Abstract
Novel two-step solution phase protocols for the synthesis of dihydroquinazolines and fused dihydroquinazoline-benzodiazepine tetracycles are reported. The methodology employs the Ugi reaction to assemble desired diversity and acid treatment enables ring closing transformations. The protocols are further facilitated by the use of microwave irradiation and n-butyl isocyanide to control the rate of each ring forming transformation.
Introduction
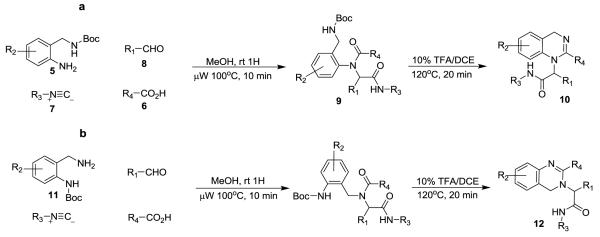
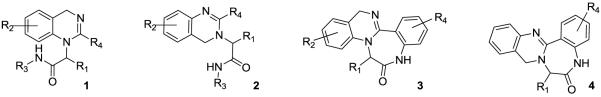
The elucidation of the complete human genome in 20011 has resulted in a dramatic increase in the demand for the identification of small molecules to validate the pharmacological potential of new macromolecular targets2,3. In particular, isonitrile based methodologies4-6, followed by a variety of secondary ring forming transformations, have shown great utility in concisely producing highly functionalized and drug-like scaffolds with high iterative efficiency potential7-9. Methodologies developed in this laboratory have proven quite productive, delivering examples where initial hits have progressed into clinical trials for the treatment of both HIV infection10,11 and pre-term labor,12-15 importantly without the need to ‘scaffold hop’. Recently, we have reported a contrite two-step synthesis of triazabenzulenones16 that represents the first post-condensation Ugi modification employing two internal amino nucleophiles and a subsequent report that utilized microwave irradiation with n-butyl isonitrile in place of a traditional ‘designer convertible isonitrile’17 to form benzodiazepines and diketopiperazines. Herein, we report two-step syntheses that yield dihydroquinazolines 1 and 2, and fused dihydroquinazoline-benzodiazepine tetracycles 3 and 4. The tetra-cyclic scaffolds represent a second example of a post-condensation Ugi modification employing two internal amino nucleophiles and all 4 syntheses rely on the reduced reactivity of an n-butyl amide carbonyl derived from n-butyl isonitrile relative to traditional convertible isonitriles to ensure the correct sequence of ring forming events. It was envisioned that the dihydroquinazoline core could be produced in two steps: an Ugi reaction with mono-Boc protected 2-aminobenzylamines 5, supporting aldehydes 8, non-convertible isonitriles 7, and carboxylic acids 6 to yield the condensation product 9 followed by acid promoted deprotection and cyclization. Both the 1,4-dihydroquinazoline (Scheme 1a, 10) and 3,4-dihydroquinazoline (Scheme 1b, 12) scaffolds can be produced from the corresponding mono-protected 2-aminobenzylamine input 5 or 11. We have previously demonstrated a similar acid promoted dehydration of an Ugi condensation product to generate an aromatic benzimidazole core16,18 and this report expands the use of such methodology to obtain non-aromatic bi-cyclic rings such as the dihydroquinazolines 1 and 2 with significantly different physicochemical properties and spacial positioning of decorating functionality.
Scheme 1.
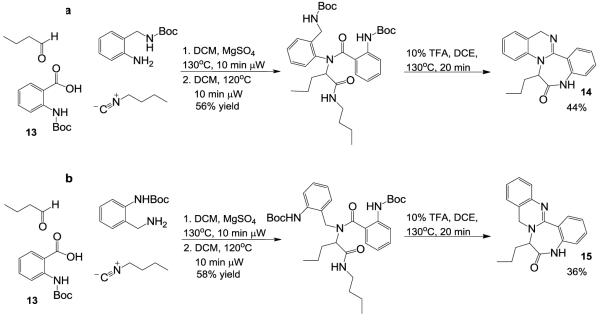
Optimal yields for the Ugi reaction were found to occur via pre-formation of the Schiff base in methanol for 30 minutes, followed by addition of the isonitrile and carboxylic acid inputs with subsequent microwave irradiation at 100°C for 10 minutes (isolated yields 48-85%). The Ugi product was then treated with 10% TFA/DCE (irradiated at 120°C) to form the desired quinazoline scaffolds 10 and 12 core in good yield (46-65% ~ isolated overall yield for two steps). With this protocol in hand, it was hypothesized addition of a second protected amine, through the use of an N-Boc protected anthranilic acid 13, would enable formation of novel fused dihydroquinazoline-benzodiazepines (Scheme 2a and 2b, 14 and 15) after deprotection and two sequential cyclization steps. However, addition of the bulky Boc-protected amine group resulted in a dramatic decrease in the yield of the Ugi reaction (<5%).
Scheme 2.
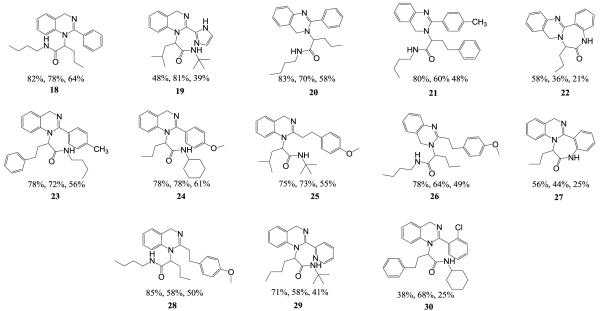
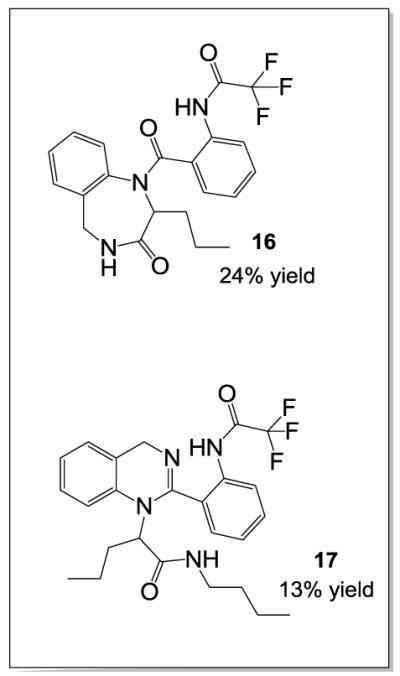
This effect was also observed in the 1,4-quinazoline series with 2-chlorobenzoic acid (Figure 3, 30). Circumventing this problem, pre-formation of the Schiff base in toluene under microwave irradiation with removal of water (MgSO4) provided the Schiff base in quantitative yields. Subsequent reaction in MeOH afforded the desired Ugi product in 44% yield [Note: observed by-products arose from methanol addition to the Schiff base and the Passerini reaction]. Interestingly, reactions in trifluoroethanol yielded similar results – a strategy that is often successful in reducing solvent participation in the Ugi reaction. A modest improvement in yield was observed by pre-forming the Schiff base in dichloromethane in the presence of MgSO4 (microwave, 120°C). Following addition of supporting reagents, the reaction was irradiated at 120°C for 10 minutes with an acceptable improvement in yield (56%, Scheme 2a). Removal of the 2 Boc groups, cyclo-dehydration to the dihydroquinazoline core and concomitant cyclization onto the n-butyl amide, afforded the desired fused 1,4-dihydroquinazoline-benzodiazepines 14 and 15 in acceptable yields upon simple acid treatment and microwave irradiation. Not surprisingly, the major side products of the cascade reaction were the benzodiazepine trifluoroacetamide 16 (24% yield), and the bicyclic trifluoroacetamide 17 (13% yield).
Figure 3.
Reported % yields = Ugi Yield, Cyclization Yield, Overall Yield
The scope of the methodology was evaluated with a selection of different reagents. Cyclization reactions of purified Ugi products were run in series on a Biotage Initiator 8 microwave and were purified in a sequential manner on a Biotage Isolera 4 system utilizing neutralized silica gel columns. The observed high polarity of dihydroquinazolines can be attributed to their relatively high pKa values (Table 1). Thirteen examples are presented 18 through 30 containing all four dihydroquinazoline cores with isolated overall yields for the two-step procedure ranging from 21% to 64% Figure 3.
Table 1.
pKa values were estimated using ACD/pKa
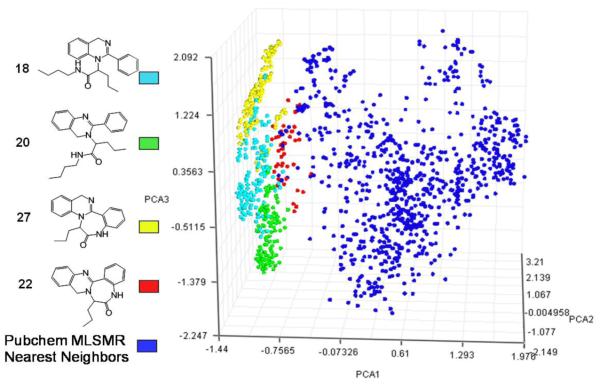
To demonstrate scaffold uniqueness, virtual libraries for 1 through 4 were enumerated (comprising scaffold 1—168 compounds; 2—168 compounds, 3—144 compounds, 4—48 compounds) and compared with the 375,000 compounds in the NIH molecular libraries small molecule repository (MLSMR). A total of 1043 nearest neighbors were indentified and a principle component analysis21 clearly demonstrates the unique diversity space occupied by expanded libraries of the four scaffolds described herein Figure 4.
Figure 4.
In summary, we have reported concise two-step solution phase syntheses that afford bi-cyclic dihydroquinazolines and fused tetra-cyclic dihydroquinazoline-benzodiazepines, which are under-represented in the literature and the MLSMR.22 With amenability to high-throughput solution phase synthesis, it is expected the methodology will be embraced by the lead generation community.
Figure 1.
Representative dihydroquinazoline bicyclic and tetracyclic scaffolds
Figure 2.
Acknowledgements
We would like to thank the Office of the Director, NIH and the National Institute of Mental Health for funding (1RC2MH090878-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bienayme H, Hulme C, Oddon G, Schmitt P. Chemistry. 2000;6:3321–9. doi: 10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Borthwick AD, Davies DE, Exall AM, Hatley RJ, Hughes JA, Irving WR, Livermore DG, Sollis SL, Nerozzi F, Valko KL, Allen MJ, Perren M, Shabbir SS, Woollard PM, Price MA. J Med Chem. 2006;49:4159–70. doi: 10.1021/jm060073e. [DOI] [PubMed] [Google Scholar]
- 3.Borthwick AD, Davies DE, Exall AM, Livermore DG, Sollis SL, Nerozzi F, Allen MJ, Perren M, Shabbir SS, Woollard PM, Wyatt PG. J Med Chem. 2005;48:6956–69. doi: 10.1021/jm050557v. [DOI] [PubMed] [Google Scholar]
- 4.Domling A. Chemical Reviews. 2005;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 5.Grenet O. Pharmacogenomics J. 2001;1:11–2. doi: 10.1038/sj.tpj.6500010. [DOI] [PubMed] [Google Scholar]
- 6.Habashita H, Kokubo M, Hamano S, Hamanaka N, Toda M, Shibayama S, Tada H, Sagawa K, Fukushima D, Maeda K, Mitsuya H. J Med Chem. 2006;49:4140–52. doi: 10.1021/jm060051s. [DOI] [PubMed] [Google Scholar]
- 7.Hulme C, Chappeta S, Dietrich J. Tetrahedron Lett. 2009;50:4054–4057. doi: 10.1016/j.tetlet.2010.06.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulme C, Chappeta S, Griffith C, Lee Y-S, Dietrich J. Tetrahedron Lett. 2009;50:1939–1942. [Google Scholar]
- 9.Hulme C, Dietrich J. Mol Divers. 2009;13:195–207. doi: 10.1007/s11030-009-9111-6. [DOI] [PubMed] [Google Scholar]
- 10.Hulme C, Gore V. Curr Med Chem. 2003;10:51–80. doi: 10.2174/0929867033368600. [DOI] [PubMed] [Google Scholar]
- 11.Hulme C, Maggiora GM. Curr Opin Chem Biol. 2008;12:257–9. doi: 10.1016/j.cbpa.2008.04.601. [DOI] [PubMed] [Google Scholar]
- 12.Hulme C, Nixey T. Curr Opin Drug Discov Devel. 2003;6:921–9. [PubMed] [Google Scholar]
- 13.Liddle J, Allen MJ, Borthwick AD, Brooks DP, Davies DE, Edwards RM, Exall AM, Hamlett C, Irving WR, Mason AM, McCafferty GP, Nerozzi F, Peace S, Philp J, Pollard D, Pullen MA, Shabbir SS, Sollis SL, Westfall TD, Woollard PM, Wu C, Hickey DM. Bioorg Med Chem Lett. 2008;18:90–4. doi: 10.1016/j.bmcl.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa R, Nishiyama T, Hisaichi K, Matsunaga N, Minamoto C, Habashita H, Takaoka Y, Toda M, Shibayama S, Tada H, Sagawa K, Fukushima D, Maeda K, Mitsuya H. Bioorg Med Chem Lett. 2007;17:727–31. doi: 10.1016/j.bmcl.2006.10.084. [DOI] [PubMed] [Google Scholar]
- 15.Reiss T. Trends in Biotechnology. 2001;19:496–499. doi: 10.1016/s0167-7799(01)01811-x. [DOI] [PubMed] [Google Scholar]
- 16.Tempest P, Ma V, Thomas S, Hua Z, Kelly MG, Hulme C. Tetrahedron Lett. 2001;42:4959–4962. [Google Scholar]
- 17.Venter JC, et al. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 18.Wyatt PG, Allen MJ, Borthwick AD, Davies DE, Exall AM, Hatley RJ, Irving WR, Livermore DG, Miller ND, Nerozzi F, Sollis SL, Szardenings AK. Bioorg Med Chem Lett. 2005;15:2579–82. doi: 10.1016/j.bmcl.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 19.General Preparation of dihydroquinazolines, 19: To a solution of butyraldehyde (164 μl, 1.800 mmol) in methanol (3 mL) in a 2.0-5.0 ml microwave vial was added tert-butyl 2-aminobenzylcarbamate (200 mg, 0.900 mmol). The reaction was stirred at room temperature for 30 minutes followed by the addition of n-butyl isocyanide (95 μl, 0.900 mmol) and benzoic acid (110 mg, 0.900 mmol). The reaction was then irradiated for 10 minutes at 100°C. The solvent was evaporated in vacuo, crude material taken up in 3 mL 10% EtOAc/Hexane and loaded onto a 12 g silica column with purification performed on a Biotage® Isolera 4 (gradient 10%-20% EtOAc/Hexane) to yield the desired Ugi product (344 mg, 0.738 mmol, 82 % yield). The Ugi product was then taken up in 5 mL 10% TFA/DCE, transferred to a 2-5 mL microwave vial and irradiated at 120°C for 20 minutes. The reaction was then poured into a separatory funnel that contained 50 mL saturated sodium carbonate and 50 mL DCM. The organic layer was collected and aqueous layer further extracted with DCM (50 ml). The combined organics were dried over MgSO4, concentrated onto neutralized silica, and purified on a Biotage Isolera (25 g neutralized column, 20% EtOAc/1.5% TEA/Hexane) to yield the desire 1,4-dihydroquinazoline product, N-butyl-2-(2-phenylquinazolin-1(4H)-yl)pentanamide 20 (209 mg, 0.576 mmol, 64 % yield). 1H NMR (300 MHz, CDCl3): 7.50 (dd, 2H, J=1.8 Hz, J=14.7 Hz), 7.31-7.45 (m, 3H), 7.12 (dt, 1H, J=7.5 Hz, J=0.9 Hz), 7.05 (dt, 1H, J=0.9 Hz, J=7.5 Hz), 6.98 (d, 1H, J=5.4 Hz), 6.78 (d, 1H, J=7.5 Hz). 6.45 (t, 1H, J=5.4 Hz), 4.83 (d, 1H, J=18.6 Hz), 4.74 (d, 1H, J=18.6 Hz), 4.25 (dd, 1H, J=4.2 Hz, J=10.2 Hz), 3.29 (m, 2H), 1.7-2.2 (m, 2H), 1.45-1.6 (m, 2H), 1.20-1.40 (m, 4H), 0.91 (t, 3H, J=4.5 Hz), 0.79 (t, 3H, J=4.2 Hz). 13C NMR (75 MHz, CDCl3): 171.05, 158.18, 137.03, 136.62, 130.05, 129.09 (2C), 128.40 (2C), 127.32, 126.78, 124.66, 123.92, 116.74, 62.99, 49.30, 39.90, 31.91, 29.89, 20.52, 20.18, 14.14, 14.03.
- 20.General procedure for preparation of dihydroquinazoline-benzodiazepine tetracycle 26: To a 2.0-5.0 mL microwave vial was added a solution of butyraldehyde (246 μl, 1.350 mmol) in DCM (3ml), tert-butyl (2-(aminomethyl)phenyl)carbamate (300 mg, 1.350 mmol), and MgSO4. The reaction was sealed and irradiated for 10 minutes at 120°C to yield the Schiff base in quantitative yields (Rf 0.82, 25% EtOAc/Hex). n-Butylisocyanide (143 μl, 1.350 mmol) and 2-((tert-butoxycarbonyl)amino)benzoic acid (320 mg, 1.350 mmol) were added and reacted at 120°C under microwave irradiation. The crude mixture was poured into a separatory funnel containing saturated sodium bicarbonate and DCM. The organic layer was collected, dried, loaded onto 2 g silica gel and purified on a Biotage Isolera (40g column, gradient 10%-35% EtOAc/Hex) to yield the Ugi product (478 mg, 0.783 mmol, 58 % yield). The Ugi product was then taken up in 10% TFA/DCE (5ml), transferred to a 2-5 mL microwave vial and irradiated at 130°C for 20 minutes. After heating, the reaction was poured into a separatory funnel that contained saturated sodium carbonate (50 mL) and DCM (50 mL). The organic layer was collected and then the aqueous layer was extracted twice more with DCM (2×50 mL). The combined organics were dried over MgSO4, concentrated onto neutralized silica, and purified on a Biotage Isolera (25g neutralized column, 50% EtOAc/2.0% TEA/Hexane) to yield the desire 3,4-dihydroquinazoline-benzodiazepine tetracyclic product, 7-propyl-7,9-dihydrobenzo[5,6]-[1,4]diazepino[7,1-b]quinazolin-6(5H)-one 27 (87 mg, 0.283 mmol, 21 % yield). 1H NMR (300 MHz, CDCl3): 8.65 (s, 1H), 8.08 (d, 1H, J=7.2 Hz), 7.45 (t, 1H, J=7.2 Hz), 7.2-7.4 (m, 3H), 7.1-7.2 (m, 1H), 7.10 (t, 1H (dd, 2H, J=1.8 Hz, J=14.7 Hz), 6.95-7.10 (m, 3H), 4.41 (d, 1H, J=13.2 Hz), 4.27 (d, 1H, J=13.2 Hz), 4.12 (t, 1H, 7.5 Hz), 1.8-2.1 (m, 2H), 1.2-1.5 (m, 4H), 0.95 (t, 3H, J=7.2 Hz). 13C NMR (75 MHz, CDCl3): 170.95, 143.533, 137.11, 132.12, 131.64, 129.16, 128.94, 126.07, 125.59, 125.46, 124.48, 122.63, 121.22, 56.41, 43.14, 27.79, 19.42, 14.32.
- 21.In an exercise to demonstrate uniqueness, virtual libraries for all four scaffolds were enumerated with Symyx Draw 3.2 (comprising scaffold 1—168 compounds; 2—168 compounds, 3—144 compounds, 4—48 compounds). The parent structure of each compound was then compared with the 375,000 compounds available in the NIH molecular libraries small molecule repository (MLSMR) described by pharmacophore fingerprints using PowerMV v0.61. A total of 1043 similar compounds were selected using a Tanimoto Similarity function as implemented in the nearest neighbor search tool in PowerMV (distance threshold for library 1 and 2 = 0.5; library 3 = 0.29; library 4 = 0.4). The libraries and the MLSMR subset (1571 compounds total) were imported into MOE (Molecular Operating Environment v2009.11, Chemical Computing Group) and represented by structural fingerprints (MACCS keys). Tanimoto similarities were computed based on the MACCS keys, which generated a 1571 by 1571 matrix. The columns of the matrix were used as input for a principle component analysis and the first three principle components were represented graphically using MOE (Figure 4).
- 22.(a) Krasavin M, Busel A, Parchinsky V. Tetrahedron Lett. 2009;50:5945–5950. [Google Scholar]; (b) Lygin Alexander V., de Meijere Armin. Org. Lett. 2009;11:389–392. doi: 10.1021/ol802659m. [DOI] [PubMed] [Google Scholar]