Abstract
Vesicular neurotransmitter transporters are required for the storage of all classical and amino acid neurotransmitters in secretory vesicles. Transporter expression can influence neurotransmitter storage and release, and trafficking targets the transporters to different types of secretory vesicles. Vesicular transporters traffic to synaptic vesicles as well as large dense core vesicles, and are recycled to synaptic vesicles at the nerve terminal. Some of the intrinsic signals for these trafficking events have been defined and include a dileucine motif present in multiple transporter subtypes, an acidic cluster in the neural isoform of the vesicular monoamine transporter (VMAT2) and a polyproline motif in the vesicular glutamate transporter VGLUT1. The sorting of VMAT2 and the vesicular acetylcholine transporter (VAChT) to secretory vesicles is regulated by phosphorylation. In addition, VGLUT1 uses alternative endocytic pathways for recycling back to synaptic vesicles following exocytosis. Regulation of these sorting events has the potential to influence synaptic transmission and behavior.
Introduction: Neurotransmitter Transporters Subtypes
Synaptic transmission requires two types of neurotransmitter transporters. Following exocytosis, plasma membrane transporters remove neurotransmitters from the synaptic cleft, to terminate signaling and recycle the neurotransmitters for another round of exocytosis (1, 2). Vesicular neurotransmitter transporters package neurotransmitters into the lumen of secretory vesicles to allow exocytotic release (3, 4). Four types of vesicular transporters have thus far been identified* and include: 1) the vesicular acetylcholine transporter or VAChT (5); 2) vesicular monoamine transporters or VMATs (5); 3) the vesicular GABA and glycine transporter, VGAT, also known as the vesicular inhibitory amino acid transporter or VIAAT (6); and 4) the vesicular glutamate transporters or VGLUTs (7). Mammals express two VMAT genes, VMAT1 and 2 (5), and three VGLUTs, VGLUT1, 2 and 3 (7, 8). VMAT2 is expressed in all aminergic neurons in the brain, mast cells and some neurons in the gut (9) whereas VMAT1 is expressed in peripheral neuroendocrine cells including adrenal chromaffin cells (9). VGLUT1 and 2 are expressed in complementary subsets of glutamatergic neurons in the CNS (10). In contrast, the most recently identified isoform, VGLUT3, is co-expressed with VAChT and VMAT2 in a number of cholinergic and aminergic cell types (11–13).
1) The Functional Impact of Vesicular Transporter Trafficking
Vesicular transporter expression can influence neurotransmitter release
The response of a post-synaptic cell to a single exocytotic event (one secretory vesicle) is defined as quantal size. Over the past ten years, it has become increasing clear that vesicular transporter expression can regulate the amount of neurotransmitter contained in secretory vesicles, and thus may influence quantal size (4, 14). In vitro overexpression of mammalian VAChT (15) VGLUT1 (16, 17) and VMAT2 (18), as well as Drosophila VGLUT increases quantal size (19). Conversely, a decrease in quantal size has been observed in VAChT knockdown mice (20), VGAT knockout heterozygotes (21) and VGLUT heterozygotes (22). These data suggest that altered transporter expression may increase or decrease the number of vesicular transporters that localize to each synaptic vesicle; it should be noted, however, this has not yet been demonstrated directly. Indeed, the number of transporters that actually reside on a synaptic vesicle in any system remains unclear, and estimates range from 1 to 14 (23, 24).
Overexpression of Drosophila VMAT increases amine dependent behavior (25) and a number of mouse knockout models indicate that decreasing transporter expression can affect a number of complex behavior in vivo (20, 22, 26–31). Similarly, it is possible that regulated changes in transporter localization have synaptic and behavioral sequelae, underscoring the potential importance of vesicular transporter trafficking in vivo (32–36).
Vesicular transporters traffic to least two distinct types of secretory vesicles
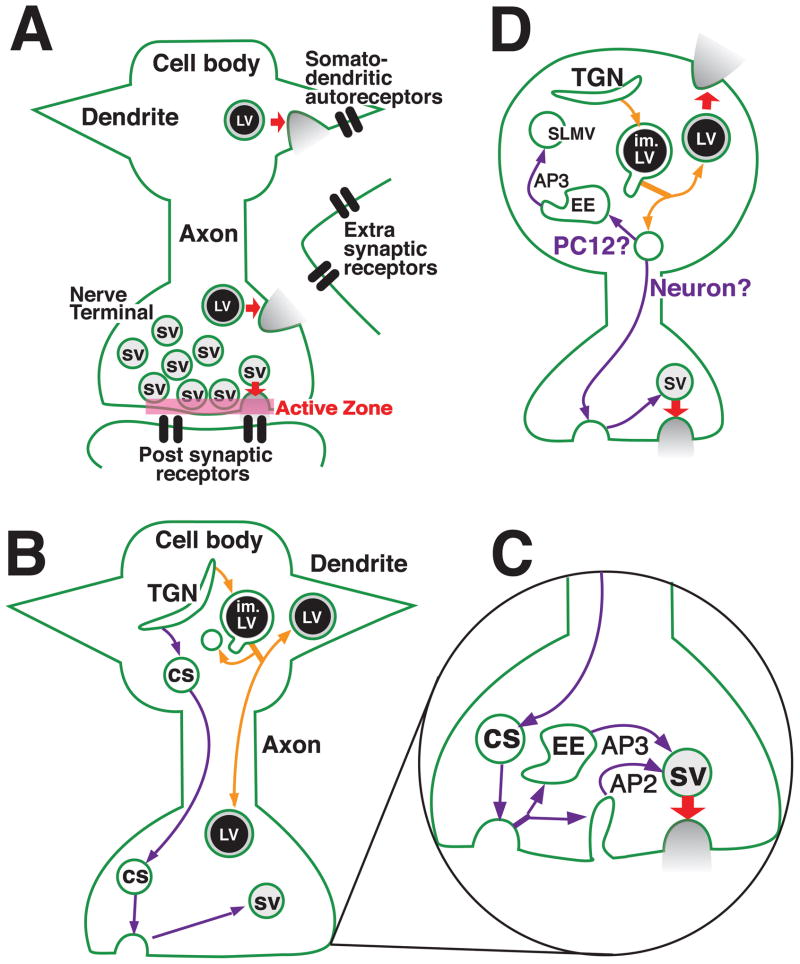
Transporter trafficking may influence synaptic transmission by directing the transporters to different types of secretory vesicles. In addition to their storage in synaptic vesicles, monoamines, and perhaps other neurotransmitters, are stored in large dense core vesicles (LDCVs) in neurons (37, 38). (LDCVs are analogous to the large dense core granules (LDCGs) found in neuroendocrine cells (37, 38)). Synaptic vesicles and LDCVs differ in several important ways, including their subcellular sites of release (see Fig. 1A for additional details). Importantly, VMAT2 localizes to both synaptic vesicles and LDCVs in neurons (39–41). Therefore, regulated changes in VMAT2 trafficking have the potential to alter the relative distribution of the transporter to synaptic vesicles versus LDCVs, and the subcellular site of monoamine release.
Fig. 1.
Trafficking Pathways Proposed for Vesicular Neurotransmitter Transporters. A) Secretory vesicle subtypes. Vesicular transporters localize to different types of secretory vesicles in neurons. These include synaptic vesicles (labeled SV in the figure), and large dense core vesicles (LDCVs, labeled LV in the figure). LVs, but not synaptic vesicles, co-release neuromodulatory peptides, Both SVs and LVs undergo calcium-mediated exocytosis (red arrows) but differ in the site and mode of release. SVs undergo regulated release in nerve terminals and more specifically, at the active zone, a discrete presynaptic region located opposite to post-synaptic receptors. Unlike SVs, LVs are found in both the nerve terminal as well as the cell body and dendrites, and do not closely appose the plasma membrane. The release of neurotransmitter from LVs activates receptors outside of the synapse (labeled “Extra synaptic receptors” in panel A) rather than postsynaptic receptors. The release of neurotransmitter from LVs can also activate somatodendritic autoreceptors, which are found on both the nerve terminal and somatodendritic membranes of most if not all neurons, and provide negative feedback to limit excess neurotransmitter release.
B) Trafficking to secretory vesicle subtypes. Vesicular transporters traffic to SVs and LVs via different routes. For sorting to SVs (purple arrows), proteins are thought the exit the trans Golgi network (TGN) in constitutive secretory (CS) vesicles, and are likely to undergo an endocytic step to generate a mature SV. In contrast, proteins destined for LVs sort into the regulated secretory pathway at the TGN (orange arrows). Immature LVs (im. LVs) are thought to mature via AP1- and PACS-1 dependent removal of some proteins, shown here as a budding event.
C) Synaptic vesicle trafficking at the synapse. The generation of mature SVs as well as SV recycling at the nerve terminal may occur via several mechanisms. Following fusion with the plasma membrane, transporters on constitutive secretory (CS) vesicles may be sorted to a mature SV directly from the plasma membrane using clathrin and the adaptor complex AP2. Alternatively, they may sort to SVs via a two-step pathway involving AP3 and budding from an early endosomal intermediate (EE). Following exocytosis, SVs and vesicular transporters may recycle via either of these pathways. It is also possible that SVs recycle using a kiss-and-run mechanism (not shown).
D) Alternative models of transporter trafficking. Alternative models have been proposed for the sorting of some vesicular transporters to SLMVs in neuroendocrine cells (64, 65) (pathway labeled “PC12?”), and possibly to SVs in neurons (pathway labeled “Neurons?”). In one alternative model, proteins such as VAChT that are removed from immature LVs are sorted to an early endosome where they are sorted into SLMVs. Vesicles that leave immature LVs may represent a “constitutive-like” secretory pathway as originally proposed for secretory granules in pancreatic islet cells (63, 150). Trafficking of constitutive-like vesicles to the nerve terminal might allow sorting to mature SVs via endocytosis.
It addition to monoamines, it is possible that other types of neurotransmitters are released from vesicle types that are similar to LDCVs (42–47) albeit without a dense proteinaceaous core. In glutamatergic, hippocampal neurons, heterologously expressed VMAT2 is sorted to clear vesicles that can be released at somatodendritic sites (44). Similarly, somatodendritic release in dopaminergic neurons is mediated by clear, pleiomorphic vesicles known as tubulovesicular bodies (39, 48, 49). At least one VGLUT isoform, VGLUT3, localizes to dendrites (8, 11) and glutamate release from dendrites regulates signaling in cortical pyramidal cells (45, 46). In addition, GABA release from dendrites has been reported to mediate retrograde signaling in the cortex (46). Since synaptic vesicles are thought to be restricted to the nerve terminal, these data suggest that both VGLUT and VGAT localize to other types of secretory vesicles, and the possibility that taransporter trafficking regulates the subcellular site of GABA and glutamate release.
2) Sorting Vesicular Transporters to Synaptic Vesicles and LDCVs
Synaptic vesicle biogenesis and the possible use of alternative endocytic pathways
During synaptic vesicle biogenesis, proteins bound for synaptic vesicles at the nerve terminal are thought to first traffic to the plasma membrane (50, 51) and vesicular transporters are likely to follow a similar pathway (52). In Fig. 1B, we show exit from the TGN via constitutive secretory vesicles (50), although it remains possible that some synaptic vesicle proteins exit the TGN on more specialized organelles (53). It is generally accepted that vesicles exiting the TGN do not contain the complete complement of proteins found on mature synaptic vesicles, and require an additional trafficking step(s) to fully mature (51), most likely at the nerve terminal (Figs. 1B and C) (51, 54) (but possibly at the soma, see (55)).
To complete the maturation of synaptic vesicles via endocytosis, they may either bud directly from invaginations at the plasma membrane (56) or pass through an endosomal intermediate (57) (Fig. 1C). Both processes may use the clathrin adaptor complex AP2, but the second model requires an additional AP3 dependent step (57). The AP3 dependent pathway is clearly active in the biogenesis of SLMVs in neuroendocrine cells (57–59). AP3 is unlikely to be required for the biogenesis of most synaptic vesicles, or the sorting of vesicular transporters onto mature synaptic vesicles in most neurons (60, 61). However, a mouse knockout of the mu3B subunit of the neuron-specific AP3B complex shows lowers number of synaptic vesicles in inhibitory terminals, and a modest change in the amount of VGAT imunoreactivity in synaptosomes (62); thus, it remains possible that AP3 plays a major role in de novo synaptic vesicle biogenesis in some neurons.
It is also possible that sorting into the regulated secretory pathway is the first step for targeting some cargo to synaptic vesicles. As previously shown in pancreatic islet cells, proteins may be removed from immature LVs and sort into a “constitutive-like” secretory pathway (63). A similar pathway has been suggested to sort VAChT to SLMVs in PC12 cells (see Fig. 1D) (64, 65).
SVs recycle at the nerve terminal via alternative endocytic pathways and localize to at least two functional pools
Endocytosis is required for recycling synaptic vesicles at the nerve terminal as well as their biogenesis (66). Importantly, both fast and slow endocytic pathways have been identified (67, 68).* The slow endocytic pathway, sometimes referred to as bulk, or compensatory endocytosis is particularly important for vesicle recycling under conditions of sustained release. Similar to the AP3 dependent mode of synaptic vesicle biogenesis (57, 71), the slower endocytic path is likely to require a cisternal, or endosomal intermediate and possibly AP3 (67, 68, 72, 73).
Interestingly, trafficking through fast versus slow endocytic pathways may determine whether a synaptic vesicle will go respectively to either a “readily releasable” or “reserve” pool of synaptic vesicles at the nerve terminal (74–77)#. Thus, it is possible that proteins such as VGLUT1 (see below) that use slow and fast modes of endocytosis could show differential sorting to different pools of vesicles.
LDCV biogenesis
LDCVs (LVs in Figs. 1 and 2) are generated via a different route than synaptic vesicles and do not require endocytosis for their biogenesis (79). LDCV proteins are sorted into the regulated secretory pathway as they exit the TGN (80). The first step in the regulated secretory pathway is the generation of an immature large dense core vesicle (“im. LV” in Fig. 1B) (81); maturation requires the removal of some soluble and membrane proteins, represented in Fig. 1 as a budding event. Exit from the TGN and maturation of the LV has been suggested to require the clathrin adaptor complex AP1 (82) and the adaptor protein PACS-1 (83–85) (however see (86)).
Fig. 2.
Sorting signals have been identified in VMAT2 (A) VAChT (B) and VGLUT1 (C). All three proteins are likely to contain twelve hydrophobic domains (5, 112, 113, 151), but only ten may completely cross the membrane in VGLUT1 (152). As in Fig. 1, signals and pathways for sorting to SVs and SLMVs are colored purple; those relevant to LVs are orange. Sorting signals in VGAT/VIAAT are not known and the prediction of ten transmembrane domains (153, 154) has not been experimentally tested. A) In VMAT2 (and VAChT) a large lumenal loop between transmembrane domains 1 and 2 is glycosylated (orange “Y”). Glycosylation at this site may facilitate sorting of VMAT2 to LVs (see text). All other known sorting signals are encoded in the C-terminal, cytoplasmic domains of the vesicular transporters. For VMAT2, this includes a dileucine motif (IL) that is required for endocytosis and sorting to SLMVs in neuroendocrine cells (95). The endocytosis motif is embedded in a larger, extended dileucine motif that includes upstream glutamate residues (EE). The upstream glutamate residues are required for sorting into the regulated secretory pathway and onto LVs (44, 102). The initial step for sorting onto LVs is likely to occur at the TGN and involve sorting away from constitutive secretory vesicles (CS) and onto immature LVs (im. LV). A phosphorylated acidic cluster at the extreme C-terminus of VMAT2 (DDEE[P]SE[P]SD) helps retain VMAT2 in the LV as it matures. B) VAChT also contains a dileucine motif (LL) required for endocytosis and sorting to SLMVs (95, 96, 110). However, a novel tyrosine based motif YNYY may direct these trafficking events under some circumstances (111). A serine upstream of the dileucine motif ([P]S) in VAChT undergoes phosphorylation by PKC (102, 129). Substitution of an acidic residue to mimic phosphorylation drives a portion of VAChT into the regulated secretory pathway and on to LVs in PC12 cells (102). D) Endocytosis and recycling of VGLUT1 to SVs in hippocampal neurons requires a dileucine-like motif (FV). Under some circumstances, a polyproline motif that binds endophilin also contributes to VGLUT1 recycling to SVs (112). A distinct pathway that does not require the polyproline motif allows compensatory recycling of VGLUT, and requires the AP3 adaptor complex (112)(not shown).
3) Vesicular Transporter Sorting Signals
Vesicular transporters use several motifs involved in trafficking other membrane proteins (for a review see (87)). Previously defined motifs include the tyrosine-based motifs NPXY and YXXphe, where phe is a bulky hydrophobic residue. Dileucine-based signals contain either two leucines (LL) or a combination of other hydrophobic residues such as isoleucine plus leucine (IL) (88, 89). For most, if not all dileucine motifs, additional upstream acidic residues are required for the function of the motif as a whole and an acidic reside at position −4 and −5 (EXXXLL) facilitates binding to clathrin adaptor proteins (APs) (90–93). Acidic residues are also required in the acidic cluster that allows binding or the endopeptidase furin and other proteins to the PACS-1 adaptor (85, 94).
VMATs
Since both the biogenesis and recycling of SVs are thought to require an endocytic step, endocytosis signals are critical for the trafficking of all vesicular transporters. For VMAT2, a dileucine motif (IL) encoded in the C-terminus is required for its efficient endocytosis in PC12 cells as well as in hippocampal neurons (44, 95) (Fig. 2A). Moreover, the dileucine motif in VMAT2 may be sufficient for sorting to Synaptic-Like Microvesicles (SLMVs) in neuroendocrine cells; however, demonstrating this effect requires that trafficking to LDCVs is blocked (96, 97). This caveat makes it difficult to predict whether the dileucine motif in VMAT2 will be sufficient for sorting to synaptic vesicles in neurons, despite its demonstrated importance for endocytosis in neurons (44). Multiple forms of endocytosis may occur in neurons (98, 99), and proteins may sort to synaptic vesicles via alternate mechanisms (100, 101). Thus, it is possible that signals other than, or in addition to, the dileucine motif could help sort VMAT2 to synaptic vesicles in neurons.
Additional signals are required for the localization of VMAT2 to LDCVs. These include acidic residues upstream of the dileucine motif EEXXXLL (Fig. 2A) (102), that conserved in VMAT1 and DVMAT (103). In PC12 cells, VMAT2 mutants lacking the upstream acidic residues show dramatic defects in sorting to LDCVs (104). Furthermore, in PC12 cells as well as hippocampal neurons, these mutants traffic directly to the to the plasma membrane rather than entering the regulated secretory pathway (44). These data are consistent with the idea that the acidic residues in the dileucine motif of VMAT2 sort the transporter away from constitutive secretory vesicles and into the regulated secretory pathway at the TGN.
In most cases, both the hydrophobic and the acidic residues in the dileucine motif are thought to function as a single unit (87). Thus, it is likely that the hydrophobic residues in the VMAT2 dileucine motif (IL) help sort VMAT2 to LDCVs. However, it has not been possible to experimentally test this possibility since the IL in VMAT is also required for endocytosis and dileucine mutants are trapped at the plasma membrane (44).
An additional acidic cluster or patch at the end of the C-terminal domain (DDEESESD) is required for the localization of VMAT2 to LDCVs in PC12 cells (104), and it is possible that this motif performs a similar function in neurons. The acidic cluster motif was originally characterized in the protease furin, and for both VMAT2 and furin, it is thought to determine whether the protein is retained in LDCVs as they mature (85, 94, 104, 105). The serine/threonine directed kinase CKII phosphorylates both furin (105) and VMAT2 (106). For VMAT2, sorting to LDCVs is dramatically reduced either by deletion of the acid patch or by mutation of the phosphorylated serines to acidic residues (104).
Additional signals for localizing VMAT2 to secretory vesicles may lie outside the C-terminus (97, 107). Glycosylation has been implicated in the trafficking of synaptotagmin (108), and for VMAT2, a decrease in glycosylation may correlate with a decrease in its localization to synaptic vesicles in vivo (107). In addition, a recently described signal for localizing VMATs to LDCVs in PC12 cells is encoded in the N-linked glycosyl groups of the lumenal loop (97). The glycosylated loop is not itself sufficient to target VMAT2 to LDCVs; however, the C-terminal domain of VMAT2 is only competent for sorting to LDCVS if the lumenal loop is present and glycosylated (97).
VAChT
In non-neuronal cells and the cholinergic cell line SN56, mutation of the dileucine motif in VACHT (LL) inhibits endocytosis (55, 95, 109, 110). In addition mutation of novel tyrosine based motif (YNYY) in the C-terminal trafficking domain (111) of VAChT was shown to cause retention at the plasma membrane in at least one study (111) (See however (110)). These data suggest that VAChT may contain two distinct endocytosis motifs (111). The C-terminus of VAChT, which contains both motifs, is both necessary and sufficient for sorting to SLMVs (65, 110, 111). Furthermore, mutation of the LL motif disrupts the steady state localization of VAChT to SLMVs in SN56 cells (110) and also blocks sorting to SLMVs in PC12 cells (96). However, it is not known how the LL motif affects the trafficking of VAChT at the synapse. It remains possible that both the LL and YNYY motifs could contribute to VAChTs localization to synaptic vesicles in neurons, either during de novo biosynthesis and/or recycling at the nerve terminal.
VGLUTs
As is the case for VMAT2 and VAChT, a dileucine-like motif in VGLUT1 (FV) appears to play an important role for the endocytosis of VGLUT1 (112). However, unlike other vesicular transporters, VGLUT1 also contains a polyproline domain (PRPPPP) that works in concert with the dileucine motif (112–114). The polyproline motif binds to endophilin, and previous studies have demonstrated an important role for endophilin in both endocytosis and the remodeling of lipid membranes (115–119). Deletion of the polyproline motif in VGLUT1 has no effect on endocytosis when neurons are briefly stimulated (1 minute). In contrast, the same deletion decreases the rate of VGLUT1 endocytosis during longer periods of exocytosis and synaptic vesicle recycling (5 minutes) (112). Since neither VGLUT2 nor VGLUT3 contain the polyproline motif, they may recycle less efficiently under similar conditions (120).
Interestingly, the polyproline mutant in VGLUT1 is required for efficient endocytosis only during extended periods of active synaptic vesicle release; the polyproline deletion mutant undergoes endocytosis at a rate equivalent to wild type after exocytosis has ceased (112). Conversely, a distinct pathway is required for VGLUT1 endocytosis following prolonged periods of exocytosis (112). This pathway is likely to depend on the AP3 adaptor complex, since 1) it is sensitive to brefeldin A, and AP3-dependent budding requires the brefeldin sensitive small GTPase ARF1 (112); and 2) endocytosis of VGLUT1 through this pathway is blocked in mocha mice, which lack the AP3 delta subunit (61, 112).
These results are important for two reasons. First, they show that AP3 may function to recycle VGLUT1 to synaptic vesicle, regardless of the controversial role for AP3 in synaptic vesicle biogenesis (58, 60). Second, they establish at least two alternate modes of VGLUT1 recycling, one relatively fast and the other slow. (We exclude here the ultrafast mode of kiss and run recycling).
Other studies suggest that fast and slow endocytic pathways at the synapse may traffic respectively to the readily released versus the reserve pool of synaptic vesicles (74–76) and a recent study in Drosophila suggests that some vesicles in the reserve pool have a larger quantal size (36). It is tempting to speculate that regulated trafficking through the slow versus fast pathways could determine how many VGLUT molecules localize to synaptic vesicles that reside in different, functional pools.
Binding Partners
Trafficking of other membrane proteins requires a complex assembly of cytosolic factors and it is likely that vesicular transporters use many of the same components. Dominant negative constructs that inhibit the function of dynamin I (K44A), and clathrin (AP180-C) also block the internalization of VAChT, supporting the idea that vesicular transporters use clathrin and dynamin-based endocytic mechanisms (109, 110). In addition, in a few cases, vesicular transporters have been shown to directly bind adaptor proteins and other elements of the trafficking machinery. As noted above, VGLUT1 binds endophilin via a polyproline motif (112–114), and VMAT2 binds the adaptor PACS-1, possibly regulating exit from immature LDCVs (104). In addition, VAChT has been suggested to bind AP1 and AP2 (109, 111) via either the dileucine motif (109) or the novel YNYY motif (111).
In addition to these well-known adaptor proteins, it is possible that vesicular transporters require other proteins to interact with the sorting machinery. Two recent reports highlight the use of invertebrate genetic systems to isolate novel binding partners. In C. elegans, genetic experiments support a direct interaction between the mutant alleles of VAChT and synaptobrevin (121). Another genetic screen in C. elegans has shown that sorting of VGAT to synaptic vesicles requires the novel interacting protein unc-46 (122). In unc-46 mutants, VGAT labeling is increased at the cell body, and the remaining protein that does enter the axon is spread diffusely over the plasma membrane rather that localizing to synaptic vesicles (122). The mechanism by which unc-46 influences VGAT trafficking is not yet clear, but has been suggested to help recruit VGAT to synaptic vesicles either at the cell body or the nerve terminal (122).
4) The Regulation of Transporter Trafficking
Phosphorylation of VAChT and VMAT2 may regulate trafficking
Vesicular transporters may undergo multiple forms of regulation. These include regulated changes in transcription (123, 124) and the inactivation of transport by heterotrimeric G proteins (125, 126). Here we focus on mechanisms that may more directly affect trafficking.
In PC12 cells, VAChT localizes primarily to SLMVs, whereas VMATs localize primarily to LDCVs (127, 128). Similar to VMATs, the VAChT dileucine motif contains an upstream acidic residue at the −4 position, but unlike VMATs, VAChT shows a serine at −5 site (RSERDVLL), which can be phosphorylated by PKC (R[Phospho-S]ERDVLL) (102, 129). Phosphoserine is functionally an acidic residue, and thus similar to acidic glutamate in the VMAT2 dileucine motif (KEEKMAIL) (102, 129). Moreover, the substitution of an acidic amino acid at the phosphorylated serine (REERDVLL) increases the localization of VAChT to LDCVs three-fold in PC12 cells, partially mimicking the trafficking pattern of VMAT2 (102). These observations raise the possibility that VAChT trafficking may be regulated by phosphorylation, presumably at the level of the TGN and the sorting of VAChT into the constitutive versus the regulated secretory pathway (Fig. 2B).
VAChT resides primary on SLMVs in PC12 cells, and on synaptic vesicles in bona fide neurons (130, 131). However, as noted above, it is possible that VAChT may localize to other types of vesicles. Indeed, acetylcholine can be released from somatodendritic sites in vivo, and may localize to LDCV fractions derived from neuronal tissue (42, 43). It is therefore conceivable that in neurons as well as PC12 cells, phosphorylation by PKC could regulate the fraction of VAChT that is targeted to the regulated secretory pathway and to LDCV-like vesicles.
It is also possible that phosphorylation of VAChT regulates other trafficking events. Blockade of VAChT phosphorylation may disrupt trafficking to SLMVs in PC12 cells (129) and phosphorylation of the extended dileucine motif has been shown to regulate the endocytosis of the CD3 gamma subunit of the T cell receptor (132). Mutation of sites upstream of the dileucine motif does not appear to effect vesicular transporter endocytosis in non-neuronal or neuroendocrine cell lines (95). However, as noted above, trafficking in neuroendocrine cells and neurons may differ, and it is possible that VAChT phosphorylation could regulate endocytosis in neurons. In hippocampal slices, activation of PKC blocks the ability of the VAChT inhibitor vesamicol to inhibit ACh release, and this effect was correlated with an increase in VAChT phosphorylation (133). This effect would seem more likely to occur during endocytosis than on exit from the TGN.
For VMAT2, phosphorylation by CKII of the acid patch motif at the extreme C-terminus may regulate its localization to LDCVs (104). Substitution of acidic residues at the phosphorylation sites increases the localization of VMAT2 to immature LDCVS (104). Thus, similar to furin, the phosphorylation state of VMAT2 may determine whether it is removed from LDCVs as they mature.
Since CKII is constitutively active, phosphatases are more likely to regulate the function of the acid patch in VMAT2. For furin, the removal of phosphate from serines in the acidic cluster regulates exit from immature LDCVs (85). In addition, furin that reaches the plasma membrane can be recycled, and dephosphorylation may regulate its return to the TGN from the plasma membrane (134, 135). Interestingly, phosphorylated furin that is endocytosed from the plasma membrane traffics to the TGN indirectly through late endosomes, whereas dephosphorylated furin is sorted directly back to the TGN (135). For VMAT2, regulation of dephosphorylation could potentially determine the amount of VMAT2 that is removed from LDCVs as they mature (Fig. 2A). Inhibition of dephosphorylation could increase sorting into either the constitutive or constitutive-like secretory pathway, and potentially increase VMATs localization to synaptic vesicles.
VGAT also undergoes phosphorylation, and interestingly, phosphorylation of VGAT has been observed in neurons but not other non-neuronal cells (136). This difference suggests the possibility of a specific neuronal function for VGAT phosphorylation, but this remains untested.
Phosphorylation may also indirectly regulate vesicular transporter trafficking. PKA does not directly phosphorylate VMATs, but is required for the localization of VMAT1 and 2 to LDCVs in PC12 cells (137). Additional phosphorylation events associated with VAChT are suggested by a series of physiological studies on cholinergic signaling at the neuromuscular junction (see (138, 139)). However, further studies will be needed to elucidate the mechanisms underlying these phenomena and their potential contribution of VAChT trafficking.
Additional Regulatory Mechanisms
Additional regulatory mechanisms relevant to transporter trafficking include alternative mRNA splicing of exons representing trafficking domains, demonstrated previously for plasma membrane transporters (140–142). The Drosophila VMAT ortholog shows variable use of a 3′ splice site in the last exon of the gene, which leads to two divergent carboxy-terminal domains (103). As a result of this difference, only one version undergoes efficient endocytosis in vitro (103).
An extensive series of studies has shown that blockade of amine uptake at the plasma membrane increases the localization of mammalian VMAT2 to synaptic vesicles (143–145). In contrast, drugs that promote efflux such as amphetamines decrease the localization of VMAT to synaptic vesicles (146). These events may involve the upstream activation of dopamine autoreceptors (147, 148). However, the mechanism by which activation of autoreceptors regulates VMAT2 trafficking remains to be determined. Finally, recent data show that the expression of VGLUT1, and possibly its localization to synaptic vesicles, follows a circadian pattern (35). Additional mechanisms are no doubt involved in the regulation of vesicular transporter trafficking, and identifying these mechanisms remains one the current challenges for the field.
Future Directions
Many other questions about vesicular transporter trafficking remain unanswered. Although some motifs have been have tentatively assigned to particular trafficking events, they cannot fully account for all of the known pathways required for sorting to secretory vesicles. Thus, it is likely that additional signals remain to be identified. For example, the potential contribution of ubiquitination to vesicular transporter trafficking is not known. It is also unlikely that all transporter trafficking pathways have been identified. In neuroendocrine cells, trafficking to SLMVs has been suggested to occur in some cases via a late endosome intermediate (100), but the extent to which neurons use similar, alternative pathways is not known. We note that at least a portion of VGLUT1 would appear to localize to synaptic vesicles in the absence of known endocytosis motifs (112), supporting the notion that alternative targeting mechanisms are active in at least some neurons.
It is unknown whether vesicular transporters use specific signals for trafficking to the axon versus the somatodendritic compartment. Moreover, there is no information on the potential mechanisms by which vesicular transporters are degraded. This extreme length of many axons highlights the consequences of degrading a transporter at the nerve terminal. To be replaced, it must be synthesized and sorted into a precursor vesicle at a Golgi stack that might be a meter away.
For all of these known and potential trafficking events it remains unclear how variations between neuronal subtypes may influence transporter trafficking. Glutamatergic hippocampal neurons have proven to be a very useful model, but it is possible that variations in neurochemical identity will have dramatic effects on transporter trafficking. This possibility is underscored by the recent finding that loss of dynamin I differentially affects endocytosis at inhibitory versus excitatory synapses (149). New mammalian model systems representing a variety of different synaptic subtypes are likely to be required to fully understand these effects. Invertebrate systems such as C. elegans and Drosophila may also be useful in this regard, although the extent to which neuronal trafficking mechanisms are conserved is somewhat controversial.
Surprisingly, the number of vesicular transporters that reside on each secretory vesicle is not known. It has been suggested that as few as one in Drosophila (23) or as many as fourteen VGLUT molecules in mammalian preparations (24) may localize to an individual synaptic vesicle. One of the most important topics for future studies will be determining this number and how it may be regulated. Despite observed variations in neurotransmitter content, it is difficult to imagine that the synapse could tolerate large, random fluctuations in quantal size.
Finally, we believe that it will be critical to determine how changes in vesicular transporter trafficking may affect the nervous system as a whole. In vitro studies have provided tantalizing hints about the potential impact of trafficking on quantal size and synaptic transmission. However, we will not be able to determine how changes in transporter trafficking may affect behavior until we can study trafficking mutants in vivo.
Table 1.
Summary of Vesicular Transporter Types and Their Trafficking Signals
| Transporter | Substrates | Established Trafficking motifs | Proposed Functions |
|---|---|---|---|
| VMAT1 | Dopamine, Serotonin, Norepinephrine | ||
| VMAT2 | Dopamine, Serotonin, Norepinephrine, Histamine | IL | Endocytosis in PC12 cells and in neurons. |
| EExxx(IL) | Sort to immature LDCVS in PC12 cells. Sort to regulated secretory pathway in neurons. |
||
| VAChT | Acetylcholine | LL | Endocytosis. Sort to SLMVs in PC12 cells. |
| YNYY | Endocytosis? | ||
| [Phospho-S]xxxLL | Induce sorting to LDCVs in PC12 cells. | ||
| VGAT | GABA, Glycine | ? | |
| VGLUT1 | Glutamate | FV (dileucine-like) | Endocytosis in neurons. |
| PPPP | Endocytosis in neurons. | ||
| VGLUT2 | Glutamate | (no PRPPPP) | |
| VGLUT3 | Glutamate | (no PRPPPP) |
Acknowledgments
The authors are funded by the NIMH and NIEHS (MH076900, ES015747) and training grants from the ARCS foundation (AG), the Hatos Center for Neuropharmacology (LB, AG), the UCLA Center for Neurobehavioral Genetics (ESB) and the UCLA Cellular and Molecular Biology Training Program (AC). We thank Esteban Dell’ Angelica, Susan Voglmaier and Felix Schweizer for their helpful comments.
Footnotes
We confine our discussion to vesicular transporters for the classical (acetylcholine, monoamines), and amino acid neurotransmitters (GABA, glycine, glutamate). Novel neurotransmitters such as zinc use other transporter subtypes, and peptide neurotransmitters do not enter secretory vesicles via transport across the vesicle membrane. Rather, they are sorted into the lumen of secretory vesicles at the level of the Golgi.
In addition some synaptic vesicles may take a third route, and undergo ultrafast “kiss and run” without complete fusion 69. Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol 1973;57(2):499–524. We do not discuss this pathway here. For a recent review of kiss and run see 70. Rizzoli SO, Jahn R. Kiss-and-run, collapse and ‘readily retrievable’ vesicles. Traffic 2007;8(9):1137–1144.).
For additional information on the functional properties of synaptic vesicle pools see 78. Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci 2005;6(1):57–69.
References
- 1.Hahn MK, Blakely RD. The functional impact of SLC6 transporter genetic variation. Annu Rev Pharmacol Toxicol. 2007;47:401–441. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- 2.Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Curr Opin Neurobiol. 2007;17(3):304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry FA, Boulland JL, Jenstad M, Bredahl MK, Edwards RH. Pharmacology of neurotransmitter transport into secretory vesicles. Handb Exp Pharmacol. 2008;(184):77–106. doi: 10.1007/978-3-540-74805-2_4. [DOI] [PubMed] [Google Scholar]
- 4.Erickson JD, De Gois S, Varoqui H, Schafer MK, Weihe E. Activity-dependent regulation of vesicular glutamate and GABA transporters: a means to scale quantal size. Neurochem Int. 2006;48(6–7):643–649. doi: 10.1016/j.neuint.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch. 2004;447(5):636–640. doi: 10.1007/s00424-003-1100-5. [DOI] [PubMed] [Google Scholar]
- 6.Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447(5):756–759. doi: 10.1007/s00424-003-1091-2. [DOI] [PubMed] [Google Scholar]
- 7.Reimer RJ, Edwards RH. Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflugers Arch. 2004;447(5):629–635. doi: 10.1007/s00424-003-1087-y. [DOI] [PubMed] [Google Scholar]
- 8.Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27(2):98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Weihe E, Eiden LE. Chemical neuroanatomy of the vesicular amine transporters. Faseb J. 2000;14(15):2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- 10.Boulland JL, Qureshi T, Seal RP, Rafiki A, Gundersen V, Bergersen LH, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA. Expression of the vesicular glutamate transporters during development indicates the widespread corelease of multiple neurotransmitters. J Comp Neurol. 2004;480(3):264–280. doi: 10.1002/cne.20354. [DOI] [PubMed] [Google Scholar]
- 11.Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99(22):14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, Gasnier B, Giros B, El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22(13):5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277(52):50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RH. The neurotransmitter cycle and quantal size. Neuron. 2007;55(6):835–858. doi: 10.1016/j.neuron.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Song H-J, Ming G-L, Fon E, Bellocchio E, Edwards RH, Poo M-M. Expression of a putative vesicular acetylcholine transporter facilitates quantal transmitter packaging. Neuron. 1997;18:815–826. doi: 10.1016/s0896-6273(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 16.Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101(18):7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25(26):6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH, Sulzer D. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20:7297–7306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24:10466–10474. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prado VF, Martins-Silva C, de Castro BM, Lima RF, Barros DM, Amaral E, Ramsey AJ, Sotnikova TD, Ramirez MR, Kim HG, Rossato JI, Koenen J, Quan H, Cota VR, Moraes MF, et al. Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron. 2006;51(5):601–612. doi: 10.1016/j.neuron.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50(4):575–587. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, Del Rio J. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1) The European journal of neuroscience. 2007;25(1):281–290. doi: 10.1111/j.1460-9568.2006.05259.x. [DOI] [PubMed] [Google Scholar]
- 23.Daniels RW, Collins CA, Chen K, Gelfand MV, Featherstone DE, DiAntonio A. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron. 2006;49:11–16. doi: 10.1016/j.neuron.2005.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127(4):831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Chang H-Y, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. Over-expression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Molecular Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- 26.Fon EA, Pothos EN, Sun B-C, Kileen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y-M, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Block CB, Miller GW, Wightman RM, Caron MC. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. doi: 10.1016/s0896-6273(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl G. VMAT2 knockout mice: Heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci USA. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall FS, Sora I, Uhl GR. Sex-dependent modulation of ethanol consumption in vesicular monoamine transporter 2 (VMAT2) and dopamine transporter (DAT) knockout mice. Neuropsychopharmacology. 2003;28(4):620–628. doi: 10.1038/sj.npp.1300070. [DOI] [PubMed] [Google Scholar]
- 30.Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, Wetsel WC. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci. 2007;27(39):10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der Spek P, Kass S, Meert T, D’Hooge R, Rosenmund C, Hampson RM. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26(46):12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naves LA, Van der Kloot W. Repetitive nerve stimulation decreases the acetylcholine content of quanta at the frog neuromuscular junction. J Physiol. 2001;532(Pt 3):637–647. doi: 10.1111/j.1469-7793.2001.0637e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Li Y, Engisch KL, Nakanishi ST, Dodson SE, Miller GW, Cope TC, Pinter MJ, Rich MM. Activity-dependent presynaptic regulation of quantal size at the mammalian neuromuscular junction in vivo. J Neurosci. 2005;25(2):343–351. doi: 10.1523/JNEUROSCI.3252-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elhamdani A, Palfrey HC, Artalejo CR. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31(5):819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- 35.Yelamanchili SV, Pendyala G, Brunk I, Darna M, Albrecht U, Ahnert-Hilger G. Differential sorting of the vesicular glutamate transporter 1 into a defined vesicular pool is regulated by light signaling involving the clock gene Period 2. J Biol Chem. 2006;281(23):15671–15679. doi: 10.1074/jbc.M600378200. [DOI] [PubMed] [Google Scholar]
- 36.Steinert JR, Kuromi H, Hellwig A, Knirr M, Wyatt AW, Kidokoro Y, Schuster CM. Experience-dependent formation and recruitment of large vesicles from reserve pool. Neuron. 2006;50(5):723–733. doi: 10.1016/j.neuron.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Krantz DE, Waites C, Edwards RH. Membrane trafficking of neurotransmitter transporters in the regulation of synaptic transmission. Trends Cell Biol. 1999;9:356–363. doi: 10.1016/s0962-8924(99)01605-0. [DOI] [PubMed] [Google Scholar]
- 38.Torrealba F, Carrasco MA. A review on electron microscopy and neurotransmitter systems. Brain Res Brain Res Rev. 2004;47(1–3):5–17. doi: 10.1016/j.brainresrev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Ultrastructural localization of the monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J Neurosci. 1996;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Vesicular monoamine transporter-2: immunogold localization in striatal axons and terminals. Synapse. 1997;26:194–198. doi: 10.1002/(SICI)1098-2396(199706)26:2<194::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 41.Nirenberg MJ, Liu Y, Peter D, Edwards RH, Pickel VM. The vesicular monoamine transporter-2 is present in small synaptic vesicles and preferentially localizes to large dense core vesicles in rat solitary tract nuclei. Proc Natl Acad Sci USA. 1995;92:8773–8777. doi: 10.1073/pnas.92.19.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agoston DV, Whittaker VP. Characterization, by size, density, osmotic fragility, and immunoaffinity, of acetylcholine-and vasoactive intestinal polypeptide-containing storage particles from neurones of the guinea-pig. J Neurochem. 1989;52:1474–1480. doi: 10.1111/j.1471-4159.1989.tb09196.x. [DOI] [PubMed] [Google Scholar]
- 43.Lundberg JM, Fried G, Fahrenkrug J, Holmstedt B, Hokfelt T, Lagercrantz H, Lundgren G, Anggard A. Subcellular fractionation of cat submandibular gland: comparative studies of the distribution of acetylcholine and vasoactive intestinal peptide (VIP) Neurosci. 1981;6:1001–1010. doi: 10.1016/0306-4522(81)90066-x. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Waites CL, Staal RG, Dobryy Y, Park J, Sulzer DL, Edwards RH. Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron. 2005;48(4):619–633. doi: 10.1016/j.neuron.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 45.Harkany T, Holmgren C, Hartig W, Qureshi T, Chaudhry FA, Storm-Mathisen J, Dobszay MB, Berghuis P, Schulte G, Sousa KM, Fremeau RT, Jr, Edwards RH, Mackie K, Ernfors P, Zilberter Y. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: involvement of vesicular glutamate transporter 3. J Neurosci. 2004;24(21):4978–4988. doi: 10.1523/JNEUROSCI.4884-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zilberter Y, Kaiser KM, Sakmann B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24(4):979–988. doi: 10.1016/s0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]
- 47.Braun M, Wendt A, Karanauskaite J, Galvanovskis J, Clark A, MacDonald PE, Rorsman P. Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic beta cells. The Journal of general physiology. 2007;129(3):221–231. doi: 10.1085/jgp.200609658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattori T, McGeer PL, McGeer EG. Dendro axonic neurotransmission. I. Morphological sites for the synthesis, binding and release of neurotransmitters in dopaminergic dendrites in the substantia nigra and cholinergic dendrites in the neostriatum. Brain Res. 1979;170:71–83. doi: 10.1016/0006-8993(79)90941-7. [DOI] [PubMed] [Google Scholar]
- 49.Heeringa MJ, Abercrombie ED. Biochemistry of somatodendritic dopamine release in substantia nigra: an in vivo comparison with striatal dopamine release. J Neurochem. 1995;65:192–200. doi: 10.1046/j.1471-4159.1995.65010192.x. [DOI] [PubMed] [Google Scholar]
- 50.Regnier-Vigouroux A, Tooze SA, Huttner WB. Newly synthesized synaptophysin is transported to synaptic-like microvesicles via constitutive secretory vesicles and the plasma membrane. EMBO J. 1991;10:3589–3601. doi: 10.1002/j.1460-2075.1991.tb04925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hannah MJ, Schmidt AA, Huttner WB. Synaptic vesicle biogenesis. Annu Rev Cell Dev Biol. 1999;15:733–798. doi: 10.1146/annurev.cellbio.15.1.733. [DOI] [PubMed] [Google Scholar]
- 52.Prado VF, Prado MA. Signals involved in targeting membrane proteins to synaptic vesicles. Cell Mol Neurobiol. 2002;22(5–6):565–577. doi: 10.1023/a:1021884319363. [DOI] [PubMed] [Google Scholar]
- 53.Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3(5):445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- 54.Nakata T, Terada S, Hirokawa N. Visualization of the dynamics of synaptic vesicles and plasma membrane proteins in living axons. J Cell Biol. 1998;140:659–674. doi: 10.1083/jcb.140.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santos MS, Barbosa J, Jr, Veloso GS, Ribeiro F, Kushmerick C, Gomez MV, Ferguson SS, Prado VF, Prado MA. Trafficking of green fluorescent protein tagged-vesicular acetylcholine transporter to varicosities in a cholinergic cell line. J Neurochem. 2001;78:1104–1113. doi: 10.1046/j.1471-4159.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol. 1997;135:445–414. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faundez V, Horng J-T, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 58.Salazar G, Craige B, Love R, Kalman D, Faundez V. Vglut1 and ZnT3 co-targeting mechanisms regulate vesicular zinc stores in PC12 cells. J Cell Sci. 2005;118(Pt 9):1911–1921. doi: 10.1242/jcs.02319. [DOI] [PubMed] [Google Scholar]
- 59.Salazar G, Love R, Werner E, Doucette MM, Cheng S, Levey A, Faundez V. The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol Biol Cell. 2004;15(2):575–587. doi: 10.1091/mbc.E03-06-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mullins C, Hartnell LM, Bonifacino JS. Distinct requirements for the AP-3 adaptor complex in pigment granule and synaptic vesicle biogenesis in Drosophila melanogaster. Mol Gen Genet. 2000;263(6):1003–1014. doi: 10.1007/pl00008688. [DOI] [PubMed] [Google Scholar]
- 61.Kantheti P, Qiao X, Diaz ME, Peden AA, Meyer GE, Carskadon SL, Kapfhamer D, Sufalko D, Robinson MS, Noebels JL, Burmeister M. Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron. 1998;21(1):111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- 62.Nakatsu F, Okada M, Mori F, Kumazawa N, Iwasa H, Zhu G, Kasagi Y, Kamiya H, Harada A, Nishimura K, Takeuchi A, Miyazaki T, Watanabe M, Yuasa S, Manabe T, et al. Defective function of GABA-containing synaptic vesicles in mice lacking the AP-3B clathrin adaptor. J Cell Biol. 2004;167(2):293–302. doi: 10.1083/jcb.200405032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuliawat R, Arvan P. Protein targeting via the “constitutive-like” secretory pathway in isolated pancreatic islets: passive sorting in the immature granule component. J Cell Biol. 1992;118:521–529. doi: 10.1083/jcb.118.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tao-Cheng JH, Eiden LE. The vesicular monoamine transporter VMAT2 and vesicular acetylcholine transporter VAChT are sorted to separate vesicle populations in PC12 cells. Adv Pharmacol. 1998;42:250–253. doi: 10.1016/s1054-3589(08)60740-1. [DOI] [PubMed] [Google Scholar]
- 65.Varoqui H, Erickson JD. The cytoplasmic tail of the vesicular acetylcholine transporter contains a synaptic vesicle targeting signal. J Biol Chem. 1998;273:9094–9098. doi: 10.1074/jbc.273.15.9094. [DOI] [PubMed] [Google Scholar]
- 66.Jahn R, Rizzoli SO. Endocytosis in neurons: editorial introduction and overview. Traffic. 2007;8(9):1121–1122. doi: 10.1111/j.1600-0854.2007.00615.x. [DOI] [PubMed] [Google Scholar]
- 67.Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Lange RP, de Roos AD, Borst JG. Two modes of vesicle recycling in the rat calyx of Held. J Neurosci. 2003;23(31):10164–10173. doi: 10.1523/JNEUROSCI.23-31-10164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;57(2):499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rizzoli SO, Jahn R. Kiss-and-run, collapse and ‘readily retrievable’ vesicles. Traffic. 2007;8(9):1137–1144. doi: 10.1111/j.1600-0854.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 71.Blagoveshchenskaya AD, Hewitt EW, Cutler DF. Di-leucine signals mediate targeting of tyrosinase and synaptotagmin to synaptic-like microvesicles within PC12 cells. Mol Biol Cell. 1999;10(11):3979–3990. doi: 10.1091/mbc.10.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rizzoli SO, Bethani I, Zwilling D, Wenzel D, Siddiqui TJ, Brandhorst D, Jahn R. Evidence for early endosome-like fusion of recently endocytosed synaptic vesicles. Traffic. 2006;7(9):1163–1176. doi: 10.1111/j.1600-0854.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 74.Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27(3):551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- 75.Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. J Neurosci. 2003;23(10):4092–4099. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Evans GJ, Cousin MA. Activity-dependent control of slow synaptic vesicle endocytosis by cyclin-dependent kinase 5. J Neurosci. 2007;27(2):401–411. doi: 10.1523/JNEUROSCI.3809-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kidokoro Y, Kuromi H, Delgado R, Maureira C, Oliva C, Labarca P. Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse. Brain Res Brain Res Rev. 2004;47(1–3):18–32. doi: 10.1016/j.brainresrev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 78.Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6(1):57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- 79.Kelly RB. Storage and release of neurotransmitters. Cell/Neuron. 1993;72/10:43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 80.Tooze SA, Huttner WB. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990;60:837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tooze SA, Flatmark T, Tooze J, Huttner WB. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991;115:1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dittie AS, Hajibagheri N, Tooze SA. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J of Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dittie AS, Thomas L, Thomas G, Tooze SA. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II. EMBO J. 1997;16:4859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crump CM, Xiang Y, Thomas L, Gu F, Austin C, Tooze SA, Thomas G. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. Embo J. 2001;20(9):2191–2201. doi: 10.1093/emboj/20.9.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 86.Lubben NB, Sahlender DA, Motley AM, Lehner PJ, Benaroch P, Robinson MS. HIV-1 Nef-induced down-regulation of MHC class I requires AP-1 and clathrin but not PACS-1 and is impeded by AP-2. Mol Biol Cell. 2007;18(9):3351–3365. doi: 10.1091/mbc.E07-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 88.Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 89.Bremnes B, Madsen T, Gedde-Dahl M, Bakke O. An LI and ML motif in the cytoplasmic tail of the MHC-associated invariant chain mediate rapid internalization. J Cell Sci. 1994;107:2021–2032. doi: 10.1242/jcs.107.7.2021. [DOI] [PubMed] [Google Scholar]
- 90.Pond L, Kuhn LA, Teyton L, Schutze M-P, Tainer JA, Jackson MR, Peterson PA. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J Biol Chem. 1995;270:19989–19997. doi: 10.1074/jbc.270.34.19989. [DOI] [PubMed] [Google Scholar]
- 91.Sandoval IV, Martinez-Arca S, Valdueza J, Palacios S, Holman GD. Distinct reading of different structural determinants modulates the dileucine-mediated transport steps of the lysosomal membrane protein LIMPII and the insulin-sensitive glucose transporter GLUT4. J Biol Chem. 2000;275(51):39874–39885. doi: 10.1074/jbc.M006261200. [DOI] [PubMed] [Google Scholar]
- 92.Dietrich J, Kastrup J, Nielsen BL, Odum N, Geisler C. Regulation and function of the CD3 gamma DXXXLL motif: A binding site for adaptor protein-1 and adaptor protein-2 in vitro. J Cell Biol. 1997;138:271–281. doi: 10.1083/jcb.138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bresnahan PA, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene WC. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8(22):1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 94.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3(10):753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan PK, Waites C, Liu Y, Krantz DE, Edwards RH. A leucine-based motif mediates the endocytosis of vesicular monoamine and acetylcholine transporters. J Biol Chem. 1998;28:17351–17360. doi: 10.1074/jbc.273.28.17351. [DOI] [PubMed] [Google Scholar]
- 96.Colgan L, Liu H, Huang SY, Liu YJ. Dileucine motif is sufficient for internalization and synaptic vesicle targeting of vesicular acetylcholine transporter. Traffic. 2007;8(5):512–522. doi: 10.1111/j.1600-0854.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- 97.Yao J, Hersh LB. The vesicular monoamine transporter 2 contains trafficking signals in both its N-glycosylation and C-terminal domains. J Neurochem. 2007;100(5):1387–1396. doi: 10.1111/j.1471-4159.2006.04326.x. [DOI] [PubMed] [Google Scholar]
- 98.Galli T, Haucke V. Cycling of synaptic vesicles: how far? How fast! Sci STKE. 2004;2004(264):re19. doi: 10.1126/stke.2642004re19. [DOI] [PubMed] [Google Scholar]
- 99.Wienisch M, Klingauf J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat Neurosci. 2006;9(8):1019–1027. doi: 10.1038/nn1739. [DOI] [PubMed] [Google Scholar]
- 100.Blagoveshchenskaya AD, Cutler DF. Sorting to synaptic-like microvesicles from early and late endosomes requires overlapping but not identical targeting signals. Mol Biol Cell. 2000;11(5):1801–1814. doi: 10.1091/mbc.11.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thoidis G, Chen P, Pushkin AV, Vallega G, Leeman SE, Fine RE, Kandror KV. Two distinct populations of synaptic vesicles from rat brain. Proc Natl Acad Sci USA. 1998;95:183–188. doi: 10.1073/pnas.95.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krantz DE, Waites C, Oorschot V, Liu Y, Wilson RI, Tan PK, Klumperman J, Edwards RE. A phosphorylation site regulates sorting of the vesicular acetylcholine transporter to dense core vesicles. J Cell Biol. 2000;149:379–395. doi: 10.1083/jcb.149.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, Chang H-Y, Houshyar R, Bainton RJ, DiAntonio A, Krantz DE. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin and octopamine. J Neurobiol. 2005;64:239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- 104.Waites CL, Mehta A, Tan PK, Thomas G, Edwards RH, Krantz DE. An acidic motif retains vesicular monoamine transporter 2 on large dense core vesicles. J Cell Biol. 2001;152:1159–1168. doi: 10.1083/jcb.152.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones BG, Thomas L, Molloy SS, Thulin CD, Fry MD, Walsh KA, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. Embo J. 1995;14(23):5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krantz DE, Peter D, Liu Y, Edwards RH. Phosphorylation of a vesicular monoamine transporter by casein kinase II. J Biol Chem. 1997;272(10):6752–6759. doi: 10.1074/jbc.272.10.6752. [DOI] [PubMed] [Google Scholar]
- 107.Cruz-Muros I, Afonso-Oramas D, Abreu P, Rodriguez M, Gonzalez MC, Gonzalez-Hernandez T. Deglycosylation and subcellular redistribution of VMAT2 in the mesostriatal system during normal aging. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 108.Han W, Rhee JS, Maximov A, Lao Y, Mashimo T, Rosenmund C, Sudhof TC. N-glycosylation is essential for vesicular targeting of synaptotagmin 1. Neuron. 2004;41(1):85–99. doi: 10.1016/s0896-6273(03)00820-1. [DOI] [PubMed] [Google Scholar]
- 109.Barbosa J, Jr, Ferreira LT, Martins-Silva C, Santos MS, Torres GE, Caron MG, Gomez MV, Ferguson SS, Prado MA, Prado VF. Trafficking of the vesicular acetylcholine transporter in SN56 cells: a dynamin-sensitive step and interaction with the AP-2 adaptor complex. J Neurochem. 2002;82(5):1221–1228. doi: 10.1046/j.1471-4159.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 110.Ferreira LT, Santos MS, Kolmakova NG, Koenen J, Barbosa J, Jr, Gomez MV, Guatimosim C, Zhang X, Parsons SM, Prado VF, Prado MA. Structural requirements for steady-state localization of the vesicular acetylcholine transporter. J Neurochem. 2005;94(4):957–969. doi: 10.1111/j.1471-4159.2005.03244.x. [DOI] [PubMed] [Google Scholar]
- 111.Kim MH, Hersh LB. The vesicular acetylcholine transporter interacts with clathrin-associated adaptor complexes AP-1 and AP-2. J Biol Chem. 2004;279(13):12580–12587. doi: 10.1074/jbc.M310681200. [DOI] [PubMed] [Google Scholar]
- 112.Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51(1):71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 113.De Gois S, Jeanclos E, Morris M, Grewal S, Varoqui H, Erickson JD. Identification of Endophilins 1 and 3 as Selective Binding Partners for VGLUT1 and Their Co-Localization in Neocortical Glutamatergic Synapses: Implications for Vesicular Glutamate Transporter Trafficking and Excitatory Vesicle Formation. Cell Mol Neurobiol. 2006 doi: 10.1007/s10571-006-9054-8. [DOI] [PubMed] [Google Scholar]
- 114.Vinatier J, Herzog E, Plamont MA, Wojcik SM, Schmidt A, Brose N, Daviet L, El Mestikawy S, Giros B. Interaction between the vesicular glutamate transporter type 1 and endophilin A1, a protein essential for endocytosis. J Neurochem. 2006;97(4):1111–1125. doi: 10.1111/j.1471-4159.2006.03821.x. [DOI] [PubMed] [Google Scholar]
- 115.Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O, De Camilli P. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron. 1999;24(1):143–154. doi: 10.1016/s0896-6273(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 116.Guichet A, Wucherpfennig T, Dudu V, Etter S, Wilsch-Brauniger M, Hellwig A, Gonzalez-Gaitan M, Huttner WB, Schmidt AA. Essential role of endophilin A in synaptic vesicle budding at the Drosophila neuromuscular junction. Embo J. 2002;21(7):1661–1672. doi: 10.1093/emboj/21.7.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109(1):101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- 118.Gallop JL, Jao CC, Kent HM, Butler PJ, Evans PR, Langen R, McMahon HT. Mechanism of endophilin N-BAR domain-mediated membrane curvature. Embo J. 2006;25(12):2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol. 2001;155(2):193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304(5678):1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- 121.Sandoval GM, Duerr JS, Hodgkin J, Rand JB, Ruvkun G. A genetic interaction between the vesicular acetylcholine transporter VAChT/UNC-17 and synaptobrevin/SNB-1 in C. elegans. Nat Neurosci. 2006;9(5):599–601. doi: 10.1038/nn1685. [DOI] [PubMed] [Google Scholar]
- 122.Schuske K, Palfreyman MT, Watanabe S, Jorgensen EM. UNC-46 is required for trafficking of the vesicular GABA transporter. Nat Neurosci. 2007;10(7):846–853. doi: 10.1038/nn1920. [DOI] [PubMed] [Google Scholar]
- 123.Watson F, Kiernan RS, Deavall DG, Varro A, Dimaline R. Transcriptional activation of the rat vesicular transporter 2 promoter in gastric epithelial cells: regulation by gastrin. J Biol Chem. 2000;276:7661–7671. doi: 10.1074/jbc.M006697200. [DOI] [PubMed] [Google Scholar]
- 124.Catlow K, Ashurst HL, Varro A, Dimaline R. Identification of a gastrin response element in the vesicular monoamine transporter type 2 promoter and requirement of 20 S proteasome subunits for transcriptional activity. J Biol Chem. 2007;282(23):17069–17077. doi: 10.1074/jbc.M611421200. [DOI] [PubMed] [Google Scholar]
- 125.Brunk I, Blex C, Rachakonda S, Holtje M, Winter S, Pahner I, Walther DJ, Ahnert-Hilger G. The first luminal domain of vesicular monoamine transporters mediates G-protein-dependent regulation of transmitter uptake. J Biol Chem. 2006;281(44):33373–33385. doi: 10.1074/jbc.M603204200. [DOI] [PubMed] [Google Scholar]
- 126.Brunk I, Holtje M, von Jagow B, Winter S, Sternberg J, Blex C, Pahner I, Ahnert-Hilger G. Regulation of vesicular monoamine and glutamate transporters by vesicle-associated trimeric G proteins: new jobs for long-known signal transduction molecules. Handb Exp Pharmacol. 2006;(175):305–325. doi: 10.1007/3-540-29784-7_15. [DOI] [PubMed] [Google Scholar]
- 127.Liu Y, Schweitzer ES, Nirenberg MJ, Pickel VM, Evans CJ, Edwards RH. Preferential localization of a vesicular monoamine transporter to dense core vesicles in PC12 cells. J Cell Biol. 1994;127:1419–1433. doi: 10.1083/jcb.127.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu Y, Edwards RH. Differential localization of vesicular acetylcholine and monoamine transporters in PC12 cells but not CHO cells. J Cell Biol. 1997a;139:907–916. doi: 10.1083/jcb.139.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cho GW, Kim MH, Chai YG, Gilmor ML, Levey AI, Hersh LB. Phosphorylation of the rat vesicular acetylcholine transporter. J Biol Chem. 2000;275(26):19942–19948. doi: 10.1074/jbc.M902174199. [DOI] [PubMed] [Google Scholar]
- 130.Gilmor ML, Nash NR, Roghani A, Edwards RH, Yi H, Hersch SM, Levey AI. Expression of the putative vesicular acetylcholine transporter in rat brain and localization in cholinergic synaptic vesicles. J Neurosci. 1996;16:2179–2190. doi: 10.1523/JNEUROSCI.16-07-02179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weihe E, Tao-Cheng JH, Schafer MK, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Nat Acad Sci USA. 1996;93:3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dietrich J, Hou X, Wegener AM, Geisler C. CD3 gamma contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO J. 1994;13(9):2156–2166. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barbosa J, Jr, Clarizia AD, Gomez MV, Romano-Silva MA, Prado VF, Prado MAM. Effect of protein kinase C activation on the release of [3H] acetylcholine in the presence of vesamicol. J Neurochem. 1997;69:2608–2611. doi: 10.1046/j.1471-4159.1997.69062608.x. [DOI] [PubMed] [Google Scholar]
- 134.Molloy SS, Thomas L, Kamibayashi C, Mumby MC, Thomas G. Regulation of endosome sorting by a specific PP2A isoform. J Cell Biol. 1998;142:1399–1411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schapiro FB, Soe TT, Mallet WG, Maxfield FR. Role of cytoplasmic domain serines in intracellular trafficking of furin. Mol Biol Cell. 2004;15(6):2884–2894. doi: 10.1091/mbc.E03-09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bedet C, Isambert MF, Henry JP, Gasnier B. Constitutive phosphorylation of the vesicular inhibitory amino acid transporter in rat central nervous system. J Neurochem. 2000;75(4):1654–1663. doi: 10.1046/j.1471-4159.2000.0751654.x. [DOI] [PubMed] [Google Scholar]
- 137.Yao J, Erickson JD, Hersh LB. Protein kinase A affects trafficking of the vesicular monoamine transporters in PC12 cells. Traffic. 2004;5(12):1006–1016. doi: 10.1111/j.1600-0854.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 138.Van der Kloot W. Down-regulation of quantal size at frog neuromuscular junctions: possible roles for elevated intracellular calcium and for protein kinase C. J Neurobiol. 1991;22(2):204–214. doi: 10.1002/neu.480220210. [DOI] [PubMed] [Google Scholar]
- 139.Van der Kloot W, Branisteanu D. Effects of activators and inhibitors of protein kinase A on increases in quantal size at the neuromuscular junction. Pflugers Arch. 1992;420:336–341. doi: 10.1007/BF00374467. [DOI] [PubMed] [Google Scholar]
- 140.Borowsky B, Hoffman BJ. Analysis of a gene encoding two glycine transporter variants reveals alternative promoter usage and a novel gene structure. J Biol Chem. 1998;273:29077–29085. doi: 10.1074/jbc.273.44.29077. [DOI] [PubMed] [Google Scholar]
- 141.Poyatos I, Ruberti F, Martinez-Maza R, Gimenez C, Dotti C, Zafra F. Polarized distribution of glycine transporter isoforms in epithelial and neuronal cells. Mol Cell Neurosci. 2000;15:99–111. doi: 10.1006/mcne.1999.0807. [DOI] [PubMed] [Google Scholar]
- 142.Hanley JG, Jones EMC, Moss SJ. GABA receptor p1 subunit interacts with a novel plice variant of the glycine transporter GLYT-1. J Biol Chem. 2000;275:840–846. doi: 10.1074/jbc.275.2.840. [DOI] [PubMed] [Google Scholar]
- 143.Rau KS, Birdsall E, Hanson JE, Johnson-Davis KL, Carroll FI, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Bupropion increases striatal vesicular monoamine transport. Neuropharmacology. 2005;49(6):820–830. doi: 10.1016/j.neuropharm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 144.Brown JM, Hanson GR, Fleckenstein AE. Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J Pharmacol Exp Ther. 2001;296:762–767. [PubMed] [Google Scholar]
- 145.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 146.Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, Ugarte YV, Gibb JW, Hanson GR, Fleckenstein AE. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. J Pharmacol Exp Ther. 2002;302(2):497–501. doi: 10.1124/jpet.302.2.497. [DOI] [PubMed] [Google Scholar]
- 147.Truong JG, Newman AH, Hanson GR, Fleckenstein AE. Dopamine D2 receptor activation increases vesicular dopamine uptake and redistributes vesicular monoamine transporter-2 protein. Eur J Pharmacol. 2004;504(1–2):27–32. doi: 10.1016/j.ejphar.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 148.Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J Neurosci. 2002;22(19):8705–8710. doi: 10.1523/JNEUROSCI.22-19-08705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hayashi M, Raimondi A, O’Toole E, Paradise S, Collesi C, Cremona O, Ferguson SM, De Camilli P. Cell- and stimulus-dependent heterogeneity of synaptic vesicle endocytic recycling mechanisms revealed by studies of dynamin 1-null neurons. Proc Natl Acad Sci U S A. 2008;105(6):2175–2180. doi: 10.1073/pnas.0712171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuliawat R, Arvan P. Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic beta-cells. J Cell Biol. 1994;126:77–86. doi: 10.1083/jcb.126.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jung SK, Morimoto R, Otsuka M, Omote H. Transmembrane topology of vesicular glutamate transporter 2. Biol Pharm Bull. 2006;29(3):547–549. doi: 10.1248/bpb.29.547. [DOI] [PubMed] [Google Scholar]
- 152.Fei H, Karnezis T, Reimer RJ, Krantz DE. Membrane topology of the Drosophila vesicular glutamate transporter. J Neurochem. 2007;101:1662–1671. doi: 10.1111/j.1471-4159.2007.04518.x. [DOI] [PubMed] [Google Scholar]
- 153.McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- 154.Sagne C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417(2):177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]