Abstract
Using electrophoresis, sequencing and enzymatic digestion, we show that the group I intron from the cyanobacterium Anabaena sp. PCC 7120 catalyzes phosphodiester bond formation using a triphosphate on the 5′-terminal nucleotide, much like protein polymerases and engineered ribozymes. In the process, this ribozyme forms a unique circular RNA that incorporates the exogenous guanosine cofactor added during self-splicing. This finding may have relevance to a prebiotic RNA world and to modern biology.
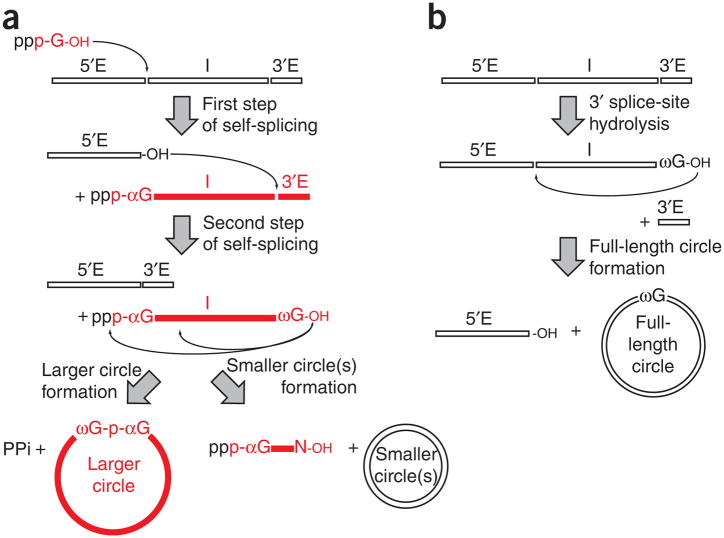
Group I introns self-splice in two chemical steps upon addition of a cofactor that can be guanosine (1) or one of its 5′-phosphorylated forms—guanosine 5′-monophosphate (GMP or pG, 2), guanosine 5′-diphosphate (GDP, 3) or guanosine 5′-triphosphate (GTP, 4)1. During the first step, this exogenous guanosine (here called ‘αG’) binds to a guanosine binding site inherent to the folded RNA structure; it then attacks the phosphorus atom at the 5′ splice site, becoming covalently attached to the 5′ end of the intron through a 3′,5′ linkage (Fig. 1a). A conformational change then brings the conserved guanosine at the 3′ end of the intron (called ‘ωG’) into the guanosine binding site in place of the αG. During the second chemical step, the free 3′ hydroxyl of the 5′ exon attacks the 3′ splice site, resulting in the formation of a phosphodiester bond that ligates the two exons. The released intron RNA is a linear RNA molecule having a guanosine at both the 5′ end (αG) and the 3′ end (ωG). This intron can undergo further transesterification reactions, thereby forming circles that contain fewer nucleotides than the linear intron2 (Fig. 1a). A parallel pathway that requires hydrolysis at the 3′ splice site leads to full-length circles (Fig. 1b). The formation of full-length circles is a general property of group I introns that may be linked to intron mobility3.
Figure 1.
Reaction pathways catalyzed by group I introns. (a) Self-splicing is followed by the formation of a larger circle (left; this work) or smaller circles (right). The exogenous [α-32P]GTP used here is shown in red. The subsequently radioactively labeled products are colored in red. (b) Full-length circle formation as described previously3,21. 5′E, 5′ exon; I, intron; 3′E, 3′ exon.
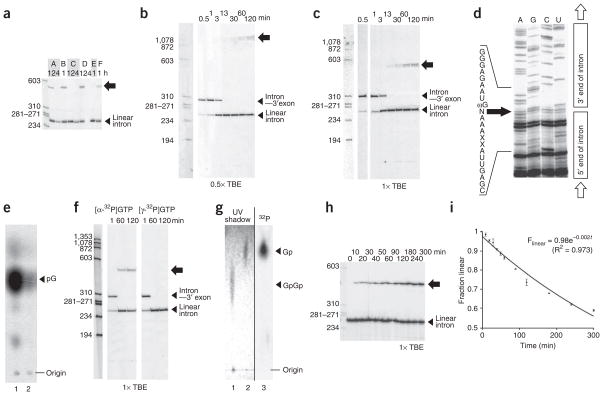
We recently assayed for self-splicing activity of 12 group I introns under 6 reaction conditions, in the presence of [α-32P]GTP (5) as the cofactor4. While screening for catalytic activity, an unexpected product was observed for a 249-nucleotide group I intron embedded in a tRNALeu gene from the cyanobacterium Anabaena sp. PCC 71205,6 (Fig. 2a). This 32P-labeled product had an apparent size around 550–600 nucleotides—larger than the initial unspliced precursor RNA (334 nucleotides) and much larger than the self-spliced linear intron, which was observed (as expected) to be around 250 nucleotides in size. No labeled product of that size could be accounted for by known self-splicing mechanisms.
Figure 2.
New circular RNA. (a) Anabaena precursor RNA containing the group I intron after incubation in 0.2 mM GTP, 1.0 μCi ml−1 [α-32P]GTP, under six reaction conditions (A: 15 mM MgCl2, 25 mM NaCl, 25 mM HEPES pH 7.5, 32 °C; B: same as A except 42 °C; C: same as A except 1.0 M NaCl; D: same as A except 200 mM MgCl2; E: 100 mM Mg(OAc)2, 1.0 M NH4OAc, 25 mM HEPES pH 7.5, 37 °C; F: 15 mM MgCl2, 0.5 M KCl, 5 mM DTT, 2 mM spermidine, 40 mM Tris pH 7.5, 50 °C) (from ref. 4). Block arrow, new species. (b) Reaction time-course (condition A), electrophoresis in 0.5× TBE. (c) Same except electrophoresis in 1.0× TBE. (d) Sequencing across the circularization site. N, incorporated residue. (e) Complete nuclease P1 digestion followed by TLC identifies αG incorporated into the circle (lane 1: P4–P6 RNA body-labeled with [α-32P]GTP and P1-digested; lane 2: gel-purified circle incorporating a single label at the circularization site and P1-digested). (f) Side-by-side time-course experiment as in b using [α-32P]GTP (left) or [γ-32P]GTP (right). (g) Complete RNase A plus RNase T1 digestion to determine the regiospecificity at the circularization site, visualized by TLC (lane 1: dinucleotide GpGp control, unlabeled; lane 2: mononucleotide Gp control, unlabeled; lane 3: gel-purified circle incorporating a single label at the circularization site, digested with both RNases). (h) Reaction time-course starting with purified linear intron (condition: A), electrophoresis in 1.0× TBE. (i) Determination of first-order rate constant of circularization. Error bars, s.d. of four experiments (10, 30, 60, 120 min) or two experiments (other data points). Full-length versions of these gels and repeat experiments are shown in Supplementary Figs. 6–9 online.
The low electrophoretic mobility of this RNA species suggested that it was either multimeric, circular or branched. In order to test for this, a time-course reaction over two hours was split and loaded onto two separate polyacrylamide gels, which were run using TBE buffer (1× TBE is 100 mM Tris-base, 83 mM boric acid, 1.0 mM EDTA) at a concentration of either 0.5× or 1× (see Supplementary Methods online) (Fig. 2b,c). This experiment first revealed that the formation of the low-mobility product followed the release of the linear intron, starting between 3 and 13 min after RNA splicing was initiated. Second, the low-mobility band had different relative mobilities in the two TBE concentrations, which indicates that it corresponded to either a branched or a circular species7. A similar time-course experiment using the gel-purified linear intron as the starting material (which contained the αG, but in the absence of any additional free [α-32P]GTP) confirmed a precursor-product relationship between the excised linear intron and the new RNA (Supplementary Fig. 1a online). The reverse reaction was also demonstrated: the linear intron could be obtained after incubation of the gel-purified low-mobility product under similar reaction conditions (Supplementary Fig. 1a).
Sequencing the gel-purified low-mobility product by reverse transcription using a primer binding to a region near the 5′ end of the Anabaena intron revealed that this product was in fact a covalently closed circle (Fig. 2d). This circle contained an additional residue between the ωG and the first residue at the 5′ end of the intron. The presence of this additional nucleotide was also apparent after sequencing of the circularization product obtained from the precursor RNA instead of the purified excised linear intron, which in fact corresponded to both this circle and the full-length circle previously observed6 (Supplementary Fig. 1b). The pausing of the reverse transcriptase observed across the sequencing gel at the position of this extra residue—likely due to circle reopening (Supplementary Fig. 1a)—masked its identity. We anticipated this nucleotide to be the αG and confirmed this prediction by performing a complete digestion of the circle using nuclease P1. Nuclease P1 cleaves any sequence and leaves a phosphate group on the 5′ end of the mononucleotide products (Supplementary Fig. 2a online). A single spot was observed next to the guanosine marker on a TLC plate, which is consistent with GMP having been incorporated into the circle (the label at the α position of the αG would be the only radioactive label in the circle) (Fig. 2e).
When splicing was performed in the presence of [γ-32P]GTP (6), the intron was excised but no labeled circle was observed, which suggests that circle formation was accompanied by inorganic pyrophosphate release (Fig. 2f). Further supporting this mechanism, the excised intron derived from body-labeled precursor RNA self-spliced using GMP was unable to circularize (Supplementary Fig. 3 online). Such a reaction mechanism is similar to that used by RNA and DNA polymerases, as well as several in vitro–evolved ribozymes (ref. 8; reviewed in ref. 9). However, certain ribozymes catalyze the formation of a 2′,5′ bond instead of the 3′,5′ bond found in most natural RNA molecules10. In order to test the regiospecificity of the linkage at the circularization site, we performed a complete digestion of the circle using a combination of RNases T1 and A, which specifically cleave 3′,5′ bonds (Supplementary Methods) after guanosine and uracil or cytosine, respectively (Supplementary Fig. 2b). In the event of a 2′,5′ bond at the circularization site, the product would have been a GpGp dinucleotide (7), whereas a 3′,5′ bond at the circularization site would have been cleaved by RNase T1, resulting in guanosine 3′-monophosphate (Gp, 8) as the final product. The product observed after migration on a TLC plate was the mononucleotide, which indicates that the circle was linked by a 3′,5′ bond (the only label in the circle came from the phosphorus atom connecting the αG and the ωG; Fig. 2g).
To further characterize the catalytic properties of this natural RNA ligase, we measured the rate of circle formation in the presence of 15 mM MgCl2, 25 mM NaCl at pH 7.5 and 32 °C. The first-order rate constant was 2.0 × 10−3 min−1, which is the same order of magnitude as that found for a ribozyme in vitro evolved from the P4–P6 domain of the Tetrahymena thermophila group I intron11 (3.0 × 10−3 min−1; Fig. 2h,i). This rate is modest compared to that achieved by some protein enzymes and in vitro–evolved ribozymes under optimal conditions (>0.1 min−1)9. Nevertheless, the rate enhancement over the uncatalyzed reaction is still 104 (using 2 × 10−7 min−1 as an estimate of the rate of the uncatalyzed reaction9) (Supplementary Fig. 4 online). Finally, this rate constant was unchanged between 2.0 and 50 nM RNA, which indicates that the reaction is unimolecular (Supplementary Fig. 5 online).
These results expand on growing observations that a common RNA scaffold can support various catalytic mechanisms12,13. Yet the catalytic site for this circularization reaction remains to be pinpointed. The simplest explanation would be that the guanosine binding site is being used, and the free 3′ hydroxyl group of the ωG (still bound to the guanosine binding site after the second step of self-splicing) attacks the α phosphate of the (α-32P)GTP at the 5′ end. Although we have no evidence that such circle formation occurs in vivo, having an additional nucleotide could be an advantage for the subsequent reinsertion of these introns into genomic sequences with no loss of information. Such a process would be analogous to a recombination event during which the extra nucleotide could serve as a leaving group for the insertion and could thus be swapped for the host sequence. Consequently, other ribozymes able to catalyze a similar reaction could be found among the numerous instances in the literature where [α-32P]GTP has been used to identify introns in cellular RNA extracts.
In fact, the original discoverers of the Anabaena group I intron had also observed a product with low electrophoretic mobility. At the time, they related it to a highly structured product of the first step of self-splicing, because the corresponding product was resistant to alkaline phosphatase5. This band may now be attributed to the new circular species characterized here. Subsequent studies of the reactions catalyzed by the Anabaena intron—see for example references 6,14—used guanosine in place of GTP. Hence, the reaction reported herein would not have occurred and thereby remained unidentified for 18 years.
Whether this reaction is a relic of a prebiotic RNA world, or simply just another illustration of the plasticity of the group I intron scaffold, is unclear. The features of using a nucleoside triphosphate and extending an RNA chain by formation of a 3′,5′ bond are expected characteristics of a prebiotic ribozyme, if it is to transition to modern biochemistry15. However, obtaining the 3′,5′ regiospecificity is difficult, as prebiotic simulations of RNA synthesis often yield 2′,5′ linkages16,17. Even though the first catalytic RNAs discovered (group I introns and the RNA component of RNase P) were considered to provide the much-anticipated evidence that an RNA world could have existed, these ribozymes only performed RNA cleavage or transesterification reactions from substrates that contained at least two nucleotides connected by phosphodiester linkages1. Only later was it discovered that ribozymes can use the 5′-terminal triphosphate of an RNA molecule for RNA ligation, in the case of a modified group II intron18 and ribozymes identified by in vitro selection (ref. 8; reviewed in ref. 9). The results presented herein illustrate how a natural group I intron is also able to perform 3′,5′ RNA ligation. Together with the earlier findings that ribozymes selected by in vitro evolution from group I introns can catalyze RNA ligation11,19, this provides additional evidence that prebiotic replication could have been catalyzed by ribozymes related to group I introns20.
Supplementary Material
Acknowledgments
We thank A. Zaug for experimental suggestions and insightful discussions; A. Gooding (Howard Hughes Medical Institute) for preparing the T7 RNA polymerase; D. Zappulla (Johns Hopkins University) for the gift of RNase A; the remaining members of the Cech laboratory, as well as H. Nielsen, O. Uhlenbeck, S. Silverman and M. Caruthers, for helpful discussions; and H. Nielsen, S. Silverman, A. Gooding and A. Zaug for careful reading of the manuscript.
Footnotes
Note: Supplementary information and chemical compound information is available on the Nature Chemical Biology website.
AUTHOR CONTRIBUTIONS
Q.V. and T.R.C. designed the study and wrote the manuscript; Q.V. performed the experiments.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Cech TR. In: The RNA World. 1. Gesteland RF, Atkins JF, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York, USA: 1993. pp. 239–269. [Google Scholar]
- 2.Zaug AJ, Grabowski PJ, Cech TR. Nature. 1983;301:578–583. doi: 10.1038/301578a0. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen H, et al. RNA. 2003;9:1464–1475. doi: 10.1261/rna.5290903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vicens Q, Paukstelis PJ, Westhof E, Lambowitz AM, Cech TR. RNA. 2008;14:2013–2029. doi: 10.1261/rna.1027208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu MQ, Kathe SD, Goodrich-Blair H, Nierzwicki-Bauer SA, Shub DA. Science. 1990;250:1566–1570. doi: 10.1126/science.2125747. [DOI] [PubMed] [Google Scholar]
- 6.Zaug AJ, McEvoy MM, Cech TR. Biochemistry. 1993;32:7946–7953. doi: 10.1021/bi00082a016. [DOI] [PubMed] [Google Scholar]
- 7.Kjems J, Egebjerg J, Christiansen J. In: Laboratory Techniques in Biochemistry and Molecular Biology: Analysis of RNA-Protein Complexes In Vitro. van der Vliet PC, editor. Elsevier; Amsterdam, The Netherlands: 1998. pp. 181–232. [Google Scholar]
- 8.Bartel DP, Szostak JW. Science. 1993;261:1411–1418. doi: 10.1126/science.7690155. [DOI] [PubMed] [Google Scholar]
- 9.Joyce GF. Angew Chem Int Edn Engl. 2007;46:6420–6436. doi: 10.1002/anie.200701369. [DOI] [PubMed] [Google Scholar]
- 10.Murray HL, et al. Mol Cell. 2001;8:201–211. doi: 10.1016/s1097-2765(01)00300-8. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka W, Ikawa Y, Jaeger L, Shiraishi H, Inoue T. RNA. 2004;10:1900–1906. doi: 10.1261/rna.7170304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammann C, Westhof E. Genome Biol. 2007;8:210. doi: 10.1186/gb-2007-8-4-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckert B, et al. EMBO J. 2008;27:667–678. doi: 10.1038/emboj.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden BL, Cech TR. Biochemistry. 1996;35:3754–3763. doi: 10.1021/bi952599z. [DOI] [PubMed] [Google Scholar]
- 15.Orgel LE. Trends Biochem Sci. 1998;23:491–495. doi: 10.1016/s0968-0004(98)01300-0. [DOI] [PubMed] [Google Scholar]
- 16.Ferris JP, Usher DA. In: Biochemistry. Zubay G, editor. Macmillan; New York: 1988. pp. 1120–1152. [Google Scholar]
- 17.Lohrmann R, Orgel LE. Tetrahedron. 1978;34:853–855. [Google Scholar]
- 18.Mörl M, Niemer I, Schmelzer C. Cell. 1992;70:803–810. doi: 10.1016/0092-8674(92)90313-2. [DOI] [PubMed] [Google Scholar]
- 19.Jaeger L, Wright MC, Joyce GF. Proc Natl Acad Sci USA. 1999;96:14712–14717. doi: 10.1073/pnas.96.26.14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cech TR. Proc Natl Acad Sci USA. 1986;83:4360–4363. doi: 10.1073/pnas.83.12.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabowski PJ, Zaug AJ, Cech TR. Cell. 1981;23:467–476. doi: 10.1016/0092-8674(81)90142-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.