Abstract
Purpose
To improve spatial and temporal resolution and signal-to-noise ratio (SNR) in 3D radial contrast-enhanced (CE) time-resolved MR angiography by means of a novel hybrid phase contrast (PC) and CE MRA acquisition and HYPR reconstruction (PC HYPR Flow).
Materials and Methods
PC HYPR Flow consists of a CE exam immediately followed by a PC scan used to constrain the HYPR reconstruction of the time series. Temporal resolution of the new method was studied in computer simulations. The feasibility of the new technique was studied in healthy subjects and patients with brain arterio-venous malformations and in a canine model of aneurysms.
Results
Simulations demonstrated preservation of contrast agent dynamics in proximal vessels, showing better performance than peer methods for acceleration up to 20 in 2D. In vivo, PC HYPR Flow yielded 3D time series with frame rate of 0.5 s and significantly outperformed two peer methods via a major increase in spatial resolution (0.8×0.8×0.8 mm3) and arterial/venous ratio, while maintaining necessary temporal waveform fidelity and high SNR.
Conclusion
This initial study indicates that PC HYPR Flow simultaneously provides 3D isotropic sub-millimeter spatial resolution, sub-second temporal reconstruction windows and high SNR level, which may benefit a wide range of CE MRA applications.
Keywords: Constrained Reconstruction, Constrast Enhanced MRA, Phase Contrast MRA, HYPR, Angiography, Radial Trajectory
INTRODUCTION
In contrast-enhanced (CE) time-resolved angiography it is desirable to obtain images with high spatial and temporal resolution as well as high signal-to-noise ratio (SNR). This is a challenging problem in MRI because rapid passage of the contrast agent limits the temporal window available for acquisition of each time frame. In Cartesian sampling schemes, limited acquisition window bounds the number of phase encodes, which, in turn, limits temporal and/or spatial resolution of the acquired time frames. Non-Cartesian acquisition schemes, such as radial or spiral, exhibit much less structured artifacts resulting from undersampling; however, the SNR of the reconstructed images is still proportional to the square root of acquisition time, making the noise level prohibitively high for rapid imaging applications. Use of view sharing or parallel imaging techniques (1-3) addressed the spatial/temporal resolution trade off to a certain degree but still did not overcome the SNR limitation (4,5), although the SNR loss, typical for parallel imaging, is not as strong in CE MR angiography (6,7).
Recently introduced HighlY constrained backPRojection (HYPR) reconstruction technique (8) for time-resolved imaging demonstrated great potential in obtaining clinical quality images from severely undersampled data in such applications as Phase Contrast (PC) angiography of the brain (9) and contrast enhanced peripheral angiography (10,11). The original algorithm was followed by several modifications, including HYPR LR (Local Reconstruction) (12) and several iterative methods (13-15). In HYPR methods, a composite image from time averaged MR signal is used to constrain the reconstruction of individual time frames. Among many advantages, HYPR methods allow for departure from the square root of imaging time SNR dependence due to their ability to transfer the SNR of the composite image into individual time frames. HYPR is tailored to radial acquisitions such as the 3D Vastly undersampled Isotropic Projection Reconstruction (VIPR) scheme (16). The ability of VIPR to sample the k-space efficiently and to provide 3D isotropic spatial resolution makes it a perfect match to the imaging task at hand.
The performance of HYPR reconstruction depends significantly on the quality of composite image. In particular, it is bounded from above by the quality of prior information from composite images including spatial resolution and SNR. Typically, the composite image is obtained from all or a subset of the projections collected in a time-resolved exam. As we demonstrate in the paper, in highly accelerated applications, such as CE MRA of AVMs, the target acceleration factors may significantly limit quality of composite image formed in this fashion because the acquisition window of the first pass of contrast agent is not long enough to provide both high spatial resolution and high SNR.
In this paper, we introduce a novel technique, PC HYPR Flow, to address the dilemma of providing high SNR and spatial resolution without sacrificing temporal resolution by acquiring composite image in a separate, non-time-resolved phase contrast (PC) scan. In this technique, a contrast agent injection is timed to a 3D interleaved VIPR acquisition with high frame rate. The CE exam is immediately followed by a longer post-contrast PC VIPR scan (17) that provides a high quality composite image used as a constraint in the HYPR LR reconstruction of the CE data. Such a scheme splits the task of providing high spatial resolution/SNR and high temporal resolution between two scans: depiction of the rapid flow dynamics is provided by the time-resolved CE exam, while high spatial resolution and high SNR result from the longer PC scan used to obtain a composite image. The acquisitions are then merged into the reconstructed time series using the HYPR LR algorithm. Thus, PC HYPR Flow combines the benefits of several 3D interleaved radial acquisitions and HYPR reconstruction.
The layout of this paper is as follows: we begin the next section by describing the acquisition scheme for PC HYPR Flow. Next, we review the HYPR LR method used for reconstruction of the acquired data and discuss the specific challenges of the PC HYPR Flow reconstruction. Finally, we discuss temporal response of the new method and provide both simulation and in-vivo results.
METHODS
As briefly explained in the Introduction, PC HYPR Flow exam consists of three continuously performed scans. Data acquisition starts with a pre-contrast scan, immediately followed by a contrast agent injection and a time-resolved first pass scan. These short exams (usually 30-60 sec long each) are instantly succeeded by a longer PC exam. The acquired time-resolved data are then reconstructed using HYPR LR processing with the composite image constraint obtained from the post-contrast PC exam. We describe both acquisition and reconstruction procedures in detail below.
Acquisition Scheme
Both pre-contrast and first pass exams are done using the same acquisition scheme. This allows using the pre-contrast data as a k-space subtraction mask to suppress the background and stationary tissue for improved vessel visualization and enforcing sparsity of underlying time frames. In order to capture rapidly changing dynamics of the contrast passage, high time frame rates need to be maintained (2). We maximize the efficiency of a 3D radial acquisition by using Multi-Echo (ME) VIPR sequence (16,17). During the central readout, the sequence acquires two half echoes at slightly different projection angles allowing four half-echoes to be acquired at four separate projection angles in each TR. Data sampling during the gradient dephasers and rephasers further increases acquisition efficiency. The increased acquisition efficiency of such a modified trajectory leads to SNR improvement, while the specific choice of the four echo times acquired in each TR to yield in- and out-of-phase images provides an added benefit of fat signal suppression (17). In order to ensure a uniform coverage of the spherical region, we use interleaved acquisition with the number of interleaves equal to the number of target time frames. The interleaves are scheduled pseudo-randomly according to the bit-reversal algorithm (18).
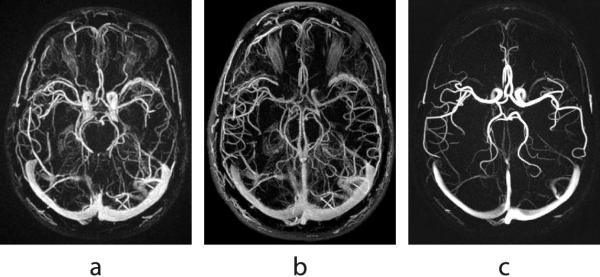
The contrast enhanced time-resolved exam is immediately followed by a phase contrast exam. Our PC scan also uses a VIPR sequence with velocity encoding in three directions (19-21). The 3D image volume is produced via a complex difference processing (22) by taking linear combinations of the raw data acquired for different velocity directions according to the Hadamard encoding scheme (23). Such complex difference processing allows for visualization of a vascular tree by suppressing background and stationary tissue. Since we are no longer bound by the need to capture the contrast passage, the PC data may be acquired much longer to reach the necessary SNR and high isotropic spatial resolution (typically 0.62 -- 0.8 mm (20,21)). Figure 1 provides an example of composite images obtained (a) from the projections acquired during the first pass of contrast in the CE exam, (b) from all the projections acquired in a CE exam during 240 s (total 480 s with mask acquisition), and (c) from a separate PC exam. As can be seen from this example, despite having a subtraction mask, both CE images suffer from background enhancement, with the soft tissues even more pronounced in the longer scan due to the contrast leakage into the neighboring tissues. Moreover, since the longer scan represents a temporal averaging of the contrast recirculation and gradual washout, the venous signal has higher intensity while the carotid arteries have poor visibility. The PC image does not suffer from these problems and overall possesses a higher level of sparsity, a feature necessary for successful application of HYPR reconstruction. The choice of velocity encoding (VENC) regulates the intensity of the vessels in the PC image depending on the speed of the blood flow in them. A typical choice of VENC for a human subject is 60-100 cm/s. As a result, the additional advantage of using a PC composite over a CE composite in the HYPR processing is a minimal signal cross-talk between veins and arteries since the venous signal in the PC image is suppressed because of the slower blood flow.
1.
Three possible choices for a composite image obtained (a) from the projections acquired during the first pass of contrast; (b) from all of the projections acquired during a 4 minute CE exam; (c) from a separate 5 minute PC exam.
Reconstruction Approach
The reconstruction of the time-resolved series is performed using HYPR LR algorithm (12). This reconstruction technique is well-suited for the given problem for the following three reasons. As it was pointed out in (12), HYPR LR significantly reduces cross-talk between spatially distinct vessels with different time courses, allowing for excellent separation of the arterial and venous phases. Secondly, HYPR LR can provide accurate temporal information about individual time frames even if it uses a composite image acquired over a long period of time. This is important since SNR of individual time frames is determined primarily by the SNR of the composite image, hence, better quality composite image results in higher quality time frames. Finally, unlike the original HYPR algorithm, HYPR LR can be easily applied to arbitrary k-space trajectories. In HYPR LR, a relevant composite image is formed from all or a portion of the k-space data acquired over the course of the exam. Then, the high resolution, high SNR composite image is multiplied by a weighting image particular to each time frame. Mathematically, the reconstruction of HYPR LR time frame is expressed by the following formula:
| [1] |
where T is a conventionally reconstructed time frame image, C is the composite image, ϕ is the convolution kernel and t and are temporal and spatial variables, respectively. The dependency of the composite image on time variable in the denominator of Eq. [1] reflects the fact that we reproject the composite image along the same trajectory that was used for the acquisition of the given time frame and then reconstruct an undersampled version of the composite image. This procedure produces undersampling artifacts with the same geometry as the ones in the undersampled time frame image in the numerator of Eq. [1] allowing for their partial cancellation in the weighting image. HYPR LR is naturally suited for use with multi-coil arrays. In this case, images T and C in Eq. [1] can be obtained either using a conventional square root of sum of squares reconstruction (the approach we chose in this paper) or any of the parallel imaging algorithms such as SENSE or GRAPPA. At high acceleration factors, it may be beneficial to use a parallel imaging algorithm prior to HYPR LR processing in order to reduce some of the undersampling artifacts. However, for non-Cartesian sampling it usually increases reconstruction time noticeably.
In PC HYPR Flow reconstruction, we apply Eq. [1] with the composite image C obtained from the PC VIPR scan and time frame data T obtained from the rapid CE exam. The convolution operation is performed in k-space: we multiply the acquired or generated k-space data for the time frame and composite images by the Fourier transform of the convolution kernel ϕ at these sampling locations. We chose the Gaussian low-pass filter for our implementation of HYPR LR because of its localization properties both in the image domain and in the k-space.
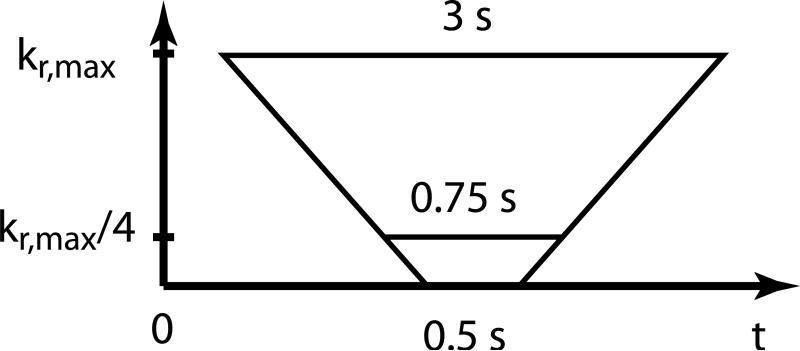
The convolution operation has a positive effect on the SNR, which increases in proportion to the norm of the convolution kernel in the case of white Gaussian noise. However, if the frame rate of the time-resolved acquisition is very high (below 0.75 sec), even the convolution process cannot mollify the high noise level and undersampling artifacts in HYPR LR weighting images. In 3D radial images, undersampling artifacts usually manifest themselves as background fog aligned with the acquisition geometry. Because we use pseudo-random interleaving of projections to ensure relatively uniform coverage of the k-space in each time frame, the noise and artifact pattern in highly undersampled HYPR LR weighting images rotates from one time frame to the next causing a flickering effect in the reconstructed time frames. In order to counteract this phenomenon, we use temporal “tornado” filter (24) for reconstruction of individual time frames prior to the convolution process. “Tornado” filtering is a view-sharing technique in which the width of the temporal window for k-space data depends on the spatial frequencies. A schematic illustration of the filter is given in Fig. 2. Experimentally, we have found that even in the cases of very severe undersampling, a linearly varying temporal window of 0.5 s at the center of k-space and 3 s wide at the highest spatial frequencies is sufficient to remove the flickering artifact from the reconstructed time frames. Moreover, since HYPR LR processing uses only densely sampled central k-space area within ¼ of full k-space acquisition radius, the effective temporal window used in so designed “tornado” filtered reconstruction does not exceed 0.75 s at all spatial frequencies.
2.
Schematic illustration of the k-space data used for reconstruction of a single time frame with spatial frequency dependent temporal tornado filtering.
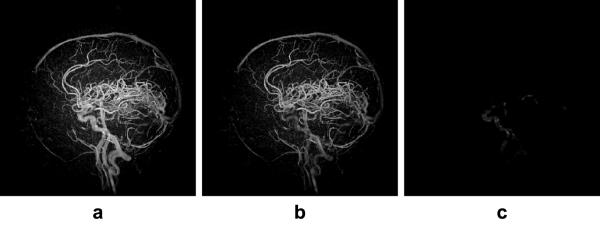
Even though we acquire pre-contrast k-space data to be used as a subtraction mask, background and stationary tissue may not cancel out completely due to residual magnetization and noise variations in the image as well as temporal instabilities of scanner electronics. As a result, early frames in PC HYPR Flow may contain low intensity imprint of the composite image. In order to alleviate this reconstruction artifact, we average temporally several of the reconstructed PC HYPR Flow frames that were acquired before contrast arrival (typically, this temporal window spans the first 5-10 seconds of the CE exam) and subtract this averaged volume from all other frames in the reconstructed time series. Both temporal averaging and subtraction are done in the image domain on the complex-valued data, followed by taking the absolute values of the difference images. This process strongly suppresses the background and reconstruction artifacts in the resultant time series at the expense of a slight decrease in the SNR. Fig. 3 illustrates the effect of the imprint subtraction from an early time frame: the image in Fig. 3a is HYPR LR reconstructed frame corresponding to the time of contrast arrival. One can clearly see the imprint of the composite image on the noisy background. The image in Fig. 3b is the subtraction mask obtained by temporally averaging 5 pre-contrast HYPR LR reconstructed frames. The image in Fig. 3c shows the final PC HYPR Flow time frame after the subtraction.
3.
(a) Imprint of the composite image on the noisy background in an early time frame. (b) Subtraction mask obtained from temporal averaging of the pre-contrast time frames. (c) Subtracted image representing the PC HYPR Flow result. All images are shown with the same window leveling.
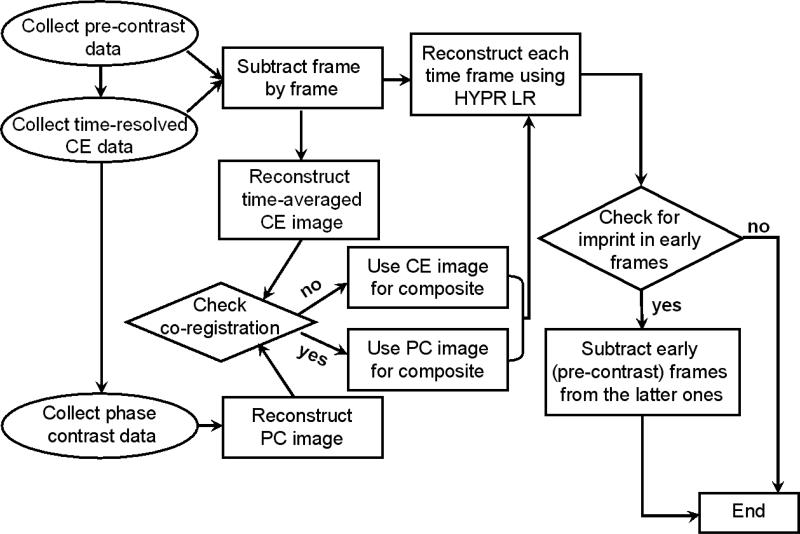
The flowchart of data acquisition and reconstruction of PC HYPR Flow is summarized graphically in Fig. 4. The goal of the described PC HYPR Flow technique is to produce a time series of 3D image volumes with high SNR, high isotropic spatial resolution, and high frame rate accurately depicting the contrast dynamics.
4.
The flowchart of the PC HYPR Flow data acquisition and reconstruction.
Simulation Studies
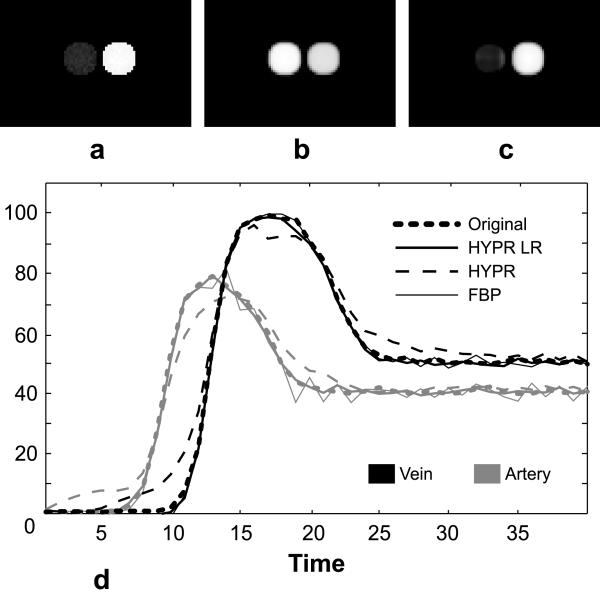
The convolution operation used in the HYPR LR processing performs local spatial averaging of the signal (12). Temporal resolution of HYPR LR was studied in detail by means of impulse response function (25). It was concluded that HYPR LR can reliably reconstruct impulse signals localized to regions as small as 3x3 pixels. In this paper, to justify the choice of HYPR LR algorithm, we evaluate the degree of cross-talk created by HYPR LR reconstruction between spatially adjacent vessels with distinct temporal behavior. For this purpose we designed a mathematical simulation using a digital phantom that contains two circular objects with a diameter of 16 pixels each. These circles, representing an artery and a vein, are separated by only two pixels as illustrated in Fig. 5a. We supply these objects with temporally distinct waveforms and add zero mean Gaussian noise to the resultant time frames. We simulated time-resolved radial acquisition of this time series by generating 10 different projections per each of the 40 frames and reconstructed the resultant data using HYPR LR algorithm with the composite image obtained from all 400 projections comprising the exam. To verify that HYPR LR correctly reflects simulated hemodynamics even in this challenging situation, we compared arterial and venous waveforms derived from the reconstructed time series with the original ones. We also compared performance of HYPR LR to that of regular HYPR and filtered backprojection (FBP), the conventional reconstruction method for radial data. A 13x13 pixel filter was used in the HYPR LR reconstruction. The size of the filter is determined by two factors: the support of the filter in k-space should be localized to the more fully sampled central portion of k-space, while in the image space the support of the filter should be comparable to the size of relevant features in the images (e.g., vessel size and distance between vessels). The first requirement allows reducing undersampling artifacts in the reconstructed images, while the second requirement is needed in order to prevent cross-talk between vessels with different temporal behavior and leaking of the signal into the background. Typically, a Gaussian filter with full width at half maximum of 8-13 pixels in each dimension preserves spatial resolution and improves image quality.
5.
(a) Peak arterial frame of a phantom in which two objects with different temporal behavior are separated by only two pixels. (b) Composite image used to constrain the HYPR LR reconstruction. (c) HYPR LR reconstruction from 10 projections of the peak arterial frame. (d) Temporal waveforms of the objects in the phantom.
In-Vivo Studies
The feasibility of PC HYPR Flow technique was explored in in-vivo exams of both healthy volunteers and patients with a brain AVM. Informed consent was obtained from all human subjects prior to the exams and the studies were carried out in compliance with the approved IRB at our institution. The exams were performed on a 3.0T GE Signa HD scanner (GE Healthcare, Waukesha, WI, USA) with an 8-channel head coil using MultiHance contrast agent (Bracco Diagnostics Inc., Princeton, NJ, USA). All exams had 260 mm field of view. The time-resolved exams had a total duration of 60 s with the 0.5 s frame rate and a TR of 3.1 ms, resulting in the acquisition of 110 four half-echo bowties in each time frame at bandwidth of 125 kHz. The length of the PC VIPR exam was 300 s during which about 7000 partial echo projections were acquired at bandwidth of 62.5 kHz. Additionally, we validated the new method in canine model of aneurysms. The acquisition scheme was identical to the one used in the human studies. The animal experiment complied with the protocol approved by the IRB at our institution.
RESULTS
Simulation studies
Figure 5 shows peak arterial frames of the original time series (Fig. 5a) and the one reconstructed with HYPR LR (Fig. 5c). Note that even though both arterial and venous signals are present in the composite image used for HYPR LR (Fig. 5b), the strong arterial signal does not contaminate the signal in the venous location despite their spatial proximity. The adequate arterial/venous separation is further confirmed by Fig. 5d that compares the original arterial and venous waveforms with the ones derived from the time series reconstructed with the FBP, HYPR LR and regular HYPR algorithms. It can be seen from the plot that HYPR LR performs better in the situations where spatially close objects in the image series have significantly different waveforms. The deviation of the reconstructed HYPR LR waveforms from the original ones is caused by noise in the projections and undersampling artifacts and does not misrepresent temporal behavior of each vessel. The simulation results confirm that the convolution operation does not result in significant cross-talk between spatially close vessels with different time courses.
In-Vivo Studies
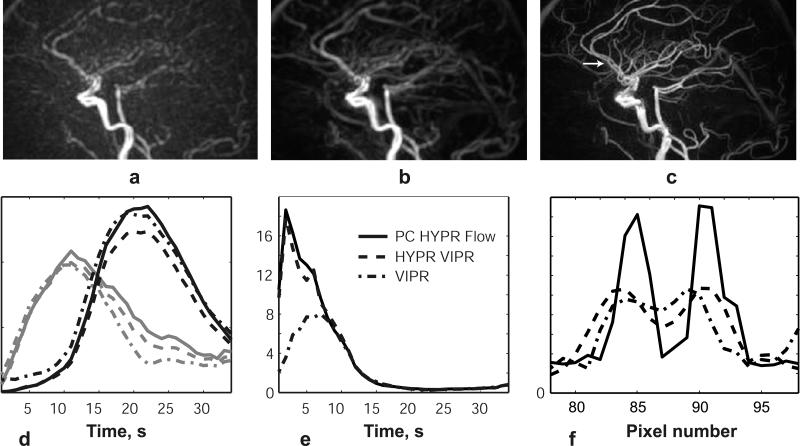
We compared three approaches to reconstruction of CE MRA data: conventional VIPR reconstruction with temporal tornado filtering, HYPR LR with composite image obtained from CE data, and proposed PC HYPR Flow method. Figures 6a-6c compare the reconstruction results of the peak arterial frame in a healthy volunteer. All images are reconstructed to isotropic 0.8x0.8x0.8 mm voxel size. It was demonstrated in (12) that SNR of HYPR LR images is largely determined by the SNR of the composite image used to constrain the reconstruction. Figure 1 provides a visual illustration of the SNR and CNR (contrast-to-noise ratio) superiority of the PC composite image over the CE one, predicting the same advantages for the PC HYPR Flow reconstructed images. This is supported by the estimation of the CNR of the images presented in Fig. 6a-6c. The CNR for the carotid artery was measured to be 59.20 for the VIPR reconstruction, 75.95 for HYPR LR reconstruction with the CE composite, and 132.02 for HYPR LR reconstruction with the PC composite.
6.
Reconstruction of a single frame of a contrast enhanced exam in a healthy volunteer using: (a) VIPR with a 3 sec tornado filter; (b) HYPR LR processing with the composite image formed from all projections in the CE exam; (c) HYPR LR processing with the composite image obtained from a separate PC exam; (d) Temporal waveforms of the three reconstructions measured in the carotid artery and sagittal sinus; (e) Arterial-to-venous ratios of the three reconstructions; (f) Vessel profiles of the three reconstructions at the horizontal cross-section marked by an arrow in (c).
The plots of the temporal waveforms in the carotid artery and sagittal sinus in Fig. 6d show that all three reconstruction approaches reflect similar temporal behavior. We calculated the arterial-venous separation ratio as the ratio of the mean values of arterial and venous signals measured in ROIs of the same size. The plots of the arterial-venous separation ratio presented in Fig. 6e illustrate that HYPR LR processing with both types of composite images provides noticeably better separation of the arterial and venous phases than the use of temporal view sharing. Finally, Fig. 6f presents image profiles of the three reconstructions, clearly demonstrating that, compared to the other two approaches, the use of the PC VIPR composite provides a significantly improved delineation of the small vessels.
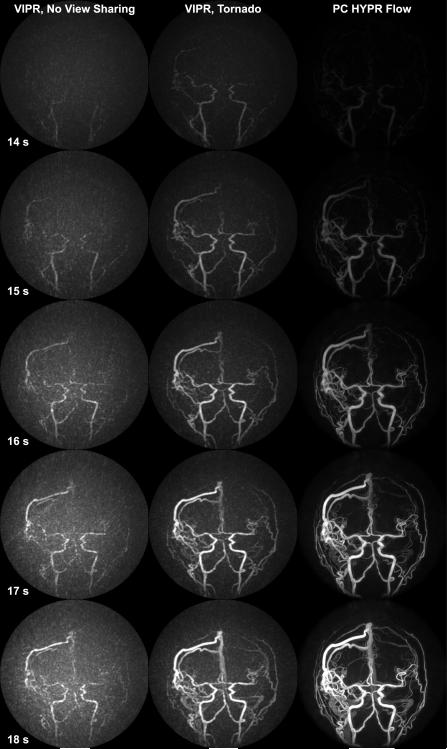
We examined the temporal fidelity of the proposed PC HYPR Flow algorithm by comparing time series of three different reconstructions of the data from an AVM patient. The first column in Fig. 7 presents VIPR reconstruction without any view sharing of 0.5 s time frames. Due to extremely high acceleration factor, these images have prohibitively low SNR and high level of undersampling artifacts manifesting themselves as background fog, which make smaller vessels virtually indiscernible. The use of tornado filtering (Fig. 7, middle column) helps improve image quality but seems to lead to some early enhancement, especially notable in the earlier frames, while small vessels are still obscured by background fog. The PC HYPR Flow time series (Fig.7, last column) significantly improves small vessel depiction, while the temporal progression of the vessel filling corresponds to the one exhibited by VIPR reconstruction.
7.
Sagittal MIPs depicting contrast passage in an AVM patient during a time window of 14-18 s after the contrast agent injection of the VIPR time series without any view sharing (1st column); VIPR time series with a 3 s tornado filter (middle column); PC HYPR Flow time series (last column).
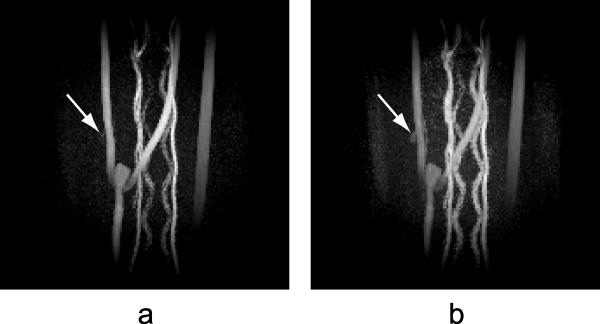
The next example demonstrates the flexibility of the PC composite image, which can be reprocessed imitating a different velocity encoding to enhance visualization of areas with significantly different blood flows. In the example of a canine model of aneurysms (Fig. 8), one of the two aneurysms that can be detected in the temporally averaged CE image is not clearly visible in the PC image obtained with the original velocity encoding of 40 cm/s due to the slow blood flow. To improve visualization of low intensity regions, we reconstructed the phase contrast data imitating a lower acquisition velocity encoding of 20 cm/s. The aneurysm became more visible on the newly reconstructed PC composite image at the expense of increased noise level, which propagated into individual time frames during HYPR LR processing. However, PC HYPR Flow images with this composite image still have sufficient image quality. Figure 8 demonstrates the results of PC HYPR Flow reconstruction of a single time frame using PC composite images with velocity encodings of 40 cm/s (Fig. 8a) and 20 cm/s (Fig. 8b). Arrows in the images indicate the location of a small aneurysm.
8.
Single frame of PC HYPR Flow reconstruction in a canine aneurysm model using phase contrast composite image with (a) VENC = 40 cm/s; (b) simulated VENC of 20 cm/s. Arrows indicate locations of aneurysms.
DISCUSSION
We proposed a novel hybrid method, PC HYPR Flow, for high resolution, high SNR 3D time resolved CE MRA. We evaluated the feasibility of PC HYPR Flow by applying it to intracranial angiography in both healthy volunteers and patients with brain arterio-venous malformations (AVMs). Using PC HYPR Flow, we were able to obtain time series of 3D image volumes depicting first pass of the contrast material with a frame rate of 0.5 s and an isotropic spatial resolution of 0.8 mm. Clinically, this method may advance imaging of patients with brain AVMs. Additional benefit of the proposed hybrid acquisition and reconstruction is that 3D blood flow velocity information obtained in the PC exam can be utilized to derive pressure gradients and wall shear stress (26,27), parameters that may be useful in medical management of stable AVM patients.
The sampling efficiency of the CE scan can be increased even further by doing partial read-out in each bowtie, thus, acquiring only lower frequency part of the k-space. This modification may further increase temporal resolution of the presented technique. We estimate that such a scheme may allow us to collect 160 four half-echo bowties in each 0.5 s time frame (compared to current 110 bowties in each 0.5 s), which results in a radial undersampling factor of about 800 relative to the Nyquist criterion for Cartesian sampling. The absence of high frequency information does not affect the quality of the reconstructed images since in HYPR LR the weighting images are formed from low resolution versions of time frames, and the composite image obtained from the PC exam contains both high and low spatial frequencies. In practice, such choice of CE acquisition is preferable if we expect good co-registration between the CE and PC exams, i.e. if the patient can be expected to lie still during the PC HYPR Flow exam. However, if motion may occur during the PC HYPR Flow exam, as may be expected, for example, when imaging pediatric patients, it is advisable to do full read-out in the ME VIPR exam, so that the CE composite image may be used instead of the PC composite image in case of misregistration between these exams. Alternatively, the data acquired in the CE and PC exams may be co-registered in post processing using image registration tools (28,29) if a patient moves between the scans. A more challenging problem is discerning and correcting for subtle effects of motion, especially during the longer PC exam. While radial acquisition intrinsically alleviates the appearance of motion artifacts compared to the Cartesian acquisition, small scale motion may result in blurring. Several approaches have been proposed in the literature to tackle this problem. First of all, a three-dimensional radial navigator can be employed during acquisition to minimize corruption of data (30). Additionally, self-navigating properties of radial sampling can be exploited to correct for rotational and translational motion by analyzing different moments of radial projections (31,32). Finally, autofocusing approach (33) can be used at high spatial resolution for correction of motion without navigators (34).
In order to evaluate whether the proposed approach can be applicable to clinical tasks other than evaluation of AVMs, we applied PC HYPR Flow to a canine model of aneurysms. Imaging aneurysms can be a challenging task because of their small size and potentially slow flow. We demonstrated that signal reduction in PC VIPR composite caused by slow flow may be alleviated by reconstruction of composite at smaller VENC values (Fig. 8). In practice, to ensure utilization of all available information, several sets of images, with low and high VENC PC VIPR composites as well as with the CE composite, may be reconstructed and supplied for radiological inspection. Another possibility is reconstruction of PC VIPR images at multiple VENC values and adaptive combination of such images into a final composite image that would combine high SNR of high VENC reconstruction and sensitivity to slow flow of low VENC reconstruction. However, while low VENC reconstruction approach enhances areas of slow flow, it provides low velocity-to-noise ratio. To ensure adequate visualization of low velocity areas in the composite image, one may need to turn to three-point PC velocimetry to increase velocity-to-noise ratio for areas with low flow velocity (35). This and other adaptive approaches are subject of the future research.
PC HYPR Flow utilizes a novel concept of using multiple data acquisitions to obtain a time series of 3D images with isotropic sub-millimeter spatial resolution, sub-second temporal resolution, and high SNR. To the best of our knowledge, this approach was not yet explored for accelerated CE MRA. Moreover, HYPR is a flexible reconstruction method that allows for incorporation of different types of prior information to constrain the reconstruction. For example, a time of flight examination (TOF) can be used as a composite image to improve spatial resolution (36). Also, composite image can admit different types of acquisition geometry, including Cartesian phase encoding, hybrid radial/Cartesian (stack of stars) or 3D radial (VIPR) acquisition. The approach introduced in the paper may be also useful to design an advanced reconstruction method based on other constrained reconstruction approaches (37-39).
In conclusion, initial trials in healthy volunteers and brain AVM patients indicate that PC HYPR Flow is a feasible technique that simultaneously provides 3D isotropic sub-millimeter spatial resolution, sub-second temporal reconstruction windows (including optional tornado filter view sharing) and high SNR level for contrast-enhanced angiography. Clinically, PC HYPR Flow has potential to advance evaluation of AVM patients. Moreover, PC HYPR Flow is a promising technique for imaging intracranial stenoses. The applicability of PC HYPR Flow is not limited to intracranial angiography; PC HYPR Flow can be used for any anatomy that admits a PC VIPR exam. We believe that the use of PC HYPR Flow can benefit, in particular, peripheral angiography and imaging of any anatomical region where rapid flow dynamics currently limit spatial resolution or SNR.
ACKNOWLEDGEMENTS
The authors would like to thank Kelly Hellenbrand for assistance in scanning volunteers.
Grant Support:
This work was supported in part by NIH RO1 HL/RR6648803A1, R01CA116380, R21EB009441, R21EB006393, and GE Healthcare.
REFERENCES
- 1.Nael K, Michaely HJ, Villablanca P, Salamon N, Laub G, Finn JP. Time-resolved contrast enhanced magnetic resonance angiography of the head and neck at 3.0 tesla: initial results. Invest Radiol. 2006;41:116–124. doi: 10.1097/01.rli.0000192416.19801.ca. [DOI] [PubMed] [Google Scholar]
- 2.Cashen TA, Carr JC, Shin W, et al. Intracranial time-resolved contrast-enhanced MR angiography at 3T. AJNR Am J Neuroradiol. 2006;27:822–829. [PMC free article] [PubMed] [Google Scholar]
- 3.Fenchel M, Nael K, Deshpande VS, et al. Renal magnetic resonance angiography at 3.0 Tesla using a 32-element phased-array coil system and parallel imaging in 2 directions. Invest Radiol. 2006;41:697–703. doi: 10.1097/01.rli.0000233319.04760.a4. [DOI] [PubMed] [Google Scholar]
- 4.Ohliger MA, Grant AK, Sodickson DK. Ultimate intrinsic signal-to-noise ratio for parallel MRI: electromagnetic field considerations. Magn Reson Med. 2003;50:1018–1030. doi: 10.1002/mrm.10597. [DOI] [PubMed] [Google Scholar]
- 5.Wiesinger F, Boesiger P, Pruessmann KP. Electrodynamics and ultimate SNR in parallel MR imaging. Magn Reson Med. 2004;52:376–390. doi: 10.1002/mrm.20183. [DOI] [PubMed] [Google Scholar]
- 6.Riedy G, Golay X, Melhem ER. Three-dimensional isotropic contrast-enhanced MR angiography of the carotid artery using sensitivity-encoding and random elliptic centric k-space filling: technique optimization. Neuroradiology. 2005;47:668–673. doi: 10.1007/s00234-005-1416-2. [DOI] [PubMed] [Google Scholar]
- 7.Riederer SJ, Houchun HH, Krueger DR, Haider CR, Campeau NG, Houston J. Intrinsic signal amplification in application of 2D SENSE parallel imaging to 3D contrast-enhanced elliptic centric MRA and MRV. Magn Reson Med. 2007;58:855–864. doi: 10.1002/mrm.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mistretta CA, Wieben O, Velikina J, et al. Highly constrained backprojection for time-resolved MRI. Magn Reson Med. 2006;55:30–40. doi: 10.1002/mrm.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velikina JV, Mistretta CA, Johnson KM, Wieben O. An Application of Highly Constrained Backprojection (HYPR) to Time-Resolved VIPR Acquisition. Procs. of the 14th Scientific Meeting & Exhibition of the International Society for Magnetic Resonance in Medicine; Seattle, WA, USA. May, 2006.p. 92. [Google Scholar]
- 10.Wu Y, Korosec FR, Mistretta CA, Wieben O. CE-MRA of the lower extremities using HYPR stack-of-stars. J Magn Reson Imag. 2009;29:917–923. doi: 10.1002/jmri.21733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong HJ, Cashen TA, Hurley MC, et al. Radial sliding-window magnetic resonance angiography (MRA) with highly-constrained projection reconstruction (HYPR) Magn Reson Med. 2009;61:1103–1113. doi: 10.1002/mrm.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KM, Velikina J, Yijing Wu, Kecskemeti S, Wieben O, Mistretta CA. Improved Waveform fidelity Using Local HYPR Reconstruction (HYPR LR) Magn Reson Med. 2008;59:456–462. doi: 10.1002/mrm.21505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griswold M, Barkauskas K, Blaimer M, Moriguchi H, Sunshine J, Duerk J. More Optimal HYPR Reconstructions using a Combination of HYPR and Conjugate-Gradient Minimization. International MR Angio Club; Basel, Switzerland. Sept. 2006. [Google Scholar]
- 14.O'Halloran RL, Wen Z, Holmes JH, Fain SB. Iterative Projection Reconstruction of Time-Resolved Images using Highly Constrained Back-Projection (HYPR) Magn Reson Med. 2008;59:132–139. doi: 10.1002/mrm.21439. [DOI] [PubMed] [Google Scholar]
- 15.Samsonov AA, Jung Y, Alexander AL, Block WF, Field AS. MRI Compressed Sensing via Sparsifying Images.. Procs. of the 15th Scientific Meeting & Exhibition of the International Society for Magnetic Resonance in Medicine; Toronto, Canada. May, 2008.p. 342. [Google Scholar]
- 16.Barger AV, Block WF, Toropov Y, Grist TM, Mistretta CA. Time-resolved contrast-enhanced imaging with isotropic resolution and broad coverage using an undersampled 3D projection trajectory. Magn Reson Med. 2002;48:297–305. doi: 10.1002/mrm.10212. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky E, Lu A, Thornton FJ, Grist TM, Block WF. Using Multiple Half-Echoes to Improve Sampling Efficiency and Fat Suppression in Time-Resolved MRA. Procs. of the 11th Scientific Meeting & Exhibition of the International Society for Magnetic Resonance in Medicine; Toronto, Canada. July 2003.p. 322. [Google Scholar]
- 18.Song HK, Dougherty L, Schnall MD. Simultaneous acquisition of multiple resolution images for dynamic contrast enhanced imaging of the breast. Magn Reson Med. 2001;46:503–309. doi: 10.1002/mrm.1220. [DOI] [PubMed] [Google Scholar]
- 19.Gu TL, Korosec FR, Block WF, Fain SB, Turk QA, Lum D, Zhou Y, Grist TM, Haughton V, Mistretta CA. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR Am J Neuroradiol. 2005;26:743–905. [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson KM, Moftakhar R, Lum D, Wieben O, Mistretta CA. 7D Flow Imaging with PC VIPR for Hemodynamical Analysis.. Procs. of 2006 ISMRM Flow and Motion Workshop.; New York, New York, USA. July 2006. [Google Scholar]
- 21.Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D Phase Contrast MRI With Off-Resonance Corrected Dual Echo VIPR. Magn Reson Med. 2008;60:1329–1336. doi: 10.1002/mrm.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein MA, Ikezaki Y. Comparison of phase-difference and complex-difference processing in phase-contrast MR angiography. J Magn Reson Imaging. 1991;1:725–991. doi: 10.1002/jmri.1880010620. [DOI] [PubMed] [Google Scholar]
- 23.Dumoulin CL, Souza SP, Darrow RD, Pelc NC, Adams WJ, Ash SA. Simultaneous acquisition of phase-contrast angiograms and stationary tissue images with Hadamard encoding of flow-induced phase shifts. J Magn Reson Imaging. 1991;1:399–404. doi: 10.1002/jmri.1880010403. [DOI] [PubMed] [Google Scholar]
- 24.Block WF, Barger AV, Toropov Y, Grist TM, Mistretta CA. 3D time-resolved vastly undersampled isotropic projection (VIPR) imaging with spatial frequency dependent temporal filtering.. ISMRM Workshop on Minimum MR Data Acquisition Methods: Making More With Less.; Marco Island, FL. 2000.pp. 54–57. [Google Scholar]
- 25.Keith LA, Kecskemeti SJ, Velikina JV, Mistretta CA. Simulation of relative temporal resolution of time-resolved MRA sequences. Magn Reson Med. 2008;60:398–404. doi: 10.1002/mrm.21658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KM, Gu TL, Wieben O, Turk QA, Mistretta CA. 4D Pressure Mapping with ECG-gated Phase Contrast Vastly Undersampled Projection Imaging. Procs. of the13th Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine.; Miami Beach, USA. May, 2005.p. 598. [Google Scholar]
- 27.Lum D, Johnson KM, Wieben O, Korosec FR, Mistretta CA, Turksi PA. Measurement of Trans-stenotic Pressure Gradients in Swine: Comparison Between Retrospective ECG-Gated 3D Phase-Contrast MRA and Endovascular Pressure-Sensing Guidewires. Radiology. 2007;245:751–760. doi: 10.1148/radiol.2453061946. [DOI] [PubMed] [Google Scholar]
- 28.Pluim JPW, Maintz JBA, Viergever MA. Mutual Information Based Registration of Medical Images: A Survey. IEEE Trans Med Imag. 2003;22:986–1004. doi: 10.1109/TMI.2003.815867. [DOI] [PubMed] [Google Scholar]
- 29.Yijing Wu, Johnson KM, Velikina JV, Turski PA, Mistretta CA. Clinical Experience of HYPR Flow. Proc. of the 16th Scientific Meeting & Exhibition of the International Society for Magnetic Resonance in Medicine; Toronto, Canada. May, 2008.p. 110. [Google Scholar]
- 30.Johnson KM, Mistretta CA, Wieben O. 3-Dimensional Navigators for Motion Tracking and Correction in 3D Radial Imaging.. Proc. of the 15th Scientific Meeting & Exhibition of the International Society for Magnetic Resonance in Medicine; Berlin, Germany. 2007.p. 3428. [Google Scholar]
- 31.Wieben O, Barger AV, Block WF, Mistretta CA. Correcting for translational motion in 3D projection reconstruction.. Proc. of the 9th Scientific Meeting & Exhibition of the International Society for Magnetic Resonance in Medicine; Glasgow, Scotland. 2001.pp. 220–221. [Google Scholar]
- 32.Khansa I, Johnson KM, Wieben O. Correcting for Translational Motion in 3D Projection Reconstruction: Revisited.. Proc. of the 15th Scientific Meeting & Exhibition of the International Society for Magnetic Resonance in Medicine; Berlin, Germany. 2007.p. 3130. [Google Scholar]
- 33.Atkinson D, Hill DL, Stoyle PN, Summers PE, Keevil SF. Automatic correction of motion artifacts in magnetic resonance images using an entropy focus criterion. IEEE Trans Med Imaging. 1997;16:903–910. doi: 10.1109/42.650886. [DOI] [PubMed] [Google Scholar]
- 34.Lin W, Ladinsky GA, Wehrli FW, Song HK. Image metric-based correction (autofocusing) of motion artifacts in high-resolution trabecular bone imaging. J Magn Reson Imaging. 2007;26:191–197. doi: 10.1002/jmri.20958. [DOI] [PubMed] [Google Scholar]
- 35.Lee AT, Pike GB, Pelc NJ. Three-Point Phase-Contrast Velocity Measurements with Increased Velocity-to-Noise Ratio. Magn Reson Med. 1995;33:122–126. doi: 10.1002/mrm.1910330119. [DOI] [PubMed] [Google Scholar]
- 36.Yijing Wu, Kecskemeti SR, Turski PA, Mistretta CA, Hybrid HYPRMRA. Proc. of the 17th Scientific Meeting & Exhibition of the International Society for Magnetic Resonance in Medicine; Honolulu, HI, USA. April, 2009.p. 3257. [Google Scholar]
- 37.Velikina JV, Samsonov AA. HYPR-L0: A Hybrid Technique for CE MRA with Extreme Data Undersampling Factors.. Proc. of the17th Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine.; Honolulu, HI, USA. April 2009.p. 4. [Google Scholar]
- 38.Velikina JV. VAMPIRE: Variation Minimizing Parallel Imaging Reconstruction.. Procs. of the13th Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine.; Miami Beach, USA. May, 2005.p. 2424. [Google Scholar]
- 39.Wu H, Block WF, Samsonov AA. HYPR-Constrained Compressed Sensing Reconstruction for Accelerated Time Resolved Imaging.. Proc. of the 16th Scientific Meeting & Exhibition of the International Society for Magnetic Resonance in Medicine; Toronto, Canada. May, 2008.p. 339. [Google Scholar]