Abstract
Recent studies have identified mutations in the leucine-rich repeat kinase2 gene (LRRK2) in the most common familial forms and some sporadic forms of Parkinson’s disease (PD). LRRK2 is a large and complex protein that possesses kinase and GTPase activities. Some LRRK2 mutants enhance kinase activity and possibly contribute to PD through a toxic gain-of-function mechanism. Given the role of LRRK2 in the pathogenesis of PD, understanding the kinetic mechanism of its two enzymatic properties is critical for the discovery of inhibitors of LRRK2 kinase that would be therapeutically useful to treat PD. In this report, by using LRRK2 protein purified from murine brain, first we characterize kinetic mechanisms for the LRRK2-catalyzed phosphorylation of two peptide substrates: PLK-derived peptide (PLK-peptide) and LRRKtide. We found that LRRK2 follows a rapid equilibrium random mechanism for the phosphorylation of PLK-peptide with either ATP or PLK-peptide being the first substrate binding to the enzyme, as evidenced by initial velocity and inhibition mechanism studies by nucleotide analogues AMP and AMP-PNP, product ADP, and analogue of peptide substrate. The binding of the first substrate has no effect on the binding affinity of the second substrate. Identical mechanistic conclusions were drawn when LRRKtide was the phosphoryl acceptor. Next, we characterize GTPase activity of LRRK2 with a kcat of 0.2 ± 0.02 s−1 and Km of 210 ± 29 µM. SKIE of 0.97 ± 0.04 was measured on kcat for the GTPase activity of LRRK2 in a D2O molar fraction of 0.86 and suggested that the product dissociation step is rate limiting of the steps governed by kcat in the LRRK2-catalyzed GTP hydrolysis. Surprisingly, binding of GTP, GDP or GMP has no effect on kinase activity, although GMP and GDP inhibit the GTPase activity. Finally, we have identified a compound LDN-73794 through screen of LRRK2 kinase inhibitors. Our study revealed that this compound is a competitive inhibitor of the binding of ATP and inhibits the kinase activity without affecting the GTPase activity.
Parkinson’s disease characterized by tremor, rigidity, bradykinesia and postural instability, is the second most common neurodegenerative disorder. It affects over 1 million Americans and more than 60,000 patients are newly diagnosed each year. PD is caused by the loss of dopaminergic neurons in the substantia nigra. Normally, these neurons produce dopamine, an essential chemical messenger in the brain. Once damaged, these neurons stop producing dopamine and compromise the brain’s ability to control movement. Mutations in several genes have been genetically linked to PD in recent years (1). Among those genes, the leucine-rich repeat kinase2 (LRRK2) has emerged as the most relevant player in PD pathogenesis. At least 20 mutations in LRRK2 have been found in the most common familial forms and some sporadic forms of PD (2–6).
LRRK2 is a large and complex protein containing several independent domains, including a leucine-rich repeat (LRR) domain, a Roc domain followed by its associated COR domain, a kinase domain, and a C-terminal WD40 domain (7, 8). LRRK2 is unusual in that it encodes two distinct but functionally linked enzymes: a protein kinase and a GTPase. Recent studies have suggested that LRRK2 is capable of undergoing both autophosphorylation and generic substrate phosphorylation, and the kinase activity is regulated by the GTPase domain (8–12). The elevated kinase activity found in some PD-associated mutations results in neuronal cell death, whereas kinase-dead variants of LRRK2 protect neuronal cells from death (8, 13, 14). Clearly, kinase activity is a critical component of LRRK2 function.
Since the emerging evidence implicates LRRK2 kinase activity in the pathogenesis of PD, identifying inhibitors of LRRK2 kinase has become a priority in the validation of kinase activity as a drug target and the search for drugs to treat PD. A detailed understanding of the kinetic mechanism underlying the two enzymatic properties of LRRK2 (kinase and GTPase) is critical for the discovery and design of inhibitors. However, such information is quite limited. As part of a program to find inhibitors of LRRK2 kinase, we conducted kinetic mechanistic studies for wild type full-length LRRK2. Specifically, we report results of studies conducted to (i) understand the kinetic mechanism of LRRK2-catalyzed phosphorylation of two peptide substrates (PLK-peptide and LRRKtide), (ii) characterize the GTPase activity, (iii) elucidate the functional relationship between GTPase activity and kinase activity, i.e., how the kinase activity is regulated, and (iv) investigate the mechanism of inhibition of a compound identified through screen.
MATERIALS AND METHODS
Materials
ATP, ADP, AMP, GDP, GMP, DTT, magnesium chloride, HEPES, and bovine serum albumin were purchased from Sigma (St. Louis, MO). GTP was from Bioline (Taunton, MA). Peptides LRRKtide (RLGRDKYKTLRQIRQ) and LRRKtideA (RLGRDKYKALRQIRQ) were purchased from American Peptide (Sunnyvale, CA). PLK-peptide (PLK-derived peptide with a motif of RRRSLLE), Eu-anti-phospho-PLK, [γ-33P]-ATP, [α-14C]-ADP, and [α-33P]-GTP were from Perkin Elmer (Boston, MA). LRRK2 was purified from BAC-transgenic mouse brain as described by Li (15).
TR-FRET Assay of LRRK2-Catalyzed PLK-peptide Phosphorylation
The kinase assay for phosphorylation of PLK-peptide (motif of RRRSLLE) was conducted in buffer containing 20 mM HEPES (pH 7.4), 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, BSA 0.5 mg/ml, 1 mM beta-Gly-PO4, PLK-peptide, and ATP. Beta-Gly-PO4 is a phosphatase inhibitor and is added to protect against phosphatases. PLK-peptide and ATP were used at various concentrations as indicated in the results section. The reactions were conducted in duplicate, initiated by the addition of 4 nM LRRK2, and incubated at room temperature for 4 h. The reaction was stopped by the addition of 10 mM EDTA and incubated with 2 nM Eu-anti-phospho-PLK for 2 h. The TR-FRET signal was read by an EnVision plate reader (PerkinElmer). In all cases, reaction progress curves for production of phospho-PLK-peptide were linear over at last 6 h, and allowed calculation of initial velocities.
Kinetic Analysis of LRRK2-Catalyzed LRRKtide Phosphorylation
The kinase assay for phosphorylation of LRRKtide (RLGRDKYKTLRQIRQ) was conducted in buffer containing 20 mM HEPES (pH 7.4), 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, BSA 0.5 mg/ml, 1 mM beta-Gly-PO4, LRRKtide, ATP, and [γ-33P]-ATP. LRRKtide and ATP were used at various concentrations as indicated in the results section, and the ratio of ATP to [γ-33P]-ATP was kept constant at all ATP concentrations (250 µM ATP/5 µCi [γ-33P]-ATP). The reactions were conducted in duplicate, initiated by the addition of 100 nM LRRK2, and incubated at room temperature for 45 min. The reaction was stopped by the addition of 20 mM EDTA, and the mixture was transferred to a multiscreen PH filtration plate (Millipore, Billerica, MA) and washed six times with 75 mM H3PO4. The plate was dried, filters were removed, and the samples were counted with a scintillation counter. Background reaction was conducted in the absence of LRRK2. In all cases, reaction progress curves for production of phospho-LRRKtide were linear over at last 60 minutes, and allowed calculation of initial velocities.
Kinetic Analysis of LRRK2-Catalyzed GTP Hydrolysis
The GTPase assay was conducted in buffer containing 20 mM Tris (pH 7.4), 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, BSA 0.5 mg/ml, GTP, and [α-33P]-GTP. The reactions were conducted in triplicate, initiated by the addition of 30 nM LRRK2, and incubated at room temperature for 20 min. The reaction was stopped by the addition of 20 mM EDTA, and the product [α-33P]-GDP was separated from [α-33P]-GTP by PEI-cellulose thin layer chromatography (TLC) (Sigma, ST. Louis, MO) developed by 0.5 M KH2PO4 (pH3.4) developing buffer and analyzed by scintillation counter. In all cases, reaction progress curves for GTP hydrolysis were linear over at last 30 minutes, and allowed calculation of initial velocities.
Data Analysis – Basic Equations
Data were analyzed by nonlinear least-squares, using either Sigma-Plot or Grafit software packages. Standard kinetic mechanisms for two-substrate reactions and their rate equations are shown below:
- Ping-Pong:
KA and KB are Michaelis constants.(1) - Rapid Equilibrium Ordered:
KA and KB are substrate dissociation constants from EA and EB, respectively.(2) - Rapid Equilibrium Random/Steady-State Ordered:
(3)
For rapid equilibrium systems, KA, KB, αKA, and αKB are substrate dissociation constants from EA, EB, and EAB; for steady-state systems, KA is substrate dissociation constant from EA, αKA and αKB are Michaelis constants. See Segel for definitions of mechanisms, substrate dissociation constants, and α (16).
RESULTS
Evaluation of the TR-FRET Assay
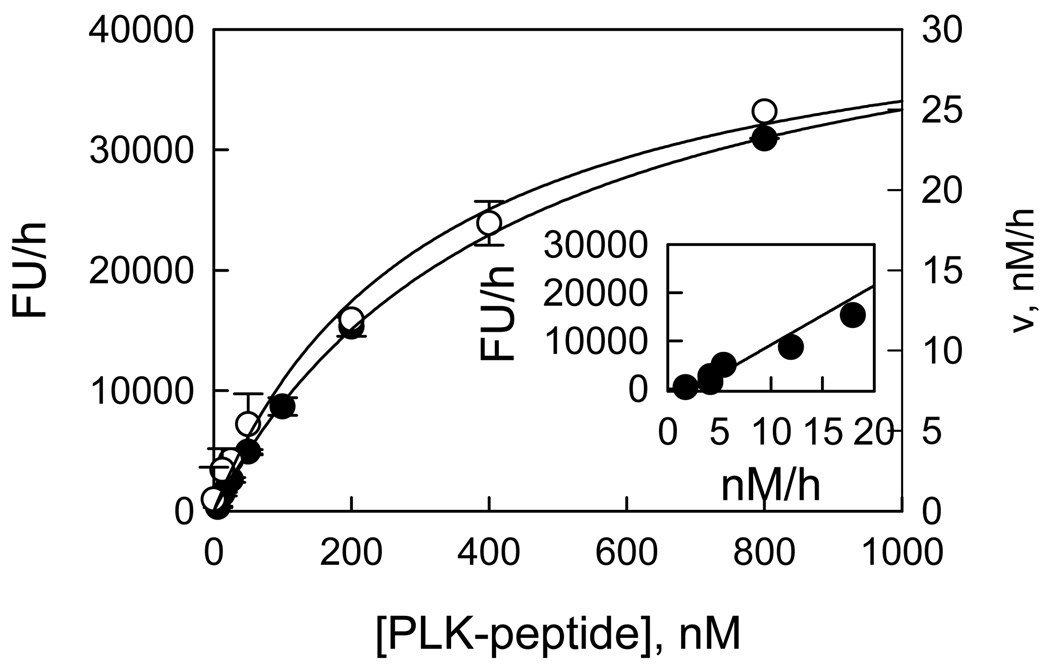
We chose PLK-peptide (based around the putative phosphorylation site of PLK protein) as the phosphoryl acceptor and developed a sensitive TR-FRET assay. To evaluate this assay, we ran a radiometric assay under the same conditions. The initial velocities were measured as a function of [PLK-peptide] at 200 µM ATP, and 24 nM LRRK2 in both assays (Figure 1). Similar kinetics were obtained from the two assays, both data sets adhered to the simple Michaelis-Menten equation, yielding steady-state kinetic parameter estimates: (kcat)app = 31870 ± 837 FU/h and (KPLK)app = 592 ± 56 nM for the TR-FRET assay, and (kcat)app = 33.6 ± 3.5 nM/h and (KPLK)app = 434 ± 16 nM for the radiometric assay. Linear correlation between unit of FU/h and unit of nM/h allows us to calculate a proportionality factor of 855 ± 108 FU/nM that can be used to convert initial velocities from unit of FU/h to unit of nM/h.
Figure 1.
Comparison of two kinase assays. The LRRK2-catalyzed PLK-peptide phosphorylation was conducted at the same condition in a TR-FRET assay and a radiometric assay. The initial velocities were measured as a function of [PLK-peptide] at 200 µM ATP, and 24 nM LRRK2. Filled circles correspond to the y-axis of FU/h on the left measured by the TR-FRET assay, while open circles correspond to the y-axis of nM/h on the right determined from the radiometric assay. The data were fit to the simple Michaelis-Menten equation. Best fit parameters are (kcat)app = 31870 FU/h and (KPLK)app = 592 nM for the TR-FRET assay; (kcat)app = 33.6 nM/h and (KPLK)app = 434 nM for the radiometric assay. The inset shows the linear correlation of unit FU/h and unit nM/h.
LRRK2-Catalyzed Phosphorylation of PLK-peptide – Initial Velocity Studies
To determine the kinetic mechanism for the LRRK2-catalyzed phosphorylation of PLK-peptide, initial velocities were measured as a function of PLK-peptide concentration, at several fixed concentrations of ATP (Figure 2A). The complete data set was subjected to global analysis by nonlinear least-squares fits to the three standard mechanisms, using eqs 1 – 3. Statistically the random mechanism (or steady-state ordered mechanism) fits the data the best and give the following estimates averaged from two independent experiments: kcat = 0.024 ± 0.002 min−1, KATP = 13 ± 3 µM, KPLK = 0.58 ± 0.1 µM, and α = 1.3 ± 0.2, as summarized in Table 1. To examine the data carefully and judge among the mechanisms, we also used the method of replots as described previously (17). The starting point in this data analysis was to calculate apparent values of (kcat)X and (kcat/Km)X (X = PLK-peptide or ATP) by nonlinear least-squares fits of each individual plot of νo vs [X] to the simple Michaelis-Menten equation. The next step in this analysis was to construct the replots of the dependences of apparent values of (kcat)A and (kcat/Km)A on [B] and the dependences of apparent values of (kcat)B and (kcat/Km)B on [A] and examine their shape. Replots will show a specific, mechanism-based pattern. For example, for the random mechanism of eq 3, apparent values of (kcat)A and (kcat/Km)A will be hyperbolically dependent on [B] according to the equations given in eqs 4 and 5, respectively:
| (4) |
| (5) |
Likewise apparent values of (kcat)B and (kcat/Km)B will be hyperbolically dependent on [A].
Figure 2.
Steady-state kinetic studies for LRRK2-catalyzed phosphorylation of PLK-peptide. A: dependence of initial velocities on [PLK-peptide] at [ATP] = 200 (●), 100 (○), 50 (▼), 25 (▽), 12.5 (■), and 6.25 µM (□). Each data point is the average of duplicate determinations. The data set was globally fit to the equation reflecting rapid equilibrium random. B & C: ATP concentration dependencies of (kcat)PLK and (kcat/Km)PLK apparent values derived from analysis of the data of panel A.
Table 1.
Initial Velocity Analysis for LRRK2-catalyzed Phosphorylation Reactions
| PLK-peptide | LRRKtide | |
|---|---|---|
| kcat, min−1 | 0.024 ± 0.002 | 0.10 ± 0.003 |
| KA, µM | 13 ± 2.9 | 67 ± 6.4 |
| KB µM | 0.58 ± 0.08 | 29 ± 2.7 |
| α | 1.3 ± 0.2 | 1.1 ± 0.1 |
The parameter estimates were calculated by globally fitting the data into the equation reflecting rapid equilibrium random/steady-state order mechanism. Each parameter estimate is the average of two independent experiments. The error limit is the deviation from the mean. A represents ATP, B represents phosphoryl acceptor.
Replots of apparent values of (kcat)PLK and (kcat/Km)PLK vs [ATP] are hyperbolic (Figures 2B and 2C) and replots of apparent values of (kcat)ATP and (kcat/Km)ATP vs [PLK] are hyperbolic as well (data not shown) and suggest that this reaction follows either a random or a steady-state ordered mechanism. Since the rate laws are mathematically equivalent for these mechanisms, they cannot be distinguished by the method of replots. However, they can be distinguished through the use of substrate analogue and product inhibition studies (see below).
LRRK2-Catalyzed Phosphorylation of LRRKtide – Initial Velocity Studies
To determine the kinetic mechanism of the LRRK2-catalyzed phosphorylation of LRRKtide, initial velocities were measured over a range of concentrations of LRRKtide at several fixed concentrations of ATP (see supplemental). The data set was globally fit to equations that reflect ping-pong, rapid equilibrium ordered, and random/steady-state ordered mechanisms. The random (or steady-state ordered) mechanism fits the data best and provides parameter estimates averaged from two independent experiments: kcat = 0.1 ± 0.003 min−1, KATP = 67 ± 6 µM, KPLK = 29 ± 3 µM, and α = 1.1 ± 0.1, as summarized in Table 1. The analysis using the method of replots confirmed that this reaction follows either a random or a steady-state ordered mechanism.
Inhibition Studies for PLK-peptide Phosphorylation
To determine the kinetic mechanism of LRRK2-catalyzed PLK-peptide phosphorylation, inhibition study was conducted with peptide substrate analogue LRRKtideA, where threonine of LRRKtide was replaced with alanine, nucleotide analogues AMP, AMP-PNP, and product ADP. Data were analyzed using methods of replots as described previously (17). As examples of this method, we analyzed inhibition by AMP. First we determined (i) at a single [PLK-peptide], the dependence of ν0 on [ATP] at several concentrations of [AMP] (Figure 3A) (ii) at a single [ATP], the dependence of ν0 on [PLK-peptide] at several concentrations of [AMP] (Figure 3D). Next, for (i), we analyzed the dependence of νo on [ATP] at each [AMP] to calculate apparent values (kcat)ATP and (kcat/Km)ATP. For (ii), we analyzed the dependence of νo on [PLK] at each [AMP] to calculate apparent (kcat)PLK and (kcat/Km)PLK. Replots of apparent values of (kcat)X (X = ATP or PLK-peptide) vs [AMP] and apparent values of (kcat/Km)X vs [AMP] were then constructed, which will show a specific, mechanism-based pattern (17).
Figure 3.
Inhibition study of the LRRK2-catalyzed phosphorylation of PLK-peptide by AMP. A: Plot of initial velocities vs [ATP] at [AMP] = 25 (●), 10 (▼), 5 (▽), 2.5 (■), and 0 µM (□) all at a fixed PLK-peptide concentration of 100 nM. B & C: AMP concentration dependencies of (kcat)ATP and (kcat/Km)ATP apparent values derived from analysis of the data of panel A. D: Plot of initial velocities vs [PLK-peptide] at [AMP] = 25 (●), 10 (○), 5 (▼), 2.5 (▽), and 0(■) mM all at a fixed ATP concentration of 200 µM. E & F: AMP concentration dependencies of (kcat)PLK and (kcat/Km)PLK apparent values derived from analysis of the data of panel D.
When ATP was the variable substrate, we found that apparent values of (kcat)ATP are independent of [AMP] (Figure 3B), but apparent values of (kcat/Km)ATP depend on [AMP] (Figure 3C) according to a simple inhibition expression of general form: νinhib = νcontrol/(1 + [I]/Ki,app). This pattern indicates that AMP is competitive with ATP. More surprisingly, the same pattern was revealed when PLK-peptide was the variable substrate, apparent values of (kcat)PLK are independent of [AMP], but apparent values of (kcat/Km)PLK depend on [AMP] according to νinhib = νcontrol/(1 + [I]/Ki,app) (Figures 3E and 3F). Thus, AMP is competitive with PLK-peptide as well.
The inhibition study of peptide substrate analogue LRRKtideA revealed that when PLK-peptide is the variable substrate, apparent values of (kcat)PLK are independent of [LRRKtideA] but apparent values of (kcat/Km)PLK depend on [LRRKtideA]; when ATP is the variable substrate, both apparent values of (kcat)ATP and (kcat/Km)ATP titrate with [LRRKtideA] (see supplemental). It suggests that LRRKtideA is competitive with PLK-peptide but noncompetitive with ATP.
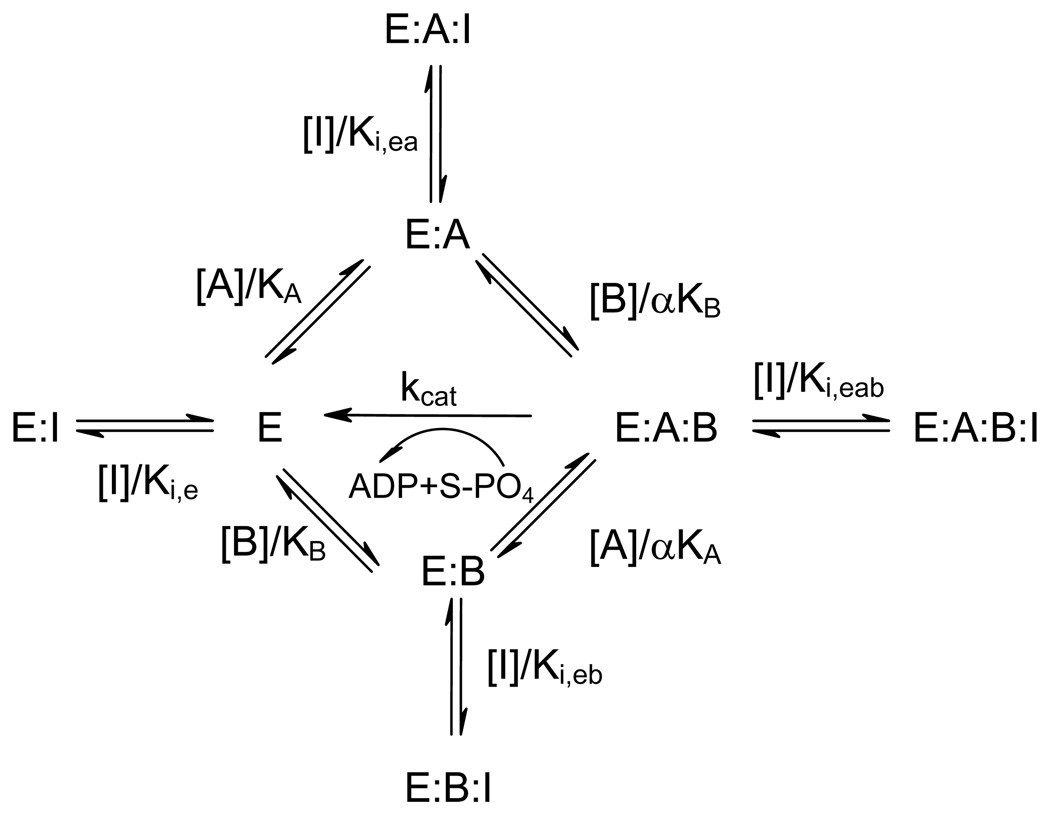
These patterns rule out a steady-state ordered mechanism, but are different from the ones well known for a random mechanism in which it has been assumed that the competitive inhibitors act as dead-end inhibitors and bind to the same enzyme form as the substrates (18). The patterns seen in this study clearly suggest that the inhibitors interact with the enzyme in a different mode. To examine these results and estimate values of inhibition constants, we used the method described in detail previously (17), where the general mechanism of Scheme I in which inhibitor can bind to all four forms of enzyme was used to derive the rate equation (eq 6) under rapid equilibrium condition.
| (6) |
Scheme I.
The rapid equilibrium random mechanism of LRRK2-catalyzed phosphorylation. A represents ATP and B represents phosphoryl acceptor, either PLK-peptide or LRRKtide.
By inspecting eqs 7–10 which describe the dependence of apparent values (Vmax)X and (Vmax/Km)X (X = ATP, or PLK-peptide) on inhibitor, we can immediately see how inhibitors interact with enzyme.
| (7) |
| (8) |
In the case of AMP inhibition where ATP is the variable substrate, apparent values of (Vmax)ATP are independence of AMP, which suggests that both Ki,ea and Ki,eab are very large and AMP cannot bind to either E:A or E:A:B complex. While on the other hand, apparent values of (Vmax/Km)ATP titrate with AMP, suggesting that AMP can bind E, E:B, or both.
| (9) |
| (10) |
In the case where PLK-peptide is the variable substrate, apparent values of (Vmax)PLK are independence of AMP, which means that both Ki,eb and Ki,eab are very large and clearly suggests that AMP cannot bind to E:B complex. While on the other hand, apparent values of (Vmax/Km)PLK titrate with AMP, suggesting that AMP can bind to E, and we can estimate Ki,e directly from eq 10 with our previous knowledge of kcat, KA, KB, and α.
Using this method, we also determined for nucleotide analogue AMP-PNP and product ADP the mechanism of inhibition and the inhibition dissociation constants (see supplemental). All these data are summarized in Table 2.
Table 2.
Inhibition of LRRK2 Kinase by Substrate Analogues
| substrate | inhibition const (mM) | ||||
|---|---|---|---|---|---|
| inhibitor | variable | mechanism | Ki,e | Ki,ea | Ki,eb |
| AMP | ATP | C | 2.2 ± 0.1 | ||
| PLK-peptide | C | 2.7 ± 0.5 | |||
| ATP | C | 2.2 ± 0.2 | |||
| LRRKtide | NC | 12 ± 0.2 | 14 ± 2.1 | ||
| AMP-PNP | ATP | C | 0.3 ± 0.03 | ||
| PLK-peptide | NC | 1.3 ± 0.3 | 2.3 ± 0.2 | ||
| ATP | C | 0.7 ± 0.2 | |||
| LRRKtide | NC | 0.7 ± 0.1 | 1.8 ± 0.2 | ||
| ADP | ATP | C | 0.02 ± 0.003 | ||
| PLK-peptide | NC | 0.1 ± 0.03 | 0.3 ± 0.1 | ||
| ATP | C | 0.2 ± 0.02 | |||
| LRRKtide | NC | 0.1 ± 0.01 | 0.2 ± 0.02 | ||
| LRRKtideA | ATP | NC | 1.3 ± 0.4 | 0.65 ± 0.06 | |
| PLK-peptide | C | 0.54 ± 0.05 | |||
| ATP | NC | 0.70 ± 0.20 | 0.90 ± 0.11 | ||
| LRRKtide | C | 0.54 ± 0.03 | |||
Inhibition Studies for LRRKtide phosphorylation
The kinetic mechanism of LRRK2-catalyzed LRRKtide phosphorylation was determined by conducting the inhibition studies with AMP, AMP-PNP, ADP, and peptide LRRKtideA as well. When ATP is the variable substrate, AMP, AMP-PNP, and ADP are competitive with ATP while peptide substrate analogue LRRKtideA is noncompetitive with ATP; when LRRKtide is the variable substrate, AMP, AMP-PNP, and ADP are noncompetitive with LRRKtide while LRRKtideA is competitive with LRRKtide (see supplemental). These inhibition patterns concur with a rapid equilibrium random mechanism with either ATP or LRRKtide being the first substrate to bind to the enzyme. Unlike the PLK-peptide phosphorylation, both AMP and LRRKtideA behave as dead-end inhibitors and bind to both E and E:X complex (X = ATP or LRRKtide). The inhibition dissociation constants were determined and summarized in Table 2.
LRRK2-Catalyzed Hydrolysis of GTP – Steady-State Kinetic Study
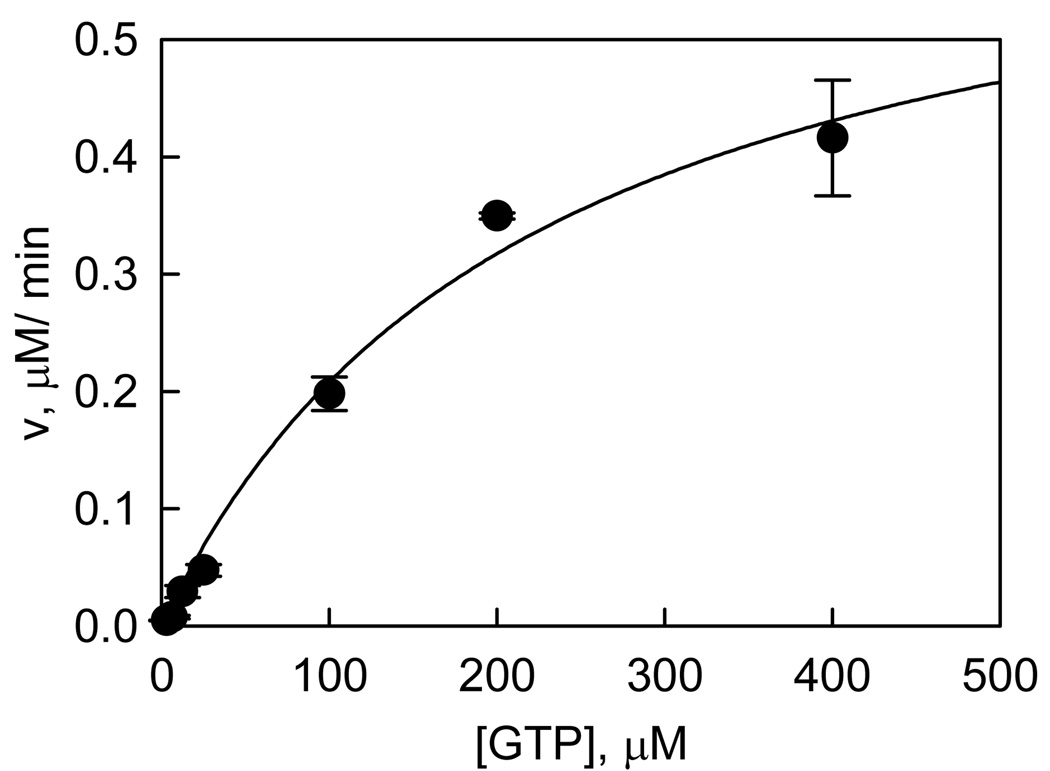
To characterize LRRK2-catalyzed hydrolysis of GTP, we have developed a radiometric assay in which [α-33P]-GDP is separated from [α-33P]-GTP by TLC and counted by a scintillation counter. The production of GDP is linearly dependent on both time and enzyme concentration (data not shown). Initial velocities were measured as a function of GTP concentration. The dependence of initial velocity on GTP concentration adheres to the simple Michaelis-Menten equation, which allowed us to calculate the steady-state kinetic parameters: kcat = 0.23 ± 0.02 s−1, and Km = 210 ± 29 µM, by nonlinear least-squares fit (Figure 4) as summarized on Table 3. These results reveal that WT LRRK2 expressed in mouse brain has GTPase activity.
Figure 4.
LRRK2-catalyzed GTP hydrolysis. The LRRK2-catalyzed GTP hydrolysis was conducted in buffer containing 20 mM Tris (pH 7.4), 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, BSA 0.5 mg/ml, GTP, and [α-33P]-GTP. The reaction was initiated by the addition of 30 nM LRRK2, and incubated at RT for 20 min. The reaction was stopped by the addition of 20 mM EDTA, and the product [α-33P]-GDP was separated from [α-33P]-GTP by PEI-cellulose TLC developed by 0.5 M KH2PO4 (pH 3.4) developing buffer and analyzed by scintillation counter. Each data point is the average of triplicate determinations, and the error bars represent standard deviation. The data set was fit to the simple Michaelis-Menten equation.
Table 3.
Kinetic Constants of LRRK2-catalyzed GTP Hydrolysis
| kcat, s−1 | KGTP, µM | Dkcat |
|---|---|---|
| 0.23 ± 0.02 | 210 ± 29 | 0.97 ± 0.04 |
Solvent Isotope Effect Study of LRRK2-Catalyzed Hydrolysis of GTP
The initial velocities were measured in H2O and D2O (the molar fraction of D2O equals 0.86) for kcat at saturating concentration of GTP (1 mM) at pH 7.4 and pD equivalent (19), respectively. Solvent isotope effect, Dkcat = 0.97 ± 0.04 is the mean of the ratio of kcat in H2O and D2O from four independent experiments.
Inhibition Studies for GTP Hydrolysis
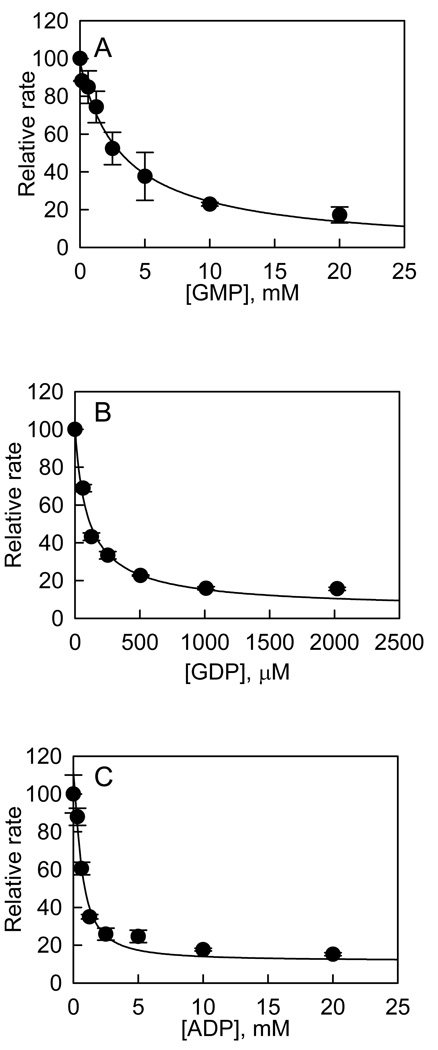
The inhibition studies of GTP hydrolysis were conducted with substrate analogues GMP and ADP, and product GDP. The initial velocities were measured as a function of inhibitor concentration at 200 µM GTP. The dependence of initial velocity on I (I = GMP, GDP, ADP) adheres to the simple inhibition expression of general form: νinhib = νcontrol/(1 + [I]/Ki,app), which allowed us to calculate the apparent dissociation constants of 2.9 ± 0.3 mM, 97 ± 16 µM, and 0.6 ± 0.1 mM for GMP, GDP, and ADP, respectively (Figure 5). We converted the apparent dissociation constants into dissociation constants of 1.5 mM, 49 µM, and 0.3 mM for GMP, GDP, and ADP, respectively, by applying the Cheng-Prusoff equation for competitive inhibition as given in eq 11 (20).
| (11) |
Figure 5.
Inhibition of the LRRK2-catalyzed GTP hydrolysis by nucleotide analogues. The GTPase reaction was carried out at 200 µM GTP in the presence of GMP (A), GDP (B), or ADP (C). The dependence of relative rate on I (I = GMP, GDP, ADP) was fit to the simple inhibition expression of general form: νinhib = νcontrol/(1 + [I]/Ki,app), which allowed us to calculate the apparent dissociation constants of 2.9 mM, 97 µM, and 0.6 mM for GMP, GDP, and ADP, respectively. Each data point is the average of triplicate determinations, and the error bars represent standard deviation.
Effects of Nucleotide on LRRK2-catalyzed Phosphorylation
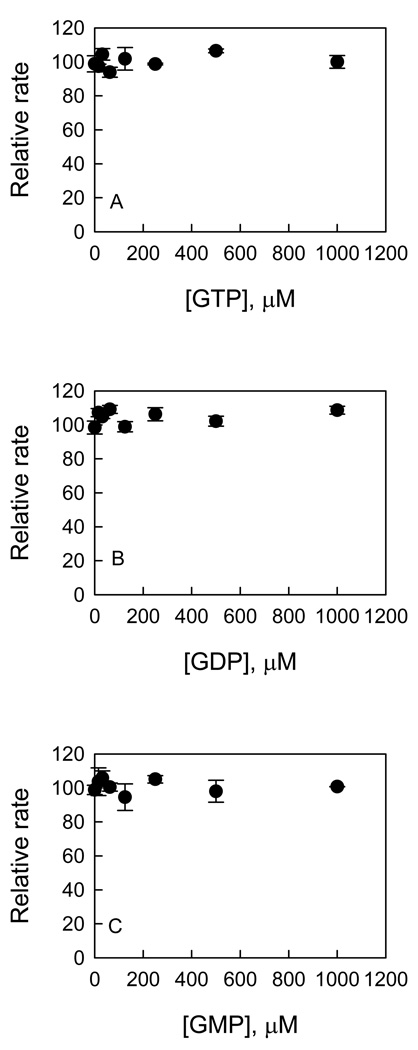
To determine the effects of nucleotides on kinase activity of LRRK2, initial velocities of LRRK2-catalyzed phosphorylation of PLK-peptide were measured over a range of concentrations of nucleotides, GTP, GDP, and GMP at conditions for kcat, (kcat/Km)ATP, and (kcat/Km)PLK. No significant activation or inhibition of kinase activity was detected at all the conditions. Figure 6 shows an example of nucleotide effect on kcat for the PLK-peptide phosphorylation. The effects of nucleotides on LRRK2-catalzyed phosphorylation of LRRKtide were also tested and no significant activation or inhibition of kinase activity was detected (data not shown).
Figure 6.
Effects of nucleotides on LRRK2-catalyzed PLK-peptide phosphorylation. The initial velocity of LRRK2-catalyzed phosphorylation of PLK-peptide were measured as a function of concentration of GTP, GDP, or GMP at saturating concentrations of ATP and PLK. Each data point is the average of triplicate determinations, and the error bars represent standard deviation.
Effects of Kinase Activity on GTPase Activity
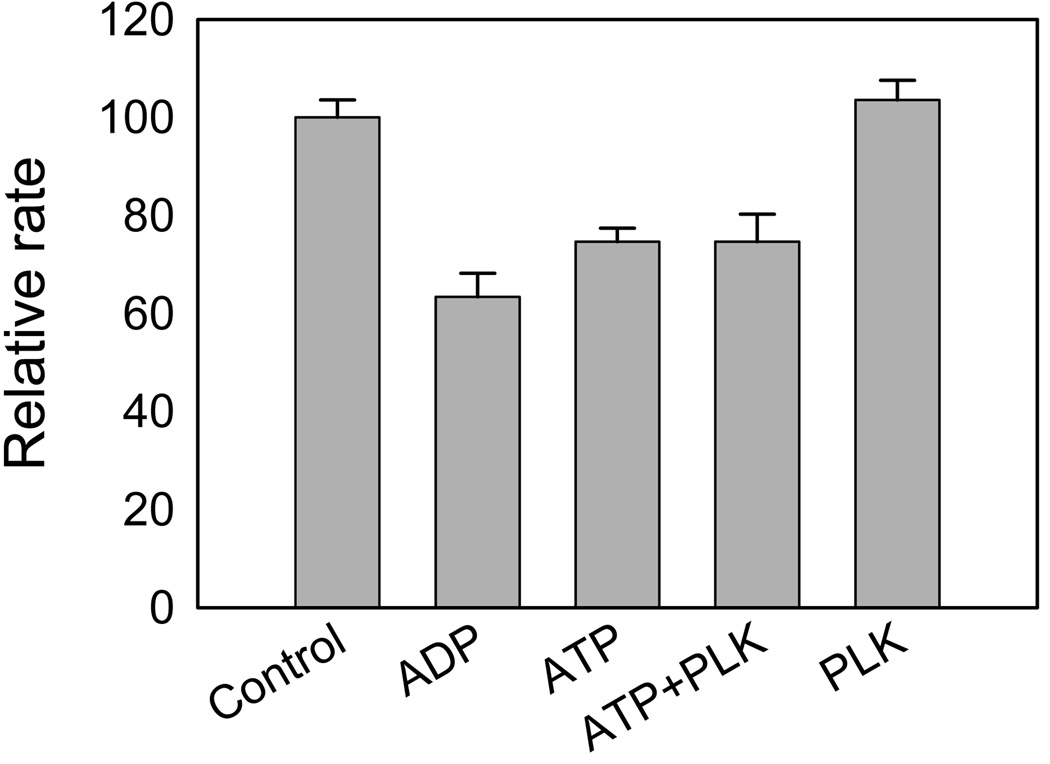
To determine the effects of kinase activity on the upstream GTPase activity of LRRK2, initial velocities of LRRK2-catalyzed GTP hydrolysis were measured in the presence of ADP, ATP, PLK-peptide at 200 µM GTP. Inhibition of GTPase activity was detected in presence of ADP or ATP, but not for PLK-peptide (Figure 7).
Figure 7.
Inhibition of the LRRK2-catalyzed GTP hydrolysis. The GTPase reaction was carried out at 200 µM GTP in the presence of 0.5 mM ADP, 0.5 mM ATP, 0.5 mM ATP & 1 µM PLK, or 1 µM PLK, respectively. The control was carried out in the absence of either ADP/ATP or PLK. Each data point is the average of triplicate determinations, and the error bars represent standard deviation.
Inhibition of LRRK2 by Compound LDN-73794
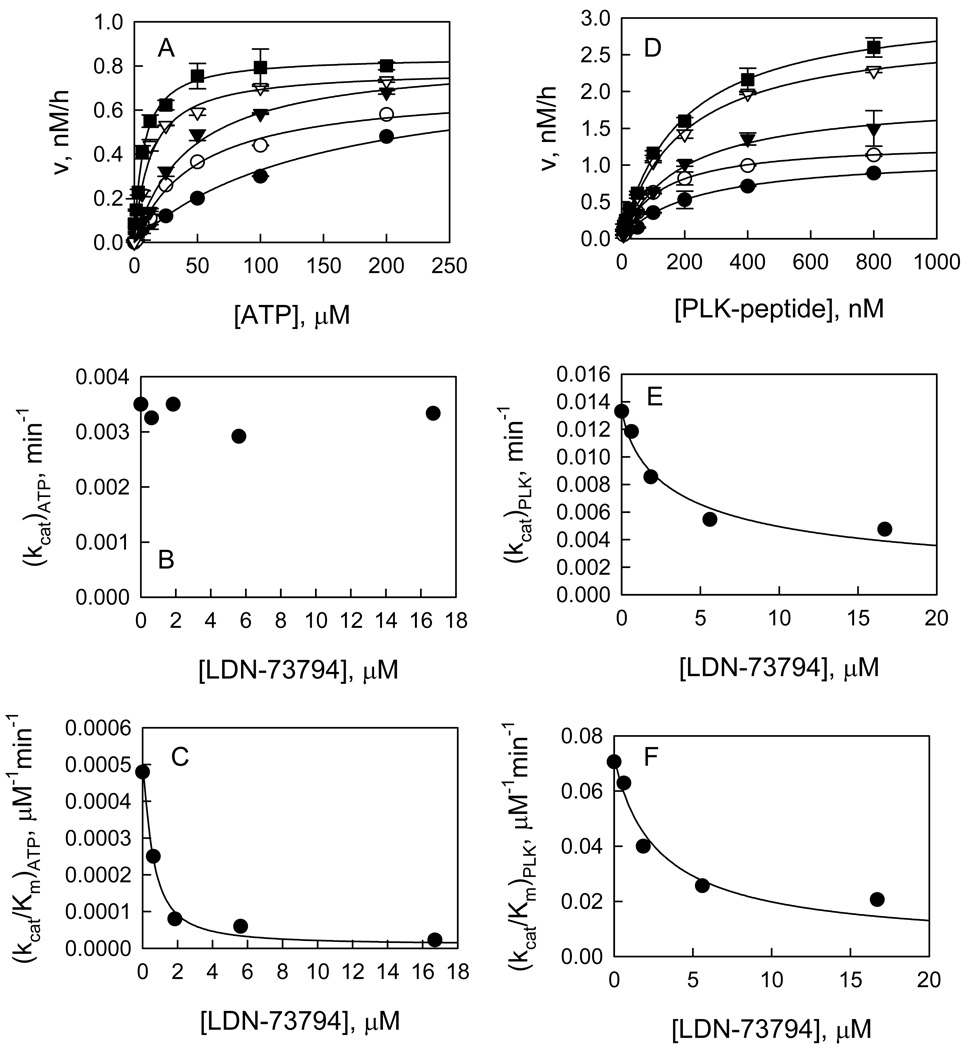
We identified compound LDN-73794 through a screen of our compound collection for inhibitors of LRRK2 kinase activity with an IC50 of 3.5 ± 0.3 µM. To characterize this inhibitor, first we studied the mechanism of inhibition of LDN-73794 in the PLK-peptide phosphorylation. The studies were done in a manner identical to that outlined above for AMP. The primary plots of the dependence of ν0 on [ATP] and the dependence of ν0 on [PLK-peptide] were determined at several concentrations of LDN-73794 (Figure 8). The replots of these data show that apparent values of (kcat)ATP are independent of the inhibitor and apparent values of (kcat/Km)ATP titrate with the inhibitor; both apparent values of (kcat)PLK and (kcat/Km)PLK titrate with the inhibitor. These patterns suggest that LDN-73794 is competitive with ATP and noncompetitive with PLK-peptide. By inspecting eqs 7–10 and using our previous knowledge of kcat, KA, KB, and α, we estimate the inhibitor dissociation constants Ki,e = 2.0 ± 0.5 µM and Ki,eb = 3.1 ± 0.5 µM. Next we carried out the inhibition study for LDN-73794 in the GTPase assay. The initial velocity was measured as a function of concentrations of LDN-73794 at 200 µM GTP and no inhibition was detected.
Figure 8.
Inhibition of the LRRK2-catalyzed phosphorylation of PLK-peptide by compound LDN-73794. A: Plot of initial velocities vs [ATP] at [LDN-73794] = 16.7 (●), 5.6 (○), 1.85 (▼), 0.62 (▽), and 0 µM (■) all at a fixed PLK-peptide concentration of 100 nM. B & C: LDN-73794 concentration dependencies of (Vmax)ATP and (Vmax/Km)ATP apparent values derived from analysis of the data of panel A. D: Plot of initial velocities vs [PLK-peptide] at [LDN-73794] = 16.7 (●), 5.6 (○), 1.85 (▼), 0.62 (▽), and 0 µM (■) all at a fixed ATP concentration of 50 µM. E & F: LDN-73794 concentration dependencies of (Vmax)PLK and (Vmax/Km)PLK apparent values derived from analysis of the data of panel D.
DISCUSSION
LRRK2 is unusual in that it encodes two distinct but functionally linked enzymes: a protein kinase and a GTPase. Recent studies have suggested that LRRK2 is capable of undergoing both autophosphorylation and generic substrate phosphorylation, and the kinase activity is regulated by the GTPase domain (8–12). But little is known about the kinetic mechanism of the two enzymatic properties. Our goal for these studies was to understand the kinetic mechanism of LRRK2 that would be useful in designing a high-throughput screening assay and to establish a kinetic background for the interpretation of the mechanism of inhibition.
Kinetic Mechanism of LRRK2 Kinase Property
There appears to be no consensus kinetic mechanism for protein kinases (21). In the majority of cases, protein kinases follow a random mechanism, while there are a number of examples of kinases following an ordered mechanism (22–27). In some cases, the nature of the substrate could influence the binding order as reported for p38α of MAPK family: the kinetic mechanism for p38α changes from one in which ATP binds to an enzyme before a peptide substrate (28) to one in which the protein ATF2 binds before ATP (29).
The physiological substrate of LRRK2 is still unclear, although recent studies reported that ezrine, radixin, and moesin (ERM), proteins which anchor the actin cytoskeleton to the plasma membrane, are efficiently phosphorylated by LRRK2 as potential substrates (30, 31). A short peptide substrate, LRRKtide, which encompasses Thr558 of moesin (RLGRDKYKTLRQIRQ) has been widely used in the characterization of LRRK2 catalysis, but the kinetic mechanism of the reaction has not been reported. In our studies of this enzyme, we sought to probe the kinetic mechanism with two peptide substrates, LRRKtide and PLK-peptide derived from PLK protein and to determine whether a common kinetic mechanism is shared by these two phosphoryl acceptors. Our initial velocity data with both PLK-peptide and LRRKtide suggest a random or a steady-state ordered mechanism. To distinguish between these two mechanisms, we conducted substrate analogue and product inhibition studies (16, 18, and 32). The results of these studies are summarized in Table 2. For LRRK2-catalyzed phosphorylation of PLK-peptide, we found that nucleotide analogue AMP is competitive with both ATP and PLK-peptide; substrate analogue LRRKtideA is PLK-peptide competitive and ATP noncompetitive. Different patterns were seen with LRRKtide as the phosphoryl acceptor: AMP is competitive with ATP and noncompetitive with LRRKtide; substrate analogue LRRKtideA is competitive with LRRKtide and noncompetitive with ATP. To elucidate the kinetic mechanism, we also tested nucleotide analogue AMP-PNP and the product ADP in the two reactions and found: AMP-PNP and ADP are competitive with ATP in both reactions and noncompetitive with the phosphoryl acceptors, PLK-peptide or LRRKtide. The substrate analogue and product inhibition patterns rule out a steady-state ordered mechanism and indicate a rapid equilibrium random mechanism for phosphorylation of both PLK-peptide and LRRKtide (18, 32). The nature of the peptide substrate does not influence the substrate binding order with LRRK2, although the binding of PLK-peptide induces a conformation change which prohibits AMP from binding. In both reactions, either ATP or phosphoryl acceptor (PLK-peptide or LRRKtide) can bind to the enzyme first to form the enzyme:substrate binary complex followed by the formation of the ternary complex. Equilibrium of these species shown in Scheme I was attained prior to the rate-limiting step, which may be the chemistry step, or some steps following the chemistry step. The binding of the first substrate does not affect the binding affinity of the second substrate as demonstrated by the cofactor α with a value close to 1.
Formation of a Nonproductive Complex
Unexpectedly, values of KATP for the reaction of LRRK2 with PLK-peptide and LRRKtide depend on the identity of the phosphoryl acceptor, equaling 13 and 67 µM, respectively. Values of Ki,e for AMP also differ 5-fold in the two reactions, equaling 2.5 and 12 µM for PLK-peptide and LRRKtide, respectively. KATP and Ki,e are dissociation constants and their magnitude are supposed to be identical regardless of the identity of the phosphoryl acceptor. A mechanism that involves the formation of a nonproductive binary complex upon the binding of the phosphoryl acceptor to free enzyme, to which nucleotides bind nonproductively, could account for these results. Thus, the measured values are complex terms including the contribution from the pathway introduced by the nonproductive binding of the phosphoryl acceptor and would be dependent on the identity of the phosphoryl acceptor.
Characterization of GTPase Activity of LRRK2
It has been reported that LRRK2 possesses a GTPase activity (10, 15). Here we characterized LRRK2-catalyzed GTP hydrolysis. The production of GDP is linearly dependent on both time and enzyme concentration. The dependence of the initial velocity of GDP production on GTP concentration adheres to the simple Michaelis-Menten equation and allowed us to calculate the steady-state kinetic parameters. No solvent kinetic isotope effect could be detected on kcat and suggests that the rate-limiting step of the reaction governed by kcat is the product GDP release. In other words, the product release step is slower than the chemical step. That was not surprising, since like all the other G-proteins, the GTP binding domain of LRRK2 undergoes a switch cycle between the GTP-bound active and GDP-bound inactive form. The exchange of GDP for GTP is promoted by either guanine nucleotide exchange factors (GEFs) or a dimerization-induced conformation change.
The Relationship of Kinase and GTPase Activities
It has been reported that the kinase activity of LRRK2 is regulated by the GTPase domain such that the binding of GTP stimulates LRRK2 kinase activity (8, 10, 11). To understand the functional regulation between the kinase and GTPase activity, we first tested the effect of nucleotides (GTP, GDP, and GMP) in a dose-response manner on LRRK2-catalyzed PLK-peptide phosphorylation under conditions for kcat and kcat/Km. No significant activation or inhibition of kinase activity in the presence of nucleotides was detected under the condition tested. To exclude that our results are substrate-dependent, we also carried out the same studies for LRRK2-catalyzed LRRKtide phosphorylation and no significant effect was detected. It might be possible that we missed the GTP activation given the fact that the reaction progress curve for GTPase reaction was linear only up to 30 min and we typically incubate the kinase reaction for at least 2h. Therefore, the effect of nucleotide binding on kinase activity was tested as a time-course. But no significant effect could be detected. The lack of GTPase domain regulation on kinase activity might suggest that the brain LRRK2 that was used in this study is already in a nucleotide-bound state, either GTP- or GDP-bound state. We hypothesized that it was likely in the inactive GDP-bound state, since the product GDP release step is slow compared with the chemical step as suggested by the SKIE study. In order to test this hypothesis, we examined the effect of product GDP and nucleotide analogue GMP on the GTPase activity, and found that both GDP and GMP inhibit the GTPase activity of LRRK2. The lack of effect of the nucleotides on kinase activity brings controversy to the hypothesis that GTP binding stimulates LRRK2 kinase activity. A previous study demonstrated that the non-hydrolyzable GTP analog, GPP(NH)p, but not GTP, increased LRRK2 kinase activity; whereas GDP had no effect on kinase activity rather than inhibits kinase activity as predicted (10). It is important to note that the effect seen here is based on in vitro assays performed on two generic substrates. There are some cellular GTPase regulators under physiological conditions, such as GTPase-activating proteins (GAP) that have been shown to stimulate GTP hydrolysis which in turn could regulate kinase activity.
To further our understanding of the functional relationship between the kinase and GTPase activity of LRRK2, i.e., the effect of the downstream kinase activity on GTPase activity, we also tested the effects of substrates or product of the kinase reaction, such as ATP, PLK-peptide, and ADP, on the GTPase activity. We found that ADP and ATP, but not PLK-peptide could inhibit the GTPase activity. There are two possibilities that ADP or ATP could inhibit GTPase activity: (i) ADP or ATP binds directly to the active site of the GTPase domain or (ii) ADP or ATP binds to the kinase domain and induces a conformational change which affects GTPase activity. The second mechanism is not likely, though, given the fact that a nonspecific kinase inhibitor, staurosporine, which inhibits LRRK2 kinase activity but does not inhibit GTPase activity at all (data not shown). To understand how ADP inhibits the GTPase activity, the dissociation constant of ADP was determined for both reactions: Ki = 20 µM in the kinase reaction and Ki = 0.6 mM in the GTPase reaction. The 30-fold difference in dissociation constants of ADP suggest that there might be two distinct binding sites of ADP on LRRK2, and ADP could bind directly to the GTPase domain and inhibit GTPase activity.
Mechanism of Inhibition of LDN-73794
With knowledge of the kinetic mechanism of LRRK2-catalyzed phosphorylation, we were able to conduct kinetic analysis for inhibitor LDN-73794 identified through a screen to understand the mechanism of inhibition, to estimate its inhibition constants, and to test its effect on the GTPase activity. Studies of inhibition by LDN-73794 revealed that this compound is a well-behaved inhibitor with no hint of time-dependence or partial inhibition. The dependence of initial velocity on inhibitor concentration can be described by the simple inhibition expression of general form: νinhib = νcontrol/(1 + [I]/Ki,app) with no need to include the higher-order terms to attain good data fitting. As a result of a previous screen of cyclin-dependent kinase5 inhibitors (cdk5), LDN-73794 did not inhibit cdk5. It is unlikely that LDN-73794 shows inhibition by chelation of magnesium, since cdk5 kinase also requires Mg2+ for the catalytic activity. The studies of mechanism of inhibition revealed that LDN-73794 is a competitive inhibitor of the binding of ATP and a noncompetitive inhibitor of PLK-peptide. It inhibits the kinase activity but has no effect on the GTPase activity. Further characterization of the mechanisms by which LDN-73794 interacts with WT LRRK2 and mutant LRRK2 is underway.
These studies establish a background of kinetic data that is essential in designing a high-throughput screening assay that allows identification of inhibitors interacting directly with the kinase domain and also allosteric inhibitors that modulate kinase activity through interaction with other LRRK2 domains. Identification of molecules that inhibit LRRK2’s kinase activity can not only become the starting point for drug development to treat PD, but also provide useful probes to further investigate the physiological functions of LRRK2 in normal cellular biology and in pathological conditions.
Supplementary Material
Figure 10.
Table 4.
Inhibition of LRRK2 Kinase by Compound LDN-73794
| substrate | inhibition const (μM) | |||
|---|---|---|---|---|
| inhibitor | variable | mechanism | Ki,e | Ki,eb |
| LDN-73794 | ATP | C | 1.2 ± 0.3 | |
| PLK-peptide | NC | 2.0 ± 0.5 | 3.1 ± 0.5 | |
ABBREVIATIONS
- PD
Parkinson’s disease
- wt LRRK2
wild type leucine-rich repeat kinase2
- PLK-peptide
PLK-derived peptide with a motif of RRRSLLE
- LRRKtide
RLGRDKYKTLRQIRQ
- LRRKtideA
RLGRDKYKALRQIRQ
Footnotes
Supporting Information Available. Data of inhibition studies of AMP, AMP-PNP, and ADP for the PLK-peptide and LRRKtide phosphorylation are provided as supplemental information. This material is available free of charge Via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu. Rev. Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 2.Paisán-Ruíz C, Jain S, Evans EW, Gilks WP, Simón J, van der Brug M, López de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Martí-Massó JF, Pérez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Müller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Berg D, Schweitzer K, Leitner P, Zimprich A, Lichtner P, Belcredi P, Brussel T, Schulte C, Maass S, Nagele T. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson's disease. Brain. 2005;128:3000–3011. doi: 10.1093/brain/awh666. [DOI] [PubMed] [Google Scholar]
- 5.Khan NL, Jain S, Lynch JM, Pavese N, Abou-Sleiman P, Holton JL, Healy DG, Gilks WP, Sweeney MG, Ganguly M, Gibbons V, Gandhi S, Vaughan J, Eunson LH, Katzenschlager R, Gayton J, Lennox G, Revesz T, Nicholl D, Bhatia KP, Quinn N, Brooks D, Lees AJ, Davis MB, Piccini P, Singleton AB, Wood NW. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson's disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128:2786–2796. doi: 10.1093/brain/awh667. [DOI] [PubMed] [Google Scholar]
- 6.Mata IF, Kachergus JM, Taylor JP, Lincoln S, Aasly J, Lynch T, Hulihan MM, Cobb SA, Wu RM, Lu CS, Lahoz C, Wszolek ZK, Farrer MJ. Lrrk2 pathogenic substitutions in Parkinson's disease. Neurogenetics. 2005;6:171–177. doi: 10.1007/s10048-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 7.Paisán-Ruíz C, Jain S, Evans EW, Gilks WP, Simón J, van der Brug M, López de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Martí-Massó JF, Pérez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 9.Greggio E, Zambrano I, Kaganovich A, Beilina A, Taymans JM, Daniëls V, Lewis P, Jain S, Ding J, Syed A, Thomas KJ, Baekelandt V, Cookson MR. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem. 2008;283:16906–16914. doi: 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL, Chen SG. The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. 2007;33– 313:3658–3670. doi: 10.1016/j.yexcr.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito G, Okai T, Fujino G, Takeda K, Ichijo H, Katada T, Iwatsubo T. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial parkinsons’s disease. Biochemistry. 2007;46:1380–1388. doi: 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 12.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith WW, Pei Z, Jiang H, Moore DJ, Liang Y, West AB, Dawson VL, Dawson TM, Ross CA. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson's disease R1441C/G mutants. J. Neurochem. 2007;103:238–247. doi: 10.1111/j.1471-4159.2007.04743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segel IH. Enzyme Kinetics. New York, NY: John Wiley& Sons; 1975. [Google Scholar]
- 17.Liu M, Choi S, Cuny GD, Ding K, Dobson BC, Glicksman MA, Auerbach K, Stein RL. Kinetic studies of Cdk5/p25 kinase: phosphorylation of tau and complex inhibition by two prototype inhibitors. Biochemistry. 2008;47:8367–8377. doi: 10.1021/bi800732v. [DOI] [PubMed] [Google Scholar]
- 18.Fromm HJ. Use of competitive inhibitors to study substrate binding order. Methods in Enzymology. 1979;63:467–486. doi: 10.1016/0076-6879(79)63020-3. [DOI] [PubMed] [Google Scholar]
- 19.Quinn DM, Sutton LD. In: Enzyme Mechanism from Isotope Effects. Cook PF, editor. Boston, MA: CRC Press; 1991. pp. 73–126. [Google Scholar]
- 20.Cheng YC, PRusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 21.Adams JA. Kinetic and catalytic mechanisms of protein kinases. Chem. Rev. 2001;101:2271–2290. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]
- 22.Songyang Z, Lu KP, Kwon YT, Tsai LH, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, DeMaggio AJ, Hoekstra MF, Blenis J, Hunter T, Cantley LC. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol Biol. Cell. 16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabatabai LB, Graves DJ. Kinetic mechanism and specificity of the phosphorylase kinase reaction. J Biol Chem. 1978;253:2196–2202. [PubMed] [Google Scholar]
- 24.Walker DH, Kuppuswamy D, Visvanathan A, Pike LJ. Substrate specificity and kinetic mechanism of human placental insulin receptor/kinase. Biochemistry. 1987;26:1428–1433. doi: 10.1021/bi00379a033. [DOI] [PubMed] [Google Scholar]
- 25.Boerner RJ, Barker SC, Knight WB. Kinetic mechanisms of the forward and reverse pp60c-src tyrosine kinase reactions. Biochemistry. 1995;34:16419–16423. doi: 10.1021/bi00050a024. [DOI] [PubMed] [Google Scholar]
- 26.Cole PA, Grace MR, Phillips RS, Burn P, Walsh CT. The role of the catalytic base in the protein tyrosine kinase Csk. J Biol Chem. 1995;270:22105–22108. doi: 10.1074/jbc.270.38.22105. [DOI] [PubMed] [Google Scholar]
- 27.Cole PA, Burn P, Takacs B, Walsh CT. Evaluation of the catalytic mechanism of recombinant human Csk (C-terminal Src kinase) using nucleotide analogs and viscosity effects. J Biol Chem. 1994;269:30880–30887. [PubMed] [Google Scholar]
- 28.Chen G, Porter MD, Bristol JR, Fitzgibbon MJ, Pazhanisamy S. Kinetic mechanism of the p38-alpha MAP kinase: phosphoryl transfer to synthetic peptides. Biochemistry. 2000;39:2079–2087. doi: 10.1021/bi9919495. [DOI] [PubMed] [Google Scholar]
- 29.LoGrasso PV, Frantz B, Rolando AM, O'Keefe SJ, Hermes JD, O'Neill EA. Kinetic mechanism for p38 MAP kinase. Biochemistry. 1997;36:10422–10427. doi: 10.1021/bi9706778. [DOI] [PubMed] [Google Scholar]
- 30.Jaleel M, Nichols RJ, Deak M, Campbell DG, Gillardon F, Knebel A, Alessi DR. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson's disease mutants affect kinase activity. Biochem J. 2007;405:307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parisiadou L, Xie C, Cho HJ, Lin X, Gu XL, Long CX, Lobbestael E, Baekelandt V, Taymans JM, Sun L, Cai H. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci. 2009;29:13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudolph FB. Product inhibition and abortive complex formation. Methods in Enzymology. 1979;63:411–436. doi: 10.1016/0076-6879(79)63018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.