Abstract
Background:
Obstructive sleep apnea (OSA) is common in children and leads to multiple end-organ morbidities induced by the cumulative burden of oxidative stress and inflammation. Leukocyte telomere length (LTL) reflects not only chronologic age but also the burden of disease. We hypothesized that LTL would be decreased in children with OSA.
Methods:
Two hundred thirteen children (mean age, 7.7 ± 1.4 years) were included after a sleep study and a morning blood sample. LTL was examined by quantitative polymerase chain reaction in a case-control setting involving 111 OSA cases and 102 controls. Myeloid-related protein (MRP) 8/14 and catestatin plasma levels also were assayed using enzyme-linked immunosorbent assay.
Results:
Log LTL was significantly increased and OSA severity dependently increased in children (P = .012), was positively associated with apnea-hypopnea index (AHI) (r = 0.236; P < .01), and was inversely correlated with age (r = −0.145; P < .05). In a multivariate regression model, LTL was independently associated with AHI (β = 0.28; P = .002) after adjusting for age, sex, BMI z score, and race. Children with OSA exhibited higher BP (P < .05), lower plasma catestatin (P = .009), and higher MRP 8/14 levels (P < .001) than controls. Of note, children with the lowest plasma catestatin levels (< 1.39 ng/mL) had 5.2-fold increased odds of moderate-to-severe OSA (95% CI, 1.19-23.4 ng/mL; P < .05) after adjusting for confounding variables.
Conclusions:
In pediatric OSA, LTL is longer rather than shorter. Children with OSA have reduced plasma catestatin levels and increased BP along with increased MRP 8/14 levels that exhibit AHI dependencies. Thus, catestatin and MRP 8/14 levels may serve as biomarkers for cardiovascular risk in the context of pediatric OSA. However, the implications of increased LTL in children with OSA remain to be defined.
Obstructive sleep apnea (OSA) is characterized by repeated events of partial or complete upper airway obstruction during sleep that result in disruption of normal ventilation, hypoxemia, and sleep fragmentation. Increasing evidence supports the concept that OSA is pathophysiologically linked to cardiovascular diseases, such as hypertension, ischemic heart disease, and cerebrovascular disease.1-4 Interestingly, hypoxia, and more prominently, intermittent hypoxia (one of the hallmark features of OSA), has been recently postulated to accelerate senescence processes5 and, therefore, could reduce life span.
Telomeres are tandem repeats of DNA sequences located at the ends of eukaryotic chromosomes.6,7 One function of these structures is to protect the telomeric regions from recombination and degradation, avoiding DNA damage due to the accruing burden of oxidative stress and systemic inflammation,8-10 which are pathophysiologic processes that are consistently activated in OSA.11,12 Recent evidence has shown that leukocyte telomere length (LTL) shortening has been linked not only with aging and senescence but also with an increased risk for age-related diseases, namely cardiovascular disease and heart failure.13-16 Interestingly, age-dependent LTL shortening appears to be much faster in early life than during adulthood, probably owing to the rapid proliferation of hematopoietic stem cell populations during growth and development.17
Catestatin is a small peptide that most likely is generated by the proteolytic enzymes serine protease plasmin and cysteine protease cathepsin L acting on chromogranin A.18,19 Recent studies have shown that a lower plasma level of catestatin is a significant risk factor for development of hypertension in humans20 and that catestatin modulates autonomic function and BP.21,22 To our knowledge, no studies on catestatin and OSA have been published to date despite the clear involvement of the cardiovascular system in OSA.
Myeloid-related protein (MRP) 8 and MRP 14 are members of the S100 family of calcium-modulated proteins that regulate myeloid cell function and control inflammation through activation of the receptor for advanced glycation end products, which in turn have been associated with OSA.23,24 MRP 8/14 recently has been shown to regulate vascular inflammation and to contribute to the biologic response to vascular injury.25 Furthermore, MRP 8/14 levels are increased in children with obesity and in children with OSA in a dose-dependent manner.26
Based on the increased oxidative stress and inflammatory load associated with pediatric OSA and the cumulative evidence indicating that LTL represents the global ability to maintain the integrity of DNA, we hypothesized that LTL would be reduced in pediatric OSA and could be used as a potential predictor of OSA-associated end-organ morbidity in children. Furthermore, we examined whether the presence of significant systemic inflammation in OSA, such as illustrated by MRP 8/14 levels,26 would be associated with LTL and, likewise, whether catestatin levels would be associated with BP alterations and LTL in the context of pediatric OSA.
Materials and Methods
Subjects
The study was approved by the University of Louisville (Louisville, KY) Human Research Committee, and informed consent was obtained from the legal caregiver of each subject. Assent also was obtained from the child if he or she was ≥ 7 years of age. Consecutive children with OSA diagnosed by polysomnographic criteria and between the ages of 5 and 10 years were invited to participate in the study. In addition, age-, sex-, and ethnicity-matched children without snoring and OSA who underwent overnight polysomnography as part of a community-based study also were invited to participate. Children were excluded if they had known diabetes or prediabetes (http://www.diabetes.org/pre-diabetes/pre-diabetes-symptoms.jsp), had any defined genetic abnormality or underlying systemic disease, or were within 1 month from any acute infectious process. The diagnosis of mild and moderate-to-severe OSA was defined by the presence of an apnea-hypopnea index (AHI) ≥ 1/hour of total sleep time (hrTST) and ≥ 5/hrTST, respectively. Control children did not snore and had an AHI < 1/hrTST.
Anthropometry
Children were weighed on a calibrated scale to the nearest 0.1 kg, and height was measured to the nearest 0.1 cm with a stadiometer (Holtain; Crymych, England). BMI was calculated, and BMI z score computed using Centers for Disease Control and Prevention 2000 growth standards (www.cdc.gov/growthcharts) and online software (www.cdc.gov/epiinfo). A BMI z score > 1.67 indicated obesity.
Sphygmomanometry
Arterial BP was measured noninvasively in all children with an automated mercury sphygmomanometer (Welch Allyn; Skaneateles, NY) at the brachial artery using a guidelines-defined appropriate cuff size on the nondominant arm.27 BP measurements were made in triplicate in the morning immediately after awakening. Systolic and diastolic BPs were first calculated as mean values, and then mean BP was calculated.
Overnight Polysomnography
Children were studied for up to 12 h in a quiet, darkened room with an ambient temperature of 24°C in the company of one of their parents. No drugs were used to induce sleep. Polysomnography was performed as previously reported.26,28 Sleep architecture was assessed by standard techniques.29 Central, obstructive, and mixed apneic events were counted. Obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for a duration of ≥ 2 breaths.30,31 Hypopneas were defined as a decrease in oronasal flow of ≥ 50% with a corresponding decrease in oxygen saturation on pulse oximetry of ≥ 4%, an arousal, or both.31,32 The AHI was defined as the number of obstructive apneas and hypopneas per hrTST. Arousals were identified as defined by the American Sleep Disorders Association Task Force report.32,33
Measurement of LTL
Genomic DNA was extracted from blood samples using the QIAmp Spin Colum protocol (Qiagen; Chatsworth, CA) according to manufacturer instructions. DNA samples were frozen at −80°C until assay. LTL was measured using the quantitative polymerase chain reaction method as described by Cawthon.34 Briefly, each sample was amplified for telomeric DNA and a single-copy gene using a 1 μL aliquot containing 100 ng template DNA. Cycle threshold was transformed into nanograms of DNA based on a standard curve. The quantitative assay determines the amount of telomeric DNA relative to the amount of single-copy control gene DNA to obtain a relative ratio, which has been previously confirmed to be highly consistent with the conventional Southern blot method on terminal restriction fragments.35,36 The primer sequences used were those described previously.34 The polymerase chain reaction was done by the ABI 7500 real-time system (Applied Biosystems; Foster City, CA) with SYBR GREEN PCR mater mix (Applied Biosystems). All measures were performed in duplicate, with a correlation coefficient for the duplicates of r = 0.98 and an average coefficient of variation for pair sets of 1.6%.
MRP 8/14 and Catestatin Levels and Serum Lipids
Fasting blood samples were drawn by venipuncture in the morning after the sleep study. Blood samples were immediately centrifuged and frozen at −80°C until assay. Plasma MRP 8/14 and catestatin levels were measured using commercial enzyme-linked immunosorbent assay kits (for MRP 8/14, ALPCO Diagnostics; Salem, NH; for catestatin, Phoenix Pharmaceuticals, Inc; Burlingame, CA) following manufacturer instructions. MRP 8/14 and catestatin assays have a sensitivity of 0.4 μg/mL and 0.15 ng/mL, respectively. The interassay and intraassay coefficients of variability for MRP 8/14 were 6.4% and 4.8%, respectively. For catestatin, the interassay and intraassay coefficients of variability were 8.2% and 5.8%, respectively. Serum levels of lipids, including total cholesterol, high-density lipoprotein, calculated low-density lipoprotein, and triglycerides, were assessed with a Flex reagent cartridge (Dade Behring; Newark, DE).
Statistical Analysis
Data are expressed as mean ± SD or mean ± standard error as indicated. Significant differences within groups were analyzed using analysis of variance for continuous variables and χ2 tests for categorical variables. Bonferroni corrections were applied for multiple comparisons. If the data were not normally distributed, they were logarithmically transformed. Spearman correlation analyses were conducted to examine potential associations among LTL, catestatin, MRP 8/14, and other variables. Univariate and stepwise multivariate linear regression analyses were then conducted while treating LTL as a dependent variable in relation to AHI and other covariates. In addition, we used a logistic regression model to estimate odds ratios and corresponding 95% CIs for risk of OSA after subdividing the cohort into groups based on tertile cut points for the distribution of LTL and catestatin levels. Statistical analyses were performed using SPSS, version 16.0, statistical software (SPPS Inc.; Chicago, IL). All P values reported are two-tailed, with statistical significance set at < .05.
Results
Study Population
Two hundred thirteen subjects were included in this study. Based on the presence or absence of habitual snoring and AHI, 85 had mild OSA, 26 had moderate-to-severe OSA, and 102 were controls. The demographic, polysomnographic, and biochemical characteristics of the three groups are shown in Table 1. Mean age, sex, and ethnic distribution were similar across the three groups (P > .05). However, both systolic and diastolic BPs were significantly elevated in the OSA groups. LTL, log MRP 8/14, and log catestatin levels also showed significant group differences (Table 1).
Table 1.
—Demographic, Respiratory, and Metabolic Characteristics of Children With Obstructive Sleep Apnea and Matched Healthy Controls
| Characteristic | Moderate-to-Severe OSA (n = 26) | Mild OSA (n = 85) | Control (n = 102) |
| Age, y | 7.19 ± 1.83 | 7.79 ± 1.57 | 7.71 ± 1.29 |
| Male, % | 53.8 | 63.5 | 54.9 |
| White, % | 53.8 | 64.7 | 64.7 |
| BMI z score | 2.08 ± 1.06a | 1.39 ± 1.37b | 1.25 ± 1.16c |
| Systolic BP, mm Hg | 113.9 ± 10.8a | 105.4 ± 10.0b | 104.1 ± 9.7c |
| Diastolic BP, mm Hg | 67.2 ± 6.3a | 62.8 ± 6.0 | 59.9 ± 7.0d |
| Mean arterial pressure, mm Hg | 82.8 ± 7.1a | 77.0 ± 6.6b | 74.6 ± 6.1d |
| AHI, events/h | 17.8 ± 10.5a | 2.25 ± 0.99e | 0.38 ± 0.27d |
| Sao2 nadir, % | 79.7 ± 10.3a | 89.5 ± 5.5e | 92.3 ± 5.3d |
| Total cholesterol, mg/dLf | 191.3 ± 43.8a | 170.1 ± 29.1 | 156.5 ± 24.8d |
| HDL cholesterol, mg/dLf | 47.9 ± 11.0 | 53.8 ± 10.6 | 49.8 ± 10.3 |
| LDL cholesterol, mg/dLf | 118.1 ± 38.5a | 100.68 ± 24.0 | 90.4 ± 22.5d |
| Tryglycerides, mg/dLf | 126.3 ± 83.5g | 77.8 ± 37.7e | 81.42 ± 43.2d |
| Log MRP 8/14 | 0.20 ± 0.24a | 0.00 ± 0.31e | −0.12 ± 0.33d |
| Actual MRP 8/14 | 1.82 ± 0.97 μg/mL | 1.28 ± 0.91 μg/mL | 1.00 ± 0.84 μg/mL |
| Log catestatin | 0.12 ± 0.22a | 0.23 ± 0.20 | 0.28 ± 0.19d |
| Actual catestatin | 1.52 ± 0.81 ng/mL | 1.94 ± 0.96 ng/mL | 2.14 ± 1.00 ng/mL |
| Log LTL (T/S ratio) | 0.10 ± 0.14a | 0.06 ± 0.11 | 0.02 ± 0.13c |
| Actual LTL (T/S ratio) | 1.34 ± 0.47 | 1.20 ± 0.33 | 1.12 ± 0.37 |
Data are presented as mean ± SD, unless otherwise indicated. BP data and catestatin levels include 115 and 147 subjects, respectively. AHI = apnea-hypopnea index; HDL = high-density lipoprotein; LDL = low-density lipoprotein; LTL = leukocyte telomere length; MRP = myeloid-related protein; OSA = obstructive sleep apnea; Sao 2 = arterial oxygen saturation; T/S = amount of telomeric DNA to amount of single-copy control gene DNA.
P < .01, control vs moderate-to-severe OSA groups.
P < .05, control vs mild OSA groups.
P < .05.
P < .01, differences among three groups (analysis of variance).
P < .01, control vs mild OSA groups.
These data were acquired in 109 children.
P < .05, control vs moderate-to-severe OSA groups.
LTL, Catestatin, and MRP 8/14 levels
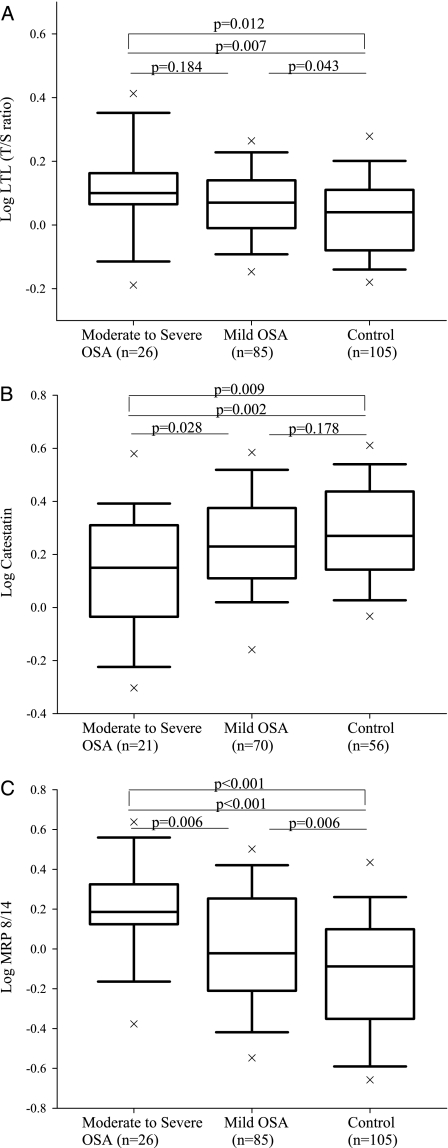
Log LTL and log catestatin levels were stratified according to the severity of OSA based on AHI (Fig 1). As shown in Figure 1, log LTL increased among groups as the magnitude of AHI increased. Subjects with moderate-to-severe OSA had the highest log LTL compared with controls (log-transformed LTL, 0.10 ± 0.14 vs 0.02 ± 0.13, respectively; actual LTL, 1.34 ± 0.47 vs 1.12 ± 0.37, respectively; P < .01) (Fig 1A). Moreover, even when we adjusted for age, log LTL still showed significant differences among the moderate-to-severe OSA, mild OSA, and control groups (age-adjusted log LTL, 0.105 ± 0.025 vs 0.067 ± 0.014 vs 0.028 ± 0.013, respectively; P = .012). Further, when we applied Bonferroni corrections for multiple comparisons, LTL only showed significant differences between the moderate-to-severe OSA and control groups (P = .021). However, log catestatin levels were decreased in terms of the severity of OSA. The lowest log catestatin levels were seen in the moderate-to-severe OSA group vs the mild OSA and control groups (log-transformed catestatin levels, 0.12 ± 0.22 vs 0.23 ± 0.20 vs 0.28 ± 0.19, respectively; actual catestatin levels, 1.52 ± 0.81 ng/mL vs 1.92 ± 0.96 ng/mL vs 2.14 ± 1.00 ng/mL, respectively; P < .01) (Fig 1B). Finally and as previously reported,26 log MRP 8/14 levels were incrementally higher with increasing AHI severity among the moderate-to-severe OSA, mild OSA, and control groups (log-transformed MRP 8/14 levels, 0.20 ± 0.24 vs 0.00 ± 0.31 vs −0.12 ± 0.33, respectively; actual MRP 8/14 levels, 1.82 ± 0.97 μg/mL vs 1.28 ± 0.91 μg/mL vs 1.00 ± 0.84 μg/mL, respectively; P < .01) (Fig 1C).
Figure 1.
Boxplots of LTL in 102 controls and 111 subjects with OSA (A). Boxplots of catestatin levels in 56 controls and 91 subjects with OSA (B). Boxplots of MRP 8/14 levels in 102 controls and 111 subjects with OSA. LTL = leukocyte telomere length; MRP = myeloid-related protein; T/S ratio = amount of telomeric DNA to amount of single-copy control gene DNA.
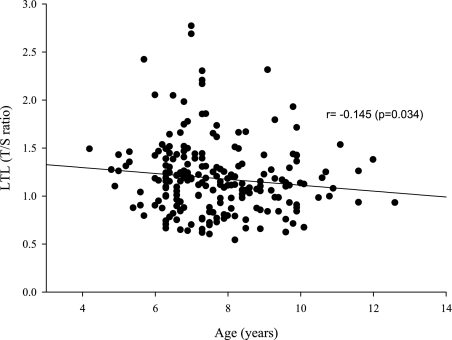
To estimate potential associations among the three biomarkers and polysomographic measures and BP levels, we performed Spearman correlation analyses. A significant linear correlation between LTL and AHI (r = 0.236; P < .01) (Table 2) and a predictable inverse correlation with age (r = −0.145; P < .05) (Fig 2) emerged. However, LTL was not significantly associated with either MRP 8/14 level (r = 0.027; P > .05) (Table 2) or catestatin concentration (r = −0.119; P > .05). Notwithstanding, catestatin plasma levels not only were inversely correlated with AHI (r = −0.226; P < .01) but also were significantly associated with mean arterial BP level (n = 115; r = −0.184; P < .05) and MRP 8/14 level (r = −0.163; P < .05) (Table 2).
Table 2.
—Correlation Coefficients Among LTL, Catestatin, and MRP 8/14 Levels and Other Variables in 213 Children
| Spearman Correlation Coefficients |
||||||
| Telomere Length (T/S Ratio) (n = 213) |
Catestatina (n = 147) |
MRP 8/14 (n = 213) |
||||
| Variable | r | P | r | P | r | P |
| Age | −0.145b | .034 | 0.054 | .519 | −0.051 | .463 |
| BMI z score | 0.122 | .075 | −0.078 | .349 | 0.391c | < .001 |
| Systolic BPa | 0.122 | .235 | −0.166 | .079 | 0.418c | < .001 |
| Diastolic BPa | 0.183b | .049 | −0.171 | .070 | 0.237b | .011 |
| Mean arterial pressurea | 0.167 | .074 | −0.184b | .049 | 0.332c | < .001 |
| AHI | 0.236c | < .01 | −0.226c | < .01 | 0.263c | < .001 |
| Sao2 nadir | −0.017 | .815 | 0.093 | .278 | −0.251c | < .001 |
| Total cholesterold | 0.001 | .996 | −0.126 | .198 | 0.168 | .082 |
| HDL cholesterold | 0.008 | .936 | 0.099 | .313 | −0.140 | .145 |
| LDL cholesterold | −0.004 | .965 | −0.115 | .237 | 0.173 | .072 |
| Triglyceridesd | −0.027 | .783 | −0.120 | .217 | 0.230b | .016 |
| MRP 8/1 4 | 0.027 | .700 | −0.163b | .048 | … | … |
| Catestatin | −0.119 | .152 | … | … | … | … |
Data were adjusted for age and BMI z score. See Table 1 for expansion of abbreviations.
These data were included for 115 children.
P < .05.
P < .01.
These data were included for 112 children.
Figure 2.
Scatterplots of LTL against chronologic age in children with obstructive sleep apnea and controls. See Figure 1 legend for expansion of abbreviations.
To further examine independent predictors of log LTL in subjects, we performed regression analyses (Table 3). In the initial univariate analysis, LTL exhibited a trend toward a positive correlation with mean arterial BP (n = 115; β ± SE, 0.003 ± 0.002; P = .070). In the multiple regression analysis, LTL was only positively associated with AHI (β ± SE, 0.28 ± 0.03; P < .01) after controlling for age, sex, BMI z score, and race. Even when adjusted for confounding factors, MRP 8/14, and catestatin levels, LTL still was related with AHI (β ± SE, 0.44 ± 0.02; P < .05).
Table 3.
—Univariate and Multivariate Analyses Between AHI and LTL and Covariates
| Telomere Length (T/S Ratio)a |
||||||
| Univariate |
Stepwise Multivariate |
|||||
| Independent Variable | β | SE | P | β | SE | P |
| Age | −0.010 | 0.006 | .114 | … | … | … |
| Sex | 0.000 | 0.019 | .983 | … | … | … |
| Race | 0.010 | 0.011 | .325 | … | … | … |
| BMI z score | 0.012 | 0.008 | .126 | … | … | … |
| AHIa | 0.042 | 0.016 | .009 | 0.28 | 0.03 | .002 |
SE = standard error. See Table 1 for expansion of other abbreviations.
Data were log transformed.
Odd Ratios for OSA According to Tertiles of LTL and Catestatin Levels in Children
In order to estimate odds ratios for OSA in relation to any given catestatin level, we performed logistic regression analysis (n = 147). Table 4 presents univariate and multivariate odds ratios on the likelihood of OSA according to decreasing tertiles of catestatin levels. In the univariate model, odds ratios of mild-to-moderate OSA (AHI ≥ 5) were 5.47 (95% CI, 1.28-23.3; P < .05) for the lowest tertile of catestatin (< 1.39 ng/mL), using the highest catestatin tertile level (> 2.13 ng/mL) as a reference. After adjusting for confounding factors such as age, sex, race, and BMI z score, subjects in the lowest tertile of catestatin levels had a 5.24-fold (95% CI, 1.19-23.4; P < .05) increased risk for moderate-to-severe OSA compared with those whose catestatin levels were within the higher range. Besides, subjects in highest tertile of LTL had 4.85-fold (95% CI, 1.26-15.3; P < .05) increased risk for moderate-to-severe OSA compared with those whose LTLs were within the lower range.
Table 4.
—Logistic Regression Analysis on the Association of OSA and Catestatin Tertile Levels in 147 Children
| Univariate Odds Ratio (95% CI) |
Multivariate Odds Ratio (95% CI)a |
||||||
| Definition of Outcome | OSA, n | Catestatin First Tertile (n = 48) | Catestatin Second Tertile (n = 49) | Catestatin Third Tertile (n = 50) | Catestatin First Tertile (n = 48) | Catestatin Second Tertile (n = 49) | Catestatin Third Tertile (n = 50) |
| Mild OSA vs control | 70 | 3.22 (0.75-13.8) | 1.11 (0.48-2.56) | 1.0 | 2.64 (0.59-11.8) | 1.13 (0.47-2.70) | 1.0 |
| Moderate-to-severe OSA vs control | 21 | 5.47 (1.28-23.3)b | 1.64 (0.68-3.92) | 1.0 | 5.24 (1.19-23.4)b | 1.58 (0.65-3.87) | 1.0 |
Logistic regression analysis was used to estimate odds ratios and 95% CIs after the cohort was divided into three groups based on tertile cut points according to the distribution of catestatin for the whole cohort. First tertile range, < 1.39 ng/mL; second tertile range, 1.39-2.13 ng/mL; third tertile range, > 2.13 ng/mL. See Table 1 for expansion of abbreviation.
Data were adjusted for age, sex, race, and BMI z score.
P < .05.
Discussion
In contrast to our original expectations, we found that children with OSA have increased LTL and exhibit a dose-dependent increase in LTL. As anticipated from previous studies, however, LTL was significantly negatively correlated with age.6 Furthermore, children with OSA had lower plasma catestatin levels than those of controls, and catestatin levels not only were inversely correlated with BP but also showed dose-dependent decreases according to AHI. As expected from our previous study,26 MRP 8/14 levels were increased in subjects with OSA, but LTL did not correlate with either catestatin or MRP 8/14 levels. However, even after adjusting for potential confounding factors, AHI was positively and independently associated with LTL.
Telomeres are tandem repeats of DNA sequences that cap and protect chromosomal integrity. Telomere dynamics exhibit an additional feature that is highly relevant to all epidemiologic studies that link LTL with aging-related diseases,37,38 namely, as telomere length becomes critically shortened, the cellular replicative machinery stops functioning, leading to replicative senescence.7,39 Oxidative stress, inflammation, and increased leukocyte turnover are major factors associated with accelerated telomere shortening and biologic aging and have been implicated in atherosclerosis and other cardiovascular diseases.8-10,16,40,41 Based on the currently proposed putative mechanisms underlying the morbid consequences of OSA, namely oxidative stress and increased activation of inflammatory processes,3,4 the hypothesis that children with OSA would exhibit reduced LTL was a logical sequel to the aforementioned considerations. However, rather than the anticipated inverse correlation between LTL and OSA severity, a positive association emerged. Although the mechanisms responsible for this surprising finding will have to be elucidated, several possibilities may account for it. First Vasan and colleagues42 recently have demonstrated that LTL is positively associated with left ventricular mass and wall thickness, especially in subjects with hypertension. Thus, LTL would be expected to be longer in consideration of the cumulative evidence showing that OSA induces increased activity and reactivity of the sympathetic nervous system43,44 and that systemic BP elevations not only are OSA severity dependent45,46 but also are associated with altered left ventricular geometry and contractibility.47,48 In this context, catestatin, a novel endogenous peptide that regulates cardiac function and BP through inhibitory activity on catecholamine-releasing chromaffin cells21,49 showed OSA severity-dependent decreases and corresponding BP increases such that the increased cardiovascular load elicited by OSA may contribute to increased LTL in children.
Second, OSA may induce early mobilization of mesenchymal stem cells and possibly recruitment of endothelial progenitor cells into peripheral blood in animal models.50-52 The protective action of mesenchymal stem cells stimulated by inflammatory mediators or hypoxia is exerted through paracrine mechanisms53,54 and by secretion of angiogenic growth factors, antiapoptotic factors, or antiinflammatory cytokines.55 Thus, LTL could reflect the replicative capacity of hematopoietic stem cells and serve as a marker of the angiogenic potential recruited as an endogenous palliative response aiming to minimize OSA-related end-organ damage.
Third, in contrast to many other human somatic cells, human lymphocytes can express the enzyme telomerase. The expression of telomerase is highly regulated during development and activation. Whereas resting mature human T cells do not express telomerase activity, proliferating T cells stimulated in vitro express high levels of telomere activity.6 Patients with OSA exhibit T cells in a highly activated state56 such that genetic variants and activation of telomerase could play an important role in the maintenance of telomere length in children with OSA.6,57
Fourth, there is a possible link between insulin-like growth factor-1 (IGF-1) and its antiinflammatory or antioxidative role in the context of telomere dynamics. Indeed, an independent association between higher IGF-1 and longer LTL has emerged that persists even after adjustment for confounding factors, suggesting a role for IGF-1 in mechanisms relating to telomere maintenance.58,59 Furthermore, circulating IGF-1 is regulated by hypoxia60 and exerts powerful antiinflammatory and antioxidant effects along with cooperative interactions with increasing numbers of endothelial progenitor cells to mitigate atherosclerosis progression.61 Moreover, IGF-1 has been shown to exert an important role in preserving cognitive function in children with OSA.62 Accordingly, higher IGF-1 levels may underlie the link between longer LTL and OSA.
Finally, telomerase expression is tightly regulated at the transcriptional and posttranscriptional level such that hypoxia would be anticipated to increase telomerase activity and thus result in longer LTL.63-65 Although we did not measure telomerase activity or find a significant correlation between LTL and nadir arterial oxygen saturation, we have previously shown that serum vascular endothelial growth factor levels, which are tightly controlled through hypoxia-inducible factor activity, are dose-dependently increased in children with OSA,66 lending support to the possibility that OSA-induced telomerase activity may have promoted the positive correlation between LTL and AHI reported herein.
Several limitations regarding the LTL portion of this study must be acknowledged. First, we did not measure IGF-1 levels or a large array of known antiinflammatory or antioxidative makers to elucidate the potential links to increased LTL in pediatric OSA. However, plasma MRP 8/14, an important vascular inflammatory marker associated with pediatric OSA,26 showed no significant relationship with LTL. Second, although a limited age range was present in our cohort by design, we found predicted reduction on LTL with advancing age. Third, we did not explore LTL in a longitudinal fashion, which may have illustrated an accelerated reduction in LTL over time among patients with OSA.
Some additional comments are necessary based on aforementioned findings. It remains unclear whether the increase of LTL is a cause or a consequence of OSA or whether it is simply an epiphenomenon. Similarly, it is uncertain whether LTL at any given age is simply a marker of the cumulative oxidative and inflammatory burden through life or, alternatively, whether LTL plays an active pathogenic role in the predisposition to adverse outcomes. Indeed, because telomere length is highly variable at birth, individuals endowed with relatively long LTL at birth are more likely to display a longer LTL at any age than those born with short LTL.67
As indicated previously, plasma catestatin levels were lower in subjects with OSA and associated with a predictive probability of BP elevation and of OSA in this cohort, suggesting that this peptide not only may be involved in the pressor response to OSA but also may serve as a risk-related biomarker for cardiovascular involvement in the context of OSA in children. Indeed, the risk of hypertension has been repeatedly demonstrated in recent studies in children.44,46,47,68 Similarly, our previous findings regarding MRP 8/14 in pediatric OSA26 were confirmed in this study, albeit in the absence of any significant association between LTL and this inflammatory protein.
In conclusion, we report a seemingly paradoxical positive association between LTL and OSA in children. Despite the relatively modest sample size, narrow age range, and other potential limitations discussed, this intriguing finding merits future confirmatory and mechanistic studies. Furthermore, this study shows OSA severity-dependent decreases in catestatin that also are associated with increased risk for elevated BP, and we confirm the increased plasma concentration of the vascular inflammatory protein MRP 8/14. We postulate that routine assessment of catestatin and MRP 8/14 in children with OSA may provide a surrogate estimate of vascular risk in these patients.
Acknowledgments
Author contributions: All authors critically reviewed and approved the final version of the manuscript.
Dr Kim: contributed to collecting samples, conducting plasma assays, analyzing data, and drafting the first version of the manuscript.
Dr Lee: contributed to conducting plasma assays.
Dr Bhattacharjee: contributed to recruiting subjects and conducting plasma assays.
Dr Khalyfa: contributed to conducting some assays and assisting with assay troubleshooting.
Dr Kheirandish-Gozal: contributed to recruiting subjects, assisting with data analyses, and manuscript editing and intellectual content.
Dr Gozal: contributed to initiating the conceptual framework of the project, recruiting subjects, assisting with data analysis and interpretation, and intellectual content in the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- AHI
apnea-hypopnea index
- CI
confidence interval
- hrTST
hours of total sleep time
- IGF-1
insulin-like growth factor-1
- LTL
leukocyte telomere length
- MRP
myeloid-related protein
- OSA
obstructive sleep apnea
Funding/Support: Dr Gozal is supported by the National Institutes of Health [Grants HL-065270 and HL-086662]. Dr Bhattacharjee was supported by a sleep fellowship from Jazz Pharmaceuticals.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Somers VK, White DP, Amin R, et al. American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology. American Heart Association Stroke Council. American Heart Association Council on Cardiovascular Nursing. American College of Cardiology Foundation Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog Cardiovasc Dis. 2009;51(5):416–433. doi: 10.1016/j.pcad.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D. Sleep, sleep disorders and inflammation in children. Sleep Med. 2009;10(suppl 1):S12–S16. doi: 10.1016/j.sleep.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177(4):369–375. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas RM, Haddad GG. Can O2 dysregulation induce premature aging? Physiology (Bethesda) 2008;23:333–349. doi: 10.1152/physiol.00023.2008. [DOI] [PubMed] [Google Scholar]
- 6.Hathcock KS, Jeffrey Chiang Y, Hodes RJ. In vivo regulation of telomerase activity and telomere length. Immunol Rev. 2005;205:104–113. doi: 10.1111/j.0105-2896.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 7.Oeseburg H, de Boer RA, van Gilst WH, et al. Telomere biology in healthy aging and disease. Pflugers Arch. 2010;459(2):259–268. doi: 10.1007/s00424-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 9.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78(2):547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 10.Vasto S, Candore G, Balistreri CR, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128(1):83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64(7):631–636. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 12.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33(6):1467–1484. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 13.Bekaert S, De Meyer T, Rietzschel ER, et al. Asklepios investigators Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6(5):639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 14.van der Harst P, Wong LS, de Boer RA, et al. MERIT-HF Study Group Possible association between telomere length and renal dysfunction in patients with chronic heart failure. Am J Cardiol. 2008;102(2):207–210. doi: 10.1016/j.amjcard.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Benetos A, Gardner JP, Zureik M, et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43(2):182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 16.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358(9280):472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 17.Rufer N, Brümmendorf TH, Kolvraa S, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190(2):157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas N, Vaingankar SM, Mahata M, et al. Proteolytic cleavage of human chromogranin a containing naturally occurring catestatin variants: differential processing at catestatin region by plasmin. Endocrinology. 2008;149(2):749–757. doi: 10.1210/en.2007-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, et al. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci U S A. 2003;100(16):9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20(7):1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Mahapatra NR. Catestatin is a novel endogenous peptide that regulates cardiac function and blood pressure. Cardiovasc Res. 2008;80(3):330–338. doi: 10.1093/cvr/cvn155. [DOI] [PubMed] [Google Scholar]
- 22.Rao F, Wen G, Gayen JR, et al. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352-372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115(17):2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 23.Robinson MJ, Tessier P, Poulsom R, Hogg N. The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J Biol Chem. 2002;277(5):3658–3665. doi: 10.1074/jbc.M102950200. [DOI] [PubMed] [Google Scholar]
- 24.Tan KC, Chow WS, Lam JC, et al. Advanced glycation endproducts in nondiabetic patients with obstructive sleep apnea. Sleep. 2006;29(3):329–333. doi: 10.1093/sleep/29.3.329. [DOI] [PubMed] [Google Scholar]
- 25.Croce K, Gao H, Wang Y, et al. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120(5):427–436. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Bhattacharjee R, Snow AB, Capdevila OS, Kheirandish-Gozal L, Gozal D. Myeloid related protein 8/14 levels in children with obstructive sleep apnoea. Eur Respir J. 2010;35(4):843–850. doi: 10.1183/09031936.00075409. [DOI] [PubMed] [Google Scholar]
- 27.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2):555–576. [PubMed] [Google Scholar]
- 28.Gozal D, Kheirandish-Gozal L, Serpero LD, Sans Capdevila O, Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116(20):2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 29.Rechstschaffen AKA. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: Brain Information Services/Brain Research Institute, University of California; 1968. [Google Scholar]
- 30.American Thoracic Society Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153(2):866–878. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 32.Schulz H. Phasic or transient? Comment on the terminology of the AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2007;3(7):752. [PMC free article] [PubMed] [Google Scholar]
- 33.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 34.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grabowski P, Hultdin M, Karlsson K, et al. Telomere length as a prognostic parameter in chronic lymphocytic leukemia with special reference to VH gene mutation status. Blood. 2005;105(12):4807–4812. doi: 10.1182/blood-2004-11-4394. [DOI] [PubMed] [Google Scholar]
- 37.Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci. 2008;63(9):979–983. doi: 10.1093/gerona/63.9.979. [DOI] [PubMed] [Google Scholar]
- 38.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35(6):1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 39.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. 1995;92(24):11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Harst P, van der Steege G, de Boer RA, et al. MERIT-HF Study Group Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49(13):1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Aviv A. Chronology versus biology: telomeres, essential hypertension, and vascular aging. Hypertension. 2002;40(3):229–232. doi: 10.1161/01.hyp.0000027280.91984.1b. [DOI] [PubMed] [Google Scholar]
- 42.Vasan RS, Demissie S, Kimura M, et al. Association of leukocyte telomere length with echocardiographic left ventricular mass: the Framingham heart study. Circulation. 2009;120(13):1195–1202. doi: 10.1161/CIRCULATIONAHA.109.853895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Brien LM, Gozal D. Autonomic dysfunction in children with sleep-disordered breathing. Sleep. 2005;28(6):747–752. doi: 10.1093/sleep/28.6.747. [DOI] [PubMed] [Google Scholar]
- 44.McConnell K, Somers VK, Kimball T, et al. Baroreflex gain in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180(1):42–48. doi: 10.1164/rccm.200808-1324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bixler EO, Vgontzas AN, Lin HM, et al. Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension. 2008;52(5):841–846. doi: 10.1161/HYPERTENSIONAHA.108.116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li AM, Au CT, Sung RY, et al. Ambulatory blood pressure in children with obstructive sleep apnoea: a community based study. Thorax. 2008;63(9):803–809. doi: 10.1136/thx.2007.091132. [DOI] [PubMed] [Google Scholar]
- 47.Amin R, Somers VK, McConnell K, et al. Activity-adjusted 24-hour ambulatory blood pressure and cardiac remodeling in children with sleep disordered breathing. Hypertension. 2008;51(1):84–91. doi: 10.1161/HYPERTENSIONAHA.107.099762. [DOI] [PubMed] [Google Scholar]
- 48.Amin RS, Kimball TR, Bean JA, et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(10):1395–1399. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- 49.Helle KB. The chromogranin A-derived peptides vasostatin-I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc Res. 2010;85(1):9–19. doi: 10.1093/cvr/cvp266. [DOI] [PubMed] [Google Scholar]
- 50.Carreras A, Almendros I, Acerbi I, Montserrat JM, Navajas D, Farré R. Obstructive apneas induce early release of mesenchymal stem cells into circulating blood. Sleep. 2009;32(1):117–119. [PMC free article] [PubMed] [Google Scholar]
- 51.de la Peña M, Barceló A, Barbe F, et al. Endothelial function and circulating endothelial progenitor cells in patients with sleep apnea syndrome. Respiration. 2008;76(1):28–32. doi: 10.1159/000109643. [DOI] [PubMed] [Google Scholar]
- 52.Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117(17):2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Woods CR, Mora AL, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 54.Patel KM, Crisostomo P, Lahm T, et al. Mesenchymal stem cells attenuate hypoxic pulmonary vasoconstriction by a paracrine mechanism. J Surg Res. 2007;143(2):281–285. doi: 10.1016/j.jss.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25(9):2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 56.Lavie L, Dyugovskaya L, Polyakov A. Biology of peripheral blood cells in obstructive sleep apnea—the tip of the iceberg. Arch Physiol Biochem. 2008;114(4):244–254. doi: 10.1080/13813450802306701. [DOI] [PubMed] [Google Scholar]
- 57.De Boeck G, Forsyth RG, Praet M, Hogendoorn PC. Telomere-associated proteins: cross-talk between telomere maintenance and telomere-lengthening mechanisms. J Pathol. 2009;217(3):327–344. doi: 10.1002/path.2500. [DOI] [PubMed] [Google Scholar]
- 58.Moverare-Skrtic S, Svensson J, Karlsson MK, et al. Serum insulin-like growth factor-I concentration is associated with leukocyte telomere length in a population-based cohort of elderly men. J Clin Endocrinol Metab. 2009;94(12):5078–5084. doi: 10.1210/jc.2009-1450. [DOI] [PubMed] [Google Scholar]
- 59.Barbieri M, Paolisso G, Kimura M, et al. Higher circulating levels of IGF-1 are associated with longer leukocyte telomere length in healthy subjects. Mech Ageing Dev. 2009;130(11-12):771–776. doi: 10.1016/j.mad.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Moromisato DY, Moromisato MY, Zanconato S, Roberts CT., Jr Effect of hypoxia on lung, heart, and liver insulin-like growth factor-I gene and receptor expression in the newborn rat. Crit Care Med. 1996;24(6):919–924. doi: 10.1097/00003246-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Sukhanov S, Higashi Y, Shai SY, et al. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 62.Gozal D, Sans Capdevila O, McLaughlin Crabtree V, Serpero LD, Witcher LA, Kheirandish-Gozal L. Plasma IGF-1 levels and cognitive dysfunction in children with obstructive sleep apnea. Sleep Med. 2009;10(2):167–173. doi: 10.1016/j.sleep.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Seimiya H, Tanji M, Oh-hara T, Tomida A, Naasani I, Tsuruo T. Hypoxia up-regulates telomerase activity via mitogen-activated protein kinase signaling in human solid tumor cells. Biochem Biophys Res Commun. 1999;260(2):365–370. doi: 10.1006/bbrc.1999.0910. [DOI] [PubMed] [Google Scholar]
- 64.Minamino T, Mitsialis SA, Kourembanas S. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol Cell Biol. 2001;21(10):3336–3342. doi: 10.1128/MCB.21.10.3336-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson CJ, Hoare SF, Ashcroft M, Bilsland AE, Keith WN. Hypoxic regulation of telomerase gene expression by transcriptional and post-transcriptional mechanisms. Oncogene. 2006;25(1):61–69. doi: 10.1038/sj.onc.1209011. [DOI] [PubMed] [Google Scholar]
- 66.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep. 2002;25(1):59–65. doi: 10.1093/sleep/25.1.59. [DOI] [PubMed] [Google Scholar]
- 67.Njajou OT, Cawthon RM, Damcott CM, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104(29):12135–12139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zintzaras E, Kaditis AG. Sleep-disordered breathing and blood pressure in children: a meta-analysis. Arch Pediatr Adolesc Med. 2007;161(2):172–178. doi: 10.1001/archpedi.161.2.172. [DOI] [PubMed] [Google Scholar]