Abstract
Background
Obstructive sleep apnea (OSA) is common after stroke and associated with poor stroke outcomes. Whether OSA after acute stroke is caused by anatomic, physiologic, or both etiologies has not been studied. We therefore used brain magnetic resonance imaging (MRI) scans to assess oropharyngeal anatomy in stroke patients with and without OSA.
Methods
Patients within 7 days of ischemic stroke underwent nocturnal polysomnography. Sagittal T1-weighted MRI performed for clinical purposes was used to measure retropalatal distance, soft palatal length, soft palatal thickness, retroglossal space, and tongue length. Nasopharyngeal area and high retropharyngeal area were measured from axial T2-weighted images, and lateral pharyngeal wall thickness from coronal T1-weighted images.
Results
Among 27 subjects, 18 (67%) had OSA (apnea/hypopnea index (AHI) ≥5). Demographics, vascular risk factors, and stroke severity were similar in the two groups. Median retropalatal distance was shorter in subjects with OSA (Wilcoxon rank-sum test, p= 0.03). Shorter retropalatal distance was associated with higher AHI (linear regression, p=0.04). None of the other morphological characteristics differed.
Conclusions
Anatomic difference between awake acute stroke patients with and without OSA shows that the sleep disorder cannot be attributed solely to sleep, sleeping position, or changes in neuromuscular control that are specific to the sleep state.
Keywords: Stroke, obstructive sleep apnea, MRI, oropharynx, brain imaging, anatomy
INTRODUCTION
Obstructive sleep apnea (OSA) may arise from any combination of contributions from constricted airway anatomy, physiologic dysfunction of muscles that line the oropharynx, and altered pharyngeal wall compliance.[1] Multiple imaging modalities have been used to study the airway and have supported anatomical differences between patients with and without OSA. The main observation has been that the oropharynx, and specifically the retropalatal region, is smaller in patients with OSA than in controls.[2] Soft tissue structures appear to encroach on the airway as evidenced by thickened lateral pharyngeal walls, larger tongue areas, and increased tongue and soft palatal fat.[2;3]
Sleep apnea is an independent risk factor for ischemic stroke and is associated with poor stroke outcomes.[4–7] Importantly, sleep apnea is much more common in stroke patients than in age-matched controls, [8] with a prevalence greater than 50%.[9] The reasons for this high prevalence and the pathophysiology of sleep apnea in stroke patients are not well understood. Infrequent case report data do exist to support the claim that stroke can cause sleep apnea. These cases are for the most part, however, strokes in particular locations in the brain stem[10] and do not speak to stroke as a cause of sleep apnea in general. Conversely, possible causes of OSA after stroke could conceivably include altered sleep physiology, neuromuscular control during sleep, or increased supine sleep that we have demonstrated previously.[11] The purpose of the current study was to investigate possible anatomic contributions to OSA in stroke patients. For the first time to our knowledge, we took advantage of brain MRI scans to compare oropharyngeal size and other anatomical characteristics in stroke patients with and without OSA. We hypothesized that stroke patients with OSA would have a smaller retropharyngeal airway and larger surrounding tissue in comparison to stroke patients without OSA. As a secondary aim, we also explored whether brain MRI scans, routinely obtained for neurological clinical care, could afford new opportunities to identify stroke patients at risk for OSA.
METHODS
Subjects
Acute ischemic stroke patients hospitalized at the University of Michigan were eligible if they were 18 or older and had a modified Rankin Scale score of 2 or greater, meaning they had at least some post-stroke disability. Patients were excluded for decompensated heart failure, cardiac or respiratory arrest, or myocardial infarction within the prior 3 months, severe pneumonia, hypertension refractory to treatment, prior exposure to continuous positive airway pressure, previous pneumothorax, bullous emphysema, or acute sinus or ear infection. These criteria were part of a parent clinical trial (Sleep Apnea Treatment After Stroke (SATS), NCT00282815) in which each subject agreed to participate. Informed consent was obtained from each subject or a proxy. The study was approved by the University of Michigan Institutional Review Board.
Study procedures
Baseline characteristics were abstracted from the medical record. Stroke severity was measured with the NIH Stroke Scale (NIHSS) by certified study staff. Weight was measured by nursing staff and height was self-reported. The presence of dysphagia was determined based on speech pathologist evaluation. All subjects who failed a bedside swallow assessment had a speech pathology evaluation. Epworth sleepiness scale was completed at the time of enrollment. Nocturnal polysomnography was performed on subjects within 7 days of stroke symptom onset. Full polysomnography using standard techniques included EEG leads (C3-A2, C4-A1, O1-A2, and O2-A1 of the International Electrode Placement System), electro-oculographic leads, chin and bilateral anterior tibialis surface electromyograms, ECG leads, oral and nasal airflow (thermocouples), nasal pressure, thoracic and abdominal excursion (piezo electric bands), snoring monitor, and finger pulse oximetry. An apnea was defined as ≥ 10 seconds of complete airflow cessation. An hypopnea was defined as a reduction in airflow, chest excursion, or abdominal excursion that led to ≥ 4% oxygen desaturation, awakening, or arousal.[12] The apnea-hypopnea index (AHI), a measure of sleep apnea severity, was calculated as the number of apneas (central and obstructive) and hypopneas per hour of sleep. A registered polysomnographic technologist scored all studies. Results were confirmed by a board-certified sleep physician. Obstructive sleep apnea was defined as ≥ 5 obstructive apneas per hour of sleep.
Brain MRIs were performed during the acute stroke hospitalization as part of the routine clinical care determined by the patients’ clinical team. Brain MR images were retrieved from the hospital PACS archives and measurements were made using the IDX Image Cast software (Version 3.3.2 Stentor Inc.). Images were obtained in the supine position with the head in a neutral position. Instructions for breath holding during MRI image acquisition were provided to each subject; however, no information on compliance with these directions is available. Acute infarctions were categorized as lacunar or non-lacunar by a board-certified vascular neurologist familiar with the patients’ clinical histories.
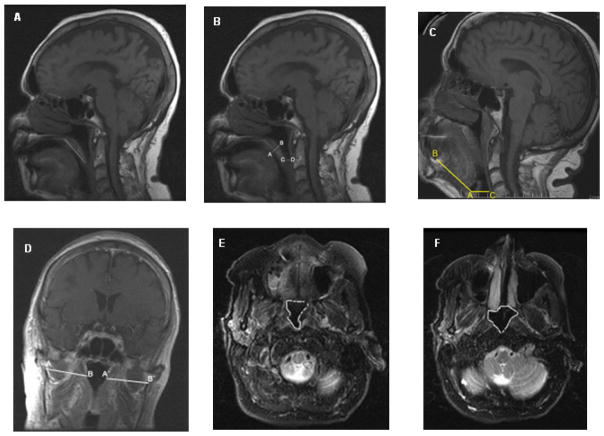
All measurements were made by an experienced neuroradiologist masked to polysomnographic data. Measurements included the retropalatal distance (mid sagittal T1), soft palatal length (mid sagittal T1), soft palatal thickness (mid sagittal T1), retroglossal space (mid sagittal T1), tongue length (mid sagittal T1), nasopharyngeal area (axial T2), high retropharyngeal area (axial T2), and lateral pharyngeal wall thickness (coronal T1). The retropalatal distance was measured as the perpendicular distance between the tip of the uvula and the posterior wall of the oropharynx. The length of the soft palate was measured from the posterior limits of the hard palate to its tip. Maximum thickness of the soft palate was measured perpendicular to the length of the soft palate. The retroglossal space, i.e., the hypopharyngeal airway space, was measured as the perpendicular distance between the anterior floor of the vallecula (postero-inferior base of tongue) and the posterior wall of the hypopharynx. The retrolingual length could not be measured as the inferior margin of the mandible was not visualized on all patients. The tongue length (vallecula–tongue base distance) was measured as the shortest distance between the anterior floor of the vallecula (postero-inferior base of tongue) and the antero-inferior corner of the tongue base. The narrowest axial cross-sectional area of the visualized nasopharynx at the level of the posterior choanae and highest level (“high retropharyngeal area”) of the oropharynx seen on T2-weighted image was also measured. The lateral pharyngeal soft tissue thickness was measured from the T1 coronal sections as the distance between the intercondylar notch of the mandible and the lateral edge of the oropharyngeal airway. Right and left were summed together as the total pharyngeal soft tissue thickness. Figure 1(A-F) depicts the regions measured.
Figure 1.
MRI measurements: (A) Palatal length on sagittal T1-weighted image. (B) Palatal thickness (A-B) and retropalatal distance (C-D) on a sagittal T1-weighted image. (C) Tongue length (A-B) and the retroglossal space (C-D) on sagittal T1-weighted image. (D) Lateral pharyngeal wall thickness on a coronal T1-weighted image. (E) Cross sectional area of high retropharyngeal region on axial T2-weighted image. (F) Cross-sectional area of nasopharynx on axial T2-weighted image.
Statistical analysis
Baseline characteristics and MRI measurements were summarized as medians and interquartile ranges (IQR) or frequencies and percents and were compared by OSA status (OSA versus no OSA) using Wilcoxon rank-sum tests or Fisher’s exact tests. Linear regression models were used to assess the association between the MRI measurements (individually) and AHI modeled as a continuous variable. The associations between retropalatal distance and age (continuous), gender, and body mass index (continuous) were also assessed using linear regression. Q-Q plots were used to assess the assumption of normally distributed residuals. Because the residuals from the linear regression models were not normally distributed, the dependent variables, AHI or retropalatal distance, were transformed using a natural log transformation. Q-Q plots using the transformed data did not reveal a departure from normality so the transformed outcomes were used in all models. A p-value < 0.05 was considered significant. A scatter plot was used to show the relationship between retropalatal distance and OSA. A receiver operating characteristic (ROC) curve was plotted and the area under it was calculated to assess the ability of retropalatal distance to discriminate OSA status. Analyses were performed using S-plus 7.0 for Windows and R: A Language and Environment for Statistical Computing version 2.10.0.
RESULTS
A total of 27 subjects met criteria and their data form the basis for all subsequent analysis. The median age was 65 years (IQR: 61, 78). Eighteen (67%) of the subjects had OSA. The median AHI in the OSA group was 31 (13, 47) with a median minimum oxygen saturation of 88% (84, 90). The median AHI in the non-OSA group was 1.6 (0.8, 2.6) and the minimum oxygen saturation was 90% (87, 92). Snoring detected during polysomnography was more common (p=0.0079) in the OSA group (17 [94%]) than in the non OSA group (4 [44%]). Additional baseline characteristics of subjects with and without OSA are listed in Table 1. The two groups did not differ in demographics, vascular risk factors, or stroke severity.
Table 1.
Baseline characteristics, expressed as median (interquartile range) or number (column percent).
| OSA (n=18) | No OSA (n=9) | p-value | |
|---|---|---|---|
| Age | 70 (61, 80) | 65 (59, 66) | 0.26 |
| Female gender | 8 (44) | 2 (22) | 0.41 |
| Race/ethnicity | |||
| African-American | 4 (22) | 2 (22) | 1.00 |
| Asian | 1 (6) | 0 (0) | |
| Non-Hispanic white | 13 (72) | 7 (78) | |
| BMI | 31 (28, 34) | 28 (24, 37) | 0.84 |
| Neck circumference (inches) | 16 (15, 18) | 17 (17, 18) | 0.29 |
| Hypertension | 14 (78) | 6 (66) | 0.65 |
| Diabetes | 6 (33) | 2 (22) | 0.68 |
| Atrial fibrillation | 3 (17) | 1 (11) | 1.00 |
| History of stroke | 6 (33) | 1 (11) | 0.36 |
| Coronary heart disease | 3 (17) | 0 (0) | 0.53 |
| Current smoking | 2 (11) | 1 (11) | 1.00 |
| Non-lacunar stroke subtype | 13 (72) | 5 (56) | 0.42 |
| Baseline NIHSS | 6 (4,8) | 6 (4,7) | 0.72 |
| Epworth Sleepiness Scale | 11 (6, 14) | 6 (5, 10) | 0.16 |
| SDQ-SA | 32 (29, 40) | 30 (27, 35) | 0.14 |
| Dysphagia | 10 (56) | 3 (33) | 0.44 |
BMI: body mass index
NIHSS: NIH stroke scale
OSA: Obstructive sleep apnea
SDQ-SA: – Sleep apnea scale of the Sleep Disorders Questionnaire
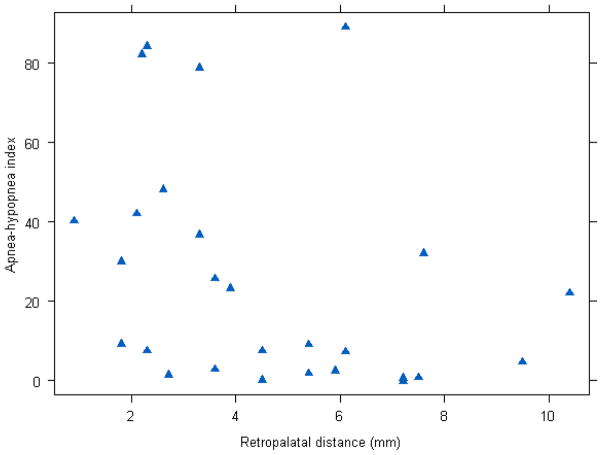
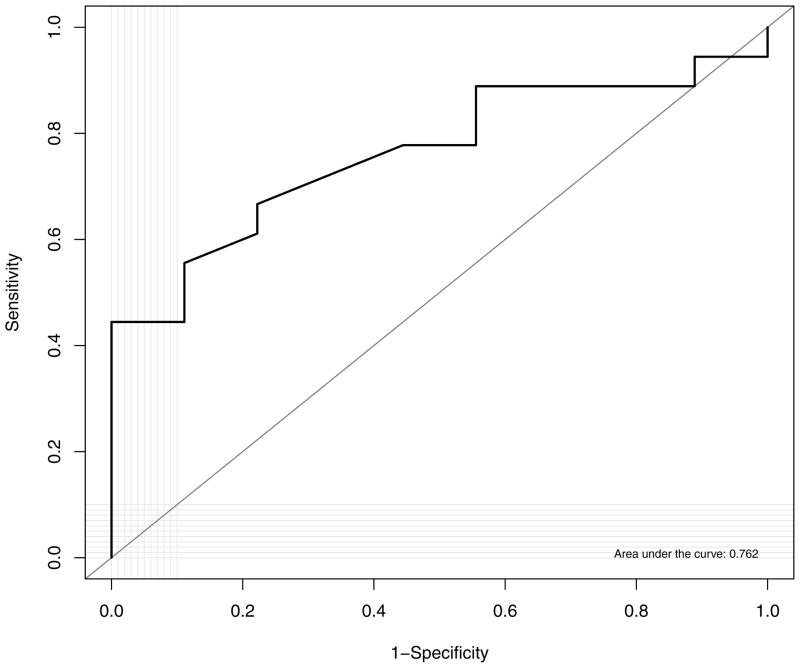
Comparisons of MRI measurements in subjects with and without OSA are shown in Table 2. Associations between MRI measurements and AHI are shown in Table 3. Median retropalatal distance was shorter in subjects with OSA (p=0.03). Similarly, shorter retropalatal distances were associated with higher AHIs (p=0.04). A scatter plot of retropalatal distance and OSA is shown in Figure 2. The area under the ROC curve for retropalatal distance as a predictor of presence or absence of sleep apnea was 0.76, which indicates moderate discrimination between groups (Figure 3).The other tested morphological characteristics did not differ by OSA status or show associations with AHI. Retropalatal distance was not associated with age (p=0.35), gender (p=0.81), or body mass index (p=0.72).
Table 2.
Comparison of MRI measures (median, in mm (interquartile range)) between ischemic stroke patients with and without sleep apnea.
| OSA | No OSA | p-value | |
|---|---|---|---|
| Retropalatal distance | 3 (2,5) | 6 (5,7) | 0.03 |
| Soft palate length | 39 (38,44) | 39 (39, 44) | 1.00 |
| Maximum palatal thickness | 10 (8, 13) | 10 (8, 11) | 0.84 |
| Retroglossal space | 12 (9,15) | 12 (8,19) | 0.70 |
| Tongue length* | 67 (60,73) | 61 (58,74) | 0.83 |
| Nasopharygeal airway (in cm2) | 4 (3, 4) | 4 (4,5) | 0.27 |
| Total lateral pharyngeal soft tissue thickness | 75 (73, 79) | 80 (79, 83) | 0.21 |
| High retro pharyngeal airway area§ | 2 (1,2) | 2 (1,2) | 0.78 |
available among n=26
available among n=24
OSA: Obstructive sleep apnea
Table 3.
Associations of MRI measures and the apnea/hypopnea index (AHI) in separatelinear regression models.
| Beta* | SE | p-value | |
|---|---|---|---|
| Retropalatal distance | −0.22 | 0.10 | 0.04 |
| Soft palate length | 0.01 | 0.05 | 0.92 |
| Maximum palatal thickness | 0.12 | 0.10 | 0.26 |
| Retroglossal space | −0.10 | 0.06 | 0.12 |
| Tongue length | 0.03 | 0.03 | 0.24 |
| Nasopharygeal airway | −0.42 | 0.25 | 0.10 |
| Total lateral pharyngeal soft tissue thickness | −0.06 | 0.04 | 0.13 |
| High retro pharyngeal airway area | −0.09 | 0.32 | 0.77 |
Note: beta is the change in AHI for a 1-log unit increase in the MRI measurement.
Figure 2.
Scatter plot of apnea-hypopnea index (Y-axis) and retropalatal distance (X-axis).
Figure 3.
Plot of receiver operating characteristic curve: discrimination of retropalatal distance for obstructive sleep apnea diagnosis.
DISCUSSION
This polysomnography and imaging study of 27 carefully characterized stroke patients with and without OSA identifies an anatomic difference—smaller retropalatal distance—likely to contribute to the sleep disorder that affects more than half of all patients after stroke. The fact that this observation could be made during wakefulness helps to establish for the first time that OSA in stroke patients is not likely to arise solely because of physiological changes in sleep cycles or stage predominance, time spent in supine sleep, or neuromuscular control of the upper airway during sleep. The similar frequency of dysphagia in our subjects with and without OSA further argues against abnormal neuromuscular control during wakefulness, as does the lack of association in other studies between OSA and either the location or severity of cerebral infarction.[13;14] The extreme amount of time spent in supine sleep post stroke, as we demonstrated in a similar cohort, could significantly contribute to OSA risk after stroke.[11] The imaging analyses performed in the current study, however, show that some anatomic contribution is also likely. Together, these data begin to explain the pathogenesis of OSA in patients with acute stroke, when damaged brain tissue may be particularly vulnerable and longer-term functional outcomes may be determined.
Although the finding of a smaller oropharynx in non-stroke patients with OSA has been identified before, [2;15–17] a smaller AP dimension has not been universally identified. Some investigators have found a smaller lateral diameter but not a smaller AP diameter on axial MRIs.[2;16] One group found a smaller AP diameter but not a smaller lateral diameter in the general OSA population using CT.[18] Others used axial MRI and found both smaller AP and lateral pharyngeal dimensions.[15] We were unable to assess the lateral airway dimension.
Unlike observations in non-stroke OSA patients, [2;15] the current results do not support thickening of the lateral pharyngeal walls in stroke patients with OSA. Thickening of the lateral walls has been postulated to explain the smaller oropharynx in OSA patients.[2;17] Studies have also identified longer vertical soft palatal dimensions in OSA patients, [2] while again the current study could not prove a difference. The palatal measurements in our patients are consistent with those found for normal subjects without OSA.[2] Several considerations could explain these disparities. Our confirmation of at least some anatomic difference between stroke patients with and without OSA does not rule out the possibility that functional contributions also increase risk for OSA even in the absence of additional anatomical compromise. Furthermore, underlying pathophysiological differences in OSA may exist between patients with and without stroke. The lack of associations between retropalatal distance and age, gender, or body mass index helps support this explanation indirectly. Alternatively, the differences may be due to the older age of our subjects compared with those of other studies. Previous morphological analyses of the oropharynx in OSA were performed with much younger patients, typically with mean ages in the 30s and 40s, [2;17] whereas our patients had a median age of 65. Older age has been associated with anatomical changes in the airway including increased fat pad thickness, longer airway length, and longer soft palate length.[19] Furthermore, technical differences in measurements could also explain the difference. We were unable to measure the lateral pharyngeal soft tissue thickness on the axial sections and therefore used the coronal images to assess this parameter. To make reliable measures in this plane, a higher level was selected compared with axial measurements and all soft tissue types were measured between the intercondylar notch and airway. The higher level, coronal plane of imaging, and inclusion of all soft tissue structures likely resulted in higher values found in the current study compared with axial measurements.
Polysomnography for acute stroke patients can be challenging and resource intensive. An alternative method to screen stroke patients for OSA would be welcome. As reported for non-stroke patients, [20] pharyngeal imaging cannot be used in acute stroke patients to identify in a definitive manner those who have OSA. Brain MRI scans, however, are commonly obtained in the evaluation of patients with stroke. Our data suggest that a simple measure of retropalatal distance, on these scans, could allow the radiologist to help predict which patients are most at risk for OSA and may deserve further assessment.
Some limitations to the current study deserve mention. The MRI studies of the brain were performed as part of routine clinical care rather than dedicated upper airway research. This precluded volumetric, dynamic, and airway shape measurements, [16;18] but also formed the basis of a strength for this study too in that the observations made are applicable to the many MRIs performed for neurological assessment already in clinical settings. The sleep status of our subjects during the MRI was not documented. Presumably, the subjects were awake during the acquisition of the T1 sagittal images, as these are the first images acquired, and would leave little time to fall asleep in the scanner. Because the sample was drawn from a parent clinical trial, there may be selection bias that influenced the demographics of subjects. Finally, the sample size for this study may not have been large enough to identify additional significant differences of smaller magnitude, between oropharyngeal anatomical features of patients with and without OSA. But despite challenges in studying acute stroke patients with polysomnography in addition to imaging, our study enrolled a sample size similar to those of previous OSA imaging studies in non-stroke patients.[16;18;21;22] Future larger studies should adjust for potential confounders such as BMI.
In short, this study identifies an anatomic difference in awake acute stroke patients that predicts OSA. We have also shown that routine brain MRIs already obtained for other clinical purposes may be helpful in identification of stroke patients at risk for OSA. We speculate that minor variations in these MRI protocols could further improve their diagnostic value with respect to OSA and perhaps shed additional light on mechanisms that make OSA so common among these vulnerable patients.
Acknowledgments
We are grateful to Suzanne M. Murphy, BBA for her technical assistance with the MRI figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Jordan AS, White DP, Fogel RB. Recent advances in understanding the pathogenesis of obstructive sleep apnea. Curr Opin Pulm Med. 2003;9:459–464. doi: 10.1097/00063198-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–1689. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 3.Fusco G, Macina F, Macarini L, Garribba AP, Ettorre GC. Magnetic resonance imaging in simple snoring and obstructive sleep apnea-hypopnea syndrome. Radiol Med. 2004;108:238–254. [PubMed] [Google Scholar]
- 4.Munoz R, Duran-Cantolla J, Martinez-Vila E, Gallego J, Rubio R, Aizpuru F, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–2321. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 6.Good D, Henkle J, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke. 1996;27:252–259. doi: 10.1161/01.str.27.2.252. [DOI] [PubMed] [Google Scholar]
- 7.Turkington PM, Allgar V, Bamford J, Wanklyn P, Elliott MW. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax. 2004;59:367–371. doi: 10.1136/thx.2003.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyken M, Somers V, Yamada T, Ren Z, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–407. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 9.Broadley SA, Jorgensen L, Cheek A, Salonikis S, Taylor J, Thompson PD, et al. Early investigation and treatment of obstructive sleep apnoea after acute stroke. J Clin Neurosci. 2007;14:328–333. doi: 10.1016/j.jocn.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Askenasy JJ, Goldhammer I. Sleep apnea as a feature of bulbar stroke. Stroke. 1988;19:637–639. doi: 10.1161/01.str.19.5.637. [DOI] [PubMed] [Google Scholar]
- 11.Brown DL, Lisabeth LD, Zupancic MJ, Concannon M, Martin C, Chervin RD. High Prevalence of Supine Sleep in Ischemic Stroke Patients. Stroke. 2008;39:2511–2514. doi: 10.1161/STROKEAHA.107.513572. (NIHMS104420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 13.Bassetti C, Aldrich M. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22:217–223. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 14.Parra O, Abroix A, Bechich S, García-Eroles L, Montserrat J, López J, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161:375–380. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 15.Ciscar MA, Juan G, Martinez V, Ramon M, Lloret T, Minguez J, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17:79–86. doi: 10.1183/09031936.01.17100790. [DOI] [PubMed] [Google Scholar]
- 16.Rodenstein DO, Dooms G, Thomas Y, Liistro G, Stanescu DC, Culee C, et al. Pharyngeal shape and dimensions in healthy subjects, snorers, and patients with obstructive sleep apnoea. Thorax. 1990;45:722–727. doi: 10.1136/thx.45.10.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa T, Enciso R, Shintaku WH, Clark GT. Evaluation of cross-section airway configuration of obstructive sleep apnea. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:102–108. doi: 10.1016/j.tripleo.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, et al. Aging Influences on Pharyngeal Anatomy and Physiology: The Predisposition to Pharyngeal Collapse. Am J Med. 2006;119:72. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayappa I, Rapoport DM. The upper airway in sleep: physiology of the pharynx. Sleep Med Rev. 2003;7:9–33. doi: 10.1053/smrv.2002.0238. [DOI] [PubMed] [Google Scholar]
- 21.Daniel MM, Lorenzi MC, da Costa LC, Lorenzi-Filho G. Pharyngeal dimensions in healthy men and women. Clinics. 2007;62:5–10. doi: 10.1590/s1807-59322007000100002. [DOI] [PubMed] [Google Scholar]
- 22.Schwab RJ, Pack AI, Gupta KB, Metzger LJ, Oh E, Getsy JE, et al. Upper airway and soft tissue structural changes induced by CPAP in normal subjects. Am J Respir Crit Care Med. 1996;154:1106–1116. doi: 10.1164/ajrccm.154.4.8887615. [DOI] [PubMed] [Google Scholar]