Abstract
Objectives
Although the effects of exercise on neurocognition have been the subject of several previous reviews and meta-analyses, they have been hampered by methodological shortcomings and are now outdated as a result of the recent publication of several large-scale randomized controlled trials (RCTs).
Methods
We conducted a systematic literature review of RCTs examining the association between aerobic exercise training on neurocognitive performance conducted between January, 1966 and July, 2009. Suitable studies were selected for inclusion according to the following criteria: randomized treatment allocation, mean age ≥ 18 years of age, duration of treatment > 1 month, incorporated aerobic exercise components, exercise training was supervised, the presence of a non-aerobic-exercise control group, and sufficient information to derive effect size (ES) data.
Results
Twenty-nine studies met inclusion criteria and were included in our analyses, representing data from 2,049 participants and 234 effect sizes. Individuals randomly assigned to receive aerobic exercise training demonstrated modest improvements in attention and processing speed (g = .158 [95% CI: .055 to .260], P = .003), executive function (g = .123 [95% CI: .021 to .225], P = .018), and memory (g = .128 [95% CI: .015 - .241], P = .026).
Conclusions
Aerobic exercise training is associated with modest improvements in attention and processing speed, executive function, and memory, although the effects of exercise on working memory are less consistent. Rigorous RCTs are needed with larger samples, appropriate controls, and longer follow-up periods.
Keywords: Cognitive performance, aerobic exercise, neuropsychological performance, executive function, randomized controlled trial, meta-analysis
INTRODUCTION
Strategies to enhance neurocognitive functioning have important public health implications as subclinical neurocognitive deficits are associated with increased risk of neurocognitive impairment (1), dementia (2), and mortality (3-7), independent of traditional risk factors. One such strategy that has gained increased attention is the use of aerobic exercise to improve neurocognitive functioning (8-12). Although the value of exercise has been critically examined in review articles (13) and meta-analytic syntheses (8-11), there has been a lack of agreement as to the magnitude of improvement in neurocognitive function associated with physical activity interventions. The current lack of consensus is due to differences in the evaluation of study methodologies, studies included in the analyses, data analytic approaches, and in the classification of various neurocognitive measures.
Cross-sectional studies have shown that physically active individuals tend to exhibit better neurocognitive function relative to inactive individuals (13-22). Prospective observational studies have reported similar findings, demonstrating that individuals who maintain greater levels of physical activity show improvements in neurocognitive function relative to their sedentary counterparts (1, 23-28). However, randomized trials have provided inconsistent results, with some reporting cognitive gains (29, 30) and others equivocal findings (31). Meta-analytic reviews of randomized controlled trials (RCTs) have also reported great variation in the magnitude of improvement in neurocognition associated with aerobic exercise (10-12), with some meta-analyses reporting moderate cognitive gains (9, 10) and others more modest improvements (8, 11, 32).
In several recent meta-analyses, including a Cochrane review (11), it was concluded that current data are insufficient to show that improvements in neurocognitive function associated with physical activity are due to improved cardiovascular fitness, and that larger studies are necessary (11, 32). However, since the publication of this review there have been several large scale RCTs examining this relationship (30, 31, 33, 34). In addition, although one previous systematic review (12) examined the effects of various forms of physical activity on boosting cognitive function (primarily general orientation) among individuals with dementia, no reviews have combined data from trials attempting to prevent dementia among vulnerable populations (i.e. individuals with cognitive impairment). The Cochrane review was limited in this sense as persons with neurocognitive impairments (e.g., mild cognitive impairment (MCI) and depression) were excluded (11). Furthermore, previous meta-analyses examining this relationship may have been influenced by the inclusion of two relatively large studies reporting substantial treatment effects but that were not truly randomized (9, 35, 36), which may have overly influenced the reported effects. Therefore, we conducted a meta-analysis that included the most recent exercise intervention trials and addressed several issues including: (1) the effects of aerobic exercise training on specific domains of neurocognitive performance including attention and processing speed, executive function, working memory, and memory; (2) the influence of specific dimensions of the exercise prescription such as the mode, duration and intensity of the exercise intervention; and (3) the issue of individual differences in response to exercise training, with a focus on baseline, pre-exercise level of cognitive functioning as a potential moderator of exercise effects (i.e., we compared individuals with Mild Cognitive Impairment (MCI)) to cognitively intact samples), as well as the age of study participants.
METHODS
In order to determine the effects of aerobic exercise interventions on neurocognitive status, an extensive literature search was conducted using the following databases between January, 1966 and July, 2009: MEDLINE, Pubmed, EMBASE, Gateway, CENTRAL, PsycINFO, Dissertation Abstracts International, Educational Research in Completion (ERIC), Sports Discus, Cochrane Register, PEDRO, Ageline, and CINAHL. The following search terms were used: cogniti*, cognitive performance, age*, elderly, mental performance, and neuropsychological in combination with fitness, aerobic, cardiovascular, VO2, and physical activity. Additional titles were identified by a manual search of relevant journals and by identifying references included in previous meta-analyses. Unpublished dissertations and conference papers were also obtained when possible.
Suitable studies were selected for inclusion according to the following criteria: (1) randomized treatment allocation; (2) mean age ≥ 18 years of age and non-demented; (3) Duration of treatment > 1 month; (4) involved aerobic exercise training (e.g. brisk walking, biking, or jogging). Age 18 was selected as a lower age limit in order to control for developmental age differences in cortical thickness and myelination, which stabilize around the second decade of life (37). Studies utilizing walking interventions that were not aerobic were not included (e.g., slow walking with frequent breaks) in order to ensure that included trials incorporated some aerobic exercise component. Combined exercise interventions with an aerobic exercise component were also included (e.g. jogging and yoga); (5) the presence of a control group that did not engage in aerobic exercise; and (6) sufficient information to derive an estimate of effect size (ES).
After initial identification and retrieval of studies, several were found to be quasi-randomized studies (36) or used case-control methodologies (15, 36, 38-44), were of insufficient duration to include (45-47), were found not to be non-randomized based on personal communication with the trial’s principal investigator (39), or did not utilize a non-aerobic exercise control group (48, 49). Another trial was conducted among adolescents and was therefore excluded (50). Several trials utilized ‘dual-task’ interventions (e.g. walking and talking) (51-53) or balance and strength-training (54, 54, 55) and were therefore not included as it could not be ascertained whether exercise was of sufficient intensity to produce aerobic changes. Several trials were not included because they utilized physical activity interventions with exclusively non-aerobic exercise components among individuals with dementia (52, 56, 57, 57, 58, 58-65). The few studies utilizing walking interventions were either explicitly non-aerobic (58) or allowed residents with limited mobility (e.g., using walkers) to rest as needed (52), thereby limiting their generalizability to more healthy samples. Accordingly, these studies were excluded from the current analyses. For two trials in which the method of randomization was unclear (39, 66) we attempted to contact the respective authors and were able to confirm that one followed a true randomization scheme (39). Results were unchanged when the remaining study was excluded and we therefore included this trial in all analyses (66).
Assessment of Study Quality
Two raters (PJS, BMH) independently extracted information from each article using an identical review protocol, which included including study identifiers (e.g., author names, year of publication, publishing journal), duration of treatment, intensity of exercise, modality of exercise, blinding of assessment personnel to treatment status, during assessments, intention to treat analyses, and time of follow-up assessment. Effect sizes were assessed independently. Interrater reliability was assessed for the outcome domains in question (i.e., in each cognitive domain as well as for study characteristics). For all areas, interrater reliability was found to be excellent (r’s > .90; Cohen’s kappa = .75).
Data Analysis
Neuropsychological test results were classified according to the cognitive domains described by Lezak (67). We considered neurocognitive tests that could be classified in the following categories: attention and processing speed (the sustained focus of cognitive resources with selective concentration and rapid processing of information (67, 68)), executive function (a set of cognitive skills responsible for the planning, initiation, sequencing, and monitoring of complex, goal-directed behavior), working memory (short-term storage and manipulation of information), and declarative memory (retention, recollection, and recognition of previously encountered information, hereafter referred to only as: “memory”). We considered including ‘complex processing speed’ as a measure of executive function as in previous analyses (9) but results were unchanged regardless of the classification of this test.
Analyses were conducted using Comprehensive Meta-analysis software (Englewood, NJ). Data were analyzed using both fixed and random effects models and Cohen’s G for between-group differences (69). Briefly, fixed effects analysis assumes that all studies are drawn from the same population, such that differences in treatment effects across studies are attributed to sampling and methodological variability (i.e. error variance). In contrast, random effects analysis allows for the possibility that studies are drawn from different populations, such that differences across studies may be due to unidentified sources of variation and provides a more conservative estimate of treatment effects (70). However, because results did not differ between fixed and random effects analyses and because random effects are generally recommended for examining treatment effects in meta-analytic studies (70), we have presented the random effects findings only. In trials reporting multiple effect sizes within the same neurocognitive domain, data were collapsed by averaging all effect sizes within each neurocognitive domain for each study, such that each study produced no more than one effect size per domain. For the purposes of the study quality analyses, treatment effects were collapsed for each study for all neurocognitive domains. In addition, two trials in our literature search produced multiple publications in either peer-reviewed journals (71-73) or book chapters (74, 75) that were combined for the purposes of analysis. Homogeneity of treatment effects was assessed using the Q statistic. Three trials collected neurocognitive data at multiple time points in which participants continued to receive treatment (30, 73, 76). However, in only one study were the effects of treatment un-contaminated by crossover between groups (30). For this study only (30), we chose data from the longest follow-up assessment for inclusion in our analyses, although results were unchanged when other time points were examined.
Exploratory sensitivity analyses (77, 78) were conducted in order to investigate sample characteristics that may have moderated the effects of treatment on neurocognitive outcomes. Specifically, three trial characteristics were examined: Duration, Intensity and Mode of exercise intervention. We also examined two important methodological characteristics associated with methodological quality: blinding of assessors of neurocognitive outcomes and use of intention-to-treat (ITT) analyses. As an additional analysis, we examined whether treatment effects varied by cognitive status of participants at baseline (i.e., ‘non-impaired’ or mild cognitive impairment [MCI]; patients with dementia [Alzheimer’s disease] were excluded) and age of study participants.
RESULTS
Our initial literature search yielded 5,538 potentially relevant studies, 68 of which were retrieved for full-text review. Twenty nine studies (N = 29) incorporating data from 2,049 participants met inclusion criteria and were included in the present analyses (Table 1), including data for 1,024 experimental participants and 997 controls. Two hundred thirty four (n = 234) effect sizes were available for analysis. Trials ranged in duration from six weeks (79) to 18 months (30). As shown in Table 1, the primary exercise modality was brisk walking and/or jogging and control groups were typically assigned to a wait-list control, although stretching and toning, health education, and relaxation exercises were also used. Rates of attrition varied widely (range 0 – 41%; mean attrition = 12.2%). Only 13 studies (44.8%) utilized blinded assessments and only seven studies (24.1%) utilized intention-to-treat (ITT) analyses. The effects of exercise on individual neurocognitive measures are presented in Table 3. Due to the substantial number and heterogeneity of neurocognitive tests, only those tests used in more than one study are presented.
Table 1.
Randomized controlled trials examining the effect of aerobic exercise on neurocognitive function
| Author / Year | Sample | Intervention | Instruments | Methodological Characteristics |
Exercise Intervention |
Hedge’s G |
|---|---|---|---|---|---|---|
| Bakken, 2001 (103) |
15, older adults, ages 72 to 91 |
Duration: 8 wks Frequency: 30min, 3/wk Intensity: - - - Combined Strength Training: N MCI: N |
Imaging (Verbal Fluency), Visual Discrimination, Raven’s Progressive Matrices, Short-Term Retention, Addition, Perception Of Ambiguous Stimuli |
Attrition: 0% ITT: N Blinding: N |
Duration: 8 wks Frequency: 30min, 3/wk Intensity: - - - |
AT = .169 |
| Blumenthal, 1989 (72)& Madden, 1989(71) |
101, sedentary, ages 60 to 83 |
Duration: 16 wks¥ Frequency: 40min, 3/wk Intensity: 70% HRR Combined Strength Training: N MCI: N |
Finger Tapping, Benton Revised Visual Retention Test, Digits Forward, Digits Backward, Selective Reminding Test, Randt Memory Test – Short Story, TMT-B, Digit Symbol, Ruff 2 & 7 Test, Stroop Color, Stroop Color-Word Interference, Nonverbal Fluency Test, Verbal Fluency Test |
Attrition: 8% ITT:Y Blinding: Y |
Duration: 16 wks Frequency: 40min, 3/wk Intensity: 70% HRR |
AT = .218 EX = −.025 WM = .114 ME = −.066 |
| Emery, 1990(110) |
48, “inner-city cohort”, ages 61 to 86 |
Duration: 12 wks Frequency: 60min, 3/wk Intensity: 70% HRR Combined Strength Training: Y MCI: N |
Digit Symbol, Digit Span, Word Copy, Number Copy |
Attrition:10% ITT: N Blinding: N |
Duration: 12 wks Frequency: 60min, 3/wk Intensity: 70% HRR |
AT = .028 EX = −.043 WM = .023 |
| Emery, 1998(111) |
79, with stable COPD, age range not reported M = 67 |
Duration: 10 wks¥ Frequency: 45min, 3/wk Intensity: - - - Combined Strength Training: N MCI: N |
Verbal Fluency, Digit Vigilance, Finger Tapping, TMT-A, TMT -B, Digit Symbol |
Attrition: 5% ITT: N Blinding: N |
Duration: 10 wks Frequency: 45min, 3/wk Intensity: - - - |
AT = .075 EX = .325 |
| Fabre, 2002(112) |
32, healthy elderly adults, ages 60 to 76 |
Duration: 8 wks Frequency: 45min, 2/wk Intensity: - - - Combined Strength Training: N MCI: N |
Weschler Memory Scale |
Attrition: 0% ITT: N Blinding: N |
Duration: 8 wks Frequency: 45min, 2/wk Intensity: - - - |
EX = −.188 WM = .878‡ME = −.339 |
| Hassmen, 1992(39) |
32, all women, ages 55 to 75 |
Duration: 12 wks Frequency: 20min, 3/wk Intensity: 9-13 RPE Combined Strength Training: N MCI: N |
Digit Span, Face Recognition, Simple Reaction Time, Choice Reaction Time |
Attrition: 7% ITT: N Blinding: N |
Duration: 12 wks Frequency: 20min, 3/wk Intensity: 9-13 RPE |
AT = .179 EX = .167 WM = .204 ME = −.145 |
| Hawkins, 1992(66) |
40, sedentary, ages 63 to 82 |
Duration: 10 wks Frequency: 45min, 3/wk Intensity: - - - Combined Strength Training: N MCI: N |
Single-Task Reaction Time, Dual Task Reaction Time, Difference Between Single- Task And Dual-Task Reaction Time |
Attrition: 10% ITT: N Blinding: N |
Duration: 10 wks Frequency: 45min, 3/wk Intensity: - - - |
AT = −.243 EX = .047 |
| Hoffman, 2008(31) |
153, sedentary and depressed, ages 41 to 87 |
Duration: 16 wks Frequency: 45min, 3/wk Intensity: 70-85% HRR Combined Strength Training: N MCI: N |
Logical Memory, Verbal Paired Associates, Digit Span, Animal Naming, COWAT, Stroop Color Word, Ruff 2 & 7 Test, Digit Symbol, TMT B-A |
Attrition: 28% ITT: Y Blinding: Y |
Duration: 16 wks Frequency: 45min, 3/wk Intensity: 70-85% HRR |
AT = .277 EX = .172 WM = −.031 ME = .072 |
| Khatri, 2001(113) |
84, sedentary and depressed, ages 50 to 72 |
Duration: 17 wks Frequency: 45min, 3/wk Intensity: 70-85% HRR Combined Strength Training: N MCI: N |
Visual Reproduction, Stroop Color-Work Interference, Digit Span, Tmt-A, Digit Symbol, Stroop Color, Stroop Word, TMT-B, Logical Memory |
Attrition: 25% ITT: Y Blinding: Y |
Duration: 17 wks Frequency: 45min, 3/wk Intensity: 70-85% HRR |
AT = .121 EX = .291 WM = −.047 ME = .186 |
| Kramer, 1999 & 2002(74, 75) |
124, sedentary, ages 60 to 75 |
Duration: 26 wks Frequency: 40 min, 3/wk Intensity: 50-70% HRR Combined Strength Training: N MCI: N |
Reaction Time Tests: Switching Trials, Non- Switching Trials, Incompatible Trials, Compatible Trials, Interference effect (difference Between Compatible And Incompatible Trials), Stop Signal Trials, |
Attrition: 29% ITT:N Blinding: N |
Duration: 26 wks Frequency: 40 min, 3/wk Intensity: 50-70% HRR |
AT = .091 EX = .196 WM = −.101 ME = .156 |
| Lautenschlager, 2008(30) |
170, elderly adults with MCI, age M = 69 |
Duration: 72 wks¥ Frequency: 50 min, 3/wk Intensity: - - - Combined Strength Training: N MCI: Y |
Simple Reaction-Time Trials Word list recall (immediate and delayed), Digit Symbol, COWAT |
Attrition: 19% ITT: Y Blinding: Y |
Duration: 72 wks Frequency: 50 min, 3/wk Intensity: - - - |
AT = .083 EX = −.071 ME = .322** |
| Masley, 2008(114) |
56, adults, age M = 45 |
Duration: 10 wks Frequency: 5/wk Intensity: 70-85% MHR Combined Strength Training: N MCI: N |
CNS Vital Signs (verbal memory, symbol digit coding, the Stroop test, shifting attention, continuous performance) |
Attrition: 16% ITT: N Blinding: N (computerized) |
Duration: 10 wks Frequency: 5/wk Intensity: 70-85% MHR |
AT = −.158 EX = .487‡ |
| Moul, 1995(115) |
30, sedentary, ages 65 to 72 |
Duration: 8 wks Frequency: 35 min, 5/wk Intensity: 60-65% HRR Combined Strength Training: N MCI: N |
Ross Information Processing Assessment Subtests: Organization, Auditory Processing, Immediate Memory, Recent Memory, Temporal Orientation, Problem Solving/ Abstract Reasoning |
Attrition: 0% ITT: N Blinding: N |
Duration: 8 wks Frequency: 35 min, 5/wk Intensity: 60-65% HRR |
EX = .780‡ ME = .351 |
| Munguia- Izquierdo, 2008(116) |
60, middle-aged women with fibromyalgia, ages 18 to 60 |
Duration: 16 wks Frequency: 50 min, 3/wk Intensity: 50-80% MHR Combined Strength Training: N MCI: N |
Paced Auditory Serial Addition Task (PASAT) |
Attrition: 12% ITT: Y Blinding: Y |
Duration: 16 wks Frequency: 50 min, 3/wk Intensity: 50-80% MHR |
AT = .922*** |
| Oken, 2004(117) |
69, multiple sclerosis, M = 49 |
Duration: 26 wks Frequency: 90 min, 1/wk Intensity: - - - Combined Strength Training: N MCI: N |
Stroop Color-Word test, Simple Reaction Time, Complex Reaction Time, Attentional Shift Task, PASAT, WMS Logical Memory, WAIS Similarities |
Attrition = 12% ITT: N Blinding: Y |
Duration: 26 wks Frequency: 90 min, 1/wk Intensity: - - - |
AT = .074 EX = .133 WM = −.354 ME = .000 |
| Oken, 2006(118) |
135, healthy adults, ages 65 to 85 |
Duration: 26 wks Frequency: 60 min, 1/wk Intensity: 70% HRR Combined Strength Training: N MCI: N |
Stroop Interference, Word List Recall, Letter- Number Sequencing, Covert Orienting, Divided Attention, Set Shifting, Simple Reaction time, Complex Reaction time |
Attrition: 13% ITT: N Blinding: Y |
Duration: 26 wks Frequency: 60 min, 1/wk Intensity: 70% HRR |
AT = −.132 EX = −.034 WM = −.029 ME = −.055 |
| Okumiya, 1996(29) |
42, healthy older adults, ages 75 to 87 |
Duration: 8 wks Frequency: 30min, 3/wk Intensity: - - - Combined Strength Training: Y MCI: N |
MMSE, Hasegawa Dementia Scale, Visuospatial Cognitive Performance Test |
Attrition: 0% ITT: N Blinding: N |
Duration: 24 wks Frequency: 60 min, 2/wk Intensity: - - - |
AT = .938** |
| Panton, 1990(119) |
39, healthy untrained older adults, ages 70 to 79 |
Duration: 16 wks Frequency: 40min, 3/wk Intensity: 70% HRR Combined Strength Training: N MCI: N |
Reaction time, Speed of Movement Time |
Attrition: 14% ITT: N Blinding: N |
Duration: 26 wks Frequency: 45min, 3/wk Intensity: 75% HRR |
AT = .111 |
| Perri, 1984(121) | 42, healthy older adults, ages 60 to 79 |
Duration: 10 wks Frequency: 45min, 3/wk Intensity: - - - Combined Strength Training: N MCI: N |
Rey Auditory Verbal Learning Task |
Attrition: 41% ITT: N Blinding: N |
Duration: 15 wks Frequency: 30 min, 3/wk Intensity: 40-50% HRR |
ME = .261 |
| Pierce, 1993(120) |
90, middle-aged adults with hypertension, ages 29-59 |
Duration: 12 wks Frequency: 60min, 3/wk Intensity: 70% HRR Combined Strength Training: N MCI: N |
Digit Symbol, Stroop Color Word test, Digit Span, TMT-B, Sternberg Memory Search Task (Slope and Y- intercept), Verbal Paired Associates, Logical Memory (immediate and delayed), Figural Memory (immediate and delayed) |
Attrition: 7% ITT: Y Blinding: Y |
Duration: 16 wks Frequency: 50 min, 3/wk Intensity: 70% HRR |
AT = .249 EX = .126 WM = −.283 ME = .233 |
| Russell, 1982(122) |
45, sedentary older adults, ages 55 to 70 |
Duration: 8 wks Frequency: 45min, 2/wk Intensity: - - - Combined Strength Training: N MCI: N |
Simple Reaction Time, Complex Reaction Time |
Attrition: 4% ITT: N Blinding: N |
Duration: 16 wks Frequency: 45min, 3/wk Intensity: - - - |
AT = .214 EX = .081 |
| Scherder, 2005(79) |
43, elderly adults with MCI, ages 76 to 94 |
Duration: 12 wks Frequency: 20min, 3/wk Intensity: 9-13 RPE Combined Strength Training: N MCI: Y |
Category Naming, TMT-A, TMT-B, Digit Span, Visual Memory Span, Rivermead Behavioral Memory Test (Faces and Pictures), Verbal Learning and Memory Test: Direct Recall, Delayed Recall, and Recognition |
Attrition: 7% ITT: N Blinding: Y |
Duration: 6 wks Frequency: 30 min, 3/wk Intensity: - - - |
EX = .441 WM = .037 ME = .413 |
| Smiley-Oyen, 2008(123) |
57, older adults, ages 65-79 |
Duration: 10 wks Frequency: 45min, 3/wk Intensity: - - - Combined Strength Training: N MCI: N |
Stroop Test, Go-No-Go Test, Simple Reaction Time, Choice Reaction Time, Wisconsin Card Sorting Test |
Attrition: 7% ITT: N Blinding: N |
Duration: 40 wks Frequency: 25- 30min, 3/wk Intensity: 65-80% HRR |
AT = .234 EX = −.092 |
| Stroth, 2009(124) |
28, young adults, age M = 20 |
Duration: 16 wks Frequency: 45min, 3/wk Intensity: 70-85% HRR Combined Strength Training: Y MCI: N |
Digit Symbol Substitution Test, Rey Auditory Verbal Learning Test, Stroop Test |
Attrition: 22% ITT: N Blinding: Y |
Duration: 6 wks Frequency: 30min, 3/wk Intensity: 70-100% aerobic threshold |
AT = −.123 ME = .650‡ |
| Wallman, 2004(125) |
61, adults with cystic fibrosis, ages 16 to 74 |
Duration: 17 wks Frequency: 45min, 3/wk Intensity: 70-85% HRR Combined Strength Training: N MCI: N |
Stroop Test (82 questions) Stroop Test (95 questions) |
Attrition: 10% ITT: N Blinding: Y |
Duration: 12 wks Frequency: Increased progressively from 5- 15 mins, 3-4/wk Intensity: based on target HR from treadmill testing |
EX = .479* |
| Whitehurst, 1991(126) |
14, sedentary older women, ages 61 to 73 |
Duration: 26 wks Frequency: 40 min, 3/wk Intensity: 50-70% HRR Combined Strength Training: N MCI: N |
Simple Reaction Time, Choice Reaction Time |
Attrition: 0% ITT: N Blinding: N |
Duration: 8 wks Frequency: 35min, 3/wk Intensity: - - - |
AT = −.551 EX = −.609 |
| Williams, 1997(104) |
187, all women, age M = 72 |
Duration: 72 wks Frequency: 50 min, 3/wk Intensity: - - - Combined Strength Training: Y MCI: N |
Digit Span, Picture Arrangement, Cattell’s Matrices |
Attrition: 20% ITT: N Blinding: N |
Duration: 42 wks Frequency: 35min, 2/wk Intensity: - - - |
AT = .501** EX = .189 WM = .348* |
| Williamson, 2009(34) |
102, elderly adults, ages 70-89 years |
Duration: 10 wks Frequency: 5/wk Intensity: 70-85% MHR Combined Strength Training: Y MCI: N |
Digit Symbol, Modified Stroop Test, 3MSE, Rey Auditory Verbal Learning Test |
Attrition: 10% ITT: N Blinding: Y |
Duration: 52 wks Frequency: 45min, 1-2/wk Intensity: - - - |
AT = .206 EX = .026 ME = .011 |
| van Uffelen, 2008(33) |
152, elderly adults with MCI, age M = 75 |
Duration: 8 wks Frequency: 35 min, 5/wk Intensity: 60-65% HRR Combined Strength Training: N MCI: Y |
Digit Symbol, Stroop Color Word Test, Verbal Fluency, Auditory Verbal Learning Test |
Attrition: 9% ITT: Y Blinding: Y |
Duration: 52 wks Frequency: 60min, 2/wk Intensity: > 3 METs |
AT = −.10 EX = −.04 ME = −.03 |
p < .001
p < .01
p < .05
p < .10
AT = attention and processing speed; EX = executive function; MET = metabolic equivalent, WM = working memory; MCI = Mild Cognitive Impairment, ME = memory; HRR = Heart Rate Reserve; MHR = maximum heart rate; RPE = Ratings of Perceived Exertion
indicates multiple time points of data.
Table 3.
Effects of aerobic exercise interventions vs. controls on neurocognitive performance for various cognitive indices
| Cognitive Test | Studies | Domain | Hedge’s G (95% CI) |
P-Value |
|---|---|---|---|---|
| Digit Symbol Substitution | 8 | Attention / Processing Speed | .146 (−.002 to .294) | .052 |
| Complex / Choice Reaction Time | 8 | Attention / Processing Speed | .112 (−.064 to .288) | .898 |
| Simple Reaction Time | 8 | Attention / Processing Speed | .088 (−.118 to .295) | .116 |
| Ruff 2 & 7 Test | 2 | Attention / Processing Speed | .052 (−.224 to .327) | .715 |
| Trail Making Test Section A | 2 | Attention / Processing Speed | .169 (−.144 to .482) | .291 |
| Stroop Interference | 7 | Executive Function | .027 (−.149 to .204) | .761 |
| Trail Making Test Section B | 5 | Executive Function | .234 (.042 to .426) | .017 |
| Animal Naming / Verbal Fluency | 4 | Executive Function | .275 (.006 to .545) | .045 |
| COWAT* | 2 | Executive Function | −.015 (−.239 to .229) | .894 |
| Logical Memory, Immediate Recall | 5 | Memory | .151 (−.050 to 352) | .140 |
| Rey Auditory Verbal Learning Test | 4 | Memory | .113 (−.082 to .308) | .255 |
| Digit Span | 6 | Working Memory | .065 (−.079 to .209) | .373 |
| WAIS Letter-Number Sequencing | 2 | Working Memory | −.134 (−.469 to .202) | .435 |
COWAT = Controlled Oral Word Association Test;
Attention and Processing Speed
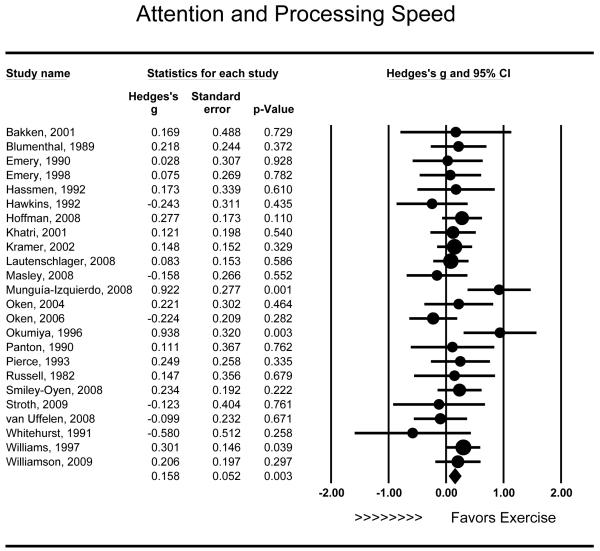
Twenty-four studies examined the effects of aerobic exercise on attention and processing speed. Exercise training was associated with modest improvements in attention and processing speed (g = .158 [95% CI: .055 to .260], P = .003) (Figure 1) and this effect was consistent across studies (Q23 = 26.249, P = .289). Moderator analyses demonstrated that trials of greater duration did not improve attention and processing speed to a greater extent than briefer interventions (r = .17, Q1 = 3.555, P = .399). Similarly, intensity was not associated with variations in attention and processing speed outcomes (r = −.375, Q1 = 1.41, P = .235). Results did not differ between individuals with MCI (g = .028, P < .001) and other samples (g = .181, P = .825) (Q1 = 1.228, P = .268). Combined interventions improved attention and processing speed to a greater extent (g = .250 [95% CI: .042 to .658], P = .026) than aerobic only interventions (g = .098 [95% CI: −.012 to .208], P = .152) (Q1 = 4.373, P = .037). There was no observed association between the mean age of study participants and improvements in attention and processing speed (r = −.047, P = .817).
Figure 1.
Effect of aerobic exercise on attention and processing speed (n = 24). Individuals randomized to aerobic exercise treatment exhibited improved attention and processing speed relative to controls (g = .158 [95% CI: .055 to .260], P = .003). Each study is denoted with a circle, with larger sample sizes corresponding to larger marks.
Executive Function
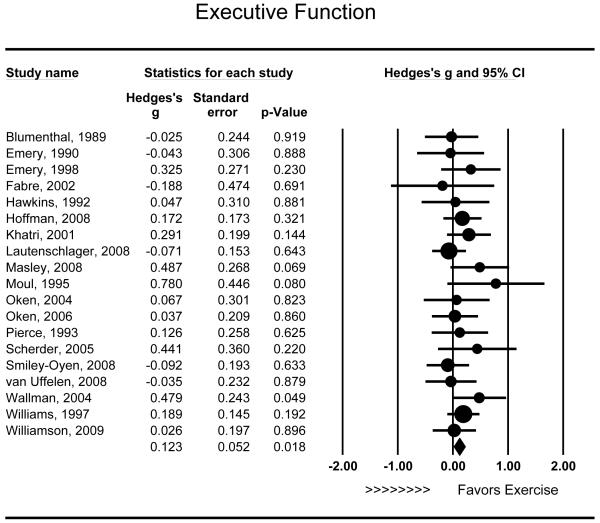
Nineteen studies assessed the effects of aerobic exercise on executive function. Aerobic exercise was associated with modest improvements in executive function (g = .123 [95% CI: .021 to .225], P = .018) (Figure 2), and effects were of similar magnitude across studies (Q18 = 13.418, P = .766). Neither duration (r = −.436, Q1 = 3.627, P = .057) nor intensity (r = −.203, Q1 = 0.413, P = .520) were related to improved executive function. Improvements in executive function were somewhat smaller among individuals with MCI (g = −.004, P = .973) relative to other samples (g = .153, P = .008) (Q1 = 1.377, P = .241), and findings did not differ between studies that included only aerobic exercise (g = .109, P = .074) or combined aerobic exercise with other exercises (e.g., strength training) (g = .163, P = .106) (Q1 = 0.214, P = .644). Finally, there was no observed association between the mean age of study participants and improvements in executive function (r = −.348, P = .130).
Figure 2.
Effect of aerobic exercise on executive function (n = 19). Individuals randomized to aerobic exercise treatment exhibited improved executive function (g = .123 [95% CI: .021 to .225], P = .018). Each study is denoted with a circle, with larger sample sizes corresponding to larger marks.
Working Memory
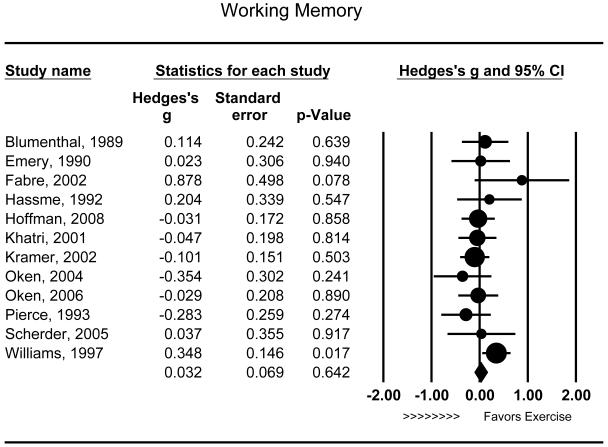
Twelve studies examined the effects of aerobic exercise on working memory. Exercise did not appear to improve working memory performance (g = .032 [95% CI: −.103 to .166], P = .642) (Figure 3) and this effect was relatively consistent across trials (Q11 = 12.241, P = .346). Similar to other cognitive domains, neither the duration of the intervention (r = .346, Q1 = 1.438, P = .230) nor the intensity of exercise (r = .109, Q1 = 0.123, P = .725) appeared to moderate the effects of treatment. Only one study examined the effects of working memory among individuals with MCI and test for moderation was therefore not examined. Combined interventions (n=2) appeared to improve working memory (Q1 = 4.817, P = .028) (g = .288 [95% CI: .030 to .546], P = .028) relative to aerobic only interventions (g = −.042 [95% CI: −.184 to .101], P = .567). In addition, a significant association was observed between mean age of study participants and improvements in working memory, with older samples demonstrating greater improvements relative to younger samples (r = .564, P = .051).
Figure 3.
Effect of aerobic exercise on working memory (n = 12). Individuals randomized to aerobic exercise treatment did not exhibit significant improvements in working memory relative to controls (g = .032 [95% CI: −.103 to .166], P = .642). Each study is denoted with a circle, with larger sample sizes corresponding to larger marks.
Memory
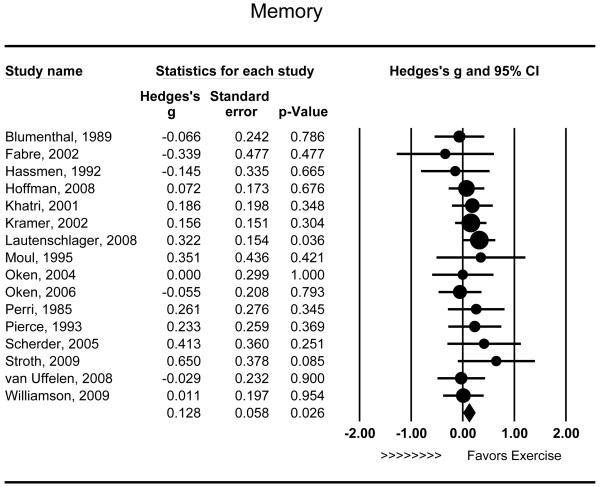
Sixteen studies assess the effects of aerobic exercise on memory function. Aerobic exercise was associated with modest improvements in memory relative to controls (g = .128 [95% CI: .015 - .241], P = .026) (Figure 4) and effects were of similar magnitude across studies (Q15 = 9.030, P = .876). Neither intensity (r = −.051, Q1 = 0.026, P = .871) nor duration (r = .373, Q1 = 1.381, P = .240) appeared to moderate the observed effects on memory. Sensitivity analyses demonstrated that the effects of exercise were stronger among individuals with MCI (g = .237 [95% CI: .000 to .474], P = .050) relative to non-cognitively compromised individuals (g = .096 [95% CI: −.032 to .224], P = .143), although the statistical test for moderation did not achieve significance (Q1 = 1.055, P = .304). Only one study assessing memory utilized a combined intervention, so this was not examined as a potential moderator. In addition, there was no observed association between the mean age of study participants and improvements in memory (r = −.222, P = .175).
Figure 4.
Effect of aerobic exercise on memory (n = 16). Individuals randomized to aerobic exercise treatment exhibited improved memory relative to controls (g = .128 [95% CI: .015 - .241], P = .026). Each study is denoted with a circle, with larger sample sizes corresponding to larger marks.
Study Quality
Analyses of whether methodological quality moderated the observed pattern of results, we examined whether treatment effects varied by 1) blinding of assessors and 2) use of ITT analyses. Studies did not differ in neurocognitive treatment effects whether they did (g = .143, P = .013) or did not use (g = .185, P = .012) blinded assessments (Q2 = .204, P = .651). Similarly, the effects of treatment on neurocognitive performance did not differ between those studies that did (g = .161, P = .004) or did not (g = .166, P = .087) utilize ITT analyses (Q2 = .002, P = .964).
DISCUSSION
Results indicate that aerobic exercise training confers modest improvements in neurocognitive function among healthy older adults, including improvements in attention and processing speed, executive function, and memory. Aerobic exercise did not appear to benefit working memory, however. Moderator analyses demonstrated that studies utilizing combined aerobic exercise and strength training interventions improved attention and processing speed and working memory to a greater extent than aerobic exercise alone. In addition, we found preliminary evidence that trials among individuals with MCI may be associated with greater improvements in memory relative to those among non-cognitively compromised samples. In contrast, neither training characteristics, such as study duration and intensity, nor methodological quality were associated with differential improvements in neurocognition.
Although previous meta-analytic reviews have reported that exercise may improve neurocognitive performance (8-12, 32), ours is one of the largest reviews to date demonstrating that aerobic exercise improves neurocognition among non-demented adults and the first to show that physical activity may enhance memory performance among individuals with MCI, a group at elevated risk for Alzheimer’s Disease (24). Several previous meta-analytic studies have examined the relationship between physical activity and cognitive function (8-12, 32). Colcombe and Kramer (9) reported that randomized controlled trials of exercise are associated with clinically meaningful improvements in executive function, processing speed, memory, and motor function. Our findings showed markedly weaker effects relative to this review, most likely as a result of excluding two decidedly positive studies trials included in Colcombe and Kramer meta-analysis (35, 36) which, upon closer examination were not truly RCTs. In a Cochrane review, Angevaren and colleagues (11) concluded that, although RCTs of aerobic exercise among individuals without cognitive impairment were associated with modest improvements in attentional processes, cognitive speed, and motor function, the existing data were insufficient to show that improvements in cognition were attributable to changes in cardiovascular fitness. Similarly, Etnier and colleagues (32) have demonstrated that, although higher levels of fitness were associated with better neurocognitive performance among cross-sectional study designs, studies examining pre-post comparisons found that larger gains in aerobic fitness were associated with lesser improvements in cognitive performance (32). Etnier and colleagues (8) have also noted that methodological limitations contributed to significant variability in treatment effects, with higher quality studies tending to show smaller effects, and studies with the highest quality rating demonstrating no effect of exercise on neurocognition. Most recently, van Uffelen and colleagues (12) reported that physical activity interventions among individuals without cognitive decline, on average, tended to report improved neurocognitive function. However, van Uffelen and colleagues (12) did not attempt to statistically combine treatment effect sizes across studies, reported that the majority of existing trials examining this question have failed to demonstrate a treatment benefit, and found that the extant literature is marked by a lack of high-quality studies. The present analyses address many of the issues raised by this previous review by including several large, high-quality RCTs not previously incorporated in systematic literature syntheses (30, 31, 33, 34).
The finding that exercise may produce larger improvements in memory for individuals with MCI than other patient groups is novel and warrants further investigation, although this must be viewed as preliminary. Although Heyn and colleagues (10) demonstrated that physical activity is associated with improvements in mental status among individuals with dementia, the majority of these trials were conducted among institutionalized adults with dementia and utilized balance and isometric exercises and did not examine the effects aerobic exercise, specifically, on neurocognition. The finding that aerobic exercise improves memory is consistent with several animal studies, which have indicated that physical activity increases brain-derived neurotrophic factor (BDNF) expression (80) in the hippocampus and peri-hippocampal structures (81, 82). In an important examination of mediators, Periera and colleagues (83) demonstrated that increased BDNF in the dentate gyrus, an area of the brain proximal to the hippocampus, was associated with dose-response improvements in memory performance among younger adults participating in an exercise intervention. In addition to plausible neurotrophic mediators, it is also possible that individual differences influenced the present findings. For example, it is possible that the MCI samples in our study were composed of a greater number of individuals with the apolipoprotein E type 4 allelic genotype (APOE-4), which has been associated with an increased risk for incident MCI and Alzheimer’s disease (84). In addition, recent evidence suggests that individuals with this genotype may exhibit relatively greater neurocognitive improvements with physical activity compared with healthy, older adults (85-87).
The finding that aerobic exercise alone did not improve working memory performance is an interesting and unpredicted finding. Although it is unclear why aerobic exercise improved other cognitive functions but did not appear to benefit working memory, this finding is somewhat consistent with previous brain imaging studies of aerobic exercise. Previous studies have demonstrated that cerebral alterations associated with exercise are preferentially in the peri-hippocampal region (83), anterior white matter tracks (88), and anterior cingulate (89). Although there is substantial overlap in the brain circuitry for carrying out complex cognitive processes, such as working memory, no imaging studies have demonstrated volumetric changes in the dorsolateral prefrontal cortex, which is primarily subserved by white matter projections from the corpus callosum and most consistently associated with working memory performance (90, 91). The finding that combined aerobic exercise and strength training interventions improved attention and working memory to a greater extent than aerobic exercise alone is consistent with previous reviews (9), as well as mechanistic studies demonstrating that strength training may improve neurocognitive function by increasing insulin growth factor, which has been implicated as a mediator of the exercise and neurocognition relationship (92-94). It is also possible that combined interventions more effective in reducing cerebrovascular risk factors (e.g. high blood pressure) (95) and improving aerobic fitness relative to aerobic training alone (96). These improvements in cardiovascular function may reduce the white matter degradation and cerebral ischemia that often results from these conditions (97-99). Alternatively, it is also possible that combined interventions may result in greater improvements in vascular health (100) and basal levels of inflammation (101, 102), although these relationships have yet to be investigated.
The present meta-analysis has several limitations. First, the literature is marked by a lack of high-quality trials examining the effects of aerobic exercise on cognitive endpoints. Trials included in our analyses differed substantially in their use of blinded evaluations, intention-to-treat analyses, and clinically validated cognitive assessment tools. Second, randomized controlled trials are limited by logistical constraints in their ability to sustain interventions over prolonged periods of time. Accordingly, the majority of studies examining cognitive end-points have done so after several months of aerobic training (79, 103) or, in some instances, incorporated follow-ups several years later (33, 104). There are limited data regarding how physical activity sustained over the course of several years may affect cognitive endpoints (2, 105), despite observational data indicating that physical activity and cardiovascular health may take years to affect brain health (106, 107). In addition, RCTs that have examined the neurocognitive effects of aerobic exercise over an extended time period have demonstrated greater improvements in memory over longer follow-up periods (30, 73). Third, the majority of extant studies have utilized interventions with frequency and intensity prescribed in accordance with the American Heart Association recommendations for cardiac rehabilitation (i.e., heart rates at 70% peak VO2 3 times per week). It is therefore possible that there was not enough of a range in exercise prescriptions to observe an effect on neurocognition. Finally, there is a lack of consensus as to which neurocognitive measures are most appropriate to examine changes in neurocognitive function associated with exercise. As shown in Table 3, there is substantial heterogeneity in treatment effects among neurocognitive measures. Accordingly, future studies would benefit from the identification of a standardized neurocognitive battery with the appropriate psychometric characteristics to examine neurocognitive measures associated with aerobic exercise.
In conclusion, aerobic exercise training results in modest improvements in cognitive performance among non-demented adults. Trials utilizing longer interventions were associated with greater gains in attention and processing speed, whereas trials conducted among individuals with MCI tended to demonstrate greater improvements in memory relative to non-MCI samples. Additional randomized trials are needed with larger samples, more extensive follow-up periods, appropriate controls, and more extensive measurement of potential mediators of cognitive change. Accordingly, future studies would benefit from the assessment of subclinical vascular health as a potential mediator of the exercise and neurocognition relationship, as this has been associated with improvements in aerobic capacity (100) and neurocognitive performance in other samples (108, 109). Future studies should also collect functional magnetic resonance imaging (fMRI) or diffusion tensor imaging (DTI) measures to track cerebral alterations following exercise, as several previous studies have demonstrated that exercise and improved fitness may increase cerebral blood flow (16) and alter blood oxygen level dependent (BOLD) response patterns to cognitive tasks (89), as well as improving structural brain health, such as by increasing white (88) and gray matter integrity (22) and brain volume (88). Finally, more rigorous studies should examine the effects of aerobic exercise training among individuals with MCI in order to determine whether this is a plausible strategy to delay or prevent incident dementia (101).
Table 2.
Classification of neurocognitive tests by domain
| Neurocognitive Domain | |||
|---|---|---|---|
| Attention | Executive Function | Working Memory | Memory |
| Accuracy Index Complex / Choice RT d2 Test of Attention Digit Matching RTe Digit Symbol Substitution Test Mental Speed Paced Auditory Serial Attention Test (PASAT) Picture Arrangement Premotor Time Response Compatibility RT Ruff 2 & 7 Test (Letters) Simple RT Single / Choice Time Sharing Spatial Attention Task Speed of Movement Stopping Task RT Stroop Color Stroop Word Task Switching RT Trail Making Test Part A Visuospatial Cognitive Performance Test Word Copying Speed |
Attentional Flexibility Categorical Fluency (Animal Naming) Cattell’s Matrices Cognitive Flexibility Covert Orienting of Attention Task Go-No-Go Test Mental Control Nonverbal Fluency Test Number Copying Speed RIPA Organization RIPA Problem Solving RIPA Abstract Reasoning Ruff 2 & 7 Test (Digits) Selective Reminding Intrusions Set Shifting Ability Stopping Task Stroop Color/Word or Interference Trail Making Test Part B Useful Field of View Verbal Fluency Test (FAS) WAIS Similarities Wisconsin Card Sorting Task |
Digit Span N-Back Spatial Task N-Back Task Self-Ordered Pointing Visual Memory span WAIS Letter Number Sequencing |
ADAS Word List Recall Auditory Verbal Learning Test Benton Visual Retention Test CERAD delayed recall RAVLT RAVLT Delay RAVLT, Temporal Order RBMT faces RBMT pictures RIPA Auditory Processing RIPA Immediate Memory RIPA Recent Memory Sternberg Memory Search Task Y-intercept Sternberg Memory Search Task, Slope Visual and Verbal Memory Test Visual Reproduction, Immediate Visual Reproductions VLMT Delayed Recall VLMT Direct Recall VLMT Recognition WMS Facial Recognition WMS Figural Memory, Immediate WMS Figural Memory, Delayed WMS Logical Memory, Immediate WMS Logical Memory, Delayed WMS Verbal Paired Associates WMS Visual Reproduction |
ADAS = Alzheimer’s Disease Assessment Scale; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; PASAT = Paced Auditory Serial Attention Test; RAVLT = Rey Auditory Verbal Learning Task; RBMT = Rivermead Behavioral Memory Test; RIPA = Ross Information Processing Test; RT = reaction time; VLMT = Verbal Learning and Memory Test; WMS = Wechsler Memory Scale; WAIS = Wechsler Adult Intelligence Scale.
Acknowledgments
The research was supported by grants MH 49679 and HL080664-01A1 from the National Institutes of Health and M01-RR-30 from the General Clinical Research Center Program, National Center for Research Resources, National Institutes of Health, awarded to James A. Blumenthal.
Acronyms
- ITT
intention-to-treat
- RCT
randomized controlled trial.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 2.Andel R, Crowe M, Pedersen NL, Fratiglioni L, Johansson B, Gatz M. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci. 2008;63(1):62–6. doi: 10.1093/gerona/63.1.62. [DOI] [PubMed] [Google Scholar]
- 3.Bassuk SS, Wypij D, Berkman LF. Cognitive impairment and mortality in the community-dwelling elderly. Am J Epidemiol. 2000;151(7):676–88. doi: 10.1093/oxfordjournals.aje.a010262. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279(8):585–92. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 5.Kuh D, Richards M, Hardy R, Butterworth S, Wadsworth ME. Childhood cognitive ability and deaths up until middle age: a post-war birth cohort study. Int J Epidemiol. 2004;33(2):408–13. doi: 10.1093/ije/dyh043. [DOI] [PubMed] [Google Scholar]
- 6.Smits CH, Deeg DJ, Kriegsman DM, Schmand B. Cognitive functioning and health as determinants of mortality in an older population. Am J Epidemiol. 1999;150(9):978–86. doi: 10.1093/oxfordjournals.aje.a010107. [DOI] [PubMed] [Google Scholar]
- 7.Whalley LJ, Deary IJ. Longitudinal cohort study of childhood IQ and survival up to age 76. BMJ. 2001;322(7290):819. doi: 10.1136/bmj.322.7290.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etnier JL, Salazar W, Landers DM, Petruzzello SJ, Han M, Nowell P. The influence of physical fitness and exercise upon cognitive functioning: a meta-analysis. Journal of Sport & Exercise Psychology. 1997;19:249–77. [Google Scholar]
- 9.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 10.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD005381.pub2. CD005381. [DOI] [PubMed] [Google Scholar]
- 12.van Uffelen JG, Chin APM, Hopman-Rock M, van MW. The effects of exercise on cognition in older adults with and without cognitive decline: a systematic review. Clin J Sport Med. 2008;18(6):486–500. doi: 10.1097/JSM.0b013e3181845f0b. [DOI] [PubMed] [Google Scholar]
- 13.McAuley E, Kramer AF, Colcombe SJ. Cardiovascular fitness and neurocognitive function in older adults: a brief review. Brain Behav Immun. 2004;18(3):214–20. doi: 10.1016/j.bbi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Hillman CH, Motl RW, Pontifex MB, Posthuma D, Stubbe JH, Boomsma DI, de Geus EJ. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Health Psychol. 2006;25(6):678–87. doi: 10.1037/0278-6133.25.6.678. [DOI] [PubMed] [Google Scholar]
- 15.Blomquist KB, Danner F. Effects of physical conditioning on information-processing efficiency. Percept Mot Skills. 1987;65(1):175–86. doi: 10.2466/pms.1987.65.1.175. [DOI] [PubMed] [Google Scholar]
- 16.Brown AD, McMorris CA, Longman RS, Leigh R, Hill MD, Friedenreich CM, Poulin MJ. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Chodzko-Zajko WJ, Moore KA. Physical fitness and cognitive functioning in aging. Exerc Sport Sci Rev. 1994;22:195–220. [PubMed] [Google Scholar]
- 18.Chodzko-Zajko WJ. Physical fitness, cognitive performance, and aging. Med Sci Sports Exerc. 1991;23(7):868–72. [PubMed] [Google Scholar]
- 19.Clarkson-Smith L, Hartley AA. Relationships between physical exercise and cognitive abilities in older adults. Psychol Aging. 1989;4(2):183–9. doi: 10.1037//0882-7974.4.2.183. [DOI] [PubMed] [Google Scholar]
- 20.Elsayed M, Ismail AH, Young RJ. Intellectual differences of adult men related to age and physical fitness before and after an exercise program. J Gerontol. 1980;35(3):383–7. doi: 10.1093/geronj/35.3.383. [DOI] [PubMed] [Google Scholar]
- 21.Etnier J, Johnston R, Dagenbach D, Pollard RJ, Rejeski WJ, Berry M. The relationships among pulmonary function, aerobic fitness, and cognitive functioning in older COPD patients. Chest. 1999;116(4):953–60. doi: 10.1378/chest.116.4.953. [DOI] [PubMed] [Google Scholar]
- 22.Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, McAuley E, Kramer AF, Colcombe S, Gratton G, Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45(5):825–38. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–61. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 24.Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le CN, Barberger-Gateau P, Dartigues JF. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59(10):1594–9. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 25.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161(7):639–51. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 26.Singh-Manoux A, Hillsdon M, Brunner E, Marmot M. Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II prospective cohort study. Am J Public Health. 2005;95(12):2252–8. doi: 10.2105/AJPH.2004.055574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56(5):425–30. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 28.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–8. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 29.Okumiya K, Matsubayashi K, Wada T, Kimura S, Doi Y, Ozawa T. Effects of exercise on neurobehavioral function in community-dwelling older people more than 75 years of age. J Am Geriatr Soc. 1996;44(5):569–72. doi: 10.1111/j.1532-5415.1996.tb01444.x. [DOI] [PubMed] [Google Scholar]
- 30.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman BM, Blumenthal JA, Babyak MA, Smith PJ, Rogers SD, Doraiswamy PM, Sherwood A. Exercise fails to improve neurocognition in depressed middle-aged and older adults. Med Sci Sports Exerc. 2008;40(7):1344–52. doi: 10.1249/MSS.0b013e31816b877c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Brain Res Rev. 2006;52(1):119–30. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 33.van Uffelen JG, Chinapaw MJ, van MW, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med. 2008;42(5):344–51. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- 34.Williamson JD, Espeland M, Kritchevsky SB, Newman AB, King AC, Pahor M, Guralnik JM, Pruitt LA, Miller ME. Changes in cognitive function in a randomized trial of physical activity: results of the lifestyle interventions and independence for elders pilot study. J Gerontol A Biol Sci Med Sci. 2009;64(6):688–94. doi: 10.1093/gerona/glp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rikli RE, Edwards DJ. Effects of a three-year exercise program on motor function and cognitive processing speed in older women. Res Q Exerc Sport. 1991;62(1):61–7. doi: 10.1080/02701367.1991.10607519. [DOI] [PubMed] [Google Scholar]
- 36.Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, Wood JS, Bradford DC. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiol Aging. 1984;5(1):35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 37.Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Brain Maturation in Adolescence and Young Adulthood: Regional Age-Related Changes in Cortical Thickness and White Matter Volume and Microstructure. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 38.Hill RD, Storandt M, Malley M. The impact of long-term exercise training on psychological function in older adults. J Gerontol. 1993;48(1):12–7. doi: 10.1093/geronj/48.1.p12. [DOI] [PubMed] [Google Scholar]
- 39.Hassmen P, Ceci R, Backman L. Exercise for older women: a training method and its influences on physical and cognitive performance. Eur J Appl Physiol Occup Physiol. 1992;64(5):460–6. doi: 10.1007/BF00625068. [DOI] [PubMed] [Google Scholar]
- 40.Bastone AC, Jacob FW. Effect of an exercise program on functional performance of institutionalized elderly. J Rehabil Res Dev. 2004;41(5):659–68. doi: 10.1682/jrrd.2003.01.0014. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka K, Quadros AC, Jr., Santos RF, Stella F, Gobbi LT, Gobbi S. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn. 2009;69(2):435–41. doi: 10.1016/j.bandc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Zlomanczuk P, Milczarek B, Dmitruk K, Sikorski W, Adamczyk W, Zegarski T, Tafil-Klawe M, Chesy G, Klawe JJ, Rakowski A. Improvement in the face/name association performance after three months of physical training in elderly women. J Physiol Pharmacol. 2006;57(Suppl 4):417–24. [PubMed] [Google Scholar]
- 43.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learn Mem. 2006 doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Brisswalter J, Arcelin R, Audiffren M, Delignieres D. Influence of physical exercise on simple reaction time: effect of physical fitness. Percept Mot Skills. 1997;85(3 Pt 1):1019–27. doi: 10.2466/pms.1997.85.3.1019. [DOI] [PubMed] [Google Scholar]
- 45.Molloy DW, Beerschoten DA, Borrie MJ, Crilly RG, Cape RD. Acute effects of exercise on neuropsychological function in elderly subjects. J Am Geriatr Soc. 1988;36(1):29–33. doi: 10.1111/j.1532-5415.1988.tb03430.x. [DOI] [PubMed] [Google Scholar]
- 46.Ploughman M, McCarthy J, Bosse M, Sullivan HJ, Corbett D. Does treadmill exercise improve performance of cognitive or upper-extremity tasks in people with chronic stroke? A randomized cross-over trial. Arch Phys Med Rehabil. 2008;89(11):2041–7. doi: 10.1016/j.apmr.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Rabadi M, Galgano M, Lynch D, Akerman M, Lesser M, Volpe B. A pilot study of activity-based therapy in the arm motor recovery post stroke: a randomized controlled trial. Clin Rehabil. 2008;22(12):1071–82. doi: 10.1177/0269215508095358. [DOI] [PubMed] [Google Scholar]
- 48.Stevenson JS, Topp R. Effects of moderate and low intensity long-term exercise by older adults. Res Nurs Health. 1990;13(4):209–18. doi: 10.1002/nur.4770130403. [DOI] [PubMed] [Google Scholar]
- 49.Cosky AC. The effect of aerobic exercise on fitness status, cognition, and health locus of control in older women. 1989. Unpublished Dissertation. [Google Scholar]
- 50.Zervas Y, Danis A, Klissouras V. Influence of physical exertion on mental performance with reference to training. Percept Mot Skills. 1991;72(3 Pt 2):1215–21. doi: 10.2466/pms.1991.72.3c.1215. [DOI] [PubMed] [Google Scholar]
- 51.Evans JJ, Greenfield E, Wilson BA, Bateman A. Walking and talking therapy: improving cognitive-motor dual-tasking in neurological illness. J Int Neuropsychol Soc. 2009;15(1):112–20. doi: 10.1017/S1355617708090152. [DOI] [PubMed] [Google Scholar]
- 52.Cott CA, Dawson P, Sidani S, Wells D. The effects of a walking/talking program on communication, ambulation, and functional status in residents with Alzheimer disease. Alzheimer Dis Assoc Disord. 2002;16(2):81–7. doi: 10.1097/00002093-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Marmeleira JF, Godinho MB, Fernandes OM. The effects of an exercise program on several abilities associated with driving performance in older adults. Accid Anal Prev. 2009;41(1):90–7. doi: 10.1016/j.aap.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Brown AK, Liu-Ambrose T, Tate R, Lord SR. The effect of group-based exercise on cognitive performance and mood in seniors residing in intermediate care and self-care retirement facilities: a randomised controlled trial. Br J Sports Med. 2009;43(8):608–14. doi: 10.1136/bjsm.2008.049882. [DOI] [PubMed] [Google Scholar]
- 55.Liu-Ambrose T, Donaldson MG, Ahamed Y, Graf P, Cook WL, Close J, Lord SR, Khan KM. Otago home-based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. J Am Geriatr Soc. 2008;56(10):1821–30. doi: 10.1111/j.1532-5415.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 56.Friedman R, Tappen RM. The effect of planned walking on communication in Alzheimer’s disease. J Am Geriatr Soc. 1991;39(7):650–4. doi: 10.1111/j.1532-5415.1991.tb03617.x. [DOI] [PubMed] [Google Scholar]
- 57.McMurdo ME, Rennie L. A controlled trial of exercise by residents of old people’s homes. Age Ageing. 1993;22(1):11–5. doi: 10.1093/ageing/22.1.11. [DOI] [PubMed] [Google Scholar]
- 58.Molloy DW, Richardson LD, Crilly RG. The effects of a three-month exercise programme on neuropsychological function in elderly institutionalized women: a randomized controlled trial. Age Ageing. 1988;17(5):303–10. doi: 10.1093/ageing/17.5.303. [DOI] [PubMed] [Google Scholar]
- 59.Nowalk MP, Prendergast JM, Bayles CM, D’Amico FJ, Colvin GC. A randomized trial of exercise programs among older individuals living in two long-term care facilities: the FallsFREE program. J Am Geriatr Soc. 2001;49(7):859–65. doi: 10.1046/j.1532-5415.2001.49174.x. [DOI] [PubMed] [Google Scholar]
- 60.Schnelle JF, MacRae PG, Giacobassi K, MacRae HS, Simmons SF, Ouslander JG. Exercise with physically restrained nursing home residents: maximizing benefits of restraint reduction. J Am Geriatr Soc. 1996;44(5):507–12. doi: 10.1111/j.1532-5415.1996.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 61.Palleschi L, Vetta F, De GE, Idone G, Sottosanti G, Gianni W, Marigliano V. Effect of aerobic training on the cognitive performance of elderly patients with senile dementia of alzheimer type. Arch Gerontol Geriatr. 1996;22(Suppl 1):47–50. doi: 10.1016/0167-4943(96)86912-3. [DOI] [PubMed] [Google Scholar]
- 62.Christofoletti G, Oliani MM, Gobbi S, Stella F, Bucken Gobbi LT, Renato CP. A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clin Rehabil. 2008;22(7):618–26. doi: 10.1177/0269215507086239. [DOI] [PubMed] [Google Scholar]
- 63.Mulrow CD, Gerety MB, Kanten D, Cornell JE, DeNino LA, Chiodo L, Aguilar C, O’Neil MB, Rosenberg J, Solis RM. A randomized trial of physical rehabilitation for very frail nursing home residents. JAMA. 1994;271(7):519–24. [PubMed] [Google Scholar]
- 64.Powell RR. Psychological effects of exercise therapy upon institutionalized geriatric mental patients. J Gerontol. 1974;29(2):157–61. doi: 10.1093/geronj/29.2.157. [DOI] [PubMed] [Google Scholar]
- 65.McMurdo ME, Rennie LM. Improvements in quadriceps strength with regular seated exercise in the institutionalized elderly. Arch Phys Med Rehabil. 1994;75(5):600–3. [PubMed] [Google Scholar]
- 66.Hawkins HL, Kramer AF, Capaldi D. Aging, exercise, and attention. Psychol Aging. 1992;7(4):643–53. doi: 10.1037//0882-7974.7.4.643. [DOI] [PubMed] [Google Scholar]
- 67.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Fourth ed. Oxford University Press; Oxford, New York, Auckland, Bangkok, Buenos Aires, Cape Town, Chenna, Dar es Sallam, Delhi, Hong Kong, Istanbul, Karachi, Kolkata, Kuala Lumpur, Madrid, Melbourne, Mexico City, Mumbai, Nairobi, Sao Paulo, Shanghai, Taipei, Tokyo, Toronto: 2004. [Google Scholar]
- 68.Anderson JR. Cognitive Psychology and Its Implications. 6th ed. Worth Publishers and W.H. Freeman and Company; 2005. [Google Scholar]
- 69.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 70.Schmidt FL, Oh IS, Hayes TL. Fixed-versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol. 2009;62(Pt 1):97–128. doi: 10.1348/000711007X255327. [DOI] [PubMed] [Google Scholar]
- 71.Madden DJ, Blumenthal JA, Allen PA, Emery CF. Improving aerobic capacity in healthy older adults does not necessarily lead to improved cognitive performance. Psychol Aging. 1989;4(3):307–20. doi: 10.1037//0882-7974.4.3.307. [DOI] [PubMed] [Google Scholar]
- 72.Blumenthal JA, Emery CF, Madden DJ, George LK, Coleman RE, Riddle MW, McKee DC, Reasoner J, Williams RS. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. J Gerontol. 1989;44(5):M147–M157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- 73.Blumenthal JA, Emery CF, Madden DJ, Schniebolk S, Walsh-Riddle M, George LK, McKee DC, Higginbotham MB, Cobb FR, Coleman RE. Long-term effects of exercise on psychological functioning in older men and women. J Gerontol. 1991;46(6):352–61. doi: 10.1093/geronj/46.6.p352. [DOI] [PubMed] [Google Scholar]
- 74.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–9. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 75.Kramer AF, Hahn S, McAuley E, Cohen NJ, Banich MT, Harrison C, Chason J, Boileau RA, Bardell L, Colcombe A, Vakil E. Exercise, aging, and cognition: healthy body, healthy mind? In: Rogers WA, Fisk AD, editors. Human Factors Interventions for the Health Care of Older Adults. Erlbaum; Mahwah, NJ: 2002. pp. 91–120. [Google Scholar]
- 76.Emery CF, Shermer RL, Hauck ER, Hsiao ET, MacIntyre NR. Cognitive and psychological outcomes of exercise in a 1-year follow-up study of patients with chronic obstructive pulmonary disease. Health Psychol. 2003;22(6):598–604. doi: 10.1037/0278-6133.22.6.598. [DOI] [PubMed] [Google Scholar]
- 77.Hedges LV, Pigott TD. The power of statistical tests for moderators in meta-analysis. Psychol Methods. 2004;9(4):426–45. doi: 10.1037/1082-989X.9.4.426. [DOI] [PubMed] [Google Scholar]
- 78.Greenhouse JB, Iyengar S. Sensitivity Analysis and Diagnostics. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. Russell Sage Foundation; New York: 1994. pp. 383–98. [Google Scholar]
- 79.Scherder EJ, Van PJ, Deijen JB, Van Der KS, Orlebeke JF, Burgers I, Devriese PP, Swaab DF, Sergeant JA. Physical activity and executive functions in the elderly with mild cognitive impairment. Aging Ment Health. 2005;9(3):272–80. doi: 10.1080/13607860500089930. [DOI] [PubMed] [Google Scholar]
- 80.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88(5):2187–95. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 81.Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29(12):2189–99. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- 82.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 83.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(13):5638–43. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60(2):246–52. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 85.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33(5):772–7. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 86.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–53. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 87.Etnier JL, Caselli RJ, Reiman EM, Alexander GE, Sibley BA, Tessier D, McLemore EC. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39(1):199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- 88.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 89.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–21. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133(1):44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- 91.Macdonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 92.Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. ScientificWorldJournal. 2006;6:53–80. doi: 10.1100/tsw.2006.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–33. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 94.Liu-Ambrose T, Donaldson MG. Exercise and cognition in older adults: is there a role for resistance training programmes? Br J Sports Med. 2009;43(1):25–7. doi: 10.1136/bjsm.2008.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stewart KJ, Bacher AC, Turner KL, Fleg JL, Hees PS, Shapiro EP, Tayback M, Ouyang P. Effect of exercise on blood pressure in older persons: a randomized controlled trial. Arch Intern Med. 2005;165(7):756–62. doi: 10.1001/archinte.165.7.756. [DOI] [PubMed] [Google Scholar]
- 96.Marzolini S, Oh PI, Thomas SG, Goodman JM. Aerobic and resistance training in coronary disease: single versus multiple sets. Med Sci Sports Exerc. 2008;40(9):1557–64. doi: 10.1249/MSS.0b013e318177eb7f. [DOI] [PubMed] [Google Scholar]
- 97.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–8. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 98.Tzourio C, Dufouil C, Ducimetiere P, Alperovitch A, EVA Study Group Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly. Epidemiology of Vascular Aging. Neurology. 1999;53(9):1948–52. doi: 10.1212/wnl.53.9.1948. [DOI] [PubMed] [Google Scholar]
- 99.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136(7):493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 100.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barnes DE, Whitmer RA, Yaffe K. Physical activity and dementia: The need for prevention trials. Exerc Sport Sci Rev. 2007;35(1):24–9. doi: 10.1097/JES.0b013e31802d6bc2. [DOI] [PubMed] [Google Scholar]
- 102.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110–8. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bakken RC, Carey JR, Di Fabio RP, Erlandson TJ, Hake JL, Intihar TW. Effect of aerobic exercise on tracking performance in elderly people: a pilot study. Phys Ther. 2001;81(12):1870–9. [PubMed] [Google Scholar]
- 104.Williams P, Lord SR. Effects of group exercise on cognitive functioning and mood in older women. Aust N Z J Public Health. 1997;21(1):45–52. doi: 10.1111/j.1467-842x.1997.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 105.Richards M, Hardy R, Wadsworth ME. Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med. 2003;56(4):785–92. doi: 10.1016/s0277-9536(02)00075-8. [DOI] [PubMed] [Google Scholar]
- 106.Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38(6):1766–73. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- 107.Rovio S, Kareholt I, Helkala EL, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–11. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 108.Smith PJ, Blumenthal JA, Babyak MA, Hoffman BM, Doraiswamy PM, Waugh R, Hinderliter A, Sherwood A. Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosom Med. 2007;69(6):578–86. doi: 10.1097/PSY.0b013e31812f7b8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cohen RA, Poppas A, Forman DE, Hoth KF, Haley AP, Gunstad J, Jefferson AL, Tate DF, Paul RH, Sweet LH, Ono M, Jerskey BA, Gerhard-Herman M. Vascular and cognitive functions associated with cardiovascular disease in the elderly. J Clin Exp Neuropsychol. 2008:1–15. doi: 10.1080/13803390802014594. [DOI] [PMC free article] [PubMed] [Google Scholar]