its relationship to respiratory symptoms. Arch Intern Med. 1995;155(16):1797–1800. [PubMed] [Google Scholar]
- 55.Shepard JW, Jr, Garrison MW, Grither DA, Evans R, Schweitzer PK. Relationship of ventricular ectopy to nocturnal oxygen desaturation in patients with chronic obstructive pulmonary disease. Am J Med. 1985;78(1):28–34. doi: 10.1016/0002-9343(85)90457-7. [DOI] [PubMed] [Google Scholar]
- 56.Fletcher EC, Luckett RA, Miller T, Costarangos C, Kutka N, Fletcher JG. Pulmonary vascular hemodynamics in chronic lung disease patients with and without oxyhemoglobin desaturation during sleep. Chest. 1989;95(4):757–764. doi: 10.1378/chest.95.4.757. [DOI] [PubMed] [Google Scholar]