Abstract
Drug motivation models postulate that attention biasing toward smoking related cues is a cognitive mechanism supporting continued or renewed drug use and predict that drug use history, deprivation, and distress should modulate the extent of this bias. The present study used the modified Stroop paradigm to extend past research regarding attention biasing toward smoking, unpleasant, pleasant, and neutral words among adult nonsmokers and daily smokers. Both nonsmokers and smokers showed differential attention toward unpleasant and pleasant cues, particularly pleasant cues, but did not show a unique bias toward smoking-related stimuli. Results suggested that, among smokers, nicotine deprivation and exogenous stress (threat of electric shock) have a non-additive effect on attention toward pleasant cues, but no effect on attention to smoking cues specifically. Similarly, instructing smokers that they would have an opportunity to smoke did not significantly increase the bias of nicotine-deprived smokers’ attention toward smoking-related cues, relative to arousing unpleasant and pleasant cues. Overall, results suggest that smokers’ attention may be biased toward both smoking-related and other salient cues when deprived of nicotine and anticipating an opportunity to smoke.
Keywords: Tobacco, modified Stroop, nicotine withdrawal, attention
Cessation failure and relapse remain the norm in tobacco cessation attempts, with fewer than 35% of people typically achieving sustained abstinence even with the aid of the most efficacious treatments (Fiore et al., 2008; Hughes, Stead, & Lancaster, 2004; Silagy, Lancaster, Stead, Mant, & Fowler, 2004). The reformulated negative reinforcement model of drug motivation posits that escape from negative affect is the dominant (although not exclusive) motive for such cessation failures and relapse (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). Negative affect (e.g., anxiety, irritability, sadness) is the primary constituent of the nicotine withdrawal syndrome (Welsch et al., 1999) and emerges within the first three hours of abstinence from smoking (Hendricks, Ditre, Drobes, & Brandon, 2006). According to the reformulated negative reinforcement model, one mechanism by which negative affect predisposes addicted individuals to smoke is by biasing information processing. Specifically, the model postulates that symptoms of withdrawal bias addicted smokers’ attention toward smoking-related cues, which may predispose them to relapse (Niaura et al., 1988; Waters & Sayette, 2006). Such biasing of attention is not a sufficient cause for smoking in most situations, however, as individuals can detect and resolve conflict between incompatible responses (i.e., smoking vs. maintaining abstinence) using inhibitory self-control mechanisms even when moderately distressed (Curtin, McCarthy, Piper, & Baker, 2006).
Research using the modified Stroop task (1935) among smokers is generally consistent with this account of the attention-modulating effects of drug motivation. In the smoking Stroop task, subjects are asked to name the color of the letters in smoking-related and neutral words. The degree to which subjects are slower to name the color of smoking-related words versus neutral words is thought to indicate interference due to attention to the meaning of the smoking-related words. Nicotine deprivation, which induces a withdrawal syndrome characterized primarily by negative affect (Welsh et al., 1999), has been shown to increase attention toward smoking-related versus neutral words in research using this task (Gross, Jarvik, & Rosenblatt, 1993; Waters & Feyerabend, 2000) and the degree of interference is positively associated with the duration of deprivation (Cox, Fadardi, & Pothos, 2006) and predictive of relapse (Waters et al., 2003). Although results have not been consistent across all studies (Cox et al., 2006; Waters & Sayette, 2006), the failures to replicate deprivation-induced increases in attention toward smoking cues are not fatal to the negative reinforcement model of drug motivation, as negative findings occurred in studies using samples low in nicotine dependence (e.g., college students) or weak manipulations of nicotine deprivation (Mogg & Bradley, 2002; Munafò, Mogg, Roberts, Bradley, & Murphy, 2003; Rusted, Caulfield, King, & Goode, 2000).
Additional research supports the notion that such attention bias is subject to top-down control, consistent with the negative reinforcement model of addiction. In the area of smoking, Wertz and Sayette (2001) demonstrated that attention is more biased toward smoking versus neutral cues during nicotine deprivation when an opportunity to smoke is anticipated. When smokers received a brief instruction that they might, versus definitely would not, have an opportunity to smoke during the experimental session, they did not show interference to smoking stimuli. When told that they would have an opportunity to smoke, a significant interference effect was observed. This moderating effect suggests that the effect of withdrawal on attention is influenced by context rather than an inevitable consequence of abstaining from drug use.
The study of smoking opportunity effects on affect and behavior is still in its infancy. Early research in this area showed that instruction that smoking opportunities would be available within 20 minutes increased urges to smoke and enhanced the urge-inducing effects of smoking cues (Juliano & Brandon, 1998). In the same study, reaction times on a simple tone detection task were longer when smokers were told they would not be able to smoke for at least three hours and were exposed to smoking-related cues (Juliano & Brandon, 1998). Manipulations of shorter time frames have suggested that the proximity of the smoking opportunity importantly influences smokers’ responses to availability manipulations. Opportunities to smoke a lit cigarette within 15 seconds elicited positive affect (as evinced by facial expression), whereas longer (e.g., 60 seconds) or ambiguous (e.g., “soon”) delays elicited negative affect, relative to a no smoking opportunity condition in laboratory studies (Sayette et al., 2003). Other research suggests that cravings, positive mood, and skin conductance increase as the probability of being able to take a puff from a lit cigarette within the next 20–30 seconds increases from 0% to 50% and 100%, while negative affect and response latencies decrease and the probability of cigarette access increases (Carter & Tiffany, 2001). Taken together, these data suggest that smokers’ attention, craving, affect, and behavior are influenced by instructions about availability in the very short term, and that longer-term availability also affects urges to smoke and reaction times. Thus, these results suggest that availability context can influence smokers in important ways. Future treatments may be able to help smokers take advantage of such contextual effects to maximize the likelihood of abstinence.
Another testable prediction derived from the reformulated negative reinforcement model of drug motivation is that repeated cycles of smoking followed by withdrawal relief should result in a narrowing of attention toward smoking-relevant stimuli in withdrawal, as smoking is the most efficient and rapid reliever of withdrawal distress. If this prediction is correct, then smokers should show an attention bias for smoking-related stimuli when deprived of nicotine, but no bias toward other pleasant stimuli that have not been associated with withdrawal relief in the past. To test this prediction, however, one needs to examine response times to color-naming to other arousing stimuli that are not related to smoking. Only one prior study has assessed nicotine deprivation effects on attention bias toward non-smoking threat and reward cues (Powell, Tait, & Lessiter, 2002). In this study, nicotine deprivation moderated attention bias for positively and negatively valenced cues among smokers such that continuing smokers showed interference to both reward- and threat-related words, relative to neutral words, whereas abstinent smokers did not. These results are consistent with the hypothesis that deprivation decreases attention to cues unrelated to smoking, even salient, motivationally relevant cues. However, the Powell et al. (2002) study cannot speak to the attention narrowing hypothesis derived from the negative reinforcement model because smoking-related stimuli were not presented.
Finally, the reformulated negative reinforcement model asserts that negative affective states that resemble withdrawal at an interoceptive level promote drug seeking (i.e., smoking) in a manner similar to that of withdrawal. That is, addicted smokers learn that smoking is negatively reinforced through the alleviation of withdrawal-induced negative affect; this learning is then generalized to similar negative affect states that arise from causes other than withdrawal. The chief symptoms of withdrawal (anxiety, anger, irritability, and sadness; Welsch et al., 1999) are influenced by myriad factors and may not be easily distinguishable from similar states elicited by different causes. For example, an individual who learns to smoke to reduce irritability due to withdrawal may experience a similar increase in smoking motivation when irritability is due to interpersonal conflict, presumably because the interoceptive affective stimuli are similar regardless of their origin. If this hypothesis is true, we should see similar increases in motivation to smoke, and associated attention bias toward smoking-related cues, following nicotine deprivation and presentation of an exogenous stressor. Stress is a potent precipitant of relapse (Kassel, Stroud, & Paronis, 2003; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). Increases in drug motivation and associated attention biasing may partially explain this relation, along with occupation of limited mental work space, according to the negative reinforcement model of addiction. Although the extent to which stress induced by exogenous stressors (e.g., interpersonal conflict, physical pain) resembles that induced by tobacco deprivation on an interoceptive level is not fully known, there is evidence that suggests that similar regions of the anterior cingulate and insula cortex are activated by both physical and emotional pain (Critchley et al., 2004; Eisenberger, Lieberman, & Williams, 2003; Seminowicz & Davis, 2007; Sewards & Sewards, 2002) and in craving for drugs of abuse, including tobacco (Bonson et al., 2002; Brody et al., 2002; Naqvi, Rudrauf, Damasio, & Bechara, 2007).
The current study compared nonsmokers’ and smokers’ attention to neutral, unpleasant, pleasant, and smoking-related words using a modified Stroop task and examined the effects of manipulations of nicotine deprivation and smoking availability (among smokers), and an exogenous stressor on attention bias. Unpredictable, uncontrollable electric shock was selected as the exogenous stressor because past research suggests that this effectively induces self-reported anxiety and increases in psychophysiological indices of negative affect, such as potentiated startle responses (Grillon, Baas, Cornwell, & Johnson, 2006; Hogle & Curtin, 2006), and can influence responding on modified Stroop tasks (e.g., Miller & Patrick, 2000). Inclusion of these manipulations allowed us to test the following hypotheses derived from the reformulated negative reinforcement model of drug motivation: 1) Smokers would show greater attention toward smoking-related versus other arousing pleasant stimuli than would nonsmokers, presumably due to the motivational relevance (and incentive salience) of smoking-relevant cues conferred by negative reinforcement learning. 2) Twenty-four hours of nicotine deprivation would result in greater interference to smoking-related vs. other salient and pleasant cues, but only when a smoking opportunity was anticipated (i.e., the effect would be eliminated by instruction that one definitely could not smoke). Although one might expect being told that smoking is not permitted might increase frustration, and therefore interference due to distress, previous research suggests that deprived smokers respond to such instructions with lower rather than greater interference (Wertz & Sayette, 2001). We sought to replicate this finding. We also examined whether deprived smokers would show the insensitivity to cues of reward (i.e., pleasant words) and threat (i.e., unpleasant words) reported by Powell and colleagues (Powell et al., 2002). 3) An exogenous stress manipulation (threat of shock) would prompt both deprived and non-deprived smokers, but not nonsmokers, to bias attention toward smoking cues, but not other arousing unpleasant or pleasant cues, presumably due to learning that smoking alleviates stress effects resembling nicotine withdrawal. In other words, we expected the exogenous threat of shock to bias smokers’ attention toward smoking-related cues in a manner similar to nicotine deprivation, although we did not assess the putative mechanism of this similarity (i.e., learned generalization across similar interoceptive and mood states induced by deprivation and stressors).
It is important to note that these predictions are not unique to the negative reinforcement model of addiction. Indeed, other models, such as Robinson and Berridge’s incentive sensitization model (1993) also predict that repeated drug use biases attention toward drug-related cues and specify that background distress levels modulate the incentive salience of drug stimuli. As such, we cannot claim that this study tests predictions that distinguish the reformulated negative reinforcement model from alternative accounts of drug motivation. Instead, we claim that the current study exposes some of the specific predictions about the effects of affective processing on smoking-relevant information processing postulated in the reformulated negative reinforcement model to risk of refutation.
Method
Participants
The final sample included 113 adults drawn from the community (see Table 1 and Table 2 for sample characteristics). All participants were at least 18 years old and literate, native speakers of English. Twenty-two nonsmokers (defined as smoking no more than 100 cigarettes over the lifetime) and 91 heavy daily smokers (defined as smoking at least 10 cigarettes per day for the past year or more and an expired air carbon monoxide level at or above 10 parts per million, see below for further details) were enrolled. Regular users of other forms of tobacco, including pipes, cigars, chew, and snuff, were excluded, as were people actively trying to quit smoking with formal assistance (e.g., nicotine replacement therapy, counseling). Individuals who endorsed colorblindness or uncorrected hearing or vision problems were excluded due to the demands of the experiment. Individuals who reported having a pacemaker or an unstable cardiac condition, or past or current diagnosis or treatment for generalized anxiety disorder, panic disorder, or chronic pain conditions such as fibromyalgia were excluded due to concerns regarding the effects of the threat manipulation. Women who reported being pregnant or at risk of becoming pregnant due to lack of protection since the last menses were excluded due to concerns about adverse effects of exposing these individuals to electric shock.
Table 1.
Demographics for the Final Sample of Subjects (N=107)a
| Nonsmokers | Continuing Smokers | Deprived Smokers | |||||
|---|---|---|---|---|---|---|---|
| (n=22) | (n=46) | (n=39) | |||||
| N | % | n | % | n | % | ||
| Gender | Female | 12 | 54.5 | 22 | 47.8 | 18 | 46.2 |
| Race | White | 18 | 81.8 | 23 | 50.0 | 18 | 46.2 |
| African American | 3 | 13.6 | 17 | 37.0 | 17 | 43.6 | |
| Other | 1 | 4.5 | 3 | 6.5 | 3 | 7.7 | |
| Ethnicity | Hispanic | 2 | 9.1 | 2 | 4.3 | 2 | 5.1 |
| Education b | 0–11 grades completed | 1 | 4.5 | 11 | 23.9 | 7 | 17.9 |
| Grade 12 or GED | 3 | 13.6 | 14 | 30.4 | 17 | 43.6 | |
| College 1–3 years | 10 | 45.5 | 17 | 37.0 | 13 | 33.3 | |
| College 4 or more years | 7 | 31.8 | 2 | 4.3 | 2 | 5.1 | |
| Employment | Working outside home | 15 | 68.2 | 21 | 45.7 | 16 | 41.0 |
| Unemployed / disabled | 3 | 13.6 | 20 | 43.5 | 23 | 59.0 | |
| Homemaker | 1 | 4.5 | 3 | 6.5 | 2 | 5.1 | |
| Student | 3 | 13.6 | 1 | 2.2 | 1 | 2.6 | |
| Retired | 0 | 0 | 1 | 2.2 | 1 | 2.6 | |
| Income | Less than $10,000 | 8 | 36.4 | 22 | 47.8 | 17 | 43.6 |
| $10,000–$19.999 | 3 | 13.6 | 11 | 23.9 | 8 | 20.5 | |
| $20,000–$24,999 | 4 | 18.2 | 4 | 8.7 | 2 | 5.1 | |
| $25,000–$34,999 | 2 | 9.1 | 7 | 15.2 | 6 | 15.4 | |
| $35,000 or more | 5 | 22.7 | 2 | 4.3 | 5 | 12.8 | |
| Marital status c | Never married | 15 | 68.2 | 17 | 37.0 | 13 | 33.3 |
| Divorced or separated | 0 | 0 | 15 | 32.6 | 12 | 30.8 | |
| Married | 5 | 22.7 | 6 | 13.0 | 7 | 17.9 | |
| Living with partner | 2 | 9.1 | 4 | 8.7 | 5 | 12.8 | |
| Widowed | 0 | 0 | 1 | 2.2 | 1 | 2.6 | |
|
| |||||||
| Age | Mean (SD) | 28.09d | (11.03) | 39.09 | (10.88) | 40.64 | (10.25) |
Percentages do not sum to 100 due to missing data;
Nonsmokers more likely than smokers to have some college education, p<.05;
Nonsmokers more likely than smokers to be never married and less likely to be separated or divorced, p<.05;
Nonsmokers younger than smokers, p<.05.
Table 2.
Descriptive Statistics for the Sample of Smokers who Complied with Experimental Instructions and Provided Reaction Time Data (N=85)
| Continuing Smokers, No Availability (n=25) |
Continuing Smokers, Availability (n=21) |
Deprived Smokers, No Availability (n=20) |
Deprived Smokers, Availability (n=19) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age | 40.32 | 11.09 | 37.62 | 10.69 | 42.40 | 8.93 | 38.79 | 11.43 |
| Cigarettes per day | 19.60 | 7.28 | 22.38 | 9.65 | 22.35 | 9.58 | 20.63 | 11.09 |
| Age first smoked | 14.16 | 2.43 | 13.33 | 3.80 | 12.80 | 6.33 | 12.74 | 2.90 |
| Age first smoked daily | 16.20 | 2.84 | 16.81 | 3.68 | 16.30 | 6.11 | 15.42 | 2.71 |
| Years smoked daily | 22.62 | 10.68 | 19.00 | 10.48 | 24.00 | 11.30 | 23.58 | 10.86 |
| Past quit attempts | 1.96 | 2.34 | 3.24 | 4.63 | 2.35 | 2.43 | 3.58 | 3.72 |
| FTND total score | 5.56 | 2.29 | 5.48 | 2.32 | 6.10 | 2.25 | 5.63 | 2.03 |
| Pre-task CO level* | 30.50 | 16.32 | 26.68 | 10.37 | 4.30 | 3.03 | 3.32 | 2.07 |
| Pre-task total WSWS | 2.19 | .63 | 2.19 | .58 | 2.48 | .50 | 2.28 | .69 |
| Pre-task WSWS Urge * | 2.42 | .94 | 2.53 | .92 | 3.29 | .68 | 3.03 | 1.06 |
| Post-task total WSWS | 1.88 | .70 | 1.93 | .57 | 2.02 | .63 | 2.03 | .87 |
| Post-task WSWS Urge | 2.25 | 1.14 | 2.36 | 1.00 | 2.70 | .94 | 2.50 | 1.28 |
| Post-task total QSU-Brief* | 3.82 | 2.26 | 3.80 | 1.92 | 4.90 | 1.71 | 4.79 | 2.05 |
p<.05
Design
The study employed a mixed design. Between subjects factors included: 1) nicotine dependence (nonsmoker vs. daily smoker); 2) among smokers, nicotine deprivation (non-deprived vs. deprived for 24 hours); and 3) among smokers, smoking opportunity during the lab session (unavailable vs. available). Thus, there were five between subjects groups, a nonsmoking group and four groups resulting from the full crossing of deprivation status and smoking opportunity among dependent smokers (22 nonsmokers, 25 non-deprived smokers not given an opportunity to smoke, 21 non-deprived smokers given an opportunity to smoke, 24 deprived smokers not given an opportunity to smoke, and 21 deprived smokers given an opportunity to smoke). Random assignment to deprivation status and smoking opportunity groups among smokers was matched on gender. Participants in each of the five groups were randomly assigned to one of eight stimulus presentation orders.
Two within-subjects factors were manipulated: 1) word set (neutral, unpleasant, pleasant, and smoking related) and 2) threat (threat of shock vs. safety). Word sets were presented in blocked format. All subjects saw each of the four word sets under both the safe and threat conditions (with the order counterbalanced across participants). The presentation order of word set blocks was constrained such that unpleasant and pleasant words were always separated by neutral words and smoking-related words always occurred in the first or last block in order to prevent carry-over effects related to seeing smoking-related words prior to neutral words documented in previous research (Waters, Sayette, & Wertz, 2003). Breaks were provided between blocks to reduce carry-over effects as well (Waters, Sayette, Franken, & Schwartz, 2005)1.
Procedure
Recruitment and Screening
Participants were recruited via television advertisements and flyers soliciting both non-smoking and daily-smoking volunteers for a research study regarding smoking. Interested individuals who telephoned the laboratory were offered a description of the study and screened for eligibility over the telephone.
Participants who met all inclusion criteria (described above) were invited to attend a one-hour group session at which informed consent for additional screening was obtained, smoking status and history were re-assessed and verified through expired alveolar carbon monoxide (CO) testing, and individual difference variables were assessed using a battery of self-report measures. Nonsmokers were considered eligible if they: confirmed smoking fewer than 100 cigarettes in their lifetime, had an expired CO concentration less than 10 parts per million, and were willing to take part in the experiment. Smokers were considered eligible if they: confirmed smoking at least 10 cigarettes per day for the last year or longer, had an expired CO concentration of at least 10 parts per million, were willing to take part in the experiment, and agreed to be randomized to either continue smoking normally or to stop smoking 24 hours prior to the experimental session.
Experimental Session
At the start of the experimental session, CO concentration was reassessed to verify smoking status and adherence to abstinence instructions. Deprived smokers instructed to abstain from smoking for 24 hours were considered abstinent if they reported no smoking in the last 24 hours and if the average of two CO readings was less than 10 parts per million and at least 50% reduced from the baseline level. Six of the 45 smokers assigned to abstain had questionable abstinence (low levels of smoking were reported at the beginning of the abstinence period or CO levels were not at least 50% reduced from baseline) and were excluded from analyses2.
At the beginning of the experimental session, subjects were informed about the nature of the experimental procedures, including the color-naming task and the electric shock and availability manipulations (although they were not told exactly how many shocks they would receive). Those who provided written informed consent to participate in the experiment and passed screening for contraindications to the administration of electric shock completed affect and withdrawal measures. All participants then completed an electric shock sensitivity assessment procedure (see below).
Modified Stroop Task
During the experiment, all participants were instructed to name aloud the script color of the words as quickly as possible. Subjects received standardized instruction in the task and then completed 20 practice trials with neutral words.
Word Sets
Each word set contained 20 words matched for initial letter and number of letters (see Appendix). Smoking-related words were adapted from previous research (Wertz & Sayette, 2001) and modified to enhance matching of stimuli. Unpleasant and pleasant words were selected from a published database (the Affect Norms for English Words; Bradley & Lang, 1999). Words with extreme valence ratings were selected and matched for arousal rating. Unpleasant words had a mean valence of 2.7 (SD=0.7) on the nine-point pleasure rating scale ranging from very unpleasant to very pleasant, whereas the pleasant words had a mean valence of 7.5 (SD=0.6). Arousal ratings made on a nine-point scale did not differ between the unpleasant (M=6.2, SD=0.8) and pleasant word sets (M=6.2, SD=0.9) and all affective words selected were rated above the midpoint on the arousal scale. Words in the neutral, unpleasant, and pleasant word sets were also matched for frequency using tables in Kucera and Francis (1967). The frequency of smoking related words was not matched due to the inherent inequality in frequencies for these words between smokers and nonsmokers, although the average frequency rating for smoking words for which frequency was available was equal to the average frequency for other types of words used in the study. Words were also matched on relation to a superordinate category. Because all smoking related words are related to smoking, similarly related classes of words were selected for the other word sets to reduce the risk of unique priming effects in smoking-related blocks (Williams, Matthews, & MacLeod, 1996). Neutral words were related to writing; unpleasant words were related to violence, pain, and death; and pleasant words were related to sex and pleasure. Sex-related words were selected because this category of positive words comprised words rated as most arousing and most pleasant in previous research (Bradley & Lang, 1999), as is true for non-verbal stimuli as well (Bernat, Patrick, Benning, & Tellegen, 2006; Bradley, Codispoti, Cuthbert, & Lang, 2001; Bradley, Codispoti, Sabatinelli, & Lang, 2001). Alternative categories, such as money and success, did not offer as many words with extreme valence and arousal ratings as did the sex/pleasure category (Bradley & Lang, 1999) and thus were more difficult to match to the other groups of words on other dimensions (i.e., length, first letter, frequency).
Appendix Table A1.
Words used in the modified Stroop task.
| Neutral | Unpleasanta | Pleasanta | Smokingb |
|---|---|---|---|
| antonym | assault | aroused | ashtray |
| byline | bomb | bold | burn |
| binder | bullet | breast | butt |
| colon | coffin | caress | carton |
| comma | crime | cute | cigarette |
| diction | drown | desire | drag |
| files | fight | fantasy | filter |
| font | flood | flirt | flavor |
| italic | injury | intercourse | inhalation |
| leaflets | leprosy | lust | lighter |
| margin | massacre | magical | matches |
| metaphor | maniac | merry | menthol |
| narrator | needle | naked | nicotine |
| printer | panic | passion | pack |
| prose | poison | pleasure | puff |
| simile | snake | sexy | smell |
| syntax | spider | spouse | smoke |
| thesis | terrorist | taste | tar |
| text | torture | terrific | taste |
| typed | trauma | thrill | tobacco |
Taken from Bradley & Lang, 1999
Adapted from Wertz & Sayette, 2001
Procedure
Words were presented individually in red, blue, or green script for 1500 ms in the center of a 21-inch CRT monitor with DMDX stimulus control software (Forster & Forster, 2003). Words were presented in blocks by category because past research suggests that the blocked version is more sensitive to interference effects than is the interspersed version (Waters & Feyerabend, 2000). Latency to the onset of a vocal response was recorded for each trial and an audio file was generated so that accuracy of the response could be coded by two independent raters (Kappa=.84, SE=.01, t=154.13, p<.001) and discrepancies could be resolved by a third rater. Stimuli were separated by varying inter-trial intervals (1000 ms, 1500 ms, 2000 ms).
Each set of 20 words was presented twice in each block of 40 trials. Each block of 40 trials was presented twice; once with the shock-delivering electrodes taped to the finger tips (threat) and once with the electrodes removed (safe), in counter-balanced order, for a total of 320 trials. Order of word presentation within each set of 20 words was randomized in each block.
Between blocks, subjects received computerized feedback regarding their average response time in order to encourage sustained effort at the color-naming task and to facilitate detection of non-responses due to failures of recording equipment (i.e., responses that were not detected yielded a response time of −2500 ms which prompted the experimenter to adjust equipment). During the inter-block interval, subjects were also asked to complete a brief self-report assessment of affect and withdrawal distress. After the fourth block, all subjects took a five-minute break and had the electrodes removed or re-applied, as needed.
Electric Shock
All participants completed a shock sensitivity assessment prior to the start of the main experiment. This procedure was used to calibrate the intensity of the shock to each individual to reduce individual differences associated with pain sensitivity. Participants were administered a series of 250 ms electric shocks of increasing intensity to the index and ring fingers on the non-dominant hand. The shocks ranged in intensity from 0.5 to 7.0 milliamperes. Subjects reported the level of shock that they first detected, first found uncomfortable, and that was the most they could tolerate. Subjects were informed that the shock sensitivity procedure would be discontinued when they rated a shock as the most they could tolerate (i.e., this would be the last shock they would receive).
Participants received a total of five electric shocks during the experimental session, with the intensity of each shock set at the midpoint between the level the participant considered uncomfortable and the maximum tolerable. Subjects were not informed of this formula for setting the shock level for the experimental task until the debriefing at the end of the experiment. As this manipulation was intended to serve as a threat condition, subjects were not informed that they would experience only five shocks prior to the experiment. Each 250 ms shock administered during the experiment was followed by a 2000 ms interval. The timing of the shocks was held constant across all participants (after the 20th word in the first block, before the first word and after the 20th word in the second block, after the 40th word in the third block, and after the 20th word in the fourth block).
Measures
At the informational meeting, participants provided self-report data regarding smoking behavior, history, and general demographic information. Among these measures was the Fagerström Test of Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Additional measures will not be discussed further in this report. Baseline characteristics of the enrolled sample are reported in Table 1.
At the start of the experimental lab session, subjects completed the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) and the Wisconsin Survey of Withdrawal Symptoms (WSWS; Welsch et al., 1999). All participants also completed the PANAS after each of the eight blocks of trials. Craving items were not administered between blocks due to concerns about reactivity to repeated questioning regarding urges to smoke. After the eighth and final block, however, smokers were asked to complete the full WSWS and the 10-item Brief Questionnaire on Smoking Urges (QSU-Brief; Cox, Tiffany, & Christen, 2001). Each of these scales has published reliability and validity information. The PANAS yields two highly internally consistent scale scores (momentary positive affect α=.89, momentary negative affect α=.85, Watson et al. 1988). The WSWS has a seven factor structure and a second-order single factor structure. Each of the seven subscales has acceptable internal consistency (α range from .75 to .90; Welsch et al., 1999). The QSU-Brief has a two-factor structure and a higher-order factor whose total scores have excellent internal consistency (α=.87 to .97; Cox et al., 2001).
Analyses of Stroop task response time was conducted for correct trials only (97.3% of trials). Extremely fast reaction times (less than or equal to 200 ms; 1.1% of correct trials) were excluded. To reduce the influence of exceptionally slow responses, trials that were slower than each participant’s mean plus three standard deviations were set to equal the mean plus three standard deviations3.
Smoking Availability Manipulation
Following Wertz and Sayette (2001), a simple instructional manipulation of smoking availability was used. Just before the first block of experimental trials, the experimenter informed the participant that he or she would have the opportunity to smoke at some point during the two-hour session, or that he or she would not be able to smoke until after the two-hour session ended. For those who were randomly assigned to have an opportunity to smoke, experimenters provided the opportunity immediately after completing the self-report measures following the eighth (final) block of trials.
Data Analytic Strategy
The nature of the three primary between-subjects factors in this experiment, smoking status (smoker vs. nonsmoker), nicotine deprivation (deprived vs. non-deprived smokers), and cigarette availability (available vs. unavailable), precluded the use of a fully crossed factorial design. For example, nonsmokers could not be included in non-deprived cells of a factorial design. Instead, we relied on orthogonal contrasts among the five smoking groups. Specifically, four orthogonal contrasts were coded to test the effects of smoking status, nicotine deprivation, and cigarette availability4:
A smoking status contrast: nonsmokers vs. the four smoker groups
A deprivation contrast: the two non-deprived smoker groups vs. the two deprived smoker groups.
A non-deprived availability contrast: non-deprived smokers with cigarettes available vs. non-deprived smokers with cigarettes unavailable.
A deprived availability contrast: deprived smokers with cigarettes available vs. deprived smokers with cigarettes unavailable.
We adopted a similar orthogonal contrast approach to test within-subjects effects of word set in the analyses of response times from the main task. Analysis of the four word sets (neutral, threat, reward, and smoking) was accomplished by the following orthogonal Helmert contrasts:
A motivationally relevant word contrast: neutral words vs. the three motivationally relevant types of words (unpleasant, pleasant, and smoking).
A reward word contrast: unpleasant words vs. pleasant and smoking words).
A smoking specific word contrast: sex/pleasure pleasant words vs. smoking words.
This contrast-based analytic strategy was employed to test the hypotheses of interest by means of orthogonal tests that partition the overall effects of the manipulation in the most meaningful way for our model, without increasing family-wise error (because the multiple planned comparisons simply partition the overall effect detected in an omnibus test without representing more tests). Complete orthogonality of the between subjects contrasts is not guaranteed, however, due to inequality in cell sizes. Hypothesis 1 is tested by the interaction between the nonsmoker versus smoker contrast and the smoking-specific word contrast which indicates whether or not smokers differentiate between smoking-related versus other reward-related words more than do nonsmokers. The first part of hypothesis 2 regarding deprivation effects on attention bias toward smoking cues is tested by the interaction between the contrast between non-deprived and deprived smokers and the contrast between smoking-related versus other pleasant, reward-related words. This will indicate whether deprivation increases bias toward smoking words specifically overall. The second part of hypothesis 2 will be tested by the interaction between the availability conditions within the deprived group and the smoking versus pleasant word contrast. This will determine whether or not the deprivation effect on attention bias toward smoking-related vs. other pleasant words depends on availability. Hypothesis 3 regarding the shock manipulation is tested by two interaction contrasts. First, the three-way interaction between the shock manipulation, the nonsmoker versus smoker contrast, and the smoking-related versus pleasant word contrasts tells us whether smokers showed greater bias toward smoking-related words under threat of shock than do nonsmokers overall, as we predicted. A second three-way interaction with the deprivation, shock, and smoking vs. pleasant word set contrasts tell us whether the effect for smokers depends on deprivation status. We predicted that this would be nonsignificant.
In addition to focusing analyses on our questions of interest, these contrasts also provide attractive controls to help establish the specificity of observed effects. For example, contrasting neutral words with the composite of unpleasant, pleasant, and smoking words allows us to determine whether or not participants seem to be responding to the broad motivational relevance or salience of words, and whether or not our manipulations alter this bias. In addition, contrasting unpleasant words with the composite of pleasant sex/pleasure and smoking words allows for a test of interference to reward words, controlling for arousal. This set of orthogonal contrasts was considered more attractive than an alternative in which reaction times to pleasant words were contrasted with the composite of reaction times to both unpleasant and smoking words, and reaction times to unpleasant versus smoking words were compared, because we expected drug cues to function as appetitive rather than aversive cues.
Results
Characteristics of smokers and nonsmokers
The nonsmoking and smoking samples were not comparable in all ways, as shown in Table 1. Nonsmokers tended to be younger, less likely to have been married, and more likely to have attended college than smokers. Removing variability related to age and college attendance in analyses did not alter the results of the hypothesis testing reported below. Marital status was found to be redundant with both of these other covariates and thus was not included as a covariate. Given the interpretation problems inherent in using covariates to attempt to reduce the influence of preexisting group differences (Miller & Chapman, 2001), we have elected to present the results of models unadjusted for covariates.
Smoking-related individual differences
The four groups of smokers were similar in terms of smoking history and nicotine dependence levels. None of the three contrasts among smokers (comparing non-deprived and deprived smokers, non-deprived smokers with vs. without an opportunity to smoke, or deprived smokers with vs. without an opportunity to smoke) was significant for any of the smoking history and nicotine dependence variables listed in Table 2.
Manipulation Checks
Nicotine Deprivation
Analyses of CO level and pre-task WSWS scores were conducted among the four smoker groups to confirm successful manipulation of nicotine deprivation among dependent smokers. Means and standard deviations by smoking group are presented in Table 2. Univariate smoking group contrasts for CO level revealed the expected significant deprivation contrast, F(1,80)= 117.51, p < .001, ηp 2=.60, with CO level lower among deprived smokers (M=3.82, SD=2.62) than among non-deprived smokers (M=28.80, SD=13.97). Neither the non-deprived nor deprived availability contrast was significant, indicating comparable deprivation status across the availability groups as expected.
Multivariate analyses of smoking group contrasts across six sub-scales of the WSWS4 revealed the expected significant multivariate deprivation contrast, F(7,75)= 2.34, p= .032, ηp 2=.18, with scale scores generally elevated in deprived smokers relative to non-deprived smokers. As expected, neither the non-deprived nor deprived availability contrast was significant in this multivariate analysis. Follow-up univariate contrasts on individual WSWS scales confirmed the expected significant deprivation contrast for the urge scale, F(1,81)= 11.78, p< .001, ηp 2=.13, with increased urge among deprived smokers (M=3.2, SD=0.9) relative to non-deprived smokers (M=2.5, SD=0.9), but no significant difference in negative affect (ηp 2=.02). In addition, total urge as assessed by the QSU-Brief was significantly higher for deprived smokers (M=4.84, SD=1.86) than non-deprived smokers (M=3.81, SD=2.10, F(1,80)=5.47, p=.02, ηp 2=.06) at the end of the task, as well, suggesting that the deprivation effects were not eliminated over the course of the experiment, although the difference between groups on the WSWS Urge scale declined (as did WSWS urge ratings overall) during the experiment. After the task, deprived smokers (M=2.60, SD=1.11) had WSWS urge scores not significantly different than those of non-deprived smokers (M=2.30, SD=1.07, F(1,81)=1.53, p=.22, ηp 2=.02). The availability manipulation did not significantly influence any of these ratings.
Threat of Shock
A Smoking Group X Threat mixed model ANOVA was conducted for all participants to confirm that the shock threat manipulation increased negative affect. There was a significant medium-sized main effect of threat, F(1,101)= 8.50, p= .004, ηp 2=.08, with negative affect scores from the PANAS higher in the shock threat (M=16.4, SD=7.0) than in the no shock conditions (M=15.1, SD=6.9). Neither the smoking group contrasts nor the interaction of these contrasts with threat was significant, confirming that the threat manipulation was comparable across all groups. Similarly, shock intensity did not differ significantly across groups6.
Modified Stroop Task
Replication of smoking Stroop effect
Prior to conducting our targeted contrast-based analyses, we conducted simple comparisons of reaction times to neutral and smoking-related words in the safety condition to determine whether our results replicated past research on smoking interference (e.g., Gross et al., 1993; Waters & Feyerabend, 2000). As expected, response latencies were greater for smoking-related than for neutral words among smokers (F(1,81)=7.07, p=.009, ηp 2=.08). This effect was significantly greater among deprived smokers (Mean difference=37.1 ms) than among continuing smokers (Mean difference=4.6 ms; F(1,81)=3.99, p=.049, ηp 2=.05). The interaction with availability condition was not significant.
Full Repeated Measure ANOVA
Next, a Smoking Group X Threat X Word Set mixed model ANOVA was conducted on response times from the modified Stroop task7. The smoking group and word set contrasts described above were used. Response time data are displayed in Table 3 as a function of smoking group, threat, and word set.
Table 3.
Reaction time data (ms) for each of eight experimental blocks, by group.
| Threat Condition |
Word Set | Nonsmokers (n=22) |
Continuing Smokers, No Availability (n=25) |
Continuing Smokers, Availability (n=21) |
Deprived Smokers, No Availability (n=20) |
Deprived Smokers, Availability (n=19) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| No shock | Neutral | 555.44 | 74.09 | 626.47 | 121.23 | 596.77 | 137.82 | 654.65 | 134.48 | 639.87 | 107.65 |
| Unpleasant | 557.31 | 71.77 | 632.01 | 109.43 | 590.04 | 135.47 | 651.28 | 125.02 | 659.02 | 137.14 | |
| Pleasant | 587.81 | 89.42 | 620.75 | 111.14 | 604.12 | 122.40 | 667.35 | 129.69 | 683.50 | 142.86 | |
| Smoking | 570.62 | 83.42 | 623.65 | 93.33 | 610.21 | 120.38 | 684.01 | 134.04 | 685.18 | 137.45 | |
| Shock | Neutral | 566.17 | 80.59 | 646.71 | 121.92 | 586.28 | 131.59 | 685.09 | 128.75 | 660.43 | 106.88 |
| Unpleasant | 573.94 | 95.16 | 645.19 | 137.61 | 590.95 | 117.38 | 680.52 | 125.23 | 677.75 | 152.20 | |
| Pleasant | 591.24 | 92.46 | 648.27 | 140.56 | 632.99 | 160.04 | 685.47 | 129.23 | 685.87 | 158.06 | |
| Smoking | 589.85 | 103.01 | 647.08 | 114.60 | 600.32 | 119.92 | 677.00 | 125.61 | 697.83 | 138.05 | |
Between-subject main effects
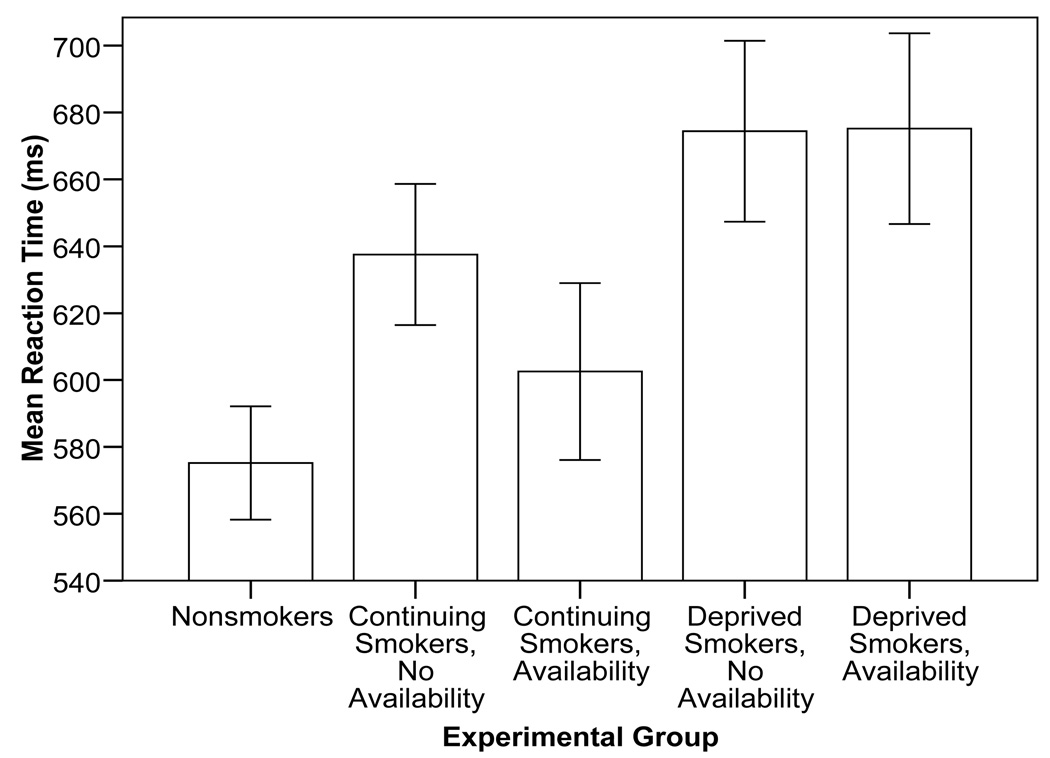
Overall smoking group contrasts (see Figure 1) revealed a significant smoking status contrast, F(1,102)= 7.41, p= .008, ηp 2=.07, with smokers (M=644.7, SD=119.0) displaying slower response times than nonsmokers overall (M=574.1, SD=79.3). A significant deprivation contrast was also observed, F(1,102)= 5.12, p= .026, ηp 2=.05, with deprived smokers displaying slower response times (M=673.4, SD=121.0) than non-deprived smokers overall (M=620.4, SD=112.9). Neither the non-deprived nor the deprived availability contrasts was significant.
Figure 1.
Mean color naming reaction time, in milliseconds, by experimental group (N=106), excluding non-responses and with extremely long reaction times corrected toward the idiographic mean. Error bars reflect the between-subjects standard error.
Within-subject main effects
With respect to overall word set contrasts collapsed across groups, a significant motivationally relevant word contrast was observed, F(1,102)= 9.62, p= .002, ηp 2=.09, with overall slower response time during motivationally relevant words (i.e., unpleasant, pleasant, and smoking; M=633.3, SD=116.6) than during neutral words (M=620.9, SD= 116.6). A significant reward word contrast was also observed, F(1,102)= 7.73, p= .006, ηp 2=.07, with slower response times overall for the composite of pleasant and smoking words; M=637.7, SD=118.0) than for unpleasant words (M=624.3, SD=120.2). The smoking specific word contrast was not significant (ηp 2=.004). A significant main effect of threat was also observed, F(1,102)= 3.90, p= .051, ηp 2=.04, with overall slower response times during shock threat (M=637.0, SD=122.0) than during no-shock blocks (M=623.3, SD=114.7).
Hypothesis testing: Hypothesis 1
The interaction between the smoking status contrast (nonsmokers vs. smokers) and the smoking vs. pleasant contrast was not significant, F(1,102)=.36, p=.55, ηp 2=.004, indicating that neither nonsmokers nor smokers showed significantly different reaction times to smoking and pleasant words.
Hypothesis testing: Hypothesis 2
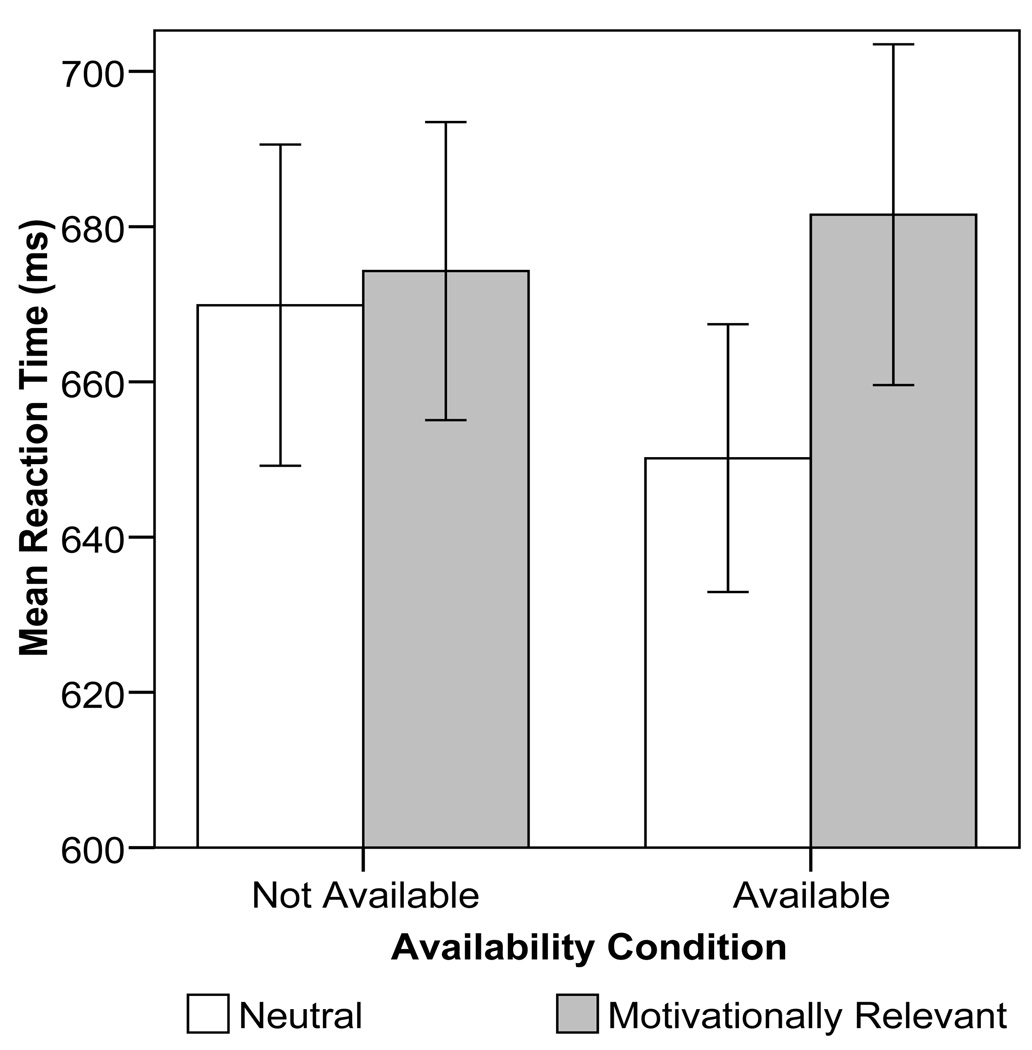
The two-way interaction between deprivation status (nondeprived vs. deprived) among smokers and word set was not significant, indicating no difference in reaction times to smoking vs. reward words as a function of deprivation status, collapsed over availability and threat conditions, F(1,102)=.75, p=.39, ηp 2=.007. Smoking availability moderated the response time to motivationally relevant (neutral vs. unpleasant, pleasant, and smoking words) words among deprived smokers, however (i.e., there was a Deprived availability X Motivationally relevant word interaction, F(1,102)= 4.40, p= .038, ηp 2=.04, see Figure 4). To follow-up this two-way interaction, pair-wise comparisons of the composite of motivationally relevant and neutral words were conducted separately within deprived smokers. Among deprived smokers with smoking availability, response time to motivationally relevant words was significantly slower than to neutral words (p= .036). In contrast, among deprived smokers with no smoking availability, the contrast between response times for motivationally relevant vs. neutral words was not significant (p= .514). Availability did not significantly affect response times and did not interact with word set among non-deprived smokers.
Figure 4.
Deprived smokers’ (N=39) mean color naming reaction times for neutral words versus all motivationally relevant words as a function of smoking availability. Error bars reflect the between-subjects standard error.
Response times to pleasant words were also contrasted against neutral words to see whether deprivation and threat differentially influenced attention to these word types (in addition to the pleasant vs. smoking contrast that was non-significant). Results revealed no significant two-way interaction between deprivation and word set, but did reveal a three way interaction between deprivation, threat condition, and word set (F(1,102)=6.15, p=.02, ηp 2=.06). Deprivation and word set interacted significantly such that interference to positive words was significantly greater among deprived than non-deprived smokers in the absence of shock (F(1,102)=4.77, 9=.03, ηp 2=.05, mean difference=27.53 ms), but not in the presence of shock (F(1,102)=.45, p=.50, ηp 2=.004, mean difference=−9.58 ms).
Hypothesis Testing: Hypothesis 3
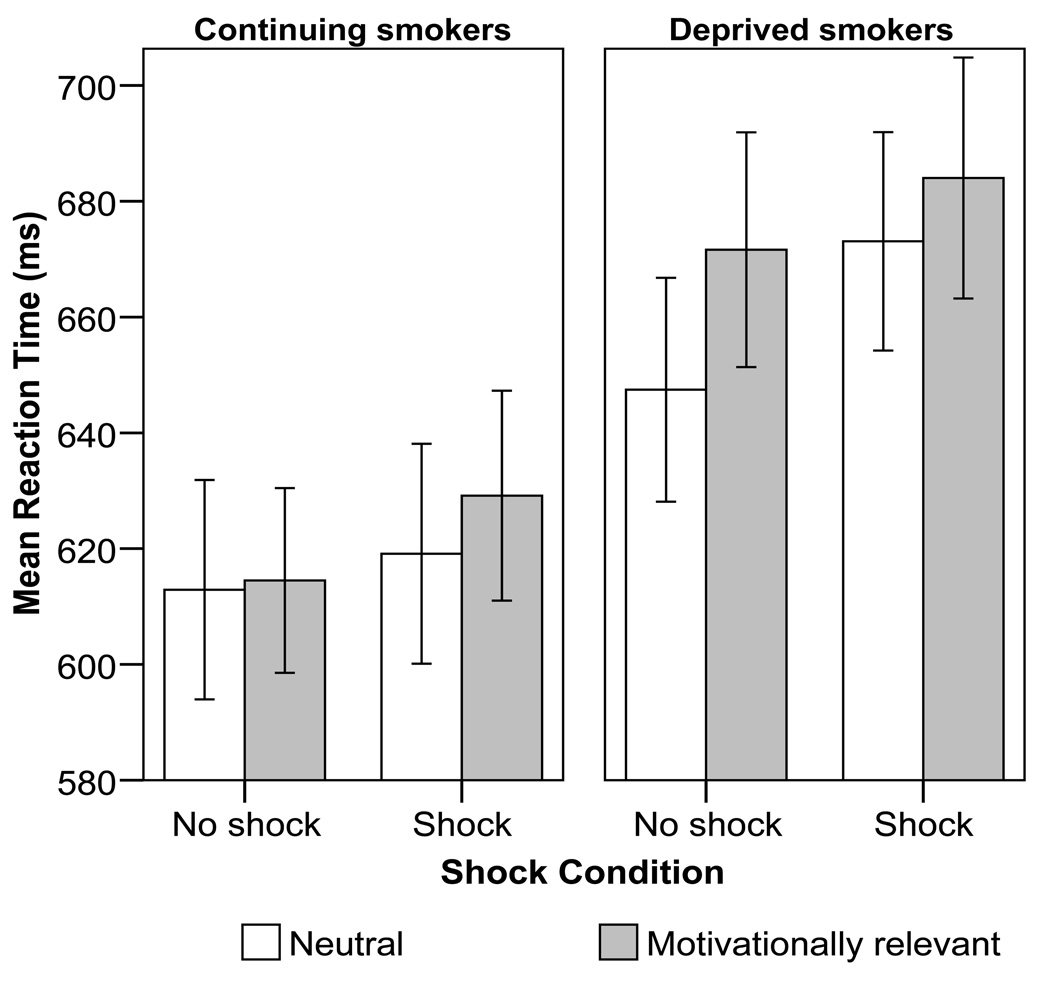
Smoking status did not interact significantly with the smoking vs. pleasant word set contrast and threat condition, F(1,102)=2.40, p=.12, ηp 2=.02, indicating that smokers were not significantly more likely to show a narrow bias of attention toward smoking-related cues under shock than were non-smokers. Nicotine deprivation and threat moderated the effect of word motivational relevance on response time, however (i.e., there was a Nicotine deprivation X Threat X Motivationally relevant word interaction, F(1,102)= 3.79, p= .054, ηp 2=.04, see Figure 2). To follow up this interaction, simple comparisons of nicotine deprivation groups were conducted for the neutral and motivationally relevant word sets in the no-shock and shock conditions. Deprived smokers displayed significantly slower response times than did non-deprived smokers to motivationally relevant word sets (all non-neutral words) in both shock (mean difference= 54.8 ms; p= .049) and no-shock conditions (mean difference= 57.1 ms; p= .028). Deprived smokers were also slower to color-name neutral words during shock than were non-deprived smokers (mean difference= 54.0 ms; p= .048). No significant deprivation effect was observed for neutral words during no-shock blocks (mean difference = 34.5 ms). To further examine the deprivation effect, pair-wise comparisons were tested between the four combinations of word type and threat among deprived smokers only. Response times among deprived smokers were significantly slower during motivationally relevant/no shock (p=.013), motivationally relevant/shock (p=.002), and neutral/shock (p=.013) conditions vs. the neutral/no shock condition. No other condition contrasts were significantly different among deprived smokers. In sum, deprived smokers displayed slower response times than non-deprived smokers during all conditions that involved a motivationally important manipulation (i.e., either presentation of motivationally relevant words or threat of electric shock). Similarly, among deprived smokers, response times were slowed to motivationally important stimuli relative to the neutral word/no-shock control condition. Non-deprived smokers did not show this sensitivity to the motivational relevance of stimuli.
Figure 2.
Smokers’ (N=85) mean color naming reaction times for neutral words versus the composite of all motivationally relevant words, by deprivation and shock condition. Error bars reflect the between-subjects standard error.
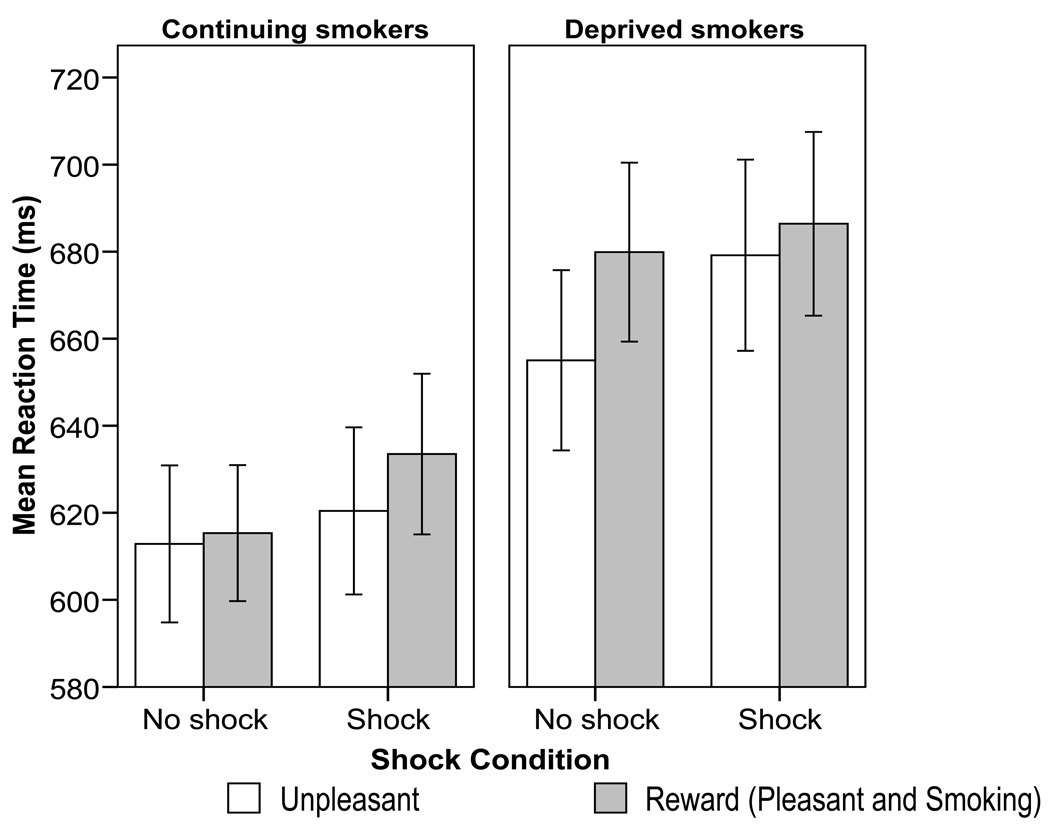
Nicotine deprivation also moderated the effect of the valence (unpleasant vs. pleasant and smoking) of words and threat on response times (i.e., there was a Nicotine deprivation X Threat X Reward word interaction, F(1,102)= 3.98, p= .049, ηp 2=.04, see Figure 3). To follow-up this interaction, simple comparisons of nicotine deprivation were conducted for the unpleasant and reward word sets in the no-shock and shock conditions. Deprived smokers displayed significantly slower response times than did non-deprived smokers to: unpleasant words during the shock condition (mean difference= 58.7 ms; p= .046), reward (pleasant and smoking) words during the no-shock condition (mean difference= 64.6 ms; p= .013), and a trend for slower responding to reward words during shock (mean difference= 52.9 ms; p= .062). No significant nicotine deprivation effect was observed for unpleasant words during no-shock (mean difference = 42.2 ms). To further examine the deprivation effect, pair-wise comparisons were tested among the four combinations of word type and threat among deprived smokers only. Relative to the unpleasant/no shock condition, response times among deprived smokers were generally slower during reward words/no shock (p=.003), reward word/shock (p=.002), and unpleasant word/shock (p=.069) conditions. No other condition contrasts were significantly different among deprived smokers (deprivation and threat did not interact to influence responses to pleasant vs. smoking words). In sum, deprived smokers displayed generally slower response times than non-deprived smokers when either viewing reward words or threatened with electric shock. Similarly, among deprived smokers, response times were generally slowed to these same manipulations relative to the unpleasant word/no shock control condition.
Figure 3.
Smokers’ (N=85) mean color naming reaction times for negative words versus the composite of pleasant and smoking-related words, by deprivation and shock condition. Error bars reflect the between-subjects standard error.
Discussion
In this study, we sought to test hypotheses regarding factors and conditions influencing attention to smoking cues derived from the reformulated negative reinforcement model of drug motivation (Baker et al., 2004). We tested the hypothesis that both tobacco deprivation and an exogenous stressor would bias smokers’ attention narrowly toward smoking-related cues in a modified smoking and emotion Stroop task. Results indicated that deprivation and stress influenced attention toward salient or motivationally relevant cues broadly, and reward-relevant cues specifically, although the biasing effects observed were not restricted to smoking-related stimuli. Results also confirmed that smoking availability moderated attention bias in nicotine-deprived smokers, such that greater bias toward all non-neutral words (but not pleasant or smoking-specific cues specifically) was observed among deprived smokers who anticipated an opportunity to smoke but not among those told that smoking would not be permitted.
Results did not support our first hypothesis, that smokers overall would show a bias toward smoking related words compared to other arousing pleasant words, whereas nonsmokers would not. Smoking words did not appear to have greater salience than other reward-related words in this sample, contrary to our expectations.
The current study found support for the second hypothesis that nicotine deprivation influences attention. The present findings run counter to Powell and colleagues’ (2002) research suggesting insensitivity to threat (unpleasant words) and reward (pleasant words) in withdrawal. The current findings show that deprived smokers showed greater interference to all motivationally relevant and both smoking and other pleasant words than did continuing smokers in this study, when not under stress. Rather than being insensitive to threat (unpleasant words) and reward (pleasant and smoking words), deprived smokers appeared to be more attentive to the motivational relevance and reward value of cues than did non-deprived smokers, even though the two groups showed roughly equivalent response latencies to neutral words in the absence of threat. These experimental reaction time data may reflect similar processes that result in increased reactivity to recent exposure to stress or smoking-related stimuli in real-time reports collected in the field (McCarthy, Piasecki, Fiore, & Baker, 2006). A similar pattern of results emerged when bias toward reward-related words was assessed relative to arousing unpleasant words. Although these results were not specific to smoking-related stimuli, they add to the research demonstrating greater interference to motivationally relevant words, particularly reward words, when deprived of nicotine than when smoking at will (e.g., Gross et al., 1993; Waters & Feyerabend, 2000; see Cox et al., 2006 for a review).
Our third hypothesis regarding the effects of an exogenous stressor on smokers’ attention requires further clarification based on these data. We predicted that both deprived and non-deprived smokers would show a bias toward smoking-related words when stressed and that deprivation and stress would have additive effects on attention. Instead, we found that deprivation status and threat had interactive effects on performance. Although the threat of shock had a medium-size effect on negative affect and slowed responses across all smoking groups, non-deprived smokers did not show greater attention toward motivationally relevant, reward, or smoking-related cues when stressed than when safe. Deprived smokers, in contrast, exhibited sensitivity to the threat manipulation, such that response times were slower to neutral cues and the bias toward non-neutral words was reduced under threat of shock compared to the safe condition. Suppression of attention bias toward concern-relevant words has been noted in research in veterans with Post-Traumatic Stress Disorder, such that anticipating a stressor after the task eliminated the usual bias toward combat-related words relative to neutral words in an emotion-Stroop task (Constans, McCloskey, Vasterling, Brailey, & Mathews, 2004). These results differed from our own, however, in that no significant slowing to all word types was noted in the threat conditions among veterans. Taken together, these results suggest that anticipating a stressor may somehow interfere with or supersede the attention bias toward concern-relevant stimuli typically exhibited on the modified Stroop task. As such, it appears as though deprivation and stress have interactive, rather than additive, effects on attention among smokers, contrary to our expectations and previous research suggesting attention bias toward threat-related words in people high in trait anxiety persists even under threat of shock (Miller & Patrick, 2000).
Taken together, the results indicate that nicotine deprivation increases smokers’ sensitivity to manipulations of word type, such that interference effects are greater when deprived than when smoking freely, at least in the absence of acute stress. Expression of deprivation effects depends on perceived drug availability, however. Interference to words with broad motivational significance was greater when deprived smokers anticipated an opportunity to smoke than when informed that smoking would be restricted for the next two hours. This result is somewhat consistent with previous research documenting interactions between word type and smoking availability (Wertz & Sayette, 2001), although the effect was not specific to stimuli related to smoking in the current study.
In summary, the results of this study provide partial support regarding factors that influence attention in addiction but also challenge the specificity of these attention processing effects. Prior modified Stroop tasks either contrasted smoking and neutral cues or emotion and neutral cues. When we compared response times between smoking and neutral words we replicated the finding that deprivation increases attention bias to smoking cues among smokers (Gross et al., 1993; Waters & Feyerabend, 2000). When we compared response times to smoking words with response times to other arousing, pleasant stimuli we found no effect, however. This null result may be due to strong association between smoking and sex-related stimuli, due to heightened salience of all stimuli (apart from motivational significance), or a more substantive reason. The bias toward sex and pleasure-related words is surprising, given the inflation of reward thresholds to non-drug stimuli that occurs in withdrawal (Epping-Jordan, Watkins, Koob, & Markou, 1998). Our results also challenge the prediction derived from the negative reinforcement model that negative affect, regardless of the source of the distress, induces a narrowing of attention toward drug-related cues. If the lack of differential bias toward smoking cues versus other highly arousing pleasant cues observed in this study is replicated with other pleasant, reward stimuli, this will challenge current accounts of the effects of drug dependence and deprivation on incentive and motivational processing and may have important clinical implications (Baker et al., 2004; Robinson & Berridge, 2003). For example, drug deprivation may not reduce interest in alternative reinforcers (such as food), although it does reduce sensitivity to the reward once obtained (Epping-Jordan et al., 1998). We must take care in interpreting these null results however, although we note that a significant bias toward pleasant versus neutral cues was observed among deprived smokers when safe from shock (i.e., the effect is not an artifact of the contrast strategy used).
Limitations
We must take care in reaching conclusions about the factors and conditions that influence smokers’ attention from these data, however, in light of the limitations of the current study. Methodological factors such as sampling strategy may have influenced results. Eliciting volunteers for a study with an explicit focus on nicotine may have attracted atypical non-smokers with particular interest in nicotine. Likewise, our requirement that smokers agree to be randomized to a deprivation condition may have deterred the most dependent or withdrawal-prone smokers from participating. Lack of balance between nonsmokers and smokers in terms of age and education may also have contributed to the overall difference in response times observed between these groups, although such differences might also be related to individual differences related to the risk of becoming a smoker.
Stimulus selection also may have contributed to the unexpected results. Several subjects commented on the sexual nature of the pleasant words and some were observed to blush during the pleasant word blocks or to make otherwise rare word-reading errors to relatively explicit words such as “breast.” The bias toward sex-related words in this study may not generalize to other pleasant words, as sex-related words may have unique salience, particularly in a laboratory setting. It is also possible that our deprivation manipulation was insufficient to induce clinically significant levels of withdrawal distress, perhaps because participants knew the deprivation would end within two-hours of the start of the experimental condition. Deprivation appeared to affect urges to smoke primarily, with marginal effects on other symptoms, despite a longer period of deprivation than that used in much prior research in this area (e.g., Gross et al., 1993) and strict biochemical verification of abstinence. Such background expectations regarding the duration of the experiment may also have weakened the availability manipulation. All explanations for the surprising similarity in the pattern of responses observed in smokers and nonsmokers and the lack of differentiation of smoking versus other reward cues must remain tentative until additional research that includes nonusers and other motivationally relevant stimuli is conducted. Similarly, comparisons across groups (i.e., nonsmokers and smokers) are stronger when cross-over patterns in reaction times to concern-relevant versus irrelevant words are compared across groups (i.e., reaction times to both tobacco-related and alcohol-related words are compared in groups of non-drinking smokers and non-smoking drinkers). Robbins and Ehrman (2004) have argued that this type of cross-over design offers a better control condition and allows stronger inferences than the nonsmoker versus smoker design employed here.
Finally, it is important to consider whether the paradigm used here is sensitive to the aspects of information processing that are most relevant to drug motivation. Other paradigms that can isolate specific information processing components, such as the orienting or shifting of attention or the detection and resolution of response conflict (Curtin et al., 2006; Robbins & Ehrman, 2004), may be more sensitive indices of processes critical to drug motivation and behavior than is the modified Stroop task.
Conclusion
Despite the limitations noted above, the current study contributes important findings to the extant research regarding the attention bias for drug-relevant cues among addicted individuals. First, the current results raise questions about the specificity of the effects of nicotine deprivation and availability reported in previous research. Our comparison conditions and contrast-based analytical strategy revealed that our manipulations affected reaction times to cues of broad motivational relevance, rather than in a smoking-specific manner as previously assumed. The current study also tests an important hypothesis about a cognitive pathway by which smoking history, deprivation, stress, and contextual variables such as smoking availability may influence addicted individuals and bias them toward smoking. The findings that deprivation and exogenous stress both appear to influence smokers’ attention toward reward cues, albeit in a non-additive manner, suggest that distress does indeed bias attention in ways that may increase the likelihood of smoking, but also in ways that may increase pursuit of alternative rewards. If sufficiently attractive, healthy alternative rewards can be identified for individuals attempting to curtail their smoking, this non-specific attentional bias may be harnessed to increase the likelihood of success. In addition, the findings regarding the availability manipulation highlight the importance of evaluating the effect of formal manipulations of smoking opportunities and assessing informal perceptions of such opportunities in future research, as this contextual variable appears to significantly affect attention toward environmental cues.
Acknowledgments
Preparation of this article was supported by a mini-grant from the University of Wisconsin Transdisciplinary Tobacco Use Research Center (P50CA084724) and the Marian Schwartz Fellowship from the University of Wisconsin-Madison Department of Psychology awarded to the first author. We thank the staff of the Center for Tobacco Research and Intervention, University of Wisconsin Medical School and the Addiction Research Laboratory, University of Wisconsin-Madison for their assistance in conducting this research. We thank Timothy B. Baker, Ph.D. for his helpful comments.
Footnotes
Order of block presentation did not interact significantly with word set, shock, or any between subject factor (i.e., smoking status, deprivation, or availability) in analyses.
Cases excluded because of questionable abstinence did not differ from the smokers who adhered to abstinence instructions in terms of smoking history or demographics. Including these cases in analyses did not change the pattern of results
A total of 1.1% of trials exceeded three standard deviations above the subject’s mean response time. The pattern of results did not change when these trials were treated as missing or set to the fence.
The pattern of results was similar when the main effects of and interaction between deprivation and availability was tested in a factorial ANOVA, although the significance level for the Threat X Wordset X Deprivation interactions for motivationally relevant and reward words were marginally significant in this approach (p=.07). The overall Threat X Wordset X Availability interaction was non-significant (p=.33).
The sleep subscale was excluded from the MANOVA because smokers were deprived for only one night and we considered it unlikely that the scale would be sensitive to deprivation lasting only 24 hours.
Excluding three individuals whose shock tolerance exceeded the maximum level of shock used in this study did not change the main or interactive effects of threat reported below, so these subjects who never reported that the shock was uncomfortable were retained in analyses.
Supplemental analyses included gender as a factor in this analysis model. However, gender did not moderate any of the significant reported effects. Therefore, it was dropped from the final report.
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bernat E, Patrick CJ, Benning SD, Tellegen A. Effects of picture content and intensity on affective physiological response. Psychophysiology. 2006;43:93–103. doi: 10.1111/j.1469-8986.2006.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson K, Grant S, Contoreggi C, Links J, Metcalfe J, Weyl H, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions to picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): Instruction manual and affective ratings. The Center for Research in Psychophysiology, University of Florida; 1999. Technical Report C-1. [Google Scholar]
- Brody A, Mandelkern M, London E, Childress A, Lee G, Bota R, et al. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Constans JI, McCloskey MS, Vasterling JJ, Brailey K, Mathews A. Suppression of attentional bias in PTSD. Journal of Abnormal Psychology. 2004;113:315–323. doi: 10.1037/0021-843X.113.2.315. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the Brief Questionnaire of Smoking Urges (QSU-Brief) in laboratory and clinical settings. Nicotine and Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Pothos EM. The addiction-Stroop test: Theoretical considerations and procedural recommendations. Psychological Bulletin. 2006;132:443–476. doi: 10.1037/0033-2909.132.3.443. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rothstein R, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Curtin J, McCarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: A model of boundary conditions. In: Wiers RW, Stacy AW, editors. Handbook of Implicit Cognition and Addiction. Thousand Oaks, CA: Sage; 2006. [Google Scholar]
- Eisenberger N, Lieberman M, Williams K. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2000. Treating Tobacco Dependence and Use. [Google Scholar]
- Forster KI, Forster JC. DMDX: A Windows display program with millisecond accuracy. Behavior Research Methods, Instruments, & Computers. 2003;35:116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: Effect of predicatability. Biological Psychiatry. 2006;60:752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Gross TM, Jarvik ME, Rosenblatt MR. Nicotine abstinence produces content-specific Stroop interference. Psychopharmacology. 1993;110:333–336. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. The Cochrane Database of Systematic Reviews, Issue 4. 2004 doi: 10.1002/14651858.CD000031. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: Evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: An electronic diary study. Journal of Abnormal Psychology. 2006;115:454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller MW, Patrick CJ. Trait differences in affective and attentional responding to threat revealed by emotional Stroop interference and startle reflex modulation. Behavior Therapy. 2001;31:757–776. [Google Scholar]
- Mogg K, Bradley BP. Selective processing of smoking related cues in smokers: Manipulation of deprivation level and comparison of three measures of processing bias. Journal of Psychopharmacology. 2002;16:385–392. doi: 10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- Munafo M, Mogg K, Roberts S, Bradley BP, Murphy M. Selective processing of smoking-related cues in current smokers, exsmokers and never-smokers on the modified Stroop task. Journal of Psychopharmacology. 2003;17:310–316. doi: 10.1177/02698811030173013. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow D, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Powell J, Tait S, Lessiter J. Cigarette smoking and attention to signals of reward and threat in the Stroop paradigm. Addiction. 2002;97:1163–1170. doi: 10.1046/j.1360-0443.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. The role of attentional bias in substance abuse. Behavioral and Cognitive Neuroscience Reviews. 2004;3:243–260. doi: 10.1177/1534582305275423. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Caulfield D, King L, Goode A. Moving out of the laboratory: Does nicotine improve everyday attention? Behavioural Pharmacology. 2000;11:621–629. doi: 10.1097/00008877-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: A facial coding analysis. Experimental and Clinical Psychopharmacology. 2003;11:218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Interactions of pain intensity and cognitive load: The brain stays on task. Cerebral Cortex. 2007;17:1412–1422. doi: 10.1093/cercor/bhl052. [DOI] [PubMed] [Google Scholar]
- Sewards T, Sewards M. The medial pain system: Neural representations of the motivational aspect of pain. Brain Research Bulletin. 2002;59:163–180. doi: 10.1016/s0361-9230(02)00864-x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Hickcox M. First lapses to smoking: Within subjects analysis of real time reports. Journal of Consulting and Clinical Psychology. 1996;2:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. The Cochrane Database of Systematic Reviews, Issue 3. 2004 doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Waters AJ, Sayette MA. Implicit cognition and tobacco addiction. In: Wiers RW, Stacy AW, editors. Handbook of Implicit Cognition and Addiction. Thousand Oaks, CA: Sage; 2006. [Google Scholar]
- Waters AJ, Feyerabend C. Determinants and effects of attentional bias in smokers. Psychology of Addictive Behaviors. 2000;14:111–120. doi: 10.1037//0893-164x.14.2.111. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA, Franken IHA, Schwartz JE. Generalizability of carry-over effects in the emotional Stroop task. Behaviour Research and Therapy. 2005;43:715–732. doi: 10.1016/j.brat.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Sayette MA, Wertz JM. Carry-over effects can modulate emotional Stroop effects. Cognition & Emotion. 2003;17:501–509. doi: 10.1080/02699930143000716. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:324–333. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter D, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychology of Addictive Behaviors. 2001;15:268–271. [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Matthews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]