Abstract
Cilia are necessary for normal tissue development and homeostasis and are generally present during interphase, but not in mitosis. The precise mechanism of pre-mitotic ciliary loss has been controversial, with data supporting either sequential disassembly through the transition zone or, alternatively, a severing event at the base of the cilia. Here we show by live cell imaging and immunofluoresence microscopy that resorbing flagella of Chlamydomonas leave remnants associated with the mother cell wall. We postulated that the remnants are the product of severing of doublet microtubules between the basal bodies and the flagellar transition zone, thereby freeing the centrioles to participate in spindle organization. We show via TEM that flagellar remnants are indeed flagellar transition zones encased in vesicles derived from the flagellar membrane. This transition zone vesicle can be lodged within the cell wall or it can be expelled into the environment. This process is observable in Chlamydomonas, first because the released flagellar remnants can remain associated with the cell by virtue of attachments to the cell wall, and second because the Chlamydomonas transition zone is particularly rich with electron-dense structure. However, release of basal bodies for spindle-associated function is likely to be conserved among the eukaryotes.
Keywords: flagellar transition zone, live cell imaging, electron microscopy, katanin, basal bodies
Introduction
Eukaryotic cilia/flagella (the terms are interchangeable) are composed of four distinct structural regions. Within the cytoplasm of the cell is the basal body, which is anchored to the plasma membrane via transitional fibers (O’Toole et al. 2003). Microtubules of the basal body are continuous with the transition zone (TZ) and ciliary microtubules, which extend typically 5-15 μm out from the cell, and along with the ciliary membrane, form the cilium proper. At the distal end of the cilium is a cap structure that bears attachments to the ciliary membrane and is the major site of ciliary subunit addition and subtraction (Dentler and Rosenbaum 1977; Johnson and Rosenbaum 1992). The TZ region is the site of several important activities (reviewed in Pazour and Bloodgood 2008): in addition to anchoring the cilium at the surface of the cell through Y fibers that connect with the base of the ciliary membrane, it is the region where the triplet microtubules of the basal body become doublet microtubules of the cilium proper; it forms a putative flagellar pore complex that regulates the traffic of proteins into and out of the cilium; and it forms a diffusion barrier that prevents mixing of flagellar and cell body membrane components. In addition, the distal end of the TZ is where the central pair microtubules of motile cilia are nucleated. This distal region of the TZ is also the site of severing during deflagellation, the stress-induced event that is the quickest mode of ciliary loss (Quarmby, 2008).
We have previously proposed that deflagellation is mediated by the microtubule-severing ATPase, katanin p60, but genetic evidence has been lacking (Lohret et al. 1998; Lohret et al. 1999). Using an RNAi knock-down approach, we recently showed that in wild-type (flagellated) Chlamydomonas cells, reduction of the levels of katanin is lethal (Rasi et al. 2009). In contrast, we observed that katanin levels can be efficiently reduced in mutant backgrounds that lack flagella, implying that katanin plays an essential function that is abrogated in cells lacking cilia (Rasi et al. 2009). One possible explanation is that katanin serves to release basal bodies from their transition zones, thus freeing them to migrate and facilitate proper placement of the spindle poles.
In this report, TEM images reveal structures encased in vesicles and embedded in post-mitotic mother cell walls. These images are consistent with the predicted TZs encased in membrane derived from the flagella. This transition zone vesicle often remains lodged within the cell wall, but occasionally it may be expelled into the environment, as we show by live cell imaging.
Materials and Methods
Chlamydomonas strains were obtained from the Chlamydomonas Genetics Center. Wild-type Chlamydomonas 137c mt(−) (CC-124) was used for all EM experiments, pf6 mutant cells (CC-1029) were used for live cell imaging experiments. Cells were maintained on TAP medium with 1.5% agar (Harris 1989).
For IFM experiments, cultures enriched for dividing cells were obtained by transferring cells growing asynchronously in TAP liquid cultures to liquid minimal medium (MI) (Harris 1989) for 24 hours in the dark. Cells were then resuspended in fresh TAP in bright light for ~12 hours, then returned to MI in the dark to induce division and assayed by phase-contrast microscopy for the presence of an abundance of large cells suggestive of imminent division.
IFM was performed as described previously (Rasi et al. 2009). For EM, synchronous cultures were fixed by adding an equal volume of 5% glutaraldehyde in MI medium. The cells were shipped overnight (from Burnaby to New Haven) and processed the following day by fixing 30 minutes at RT in 2.5% glutaraldehyde in MI with 0.1% tannic acid, postfixed in 1% osmium tetroxide in 100 mM cacadylate buffer pH 7.2 for 1 hour at 4°C, and stained en bloc with 1% uranyl acetate. The fixed cells were sedimented and embedded in 1% agarose, dehydrated, and embedded in Epon resin. Sections were viewed with a JEOL 1230 electron microscope and images were collected with an Orca HR digital camera (Hamamatsu). Micrographs were rotated, cropped, and their brightness and contrast were adjusted in Photoshop (Adobe).
For live cell imaging, cultures enriched for dividing cells were obtained by transferring cells growing asynchronously in TAP liquid cultures to liquid minimal medium (MI) (Harris 1989) for 24 hours in the dark. Cells were then resuspended in fresh TAP in bright light for 6 hours, then embedded in 0.5% low melting point agarose in MI and imaged in the dark on a Delta Vision system (Applied Precision, Issaquah WA). Images were captured once every minute for 6 hours. Flagellar length was measured in five-minute intervals using SoftWorx software (Applied Precision). Only cells with flagella longer than 4 μm at the start of imaging were included in our analyses.
Results
In the Chlamydomonas cell cycle, a haploid vegetative cell grows until it passes a commitment point, then some time later divides N times to produce 2N daughter cells (N is typically 1-4 (Umen and Goodenough 2001)). The parental cell is surrounded by a cell wall with two tunnels at the anterior end through which the flagella pass into the surrounding medium. Prior to the first division the flagella resorb, though details of how this occurs are sketchy, and the cell rotates within the cell wall. The progeny cells remain encased in the cell wall of the parental cell (the “mother cell wall”) until they assemble flagella of their own, become motile and “hatch”.
To more carefully examine the resorption of flagella prior to division, live cells were imaged with time-lapsed photomicroscopy as they approached and completed mitosis. Imaging was facilitated by using a mutant with immotile flagella (pf6). To enrich for cells entering cell division, cells were held in the dark in minimal medium, then released to light in rich medium. Cells were observed over the next 6 hours and when a substantial fraction of the population of cells were greater than ~9 μm in diameter, live cells were imaged as described in materials and methods.
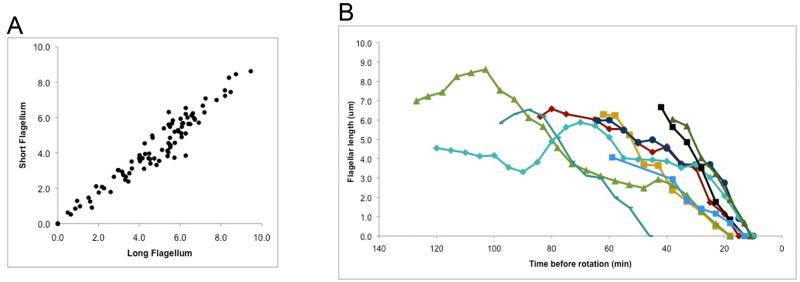
Using this technique, we captured video sequences of twenty-one cells that remained in focus through flagellar resorption and cell division (see Supplemental Movie for an example) allowing us to describe this process quantitatively. Comparing the lengths of the two flagella on a given cell revealed that the initiation and rate of resorption of the pair was tightly coupled (Fig. 1A). As is commonly observed for paralyzed mutants, the pf6 flagella are generally shorter than those of wild-type, and we did not observe any flagella greater than 10 μm in length. It is important to note that most of the cells in these samples were entering the division cycle and we cannot know a priori which ones had already begun to resorb their flagella. For this reason, we used the onset of cytoplasmic rotation as our time anchor (Fig. 1B). This period was generally 10-20 min in the cells captured on video, though one was much longer (45 min) and two were not seen to rotate. We observed an average rate of resorption of 0.17 μm/min in the final 20 minutes of resorption where the flagellar length decreased in a linear fashion (N= 9 cells from 3 independent experiments, using only one flagellum per cell; standard deviation of 0.05; range 0.09 to 0.26). We note that although there is variability in previously reported resorption rates, our data supports the conclusion that pre-mitotic flagellar resorption is substantially faster than flagellar resorption induced by NaPPi or by a shift to the restrictive temperature of the fla10-1 anterograde IFT mutant (see for example: Pan and Snell, 2005; Marshall et al., 2005).
Figure 1. Flagellar length measurements during resorption.
A. The length of the longer flagellum is plotted against the length of the shorter flagellum during resorption for each of six mitotic cells from two separate experiments.
B. The length of a single flagellum from each of 9 mitotic cells from 3 separate experiments was measured once every five minutes during resorption. The beginning of cortical rotation is set as time 0.
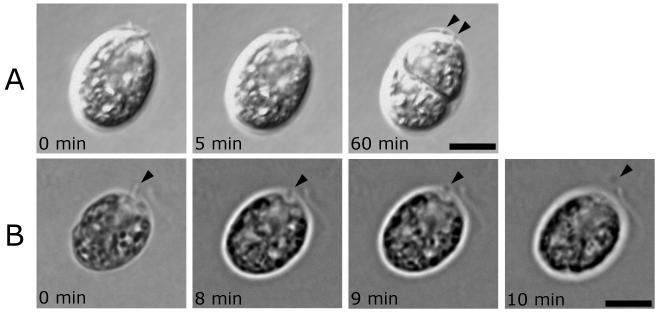
Although we and others have documented flagella of various lengths (presumably partially resorbed) detached from the basal bodies yet still adhering to cells during division, in all twenty-one cells observed here, the flagella resorbed completely, leaving only a small round spot visible, typically associated with the mother cell wall in a position consistent with the flagellar tunnels (Fig. 2A). In two of the twenty-one cells recorded by live cell imaging, the cell appeared to eject a small particle from cell wall tunnel and these particles were lost to the surrounding media (e.g. Figure 2B). We speculate that these spots were remnants of the resorbed flagella, which were lodged in the flagellar tunnel in the cell wall, and were dislodged by the rotation of the protoplast. If these, and the small spots seen associated with the mother cell wall of most cells entering mitosis, are in fact small vesicular remnants of the resorbed flagella, they may be generated by severing from the basal body and may contain residual microtubules or tubulin. Consistent with this hypothesis, we previously reported pairs of “dots” observed by anti-acetylated tubulin IFM in locations consistent with the flagellar tunnels of the mother cell wall (Rasi et al. 2009) and proposed that these were isolated TZs.
Figure 2. Imaging of dividing Chlamydomonas cells reveals formation of remnant vesicles in living cells.
Panels are time-lapse DIC (A) and phase contrast (B) images of flagellar resorption of single cells (see supplemental data for video of cell shown in A). In all cells examined thus far, remnants are visible upon completion of resorption. Arrowheads indicate remnants and times indicate minutes after the first image shown. Scale bars, 5 μm.
A. Resorption is gradual until the entire axoneme is gone except for two remnants left in association with the cell wall. The remnants (indicated by arrowheads) remain in this position even after cytoplasmic rotation and cytokinesis, indicating that they are associated with the mother wall and not with a daughter cell.
B. The left flagellum shortens gradually until it is not visible, then a remnant is ejected through the flagellar tunnel in the cell wall and comes to rest near the cell.
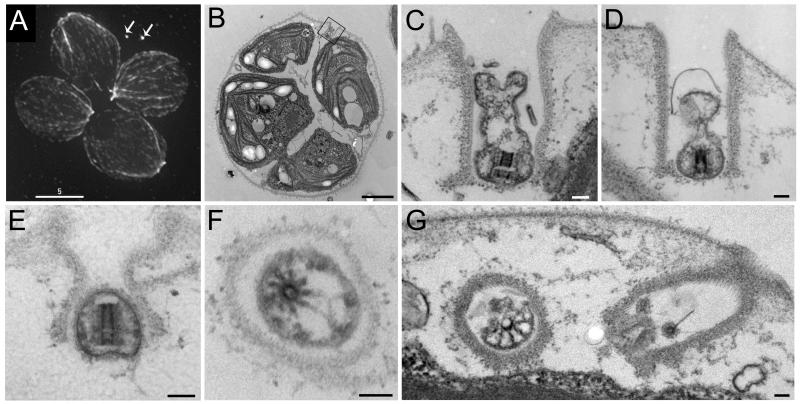
To determine whether these dots were indeed shed TZs, we grew cultures enriched for dividing cells and processed duplicate aliquots for immunofluorescence microscopy (IFM) and TEM. Taken together with our earlier published observations (Rasi et al., 2009) we have observed greater than 300 unhatched daughter clusters by IMF. In 80% of these, the anti-acetylated tubulin antibody recognized two small dots near unhatched daughter cells, in a position consistent with the flagellar tunnels of the mother cell wall (for example see Fig. 3A, arrows). The remaining 20% of cells scored were clearly in mitosis as determined by phosphohistone H3 and/or spindle staining, but didn’t have paired remnant spots (single spots were not scored because in the absence of a pair the identity of a single spot becomes ambiguous). We do not know why 20% of the cells lacked visible pairs of spots; it is likely that in some if not all cases, one or both of the remnants were expelled from the wall, as observed in some of our videos (data not shown). Though the tunnels in the cell wall were only occasionally seen in TEM section, these often contained small vesicles that enveloped electron dense material with characteristics of a TZ (Fig. 3B-G). In none of these cases were microtubules still evident, but in longitudinal section the central part of the “H” of the TZ was clearly visible (Fig. 3C-E) and in some cases (e.g. Fig. 3E) the vesicle appeared to include little more than the central hub of the TZ. In cross section the central circle of the TZ could be seen (Fig. 3F and G) and even though the microtubule doublets were no longer present the 9 fold symmetry sometimes was detectable (Fig. 3F) as were links between the central hub and the surrounding membrane (Fig. 3G).
Figure 3. Flagellar remnants visualized by immunofluorescence microscopy appear to be flagellar transition zones (TZs) when visualized by electron microscopy (EM).
A. Unhatched Chlamydomonas daughter cells stained for anti-acetylated tubulin and anti-α-tubulin. We typically see two flagellar remnants in a position consistent with the anterior flagellar tunnels of the mother cell wall (arrows). A pair of spots such as shown here are clearly visible in 80% of unhatched cells in our preparations (N > 300). Scale bar, 5μm.
B-G. Cells from the same (E and F) culture as A, or a similar culture (B-D and G) visualized by thin-section EM.
B. These cells are completing their second round of division and remain encased in their mother cell wall. The box surrounds a flagellar tunnel in the mother cell wall. Scale bar, 2 μm.
C. This magnified view of the boxed area from B shows a vesicle that contains a flagellar transition zone seen in longitudinal section. This remnant vesicle clearly sits within the flagellar tunnel of the cell wall. The TZ appears to be split in two along the cross-piece of the canonical electron-dense H. Scale bar, 100 nm.
D. A remnant vesicle lies within its mother cell wall containing the core of a transition zone. Scale bar, 100 nm.
E. In this case the remnant vesicle closely encases the remnant transition zone. Scale bar, 100 nm.
F. A cross-section through a remnant vesicle reveals a disintegrating transition zone. No microtubule doublets are present, but nine projections remain attached to the central core of the TZ. The hub has an eccentric location in the vesicle. Electron dense masses on the inside of the membrane are probably the remains of the Y links that once connected the microtubules to the flagellar membrane. Scale bar, 100 nm.
G. The two flagellar tunnels of one cell are seen, one containing a cross section of a partial transition zone, the other, with no clearly identifiable flagellar remnant. Scale bar, 100 nm.
Discussion
Due to the small size of the basal body and attached transition zone, and the transient nature of flagellar resorption, the exact sequence of events late in flagellar resorption in Chlamydomonas has been controversial. Johnson and Porter (1968) observed by TEM, flagella attached to transition zones, but severed from basal bodies, in agreement with our previous IMF observations and with the TEM images presented here. Cavalier-Smith (1974) and Gaffal (1988), however, made observations that led to the widely-held interpretation that flagella are sequentially disassembled via resorption, starting at the distal end and proceeding proximally until the entire flagellum and transition zone are gone, and the basal bodies are free of flagellar microtubules. Subtle differences in strains, culture conditions, or fixation may be contributing to these conflicting observations, but the greatest limitation of all three studies with respect to this issue is that very few dividing cells were observed. To resolve this question, we enriched for dividing cells, which enabled us to observe hundreds of cells by immunofluorescent microscopy in addition to numerous by TEM and twenty-one by live cell video microscopy. Consequently, our data is less sensitive to rare events.
We and others have documented flagellar remnants that were microns long still attached to dividing Chlamydomonas though, as we now appreciate, they were no longer attached to the basal bodies. This observation demonstrated that severing the flagella can occur before resorption is complete. Rare TEM images also supported this idea and suggested that pre-mitotic severing occurred between the basal body and the TZ (Johnson and Porter, 1968). For this to be true there must be two sites where the axoneme can be severed: just distal to the TZ, as occurs during pH-induced autotomy (Quarmby, 2008); and, between the basal body and the TZ (Rasi et al., 2009). The appearance of remnants in dividing cells of the fa2 flagellar autotomy mutant indicate that two different molecular machineries are involved the two different sites of severing, but it is likely that they share components (Parker and Quarmby, 2003). A requirement for the microtubule-severing ATPase, katanin in severing proximal to the TZ may be the reason a knockdown of the 60 kD subunit of katanin is lethal in flagellated cells (Rasi et al., 2009).
Even though severing of partially resorbed flagella from the basal bodies before division has been documented (Piasecki et al., 2008; Rasi et al., 2009), this relatively rare event was not observed in the experiments reported here. All of the cells captured on video fully resorbed their flagella before division. The cell must send two different signals to its flagella prior to cell division; one to resorb, possibly so that the axonemal tubulin can be reutilized during mitosis; and a second to abscise, perhaps to free the basal body to perform other roles in mitosis and cell division. We hypothesize that the signal to resorb is given long enough before abscission that normally the flagella resorb fully before severing of the TZ. Occasionally, resorption may not begin early enough and severing occurs before resorption is complete to rid the cell of its flagella before division starts. This may explain the perplexing images presented in earlier works (Piasecki et al. 2008; Rasi et al. 2009).
The most generally cited speculation for why flagella are lost before mitosis is to free the basal bodies to participate as centrioles in organizing the spindle. Ciliary loss is common and is consistently observed when the presence of cilia would otherwise restrict the plane of division, for example, in highly organized tissues. It should be emphasized that we do not fully understand the roles of the Chlamydomonas basal bodies during mitosis and cytokinesis. However, it is likely that migration and positioning of the basal bodies is critical for one or both of these processes and thus, in this cell, it is likely that the cell wall necessitates flagellar loss and release of the basal body. Because the flagella exit the cell through two tunnels in the cell wall, the basal bodies cannot separate and the cell can not rotate while the flagella are in place.
We propose that the TZ vesicle is abandoned by the cell because the transition zone and its associated fibers and plasma membrane and cell wall connections are not easily disassembled for the recycling of component molecules, as happens with components of the flagellum proper (Lefebvre et al. 1978). Consistent with this idea, we observed several TZ vesicles which retained visible connections with the surrounding membrane. This model is conceptually similar to how the microtubules of cytokinetic midbodies are not recycled, but rather (topologically) cast outside the cytoplasm (Mullins and McIntosh 1982). Intriguingly, Rab11 is implicated in both formation of both midbodies (Wilson et al. 2005) and cilia (Mazelova et al. 2009), leading us to propose that the abscission events of premitotic cilia loss and cytokinesis may have common components.
Understanding the mechanism of pre-mitotic flagellar resorption gives some insight into flagellar regrowth under different circumstances. Post-deflagellation, the transition zone remains in the cell and the basal bodies remain docked to the membrane; thus the cell can quickly regrow new flagella (Rosenbaum and Child 1967). During experimentally induced resorption, for example using a medium low in calcium and high in monovalent cations, flagella shorten via an unknown mechanism, but do not fully disappear (data not shown). Thus, the basal bodies and transition zones are not likely to be extensively modified by the induction of resorption, explaining the rapid regrowth of flagella when these cells are returned to normal medium (data not shown). In contrast to regrowth after deflagellation or induced resorption, assembly of a new flagellum post-mitosis requires apical positioning of the basal bodies as well as assembly of a TZ.
Some Chlamydomonas mutants with defects in flagellar assembly (the fla mutants) deflagellate precociously: they tend to deflagellate in the absence of the stress stimuli that are normally used to induce deflagellation (Parker and Quarmby 2003). At the time, these data suggested to us that there was a relationship between flagellar resorption and the severing of axonemal microtubules. The new data presented here provides direct evidence that in Chlamydomonas, normal pre-mitotic flagellar disassembly involves a microtubule-severing event between a basal body and its flagellar transition zone. However, the relationship between this event and deflagellation remains enigmatic. Release of basal bodies by severing from TZs has been observed in another green alga, Chlorogonium (Hoops and Witman 1985). In the case of Chlorogonium, the TZ and flagella remain attached to the cell and functional while the basal bodies migrate and serve as spindle pole foci. Although the retention of functional flagella makes the Chlorogonium situation unusual, it does reflect the common theme of pre-mitotic release of basal bodies from the TZ. We expect that a similar severing event occurs in all cells that bear complex TZs, and may occur in others as well.
Conclusions
Here we provide evidence that pre-mitotic flagellar resorption in Chlamydomonas involves microtubule severing between the flagellar TZ and the basal bodies, and that the TZ is not itself resorbed or otherwise recycled, as is the majority of the axoneme. It is not yet known whether a similar severing event is required to release the basal bodies of other ciliated cells that divide, such as mammalian cells bearing primary cilia. Although all cilia have a TZ that mediates attachment to the ciliary membrane, not all contain TZs that are as structurally elaborate as those in Chlamydomonas (O’Toole et al. 2003; Sanders and Salisbury 1989). Thus, it is not clear whether all will require a severing event to release the basal bodies prior to mitosis or whether some of the simpler transition zones might be amenable to disassembly akin the resorption of the cilium proper.
Supplementary Material
Acknowledgements
This work was funded by a Natural Sciences and Engineering Research Council Discovery Grant to L.M.Q. and by National Institutes of Health grant GM-14642 to J.L.R.
References
- Cavalier-Smith T. Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii. J Cell Sci. 1974;16(3):529–56. doi: 10.1242/jcs.16.3.529. [DOI] [PubMed] [Google Scholar]
- Dentler WL, Rosenbaum JL. Flagellar elongation and shortening in Chlamydomonas. III. structures attached to the tips of flagellar microtubules and their relationship to the directionality of flagellar microtubule assembly. J Cell Biol. 1977;74(3):747–59. doi: 10.1083/jcb.74.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffal KP. The Basal Body-Root Complex of Chlamydomonas-Reinhardtii during Mitosis. Protoplasma. 1988;143(2-3):118–129. [Google Scholar]
- Harris E. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press; 1989. [DOI] [PubMed] [Google Scholar]
- Hoops HJ, Witman GB. Basal bodies and associated structures are not required for normal flagellar motion or phototaxis in the green alga Chlorogonium elongatum. J Cell Biol. 1985;100(1):297–309. doi: 10.1083/jcb.100.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Rosenbaum JL. Polarity of flagellar assembly in Chlamydomonas. J Cell Biol. 1992;119(6):1605–11. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson UG, Porter KR. Fine structure of cell division in Chlamydomonas reinhardi. Basal bodies and microtubules. J Cell Biol. 1968;38(2):403–25. doi: 10.1083/jcb.38.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle KL, Schnell RA, Fernandez E, Lefebvre PA. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J Cell Biol. 1989;109(6 Pt 1):2589–601. doi: 10.1083/jcb.109.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre PA, Nordstrom SA, Moulder JE, Rosenbaum JL. Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis. J Cell Biol. 1978;78(1):8–27. doi: 10.1083/jcb.78.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohret TA, McNally FJ, Quarmby LM. A role for katanin-mediated axonemal severing during Chlamydomonas deflagellation. Mol Biol Cell. 1998;9(5):1195–207. doi: 10.1091/mbc.9.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohret TA, Zhao L, Quarmby LM. Cloning of Chlamydomonas p60 katanin and localization to the site of outer doublet severing during deflagellation. Cell Motil Cytoskeleton. 1999;43(3):221–31. doi: 10.1002/(SICI)1097-0169(1999)43:3<221::AID-CM5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL. Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell. 2005;16:270–8. doi: 10.1091/mbc.E04-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. Embo J. 2009;28(3):183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J, McIntosh J. Isolation and initial characterization of the mammalian midbody. J. Cell Biol. 1982;94(3):654–661. doi: 10.1083/jcb.94.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole ET, Giddings TH, McIntosh JR, Dutcher SK. Three-dimensional organization of basal bodies from wild-type and delta-tubulin deletion strains of Chlamydomonas reinhardtii. Mol Biol Cell. 2003;14(7):2999–3012. doi: 10.1091/mbc.E02-11-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking and blocking anterograde cargo loading. Dev. Cell. 2005;9:431–8. doi: 10.1016/j.devcel.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Parker JD, Quarmby LM. Chlamydomonas fla mutants reveal a link between deflagellation and intraflagellar transport. BMC Cell Biol. 2003;4(1):11. doi: 10.1186/1471-2121-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:115–49. doi: 10.1016/S0070-2153(08)00805-3. [DOI] [PubMed] [Google Scholar]
- Piasecki BP, LaVoie M, Tam LW, Lefebvre PA, Silflow CD. The Uni2 phosphoprotein is a cell cycle regulated component of the basal body maturation pathway in Chlamydomonas reinhardtii. Mol Biol Cell. 2008;19(1):262–73. doi: 10.1091/mbc.E07-08-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby LM. Deflagellation. In: Witman G, editor. The Chlamydomonas Sourcebook III. Academic Press, Inc.; 2008. pp. 43–69. [Google Scholar]
- Rasi MQ, Parker JD, Feldman JL, Marshall WF, Quarmby LM. Katanin knockdown supports a role for microtubule severing in release of basal bodies before mitosis in Chlamydomonas. Mol Biol Cell. 2009;20(1):379–88. doi: 10.1091/mbc.E07-10-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Child FM. Flagellar regeneration in protozoan flagellates. J Cell Biol. 1967;34(1):345–64. doi: 10.1083/jcb.34.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MA, Salisbury JL. Centrin-mediated microtubule severing during flagellar excision in Chlamydomonas reinhardtii. J Cell Biol. 1989;108(5):1751–60. doi: 10.1083/jcb.108.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umen JG, Goodenough UW. Control of cell division by a retinoblastoma protein homolog in Chlamydomonas. Genes Dev. 2001;15(13):1652–61. doi: 10.1101/gad.892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 Protein Complex Regulates Recycling Endosome Targeting to the Cleavage Furrow during Late Cytokinesis. Mol. Biol. Cell. 2005;16(2):849–860. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.