Abstract
Biliary tract cancers, encompassing the gallbladder, extrahepatic bile duct, and ampulla of Vater, are uncommon, yet highly fatal malignancies. Gallstones, the primary risk factor for biliary cancers, are linked with hyperlipidemia. We examined the associations of 12 single nucleotide polymorphisms (SNPs) of five genes in the lipid metabolism pathway with the risks of biliary cancers and stones in a population-based case-control study in Shanghai, China. We included 235 gallbladder, 125 extrahepatic bile duct, and 46 ampulla of Vater cancer cases, 880 biliary stone cases, and 779 population controls. Subjects completed an in-person interview and gave blood. Genotyping was conducted by Taqman assay using DNA from buffy coats. The effects of APOE IVS1+69 (rs440446) and APOB IVS6+360C>T (rs520354) markers were limited to men. Men carrying the G allele of APOE IVS1+69 had a 1.7-fold risk of stones (95% confidence interval (CI) =1.2–2.4), a 1.8–fold risk of gallbladder cancer (95% CI=1.0–3.3), a 3.7–fold risk of bile duct cancer (95% CI=2.0–7.0), and a 4-fold risk of ampullary cancer (95% CI=1.4–12.4). Male carriers of the T allele of APOB IVS6+360C>T had a 2-fold risk of bile duct cancer (95% CI=1.2–3.4). The APOB T-T haplotype (APOB IVS6+360C>T, EX4+56C>T) was associated with a 1.6-fold risk of bile duct cancer (95% CI=1.1–2.3). Male and female carriers of the T allele of LDLR IVS9-30C>T (rs1003723) had 1.5-fold risks of bile duct cancer. Our findings suggest that gene variants in the lipid metabolism pathway contribute to the risk of biliary tract stones and cancers, particularly of the bile duct.
Keywords: single nucleotide polymorphisms, lipid metabolism, gallstones, biliary tract cancer
Introduction
Biliary tract cancers, consisting of tumors of the gallbladder, extrahepatic bile ducts, and ampulla of Vater, are rare, but highly fatal malignancies (1, 2). Cholesterol gallstones are the most important known risk factor for biliary tract cancers, especially gallbladder cancer (3, 4). Hyperlipidemia, characterized by increased total cholesterol, triglycerides, and low-density lipoprotein (LDL), and decreased high-density lipoprotein (HDL), has been associated with gallstones (5, 6) and with biliary tract cancer (2, 7).
Serum lipids are affected by both genetic and lifestyle factors, such as diet, body weight, and physical activity. In addition to a familial tendency, hyperlipidemia has been associated with several genetic polymorphisms in the lipid metabolism pathway (8, 9). For example, variants of the LDLR (low-density lipoprotein receptor) gene encoding the LDL receptor which regulates the uptake of LDL have been linked to familial hyperlipidemia (8, 9). Variants of the APOB and APOE genes which encode the major carrier and binding proteins of LDL, apolipoprotein B and E (apo B and E), have been associated with higher serum levels of total cholesterol, LDL, apo B, and lower HDL (10, 11). Also, variants of the LPL gene, which encodes the lipoprotein lipase enzyme, have been associated with higher serum LDL levels (12), and variants of ALOX5, which is involved in the synthesis of inflammatory lipid mediators, have been linked to cancer and heart disease (13,14).
The role of genetic factors in biliary tract cancers and stones has been suggested by reports of ethnic and familial predisposition, but the genetic pathways are unclear (1, 15). Although some gene variants in the lipid metabolism pathway have been linked to gallstones (16, 17), few studies have examined their role in biliary tract cancers. In this population-based case-control study conducted in Shanghai, China, we examined the association of 12 polymorphic variants of 5 genes (APOB, APOE, LDLR, LPL, and ALOX5) in the lipid metabolism pathway with the risks of biliary tract stones and cancers, and also assessed whether the variants were related with biliary tract cancers through their association with gallstones.
Materials and Methods
Study Subjects
Details of the study methods have been reported elsewhere (4, 15, 18). Briefly, cancer cases were identified by a rapid reporting system established by the Shanghai Cancer Institute (SCI) and 42 collaborating hospitals in Shanghai. Through this system we identified more than 95% of all incident biliary tract cancer cases (ICD9 code 156) diagnosed among Shanghai residents between June 1997 and May 2001. A total of 627 incident biliary tract cancer cases (368 gallbladder, 191 extrahepatic bile duct, and 68 ampulla of Vater cases) between 35–74 years of age, were included in this study. In order to evaluate the risk of biliary stones independently of biliary tract cancer, 1,037 patients with biliary stones, (774 gallstones and 263 extrahepatic bile duct stones) without a history of cancer, were included and were frequency-matched to the cancer cases on age, (5-year groups) gender, and diagnosing hospital. Population controls, without a history of biliary tract cancer, were randomly selected from the Shanghai Resident Registry that includes records of approximately 6 million Shanghai residents. We included a total of 959 population controls who were frequency-matched to cancer cases on age (5-year groups) and gender. Participation rates among eligible cancer patients and control subjects were 95% and 82%, respectively.
Case Confirmation
Biliary tract cancer and biliary stone diagnoses were confirmed by a panel of clinicians, ultrasonographers, and pathologists. Seventy percent of biliary tract cancer cases were confirmed by histopathologic assesment, while the remaining 30% of cases, for whom we did not have histopathologic material, were confirmed using medical records, surgical reports, and imaging data, including magnetic resonance imaging (MRI), endoscopic retrograde cholangiopancreatography (ERCP), or computed tomography (CT). Biliary stone cases were confirmed by review of abdominal ultrasound, ERCP films, medical records, and surgical records, or by pathologic material for those who underwent a cholecystectomy.
Gallstone Assessment
Gallstone status was assessed in nearly all biliary tract cancer cases and population controls. Among cancer cases, gallstones were identified by self-reported history, surgical reports, or imaging results from MRI, ERCP, CT, or ultrasound. Among population controls, gallstones were identified by self-reported history or by abdominal ultrasound among those who gave consent for the procedure (85% of all population controls).
Interview
Information on demographic characteristics, medical histories, and lifestyle factors was obtained through in-person interviews conducted by trained interviewers, using a structured questionnaire. Cases were interviewed within 3 weeks of diagnosis. Weight and height were measured at the time of interview. The response rate for interviews was over 95% among cases and 82% among controls. Five percent of the study subjects were randomly selected for re-interview three months after the initial interview to assess the reproducibility of responses. The concordance of responses to key questions between the original and follow-up interviews was greater than 90%.
Genotyping
Five candidate genes were selected for their role in lipid metabolism, their effects on serum lipid levels, and their potential effects on biliary disease pathogenesis. SNPs were selected based on their putative functional significance, having a variant allele frequency of at least 5% among Asians as reported by NCI’s SNP500Cancer Database (http://snp500cancer.nci.nih.gov) (19), and a validated assay at the NCI Core Genotyping Facility (CGF) (http://cgf.nci.nih.gov/home.cfm). The following 12 SNPs in 5 genes were selected: APOB [EX26–3573T>C (rs676210), IVS23–79T>C (rs673548), IVS6+360C>T (rs520354), EX4+56C>T (rs1367117)], APOE [IVS1+69C>G (rs440446)], LDLR [IVS9–30C>T (rs1003723), EX10+55G>A (rs5930), EX15–80G>A (rs5927), IVS17–42A>G (rs6413504), EX18+88G>A (rs14158)] and LPL [IVS5–540C>T (rs263)], and ALOX5 [IVS3+100G>A (rs2029253)]. In total, we included 7 SNPs in introns and 5 in exons (2 non-synonymous, 2 synonymous, and 1 non-coding 3’UTR). Overnight fasting blood samples were collected from all subjects who gave consent (over 80%). Genomic DNA was extracted from buffy coat by the standard phenol chloroform method at the NCI laboratory. Genotyping was conducted at the CGF using the TaqMan assay (Applied Biosystems, Foster City, CA, http://snp500cancer.nci.nih.gov). The genotyping failure rate among all samples was less than 2%. The quality and potential misclassification of the genotyping results were assessed by evaluating 20 duplicate DNA samples from 4 quality control subjects (80 total samples) that were randomly placed within the same reaction plates used for study subjects. The concordance between duplicate samples was over 99%.
Statistical Analysis
The final analysis included subjects who completed the interview and for whom we had DNA, which totaled 406 incident biliary tract cancer cases (235 gallbladder, 125 extrahepatic bile duct, 46 ampulla of Vater), 880 biliary stone cases (663 gallstone, 217 bile duct), and 779 healthy controls. In order to make the appropriate case-control comparisons, for all analyses, gallbladder cancer cases were compared to controls without a history of cholecystectomy (n=730); bile duct cancer cases and ampulla of Vater cancer cases were compared to all controls (n=779); and biliary stone cases were compared to controls without biliary stones (n=586). Distributions of selected characteristics (gender, age group, education status, cigarette smoking status, alcohol drinking status, body mass index (BMI), hypertension, diabetes, and gallstone status (among cancer cases) were evaluated among cases and controls; characteristics with statistically significant different distributions between cases and controls (Fishers exact p<0.05) were examined for their associations with SNPs among controls.
The associations between SNPs and biliary tract stone and cancer risk were estimated by odds ratios (ORs) and 95% confidence intervals (95% CIs), using unconditional logistic regression. Risk estimates were calculated for the heterozygous and homozygous variant genotypes relative to the common homozygous genotype, as well as for the presence and absence of the variant allele, using indicator variables in the regression model. An initial model was adjusted for age, and additional models were further adjusted for gender, BMI, waist-to-hip ratio (WHR), cigarette smoking, alcohol drinking, hypertension, diabetes and gallstone status (cancer risk only) in order to evaluate the potential confounding by these factors. Associations between SNPs and biliary stones and cancers were assessed using the linear test of trend for the number of copies of the variant allele (0, 1, 2) assuming an additive model, or with the Wald chi-square test for the presence or absence of the variant allele (0, 1). In addition, the likelihood ratio test was used to formally test for multiplicative interactions between the above mentioned characteristics and the SNPs on stone and cancer risk. Although gallstones may be an intermediate step in the causal pathway between genetics and biliary tract cancer, it is also possible that genetic variants may affect cancer risk independent of stones, especially for bile duct and ampulla of Vater cancers, which have a much lower prevalence of stones than gallbladder cancer. Thus, in subsequent analysis, we examined the association between SNPs and biliary tract cancer after adjustment for biliary stones.
In order to assess the overall gene effects on biliary tract cancer and stone risk, the Simes global test was used to calculate a summary p-value for each gene for which we examined multiple SNPs (APOB and LDLR). The Simes global test utilizes the p trend values of each SNP and accounts for multiple SNP comparisons within each gene by controlling for the gene-wide type I error rate (20, 21). Similarly, the Simes global test was used to obtain an overall pathway p-value adjusting for all 12 SNPs and controlling for the familywise error rate.
We also examined the associations between the haplotypes of the APOB and LDLR genes and the risks of biliary stones and cancers. Among population controls, linkage disequilibrium (LD) between markers was assessed by calculating pariwise Lewontin’s D` and r2 using Haploview version 3.11 (22). Haplotypes were reconstructed for cases and controls separately from genotype data using Haplostats in R version 2.0.1 (23), which uses an expectation maximization algorithm to calculate maximum likelihood estimates of haplotype frequencies while taking into account phase ambiguity (24). Associations between haplotypes (>1% frequency) and the risks of biliary tract stones and cancer were evaluated by computing ORs and 95% CI, using Haplostats, assuming an additive model, and using the most common haplotype as the referent category. Global differences in haplotype frequencies between cases and controls were assessed for each gene using the Score test in Haplostats (23).
Results
Selected characteristics of the study subjects are shown in Table 1. The frequency of selected characteristics did not differ considerably between the 3 subgroups of controls (all controls, controls without cholecystectomy, and controls without biliary stones). Biliary stone cases were more likely to be women, younger, overweight, and diabetic, but less likely to drink alcohol or have hypertension, compared to controls without stones. Gallbladder cancer cases were predominantly women (72.3%), while bile duct cancer cases were more common in men (60%). In general, biliary cancer cases were more likely to have a higher WHR (>0.92) and gallstones than controls. Bile duct and ampulla of Vater cancer cases were more likely to ever smoke cigarettes and drink alcohol, but less likely to have hypertension, while gallbladder cancer cases were more likely to be overweight (41.3%) and diabetic (13.2%) compared to controls.
Table 1.
Selected characteristics of subjects by case-control status
| Biliary Tract Cancers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Selected Characteristics | Controls | Biliary Stones1 | Gallbladder2 | Bile duct3 | Ampulla of Vater3 |
|||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| All subjects | 779 | (100.0) | 880 | (100.0) | 235 | (100.0) | 125 | (100.0) | 46 | (100.0) |
| Sex | ||||||||||
| Male | 302 | (38.8) | 323 | (36.7) | 65 | (27.6) | 75 | (60.0) | 23 | (50.0) |
| Female | 477 | (61.2) | 557 | (63.3)* | 170 | (72.3)* | 50 | (40.0)* | 23 | (50.0) |
| Age at interview (yrs) | ||||||||||
| 34–54 | 105 | (13.5) | 263 | (29.9) | 32 | (13.6) | 17 | (13.6) | 4 | (8.7) |
| 55–64 | 222 | (28.5) | 251 | (28.5) | 61 | (25.9) | 32 | (25.6) | 9 | (19.6) |
| 65–75 | 452 | (58.0) | 366 | (41.6)* | 142 | (60.4) | 76 | (60.8) | 33 | (71.7) |
| Education | ||||||||||
| Elementary | 313 | (40.2) | 267 | (30.3) | 125 | (53.2) | 52 | (41.6) | 22 | (47.8) |
| High school | 347 | (44.5) | 463 | (52.6) | 88 | (37.4) | 55 | (44.0) | 19 | (41.3) |
| Above high school | 119 | (15.3) | 150 | (17.0)* | 22 | (9.4)* | 18 | (14.4) | 5 | (10.8) |
| Ever Smoked Cigarettes 4 | ||||||||||
| No | 545 | (69.9) | 644 | (73.2) | 170 | (72.3) | 70 | (56.0) | 26 | (56.5) |
| Yes | 234 | (30.0) | 236 | (26.8) | 64 | (27.2) | 55 | (44.0)* | 20 | (43.5) |
| Ever Drank Alcohol | ||||||||||
| No | 620 | (79.6) | 741 | (84.3) | 199 | (84.7) | 84 | (67.2) | 34 | (73.9) |
| Yes | 159 | (20.4) | 138 | (15.7)* | 36 | (15.3) | 41 | (32.8)* | 12 | (26.1) |
| Body Mass Index 5 (kg/m2) | ||||||||||
| <18.5 | 65 | (8.3) | 40 | (4.6) | 11 | (4.7) | 6 | (4.8) | 1 | (2.2) |
| 18.5–22.9 | 319 | (40.9) | 295 | (33.6) | 79 | (33.6) | 57 | (45.6) | 20 | (43.5) |
| 23.0–24.9 | 166 | (21.3) | 213 | (24.2) | 47 | (20.0) | 33 | (26.4) | 10 | (21.7) |
| ≥25 | 228 | (29.3) | 331 | (37.7)* | 97 | (41.3)* | 29 | (23.2) | 15 | (32.6) |
| Waist to Hip Ratio 6 | ||||||||||
| <0.80 | 145 | (18.6) | 105 | (11.9) | 19 | (8.1) | 8 | (6.4) | 7 | (15.2) |
| 0.80–0.83 | 134 | (17.2) | 96 | (10.9) | 14 | (5.9) | 9 | (7.2) | 3 | (6.5) |
| 0.84–0.87 | 175 | (22.5) | 195 | (22.2) | 48 | (20.4) | 20 | (16.0) | 8 | (17.4) |
| 0.88–0.91 | 176 | (22.6) | 212 | (24.1) | 57 | (24.3) | 31 | (24.8) | 10 | (21.7) |
| ≥0.92 | 149 | (19.1) | 272 | (30.9)* | 97 | (41.3) | 57 | (45.6) | 18 | (39.1) |
| Hypertension | ||||||||||
| No | 444 | (57.0) | 591 | (67.2) | 149 | (63.4) | 88 | (70.4) | 34 | (73.9) |
| Yes | 335 | (43.0) | 289 | (32.8)* | 86 | (36.6) | 37 | (29.6)* | 12 | (26.1)* |
| Diabetes | ||||||||||
| No | 714 | (91.7) | 780 | (88.7) | 204 | (86.8) | 113 | (90.4) | 43 | (93.5) |
| Yes | 65 | (8.3) | 99 | (11.3)* | 31 | (13.2)* | 12 | (9.6) | 3 | (6.5) |
| Gallstone status | ||||||||||
| No | 586 | (75.2) | - | - | 36 | (15.3) | 40 | (32.0) | 18 | (39.1) |
| Yes | 193 | (24.8) | 880 | (100.0) | 199 | (84.7)* | 85 | (68.0)* | 28 | (60.9)* |
Biliary stone cases include gallstone and bile duct stones. Biliary stone cases compared with controls without biliary stones (n=586)
Gallbadder cancer cases compared with population controls who had a gallbladder (n=730)
Bile duct and ampulla of Vater cancer cases compared to all population controls (n=779)
Ever smoked cigarettes for at least 6 consecutive months
Body mass index = weight/(height2); Distribution based on WHO classification for obesity among Asians
Cutoffs for waist-to-hip-ratio based on quintiles among population controls
p<0.05 for Fisher's exact test for difference between cases and controls
Note: Total number of subjects may vary because of missing values
The risk of biliary stones and cancers in relation to the 12 SNPs in the lipid metabolism pathway are shown in Table 2. Among population controls, the minor allele frequencies of the 12 SNPs ranged from 5% to 39%, and the genotype frequencies showed no deviation from the expected Hardy-Weinberg equilibrium proportions (p > 0.05). Of the 12 SNPs examined, only the APOE IVS1+69C>G marker was significantly associated with biliary stones. Specifically, carriers of the G allele had a 1.3-fold risk of biliary stones (95% CI=1.03–1.60, p trend=0.02), compared with those having the CC genotype. Variants in the APOE, APOB, and LDLR genes were associated with either bile duct or ampulla of Vater cancers, while none of the markers examined was significantly associated with gallbladder cancer. Carriers of the G allele of the APOE IVS1+69C>G marker had a 2.0-fold risk of bile duct cancer (95% CI=1.32–3.11, p trend=0.003) and a 2.5-fold risk of ampulla of Vater cancer (95% CI=1.21–5.08, p trend=0.002), compared with those having the CC genotype. We also found that carriers of the T allele of the APOB IVS6+360C>T marker and the T allele of the LDLR IVS9–30C>T marker each had a 1.5-fold risk of bile duct cancer, compared with those having the CC genotype of each marker. In addition, carriers of the GA genotype of the LDLR EX18+88G>A marker had a 2.7-fold risk of ampulla of Vater cancer, relative to those having the GG genotype. The associations between the APOE, APOB, and LDLR gene variants with stone and cancer risks were independent of gender, BMI, WHR, hypertension, diabetes, cigarette smoking, and alcohol drinking, while the associations with cancer were independent of gallstones.
Table 2.
Odds Ratios (ORs) and 95% Confidence Intervals (CIs) for Biliary Stones and Cancer in Relation to Polymorphisms of Lipid Metabolism Genes
| Biliary Tract Cancers | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Controls | Biliary Stones 1 | Gallbladder2 | Bile duct3 | Ampulla of Vater3 | |||||||||||||
| n | (%) | n | (%) | OR4 | (95% CI)4 | n | (%) | OR4 | (95% CI)4 | n | (%) | OR4 | (95% CI)4 | n | (%) | OR4 | (95% CI)4 | |
| TOTAL | 779 | (100.0) | 880 | (100.0) | - | - | 235 | (100.0) | - | - | 125 | (100.0) | - | - | 46 | (100.0) | - | - |
| APOE | ||||||||||||||||||
| IVS1+69C>G (rs440446) | ||||||||||||||||||
| CC | 319 | (41.4) | 326 | (37.4) | 1.00 | - | 85 | (36.8) | 1.00 | - | 32 | (25.8) | 1.00 | - | 10 | (21.7) | 1.00 | - |
| GC | 355 | (46.1) | 425 | (48.7) | 1.25 | (0.99–1.57) | 113 | (48.9) | 1.21 | (0.87–1.67) | 72 | (58.1) | 2.01 | (1.29–3.14) | 24 | (52.2) | 2.09 | (0.98–4.44) |
| GG | 96 | (12.5) | 121 | (13.9) | 1.43 | (1.01–2.02) | 33 | (14.3) | 1.32 | (0.83–2.10) | 20 | (16.1) | 2.07 | (1.13–3.79) | 12 | (26.1) | 3.96 | (1.66–9.46) |
| GC+GG | 451 | (58.6) | 546 | (62.6) | 1.29 | (1.03–1.60) | 146 | (63.2) | 1.23 | (0.91–1.67) | 92 | (74.2) | 2.03 | (1.32–3.11) | 36 | (78.3) | 2.48 | (1.21–5.08) |
| P 5 | 0.02 | 0.18 | 0.003 | 0.002 | ||||||||||||||
| APOB | ||||||||||||||||||
| EX26-3573T>C (rs676210) | ||||||||||||||||||
| TT | 439 | (56.7) | 474 | (53.9) | 1.00 | - | 137 | (58.5) | 1.00 | - | 63 | (50.4) | 1.00 | - | 26 | (57.8) | 1.00 | - |
| TC | 288 | (37.2) | 348 | (39.6) | 1.12 | (0.89–1.40) | 80 | (34.2) | 0.91 | (0.66–1.25) | 57 | (45.6) | 1.38 | (0.94–2.04) | 14 | (31.1) | 0.84 | (0.43–1.64) |
| CC | 47 | (6.1) | 57 | (6.5) | 1.02 | (0.66–1.58) | 17 | (7.3) | 1.15 | (0.64–2.08) | 5 | (4.0) | 0.74 | (0.29–1.94) | 5 | (11.1) | 1.89 | (0.69–5.18) |
| TC+CC | 335 | (43.3) | 405 | (46.1) | 1.10 | (0.89–1.37) | 97 | (41.5) | 0.94 | (0.69–1.27) | 62 | (49.6) | 1.30 | (0.89–1.89) | 19 | (42.2) | 0.99 | (0.54–1.81) |
| P 5 | 0.50 | 0.94 | 0.47 | 0.67 | ||||||||||||||
| IVS23-79T>C (rs673548) | ||||||||||||||||||
| TT | 407 | (56.4) | 446 | (54.5) | 1.00 | - | 129 | (58.6) | 1.00 | - | 59 | (50.9) | 1.00 | - | 26 | (60.5) | 1.00 | - |
| TC | 266 | (36.9) | 320 | (39.1) | 1.09 | (0.86–1.37) | 76 | (34.5) | 0.92 | (0.66–1.27) | 52 | (44.8) | 1.35 | (0.90–2.02) | 13 | (30.2) | 0.78 | (0.40–1.56) |
| CC | 48 | (6.7) | 53 | (6.5) | 0.92 | (0.59–1.42) | 15 | (6.8) | 0.97 | (0.52–1.80) | 5 | (4.3) | 0.72 | (0.27–1.87) | 4 | (9.3) | - | - |
| TC+CC | 314 | (40.6) | 373 | (45.5) | 1.06 | (0.85–1.32) | 91 | (41.4) | 0.93 | (0.68–1.26) | 57 | (49.1) | 1.25 | (0.85–1.86) | 17 | (39.5) | 0.87 | (0.46–1.63) |
| P 5 | 0.86 | 0.69 | 0.60 | 0.62 | ||||||||||||||
| IVS6+360C>T (rs520354) | ||||||||||||||||||
| CC | 515 | (67.2) | 602 | (69.6) | 1.00 | - | 157 | (67.1) | 1.00 | - | 71 | (57.7) | 1.00 | - | 32 | (69.6) | 1.00 | - |
| TC | 234 | (30.6) | 242 | (28.0) | 0.82 | (0.65–1.04) | 69 | (29.5) | 0.93 | (0.67–1.29) | 46 | (37.4) | 1.41 | (0.94–2.10) | 14 | (30.4) | 0.96 | (0.50–1.84) |
| TT | 17 | (2.2) | 21 | (2.4) | 0.97 | (0.48–1.95) | 8 | (3.4) | 1.51 | (0.63–3.60) | 6 | (4.9) | 2.51 | (0.96–6.57) | 0 | 0 | - | - |
| TC+TT | 251 | (32.8) | 263 | (30.4) | 0.83 | (0.66–1.05) | 77 | (32.9) | 0.97 | (0.71–1.33) | 52 | (42.3) | 1.48 | (1.01–2.18) | 14 | (30.4) | 0.90 | (0.47–1.71) |
| P 5 | 0.15 | 0.95 | 0.02 | 0.74 | ||||||||||||||
| EX4+56C>T (rs1367117) | ||||||||||||||||||
| CC | 564 | (73.6) | 650 | (75.3) | 1.00 | - | 182 | (78.1) | 1.00 | - | 81 | (65.9) | 1.00 | - | 32 | (71.1) | 1.00 | - |
| TC | 192 | (25.1) | 195 | (22.6) | 0.84 | (0.66–1.08) | 43 | (18.5) | 0.68 | (0.47–0.99) | 40 | (32.5) | 1.44 | (0.95–2.17) | 12 | (26.7) | 1.13 | (0.57–2.24) |
| TT | 10 | (1.3) | 18 | (2.1) | 1.39 | (0.61–3.17) | 8 | (3.4) | 2.54 | (0.97–6.70) | 2 | (1.6) | - | - | 1 | (2.2) | - | - |
| TC+TT | 202 | (26.4) | 213 | (24.7) | 0.87 | (0.69–1.11) | 51 | (21.9) | 0.77 | (0.54–1.09) | 42 | (34.1) | 1.44 | (0.96–2.15) | 13 | (28.9) | 0.85 | (0.65–1.10) |
| P 5 | 0.44 | 0.44 | 0.08 | 0.67 | ||||||||||||||
| LDLR | ||||||||||||||||||
| IVS9-30C>T (rs1003723) | ||||||||||||||||||
| CC | 565 | (72.6) | 636 | (72.6) | 1.00 | - | 170 | (72.3) | 1.00 | - | 80 | (64.5) | 1.00 | - | 39 | (84.8) | 1.00 | - |
| TC | 199 | (25.6) | 223 | (25.5) | 0.98 | (0.77–1.25) | 61 | (26.0) | 1.02 | (0.73–1.44) | 39 | (31.5) | 1.38 | (0.91–2.10) | 7 | (15.2) | 0.50 | (0.22–1.14) |
| TT | 14 | (1.8) | 17 | (1.9) | 1.02 | (0.47–2.22) | 4 | (1.7) | - | - | 5 | (4.0) | 2.53 | (0.89–7.22) | 0 | 0 | - | - |
| TC+TT | 213 | (27.4) | 240 | (27.4) | 0.98 | (0.78–1.25) | 65 | (27.7) | 1.02 | (0.73–1.41) | 44 | (35.5) | 1.46 | (0.98–2.18) | 7 | (15.2) | 0.47 | (0.21–1.06) |
| P 5 | 0.92 | 0.93 | 0.04 | 0.07 | ||||||||||||||
| EX10+55G>A (rs5927) | ||||||||||||||||||
| GG | 307 | (39.9) | 356 | (41.0) | 1.00 | - | 79 | (33.9) | 1.00 | - | 54 | (43.2) | 1.00 | - | 17 | (37.0) | 1.00 | - |
| GA | 348 | (45.2) | 382 | (44.0) | 0.94 | (0.74–1.18) | 120 | (51.5) | 1.34 | (0.97–1.85) | 52 | (41.6) | 0.85 | (0.56–1.28) | 17 | (37.0) | 0.88 | (0.44–1.76) |
| AA | 115 | (14.9) | 130 | (15.0) | 0.94 | (0.68–1.30) | 34 | (14.6) | 1.10 | (0.70–1.74) | 19 | (15.2) | 0.94 | (0.53–1.65) | 12 | (26.0) | 1.88 | (0.87–4.07) |
| GA+AA | 463 | (60.1) | 512 | (59.0) | 0.94 | (0.75–1.17) | 154 | (66.1) | 1.28 | (0.95–1.75) | 71 | (56.8) | 0.90 | (0.61–1.32) | 29 | (63.0) | 1.16 | (0.63–2.15) |
| P 5 | 0.62 | 0.35 | 0.66 | 0.18 | ||||||||||||||
| EX15-80G>A (rs14158) | ||||||||||||||||||
| GG | 696 | (89.6) | 794 | (90.7) | 1.00 | - | 204 | (86.8) | 1.00 | - | 114 | (91.9) | 1.00 | - | 42 | (91.3) | 1.00 | - |
| GA | 76 | (9.8) | 79 | (9.0) | 0.85 | (0.59–1.22) | 30 | (12.8) | 1.29 | (0.82–2.02) | 10 | (8.1) | 0.80 | (0.40–1.60) | 4 | (8.7) | 0.87 | (0.30–2.49) |
| AA | 5 | (0.6) | 2 | (0.2) | 0.30 | (0.06–1.55) | 1 | (0.4) | - | - | 0 | 0 | - | - | 0 | 0 | - | - |
| GA+AA | 81 | (10.4) | 81 | (9.3) | 0.81 | (0.57–1.15) | 31 | (13.2) | 1.24 | (0.80–1.94) | 10 | (8.1) | 0.75 | (0.38–1.50) | 4 | (8.7) | 0.81 | (0.28–2.31) |
| P 5 | 0.16 | 0.33 | 0.42 | 0.70 | ||||||||||||||
| IVS17-42A>G (rs6413504) | ||||||||||||||||||
| AA | 411 | (53.4) | 448 | (51.2) | 1.00 | - | 120 | (51.3) | 1.00 | - | 59 | (47.2) | 1.00 | - | 25 | (54.3) | 1.00 | - |
| GA | 291 | (37.8) | 359 | (41.0) | 1.19 | (0.95–1.49) | 98 | (41.9) | 1.19 | (0.87–1.62) | 56 | (44.8) | 1.34 | (0.90–1.99) | 15 | (32.6) | 0.84 | (0.43–1.62) |
| GG | 67 | (8.8) | 68 | (7.8) | 0.94 | (0.63–1.39) | 16 | (6.8) | 0.81 | (0.45–1.46) | 10 | (8.0) | 1.04 | (0.51–2.13) | 6 | (13.0) | 1.42 | (0.56–3.60) |
| GA+GG | 358 | (46.6) | 427 | (48.8) | 1.14 | (0.92–1.41) | 114 | (48.7) | 1.12 | (0.83–1.50) | 66 | (52.8) | 1.28 | (0.88–1.87) | 21 | (45.7) | 0.95 | (0.52–1.72) |
| P 5 | 0.52 | 0.87 | 0.38 | 0.78 | ||||||||||||||
| EX18+88G>A (rs5930) | ||||||||||||||||||
| GG | 290 | (37.5) | 320 | (36.7) | 1.00 | - | 86 | (36.9) | 1.00 | - | 49 | (39.5) | 1.00 | - | 12 | (26.1) | 1.00 | - |
| GA | 364 | (47.2) | 416 | (47.7) | 1.05 | (0.83–1.33) | 108 | (46.4) | 1.01 | (0.73–1.40) | 56 | (45.2) | 0.91 | (0.60–1.38) | 21 | (45.7) | 1.38 | (0.67–2.86) |
| AA | 118 | (15.3) | 136 | (15.6) | 1.04 | (0.75–1.44) | 39 | (16.7) | 1.12 | (0.72–1.73) | 19 | (15.3) | 0.95 | (0.54–1.69) | 13 | (28.3) | 2.71 | (1.19–6.12) |
| GA+AA | 482 | (62.4) | 552 | (63.3) | 1.05 | (0.84–1.31) | 147 | (63.1) | 1.04 | (0.76–1.41) | 75 | (60.5) | 0.92 | (0.62–1.36) | 34 | (73.9) | 1.70 | (0.87–3.34) |
| P 5 | 0.74 | 0.66 | 0.78 | 0.02 | ||||||||||||||
| LPL | ||||||||||||||||||
| IVS5-540C>T (rs263) | ||||||||||||||||||
| CC | 485 | (62.6) | 539 | (61.3) | 1.00 | - | 151 | (64.3) | 1.00 | - | 79 | (63.7) | 1.00 | - | 28 | (60.9) | 1.00 | - |
| TC | 254 | (32.8) | 308 | (35.0) | 1.17 | (0.93–1.47) | 76 | (32.3) | 0.97 | (0.71–1.34) | 39 | (31.5) | 0.94 | (0.62–1.42) | 18 | (39.1) | 1.20 | (0.65–2.21) |
| TT | 36 | (4.6) | 32 | (3.6) | 0.68 | (0.41–1.13) | 8 | (3.4) | 0.69 | (0.31–1.52) | 6 | (4.8) | 1.02 | (0.42–2.51) | 0 | 0 | - | - |
| TC+TT | 290 | (37.4) | 340 | (38.7) | 1.10 | (0.88–1.36) | 84 | (35.7) | 0.94 | (0.69–1.27) | 45 | (36.3) | 0.95 | (0.64–1.41) | 18 | (39.1) | 1.05 | (0.57–1.94) |
| P 5 | 0.95 | 0.50 | 0.86 | 0.85 | ||||||||||||||
| ALOX5 | ||||||||||||||||||
| IVS3+100G>A (rs2029253) | ||||||||||||||||||
| GG | 286 | (37.0) | 319 | (36.4) | 1.00 | - | 84 | (35.9) | 1.00 | - | 48 | (38.7) | 1.00 | - | 15 | (32.6) | 1.00 | - |
| GA | 376 | (48.5) | 432 | (49.3) | 1.09 | (0.86–1.37) | 113 | (48.3) | 1.05 | (0.76–1.44) | 60 | (48.4) | 0.95 | (0.63–1.43) | 25 | (54.3) | 1.24 | (0.64–2.39) |
| AA | 112 | (14.5) | 125 | (14.3) | 1.12 | (0.80–1.57) | 37 | (15.8) | 1.16 | (0.74–1.82) | 16 | (12.9) | 0.85 | (0.46–1.56) | 6 | (13.0) | 0.98 | (0.37–2.59) |
| GA+AA | 488 | (63.1) | 557 | (63.6) | 1.09 | (0.88–1.36) | 150 | (64.1) | 1.07 | (0.79–1.46) | 76 | (61.3) | 0.93 | (0.63–1.37) | 31 | (67.4) | 1.17 | (0.62–2.22) |
| P 5 | 0.43 | 0.53 | 0.60 | 0.85 | ||||||||||||||
Biliary stone cases include gallstone and bile duct stones. Biliary stone cases compared with controls without biliary stones (n=586)
Gallbadder cancer cases compared with population controls who had a gallbladder (n=730)
Bile duct and ampulla of Vater cancer cases compared to all population controls (n=779)
Adjusted for age
Test of trend for the number of copies of the variant allele (0,1,2);
Pearson chi-square for absence or presence of the variant allele if variant genotype has n < 5
Note: Total number of subjects per SNP may vary because of missing values
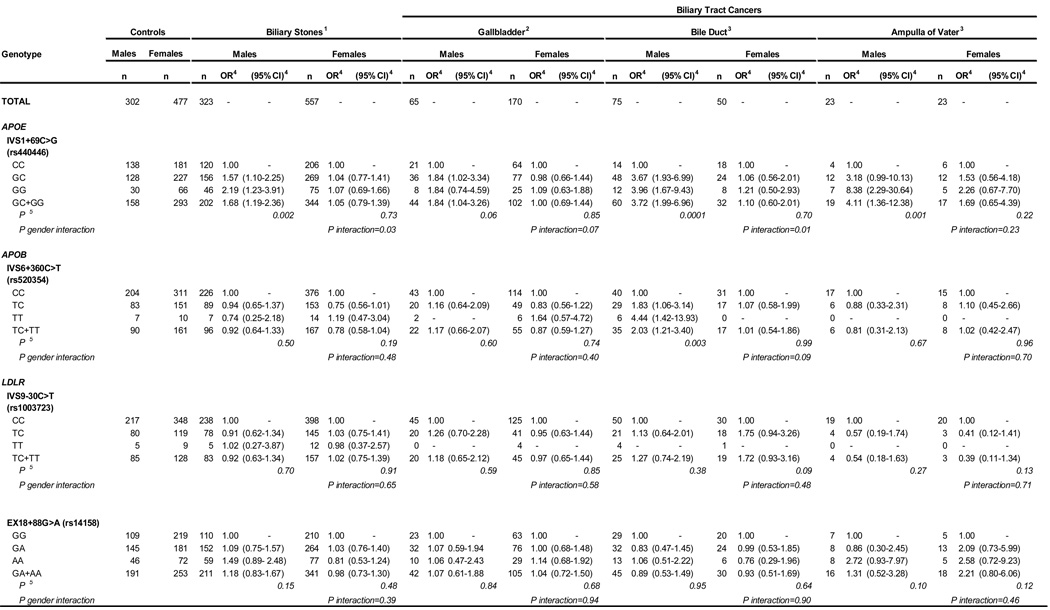
Because of gender differences in the incidence of biliary stones and biliary tract cancers, we evaluated the gender specific effects of the APOE, APOB, and LDLR markers for which we found significant main effects (Table 3). The effects of the APOE IVS1+69C>G marker were limited to men, with male carriers of the G allele having a 1.7-fold risk of biliary stones (95% CI=1.19–2.36), 1.8-fold risk of gallbladder cancer (95% CI=1.04–3.26), a 3.7-fold risk of bile duct cancer (95% CI=1.99–6.96), and a 4.1-fold risk of ampulla of Vater cancer (95% CI=1.36–12.38). A statistically significant interaction between gender and the APOE IVS1+69C>G marker was seen for biliary stones (P interaction =0.03) and bile duct cancer (P interaction =0.01). The effect of the APOB IVS6+360C>T marker was also limited to men, with male carriers of the T allele having a 2-fold risk of bile duct cancer (95% CI=1.21–3.40), although the interaction between gender and the APOB IVS6+360C>T marker was not statistically significant (P interaction=0.09). The effects of these two SNPs among men were independent of BMI, WHR, hypertension, diabetes, cigarette smoking, alcohol drinking, and gallstones (for biliary cancer). No gender differences were seen in the association between the LDLR markers, IVS9–30C>T and EX18+88G>A, and the risks of biliary stones or cancers.
Table 3.
Odds Ratios (ORs) and 95% Confidence Intervals (CIs) for Biliary Stones and Cancer in Relation to theAPOE, APOB and LDLR markers by Gender
 |
Biliary stone cases include gallstone and bile duct stones. Biliary stone cases compared with controls without biliary stones (n=586)
Gallbadder cancer cases compared with population controls who had a gallbladder (n=730)
Bile duct and ampulla of Vater cancer cases compared to all population controls (n=779)
Adjusted for age
Test of trend for the number of copies of the variant allele (0,1,2);
Pearson chi-square for absence or presence of the variant allele if variant genotype has n < 5
Note: Total number of subjects per SNP may vary because of missing values
Using the Simes test to adjust for multiple SNP comparisons within the APOB and LDLR genes, we found that bile duct cancer had a borderline association with APOB (P Simes=0.08), and a non-significant association with LDLR (P Simes =0.20). Also, adjusting for all 12 SNPs, the combined effect of these gene variants was significantly associated with bile duct cancer (P Simes =0.04). Among men, but not women, the associations of bile duct cancer with the APOB gene (P Simes =0.01) and all variants examined in the lipid metabolism pathway (P Simes =0.001) remained significant.
Of the 4 APOB SNPs examined in the study, strong linkage disequilibrium was present among the APOB EX26–3573T>C and IVS23–79T>C markers (D’=1.0) and the APOB IVS6+360C>T and EX4+56C>T markers (D’=0.81). Thus, we inferred common haplotypes separately for these two sets of APOB SNPs. Two major haplotypes (frequency ≥ 1.0%) were inferred from the APOB EX26–3573T>C and IVS23–79T>C markers (TT=75.7%, CC=24.3%) and three from the APOB IVS6+360C>T and EX4+56C>T markers (CC=80.3%, TT=11.8%, TC=5.8%) (Table 4). Consistent with our single marker results, the APOB TT haplotype, which contains the risk-conferring alleles of the IVS6+360C>T and EX4+56C>T markers, was associated with a 1.6-fold risk of bile duct cancer (95% CI=1.07–2.33), compared with the most frequent CC haplotype. The CT haplotype was associated with a significant risk of ampulla of Vater cancer (OR=3.10, 95% CI=1.12–8.61); however, this estimate was based on very small number of cases (n=5). Although the Omnibus score test was significant for the APOB (EX26–3573T>C - IVS23–79T>C) and LDLR haplotypes, these p-values may reflect the small numbers of the uncommon haplotypes since none of the common haplotypes was associated with biliary tract cancers.
Table 4.
Odds Ratios (ORs) and 95% Confience Intervals (CIs) for Biliary Tract Cancer in relation to APOB and LDLR haplotypes
| Biliary Tract Cancers | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Haplotype | Controls | Gallbladder1 | Bile Duct2 | Ampulla of Vater2 | ||||||||||||
| n | (%) | n | (%) | OR3 | (95%CI)3 | n | (%) | OR3 | (95%CI)3 | n | (%) | OR3 | (95%CI)3 | ||||
| Total # | Haplotypes | 1558 | (100.0) | 470 | (100.0) | - | - | 250 | (100.0) | - | - | 92 | (100.0) | - | - | ||
| APOB | EX26-3573T>C - IVS23-79T>C | ||||||||||||||||
| T-T | 1165 | (74.8) | 356 | (75.7) | 1.00 | - | 183 | (73.2) | 1.00 | - | 66 | (71.7) | 1.00 | - | |||
| C-C | 385 | (24.7) | 114 | (24.3) | 0.98 | (0.77–1.25) | 67 | (26.8) | 1.11 | (0.82–1.51) | 25 | (27.0) | 1.13 | (0.71–1.82) | |||
| Other 4 | 8 | (0.5) | 0 | (0.0) | - | - | 0 | (0.0) | - | - | 1 | (1.3) | - | - | |||
| P 5 | 0.40 | 0.50 | <0.001 | ||||||||||||||
| APOB | IVS6+360C>T - EX4+56C>T | ||||||||||||||||
| C-C | 1251 | (80.3) | 376 | (80.0) | 1.00 | - | 189 | (75.5) | 1.00 | - | 73 | (79.4) | 1.00 | - | |||
| T-T | 184 | (11.8) | 51 | (10.9) | 1.18 | (0.77–1.82) | 43 | (17.0) | 1.58 | (1.07–2.33) | 9 | (9.8) | 0.87 | (0.42–1.81) | |||
| T-C | 90 | (5.8) | 34 | (7.3) | 0.91 | (0.65–1.27) | 17 | (6.6) | 1.21 | (0.70–2.10) | 5 | (5.4) | 0.84 | (0.30–2.39) | |||
| Other 4 | 33 | (2.1) | 8 | (1.8) | - | - | 2 | (0.9) | - | - | 5 | (5.4) | 3.1 | (1.12–8.61) | |||
| P 5 | 0.76 | 0.05 | 0.12 | ||||||||||||||
| IVS9-30C>T - EX10+55G>A - | |||||||||||||||||
| EX15-80G>A - IVS17-42A>G - | |||||||||||||||||
| LDLR | EX18+88G>A | ||||||||||||||||
| C-A-G-A-A | 458 | (29.4) | 145 | (30.8) | 1.00 | - | 76 | (30.5) | 1.00 | - | 33 | (35.8) | 1.00 | - | |||
| C-G-G-A-G | 430 | (27.6) | 116 | (24.6) | 0.86 | (0.65–1.14) | 70 | (27.8) | 0.96 | (0.68–1.37) | 10 | (10.8) | 0.38 | (0.19–0.76) | |||
| C-G-G-G-G | 168 | (10.8) | 67 | (14.2) | 1.07 | (0.76–1.51) | 27 | (10.8) | 0.80 | (0.50–1.26) | 20 | (21.7) | 1.16 | (0.65–2.06) | |||
| T-G-G-G-G | 185 | (11.9) | 54 | (11.4) | 0.89 | (0.62–1.29) | 45 | (17.8) | 1.43 | (0.95–2.17) | 7 | (7.6) | 0.46 | (0.19–1.09) | |||
| C-G-G-A-A | 109 | (7.0) | 27 | (5.8) | 0.83 | (0.51–1.35) | 16 | (6.3) | 0.83 | (0.45–1.55) | 14 | (15.2) | 1.60 | (0.79–3.22) | |||
| C-A-A-A-G | 50 | (3.2) | 28 | (6.0) | 1.06 | (0.67–1.68) | 8 | (3.2) | 0.64 | (0.30–1.33) | 4 | (4.3) | - | - | |||
| Other 4 | 157 | (10.1) | 34 | (7.2) | 1.38 | (0.85–2.24) | 9 | (3.6) | 0.70 | (0.31–1.57) | 4 | (4.6) | - | - | |||
| P 5 | 0.04 | 1.00 | 0.001 | ||||||||||||||
Gallbadder cancer cases compared with population controls who had a gallbladder (n=730)
Bile duct and ampulla of Vater cancer cases compared to all population controls (n=779)
Adjusted for age
Combined haplotypes having frequency <1% among population controls
Omnibus score test
Discussion
In this population-based study, variants in lipid metabolism genes, including APOE, APOB, and LDLR, were associated with excess risks of biliary stones and cancers, although most of the effects were limited to men. The APOB TT haplotype, containing the risk-conferring alleles of the APOB IVS6+360C>T and EX4+56C>T markers, was associated with an excess risk of bile duct cancer, supporting further a relationship between APOB variants and bile duct cancer. These results, although needing confirmation, suggest that polymorphic variants in genes involved in lipid metabolism may play a role in the etiology of biliary stones and biliary tract cancers.
Our finding of an association between the APOE IVS1+69 marker and biliary stones corroborates previous studies relating gallstones to APOE gene polymorphisms. Several population-based studies of APOE have reported higher risks of gallstones among carriers of the e4 allele, a variant with a single amino acid change, compared to non-e4 carriers (25, 26). The APOE e4 allele has been associated with high cholesterol content in gallstones, faster crystallization, and frequent stone recurrence (26). Another study reported an excess risk of gallstones mainly among APOE e2 allele carriers who also had lower serum LDL levels (27). Since apo E is a major component of lipoproteins and plays a critical role in the transport of cholesterol to the liver, our findings, together with previous studies, suggest that the APOE gene may predispose to biliary stones through effects on serum lipid levels (28).
The observed effects of the APOE IVS1+69 marker on bile duct and ampulla of Vater cancer risks were independent of gallstones, suggesting that mechanisms in addition to stones may be involved in biliary carcinogenesis. Apart from its role in lipid metabolism, APOE has important effects on the inflammatory response. Specifically, serum apo E acts as a modulator of proinflammatory cytokines, including TNF-α and IL-1-β (29, 30), which are expressed in biliary tract tumor tissue (31, 32). In addition, apo E’s inhibitory effect on endothelial and tumor cell proliferation suggests its possible involvement in angiogenesis, tumor cell growth, and metastasis (33, 34). Although the APOE gene has not been investigated previously in biliary tract cancer, it has been related to increased risk of other malignancies, including breast, colon/rectum, and prostate (35–37).
In our study, the effects of the APOE IVS1+69C>G marker on biliary stones and cancer were limited to men, suggesting a gender-specific role of APOE. These findings are noteworthy since previous studies have noted stronger positive associations between the APOE e4 allele and triglyceride levels among men than women (38, 39), raising the possibility that the metabolic effect of APOE may contribute to the higher incidence of bile duct and ampullary cancers reported among men than women (1).
Of the 4 APOB SNPs examined, the APOB IVS6+360C>T marker was associated with an excess risk of bile duct cancer, independently of gallstones and the APOE IVS1+69C>G marker. Supporting this observation, we also found a relation between bile duct cancer and the APOB TT haplotype containing the at-risk allele of the APOB IVS6+360C>T marker. The mechanism by which APOB confers risk to bile duct cancer through a pathway independent of gallstones is unclear, but it may be linked to its relationship with serum apo B, the major carrier and binding protein of LDL (40, 41). Variants of APOB have been linked to higher levels of serum apo B (27, 42), which are correlated with higher levels of serum LDL. The resulting elevation in oxidized LDL may trigger an increase in reactive oxygen species (43, 44), DNA damage, activation of proto-oncogenes, or inactivation of tumor suppressor genes (45), all of which may play a role in biliary tract carcinogenesis (46).
Independent of gallstones and the APOE and APOB markers, two of the five LDLR markers, LDLR IVS9–30C>T and LDLR EX18+88G>A were associated with biliary cancers. The mechanisms are unclear, but may relate to the role of the LDL receptor in the uptake of LDL by the liver and extrahepatic tissue. Decreased activity of the LDL receptor has been associated with increased circulating levels of LDL, triglycerides, and total cholesterol (47), as well as proinflammatory cytokines, including TNF-α, IL-1, and IL-6, which are expressed in biliary tract cancer tissue (31, 32).
Our observation that the effect of lipid metabolism genes varied by biliary cancer subsite is consistent with the etiologic heterogeneity suggested by subsite differences in the epidemiologic (1, 2, 48) and molecular characteristics of biliary tumors (46, 49, 50). In our study, the effects of lipid metabolism genes on biliary tumors were most consistently associated with bile duct cancer, despite the fact that gallstones are most closely linked to gallbladder cancer. Reasons for this apparent anomaly are unclear, but it is possible that in addition to gallstones and lipid metabolism, the etiology of gallbladder cancer involves a number of major risk factors, including obesity and reproductive variables, which may have obscured detection of a modest genetic effect.
As with most epidemiologic studies measuring the effect of many candidate genes, false positive findings due to multiple comparisons is a concern. To minimize this potential bias, we implemented rigorous procedures for statistical adjustment. For each gene we used the Simes global test to adjust for multiple SNPs, and found that the associations of APOB and LDLR with bile duct cancer persisted. The significant association between the TT APOB haplotype and bile duct cancer also persisted after adjustments for multiple comparisons. Even though the Simes p-value obtained for all 12 SNPs suggests an effect of lipid metabolism genes on bile duct cancer, it is possible that the observed associations may be due to linkage disequilibrium with causative SNPs that were not evaluated in this study, particularly since the functional significance of the SNPs examined is unknown and since the three SNPs with significant associations were in non-coding regions. For example, moderate to high linkage disequilibrium has been previously reported between APOE IVS1+69C>G and the markers responsible for the e2 allele (51, 52), suggesting that the observed effects of APOE IVS1+69C>G may be due to the e2 allele. Nevertheless, the totality of our data suggests that APOE, APOB, and LDLR genes, and possibly other genes in the lipid metabolism pathway may play an important etiologic role in biliary tract cancers, especially of the bile duct.
Several strengths of this study should be noted: 1) Selection bias was minimal due to the population-based design and very high case ascertainment (>95%) and high response rates (>85%) ; 2) Misclassification of cancer and stone cases was also minimal due to the detailed review of pathology and clinical data; 3) The nearly complete assessment of gallstone status among cancer cases and controls made it possible to evaluate cancer risk while controlling for gallstones; 4) Misclassification of genotypes was unlikely given the good quality and purity of the extracted DNA, the 99% concordance between duplicate samples, and the 99% reproducibility of genotyping results among quality control samples; and 5) We were able to adjust for multiple comparisons, and provide confirmation of our single-locus results.
However, our study had some limitations: 1) We may have missed the effect of some important markers, since gene coverage was limited and SNP selection was not based on complete sequencing data; 2) The functional effect of the SNPs examined was unknown, and may actually represent markers for other functional SNPs that are in LD; 3) Although our study of biliary tract cancer is the largest to date, some small sample sizes limited the statistical power to evaluate certain main effects, especially of ampullary cancer, as well as gene-gene and gene-environment interactions; and 4) Our findings may have limited generalizability due to the homogeneous Chinese study population, although an effect of population stratification would be minimal.
In conclusion, this population-based study in Shanghai showed that among men, APOE was associated with the risk of biliary stones and biliary tract cancers, while APOB was associated with bile duct cancer. In addition, LDLR was related to the risk of bile duct cancer in both sexes. Further studies with greater statistical power and broader coverage of genes in the lipid metabolism pathway and other pathways are needed to identify the causal gene variants and mechanisms involved in the formation of biliary stones and cancers.
Acknowledgments
We thank the collaborating surgeons and pathologists in Shanghai for assistance in patient recruitment and pathology review; Chia-Rong Cheng, Lu Sun, and Kai Wu of the Shanghai Cancer Institute for coordinating data and specimen collection; and Shelley Niwa, of Westat for support with study and data management. This research was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute.
References
- 1.Hsing AW, Gao YT, Devesa SS, Jin F, Fraumeni JF., Jr Rising incidence of biliary tract cancers in Shanghai, China. Int J Cancer. 1998;75:368–370. doi: 10.1002/(sici)1097-0215(19980130)75:3<368::aid-ijc7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Hsing AW, Rashid A, Devesa SS, Fraumeni JF., Jr . Biliary tract cancer. In: D Schottenfeld, JF Fraumeni., Jr, editors. Cancer Epidemiology and Prevention. edition III. Oxford University Press; 2006. pp. 787–800. [Google Scholar]
- 3.Lowenfels AB, Walker AM, Althaus DP, Townsend G, Domellof L. Gallstone growth, size, and risk of gallbladder cancer: an interracial study. Int J Epidemiol. 1989;18:50–54. doi: 10.1093/ije/18.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Hsing AW, Rashid A, Sakoda L, et al. Gallstone and the risk of biliary tract cancer: a population-based study. Br J Cancer. 2007;97:1577–1582. doi: 10.1038/sj.bjc.6604047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attili AF, Capocaccia R, Carulli N, et al. Factors associated with gallstone disease in the MICOL experience. Multicenter Italian Study on Epidemiology of Cholelithiasis. Hepatology. 1997;26:809–818. doi: 10.1002/hep.510260401. [DOI] [PubMed] [Google Scholar]
- 6.Singh V, Zaidi SA, Singh VS. Lipids in biliary lithogenesis. J Pak Med Assoc. 1997;47:253–255. [PubMed] [Google Scholar]
- 7.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 8.Humphries SE, Kessling AM, Horsthemke B, et al. A common DNA polymorphism of the low-density lipoprotein (LDL) receptor gene and its use in diagnosis. Lancet. 1985;1:1003–1005. doi: 10.1016/s0140-6736(85)91611-3. [DOI] [PubMed] [Google Scholar]
- 9.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Miranda J, Ordovas JM, Ostos MA, et al. Dietary fat clearance in normal subjects is modulated by genetic variation at the apolipoprotein B gene locus. Arterioscler Thromb Vasc Biol. 1997;17:1765–1773. doi: 10.1161/01.atv.17.9.1765. [DOI] [PubMed] [Google Scholar]
- 11.Aalto-Setala K, Palomaki H, Miettinen H, et al. Genetic risk factors and ischaemic cerebrovascular disease: role of common variation of the genes encoding apolipoproteins and angiotensin-converting enzyme. Ann Med. 1998;30:224–233. doi: 10.3109/07853899808999408. [DOI] [PubMed] [Google Scholar]
- 12.Skoglund-Andersson C, Ehrenborg E, Fisher RM, Olivecrona G, Hamsten A, Karpe F. Influence of common variants in the CETP, LPL, HL APO E genes on LDL heterogeneity in healthy middle-aged men. Atherosclerosis. 2003;167:311–317. doi: 10.1016/s0021-9150(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 13.Poole EM, Bigler J, Whitton J, Sibert JG, Potter JD, Ulrich CM. Prostacyclin synthase and arachidonate 5-lipoxygenase polymorphisms and risk of colorectal polyps. Cancer Epidemiol Biomarkers Prev. 2006;15:502–508. doi: 10.1158/1055-9965.EPI-05-0804. [DOI] [PubMed] [Google Scholar]
- 14.Carlson CS, Heagerty PJ, Nord AS, Pritchard DK, Ranchalis J, Boguch JM, Duan H, Hatsukami TS, Schwartz SM, Rieder MJ, Nickerson DA, Jarvik GP. TagSNP evaluation for the association of 42 inflammation loci and vascular disease: evidence of IL6, FGB, ALOX5, NFKBIA, and IL4R loci effects. Hum Genet. 2007;121:65–75. doi: 10.1007/s00439-006-0289-8. [DOI] [PubMed] [Google Scholar]
- 15.Hsing AW, Bai Y, Andreotti G, Rashid A, et al. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer. 2007;121:832–838. doi: 10.1002/ijc.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lusis AJ. Genetic factors affecting blood lipoproteins: the candidate gene approach. J Lipid Res. 1988;29:397–429. [PubMed] [Google Scholar]
- 17.Juvonen T, Savolainen MJ, Kairaluoma MI, Lajunen LH, Humphries SE, Kesaniemi YA. Polymorphisms at the apoB, apoA-I, and cholesteryl ester transfer protein gene loci in patients with gallbladder disease. J Lipid Res. 1995;36:804–812. [PubMed] [Google Scholar]
- 18.Hsing AW, Gao YT, McGlynn KA, et al. Biliary tract cancer and stones in relation to chronic liver conditions: a population-based study in Shanghai, China. Int J Cancer. 2007;120:1981–1985. doi: 10.1002/ijc.22375. [DOI] [PubMed] [Google Scholar]
- 19.Packer BR, Yeager M, Burdett L, et al. SNP500Cancer: a public resource for sequence validation, assay development, and frequency analysis for genetic variation in candidate genes. Nucleic Acids Res. 2006;34(Database issue):D617–D621. doi: 10.1093/nar/gkj151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- 21.Rosenberg PS, Che A, Chen BE. Multiple hypothesis testing strategies for genetic case-control association studies. Stat Med. 2006;25:3134–3149. doi: 10.1002/sim.2407. [DOI] [PubMed] [Google Scholar]
- 22.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 23.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Excoffier L, Slatkin M. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol. 1995;12:921–927. doi: 10.1093/oxfordjournals.molbev.a040269. [DOI] [PubMed] [Google Scholar]
- 25.Bertomeu A, Ros E, Zambon D, et al. Apolipoprotein E polymorphism and gallstones. Gastroenterology. 1996;111:1603–1610. doi: 10.1016/s0016-5085(96)70023-9. [DOI] [PubMed] [Google Scholar]
- 26.Portincasa P, van Erpecum KJ, van De Meeberg PC, Dallinga-Thie GM, de Bruin TW, van Berge-Henegouwen GP. Apolipoprotein E4 genotype and gallbladder motility influence speed of gallstone clearance and risk of recurrence after extracorporeal shock-wave lithotripsy. Hepatology. 1996;24:580–587. doi: 10.1002/hep.510240320. [DOI] [PubMed] [Google Scholar]
- 27.Boland LL, Folsom AR, Boerwinkle E. Apolipoprotein E genotype and gallbladder disease risk in a large population-based cohort. Ann Epidemiol. 2006;16:763–769. doi: 10.1016/j.annepidem.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 29.Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J Neuroimmunol. 1997;76:70–74. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 30.Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114:107–113. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- 31.Park J, Tadlock L, Gores GJ, Patel T. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999;30:1128–1133. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 32.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 33.Hayek T, Oiknine J, Brook JG, Aviram M. Increased plasma and lipoprotein lipid peroxidation in apo E-deficient mice. Biochem Biophys Res Commun. 1994;201:1567–1574. doi: 10.1006/bbrc.1994.1883. [DOI] [PubMed] [Google Scholar]
- 34.Vogel T, Guo NH, Guy R, et al. Apolipoprotein E: a potent inhibitor of endothelial and tumor cell proliferation. J Cell Biochem. 1994;54:299–308. doi: 10.1002/jcb.240540306. [DOI] [PubMed] [Google Scholar]
- 35.Lehrer S. Possible relationship of the apolipoprotein E (ApoE) epsilon4 allele to prostate cancer. Br J Cancer. 1998;78:1398. doi: 10.1038/bjc.1998.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson MA, Gay L, Stebbings WS, Speakman CT, Bingham SA, Loktionov A. Apolipoprotein E gene polymorphism and colorectal cancer: gender-specific modulation of risk and prognosis. Clin Sci. 2003;104:537–545. doi: 10.1042/CS20020329. [DOI] [PubMed] [Google Scholar]
- 37.Chang NW, Chen DR, Wu CT, et al. Influences of apolipoprotein E polymorphism on the risk for breast cancer and HER2/neu status in Taiwan. Breast Cancer Res Treat. 2005;90:257–261. doi: 10.1007/s10549-004-4656-7. [DOI] [PubMed] [Google Scholar]
- 38.Reilly SL, Ferrell RE, Kottke BA, Kamboh MI, Sing CF. The gender-specific apolipoprotein E genotype influence on the distribution of lipids and apolipoproteins in the population of Rochester, MN. I. Pleiotropic effects on means and variances. Am J Hum Genet. 1991;49:1155–1166. [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez-Coronado D, Alvarez JJ, Entrala A, Olmos JM, Herrera E, Lasuncion MA. Apolipoprotein E polymorphism in men and women from a Spanish population: allele frequencies and influence on plasma lipids and apolipoproteins. Atherosclerosis. 1999;147:167–176. doi: 10.1016/s0021-9150(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 40.Kane JP. Apolipoprotein B: structural and metabolic heterogeneity. Annu Rev Physiol. 1983;45:637–650. doi: 10.1146/annurev.ph.45.030183.003225. [DOI] [PubMed] [Google Scholar]
- 41.Law SW, Lackner KJ, Fojo SS, Hospattankar A, Monge JC, Brewer HB., Jr The molecular biology of human apoA-I, apoA-II, apoC-II and apoB. Adv Exp Med Biol. 1986;201:151–162. doi: 10.1007/978-1-4684-1262-8_14. [DOI] [PubMed] [Google Scholar]
- 42.Pallaud C, Gueguen R, Sass C, et al. Genetic influences on lipid metabolism trait variability within the Stanislas Cohort. J Lipid Res. 2001;42:1879–1890. [PubMed] [Google Scholar]
- 43.Wells BJ, Mainous AG3rd, Everett CJ, Gill JM. Iron, cholesterol, and the risk of cancer in an 18 year cohort. Asian Pac J Cancer Prev. 2005;6:505–509. [PubMed] [Google Scholar]
- 44.Benitez S, Camacho M, Bancells C, Vila L, Sanchez-Quesada JL, Ordonez-Llanos J. Wide proinflammatory effect of electronegative low-density lipoprotein on human endothelial cells assayed by a protein array. Biochim Biophys Acta. 2006;1761:1014–1021. doi: 10.1016/j.bbalip.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Feig DI, Sowers LC, Loeb LA. Reverse chemical mutagenesis: identification of the mutagenic lesions resulting from reactive oxygen species-mediated damage to DNA. Proc Natl Acad Sci. 1994;91:6609–6613. doi: 10.1073/pnas.91.14.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rashid A, Ueki T, Gao YT, et al. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002;8:3156–3163. [PubMed] [Google Scholar]
- 47.Brown MS, Goldstein JL. How LDL receptors influence cholesterol and atherosclerosis. Sci Am. 1984;251:58–66. doi: 10.1038/scientificamerican1184-58. [DOI] [PubMed] [Google Scholar]
- 48.Devesa SS, Silverman DT, Young JL, Jr, et al. Cancer incidence and mortality trends among whites in the United States, 1947–84. J Natl Cancer Inst. 1987;79:701–770. [PubMed] [Google Scholar]
- 49.Wistuba II, Miquel JF, Gazdar AF, Albores-Saavedra J. Gallbladder adenomas have molecular abnormalities different from those present in gallbladder carcinomas. Hum Pathol. 1999;30:21–25. doi: 10.1016/s0046-8177(99)90295-2. [DOI] [PubMed] [Google Scholar]
- 50.Rashid A, Gao YT, Bhakta S, et al. Beta-catenin mutations in biliary tract cancers: a population-based study in China. Cancer Res. 2001;61:3406–3409. [PubMed] [Google Scholar]
- 51.Woo D, Kaushal R, Chakraborty R, et al. Association of apolipoprotein E4 and haplotypes of the apolipoprotein E gene with lobar intracerebral hemorrhage. Stroke. 2005;36:1874–1879. doi: 10.1161/01.STR.0000177891.15082.b9. [DOI] [PubMed] [Google Scholar]
- 52.Long JR, Liu PY, Liu YJ, et al. APOE haplotypes influence bone mineral density in Caucasian males but not females. Calcif Tissue Int. 2004;75:299–304. doi: 10.1007/s00223-004-0034-z. [DOI] [PubMed] [Google Scholar]


