Summary
Cyclin E-Cdk2 is known to regulate both DNA replication and centrosome duplication during the G1-S transition in the cell cycle [1–4], and disruption of centrosomes results in a G1 arrest in some cell types [5–7]. Localization of cyclin E on centrosomes is mediated by a 20 amino acid domain termed the centrosomal localization sequence (CLS), and expression of the GFP-tagged CLS displaces both cyclin E and cyclin A from the centrosome [8]. In asynchronous cells CLS expression inhibits the incorporation of bromodeoxyuridine (BrdU) into DNA, an effect proposed to reflect a G1 arrest. Here we show in synchronized cells that the reduction in BrdU incorporation reflects not a G1 arrest but rather direct inhibition of the initiation of DNA replication in S phase. The loading of essential DNA replication factors such as Cdc45 and PCNA onto chromatin is blocked by CLS expression, but DNA synthesis can be rescued by retargeting active cyclin E-Cdk2 to the centrosome. These results suggest that initial steps of DNA replication require centrosomally localized Cdk activity and link the nuclear cycle with the centrosome cycle at the G1-S transition.
Keywords: centrosomes, cyclin E-Cdk2, DNA synthesis
Results and Discussion
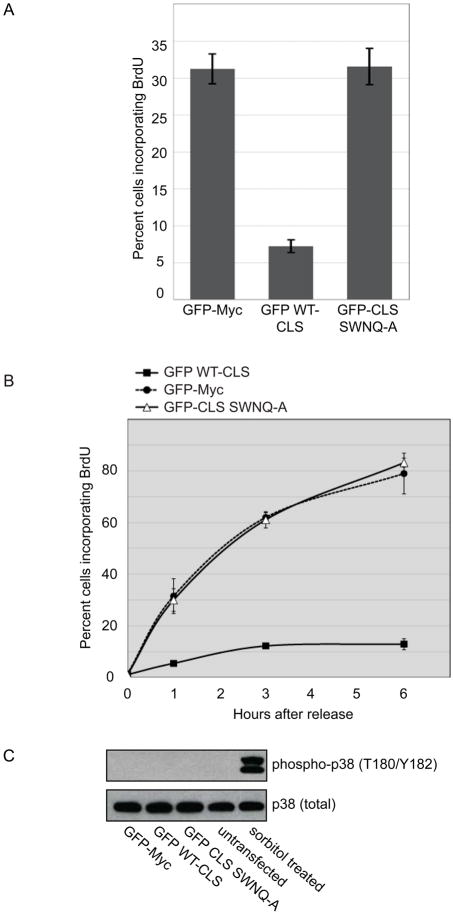
In asynchronous CHO cells, expression of the GFP-tagged 20 amino acid CLS domain of cyclin E (GFP WT-CLS) reduced the fraction of cells incorporating BrdU from approximately 30% to less than 10% (Fig. 1A), as previously reported [8]. Lack of BrdU incorporation after CLS expression could in principle reflect either a G1 arrest or direct inhibition of DNA synthesis. To determine precisely what phase of the cell cycle is affected by expression of the cyclin E CLS, CHO cells were synchronized at the G1-S boundary by double thymidine treatment and transiently transfected with GFP, the GFP WT-CLS, or a mutant CLS (SWNQ-A) that neither localizes to centrosomes nor affects endogenous cyclin localization [8]. After release into S phase, wild-type CLS expression reduced BrdU incorporation from 80% to less than 10% of the cells, indicating direct inhibition of DNA synthesis, whereas the SWNQ-A mutant had no effect (Fig. 1B).
Figure 1. Expression of the GFP-CLS of cyclin E directly inhibits DNA synthesis.
(A) BrdU incorporation was assessed as described in Experimental Procedures in asynchronous CHO-K1 cells expressing either the wild-type GFP WT-CLS, GFP-Myc (control) or the GFP CLS SWNQ-A mutant. Error bars represent mean±SD (n=3).
(B) BrdU incorporation was assessed in CHO-K1 cells synchronized by double thymidine treatment. Cells were transfected with the indicated CLS constructs during the second thymidine treatment, released from the G1-S boundary by thymidine washout, and harvested at the indicated times as described in Experimental Procedures. Error bars represent mean ± SD (n=3).
(C) Phosphorylation of p38 MAPK at activating sites was analyzed by western blotting (Upper) in CHO-K1 cells synchronized by double thymidine treatment and transfected with the indicated CLS constructs as in Panel B, or treated with 500 mM sorbitol for 30 mm. Total p38 served as a loading control (Lower).
Recent reports have described a G1 checkpoint that monitors centrosome integrity and regulates G1-S progression [7, 9]. Activation of this checkpoint and cell cycle arrest are dependent on the p38 stress-activated kinase pathway and can be suppressed by loss of p38, p53, or p21. In mammalian cells, p38 kinase can be activated by cellular stress and requires the phosphorylation of residues Thr180 and Tyr182 [10]. To determine if the p38 stress activated pathway is mediating CLS-dependent inhibition of S phase progression, the phosphorylation of p38 kinase was assessed by western blotting in double thymidine-synchronized cells expressing GFP-Myc, the GFP WT-CLS or the mutant SWNQ-A CLS (Fig. 1C). Sorbitol-induced osmotic stress resulted in robust p38 activation, but phosphorylation of p38 was not detectable in CLS-expressing cells, indicating S phase inhibition is not a result of p38 activation.
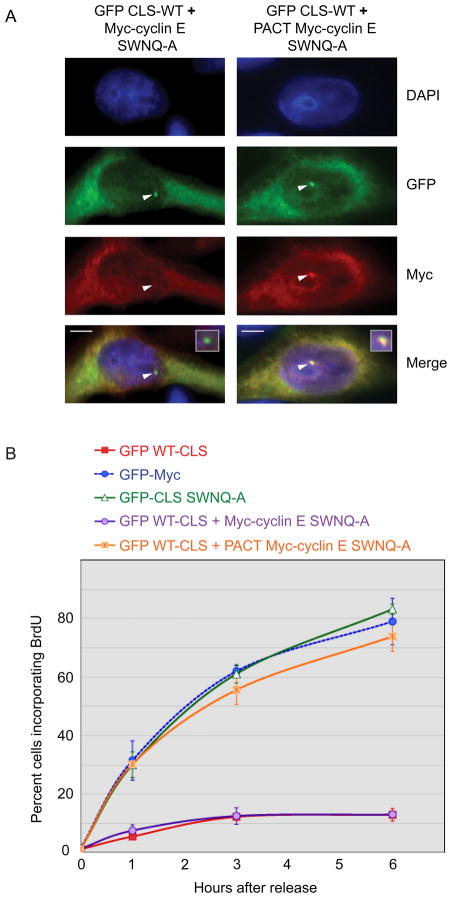
Since expression of the wild-type cyclin E CLS displaces not only endogenous cyclin E but also cyclin A, MCM5 and potentially other unidentified proteins from the centrosome [8, 11], loss of BrdU incorporation might not be directly linked to mislocalization of cyclin E. To determine if displacement of cyclin E from centrosomes was indeed responsible for inhibition of DNA synthesis, we monitored BrdU incorporation in synchronized cells co-expressing the GFP WT-CLS and 6 Myc-tagged cyclin E constructs that were centrosomally localized via a different targeting motif, the 91 amino acid pericentrin-AKAP450 centrosomal targeting (PACT) domain that is sufficient for targeting GFP to centrosomes [12].
CHO cells were co-transfected with GFP WT-CLS and the indicated 6 Myc-tagged cyclin E constructs, methanol-fixed, and analyzed for co-expression of both plasmids as well as for localization of the PACT-cyclin E protein. Transfection efficiencies were optimized to ensure that approximately 99% of the GFP positive cells were also Myc positive. Importantly, cells expressing the GFP WT-CLS also displayed centrosomal co-localization of the PACT-cyclin E protein, indicating expression of the CLS does not interfere with localization mediated by the PACT domain (Fig. 2A and 3A). In synchronized CHO cells expressing the GFP WT-CLS, co-expression of 6 Myc-tagged cyclin E containing the SWNQ-A CLS mutations that prevent localization to centrosomes did not overcome inhibition of DNA replication, whereas the same mutant fused to the PACT domain restored BrdU incorporation nearly to control levels (Fig. 2B).
Figure 2. DNA synthesis requires centrosomally-localized cyclin E.
(A) The localization of GFP WT-CLS and Myc-tagged cyclin E SWNQ-A without (left) and with (right) fusion to the PACT domain was analyzed by immunofluorescence in cells expressing the GFP-WT-CLS. Staining was performed with the indicated antibodies as described in Experimental Procedures: DAPI (blue), anti-GFP (green), and anti-Myc (red). Arrowheads mark centrosomes. Inset: enlarged image of the merged centrosomal area. Scale bars: 5 μM.
(B) DNA synthesis is restored in CLS-expressing cells by co-expression of PACT-fused Myc-cyclin E SWNQ-A. Cells were synchronized by double thymidine treatment, transfected with the indicated constructs, and assessed for BrdU incorporation at 1, 3, and 6 hr after release of G1-S arrest by thymidine washout. Error bars represent mean ± SD (n=5).
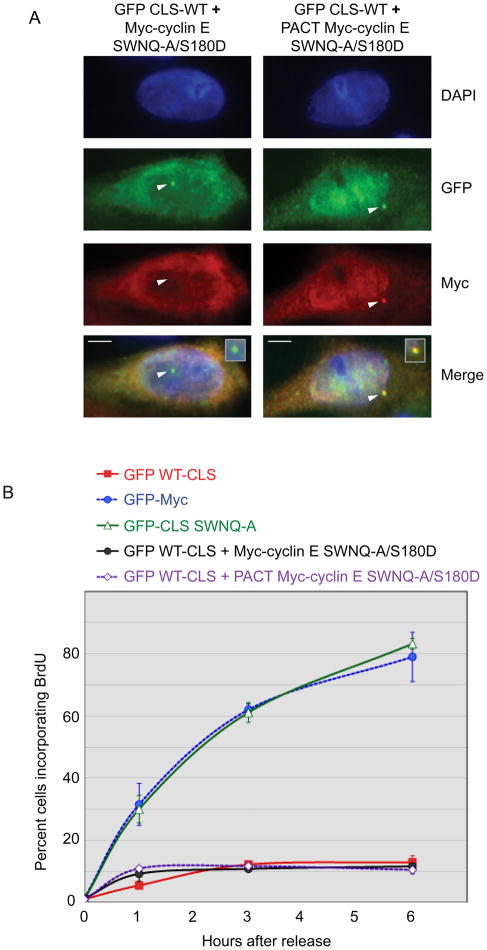
Figure 3. DNA synthesis requires active centrosomal cyclin E-Cdk2.
(A) In cells expressing the wild-type GFP-CLS, localization of Myc-tagged cyclin E SWNQ-A-S180D without (left) and with (right) fusion to the PACT domain was analyzed by immunofluorescence as in Fig. 2A. Arrowheads mark centrosomes. Inset: enlarged image of merged centrosomal area. Scale bars: 5μM.
(B) DNA synthesis requires Cdk activity at the centrosome. The cyclin E double mutant SWNQ-A-S180D deficient in both centrosomal localization and Cdk binding was assessed for restoration of BrdU incorporation in CLS-expressing cells with or without fusion to the PACT domain, as indicated. Error bars represent mean ± SD (n=5).
Previous work from this laboratory demonstrated the SWNQ-A mutant of cyclin E binds Cdk2 and has kinase activity [8]. We therefore sought to determine if Cdk2 binding and activity at the centrosome is necessary for restoring DNA synthesis. Co-expression of the GFP WT-CLS and a cyclin E double mutant containing the SWNQ-A CLS mutations and an additional point mutation, S180D, that blocks Cdk2 binding [8], did not overcome CLS-mediated inhibition of BrdU incorporation, with or without the PACT domain (Fig. 3B). These data indicate that Cdk activity specifically localized at centrosomes is an essential step in DNA synthesis. However, this requirement may not be universal, as some cells have been reported to undergo DNA synthesis in the absence of centrioles [9, 13]. Cells without centrioles may only express PCM proteins in close apposition to the nuclear periphery [14] and potentially support cyclin E-dependent regulation of DNA synthesis.
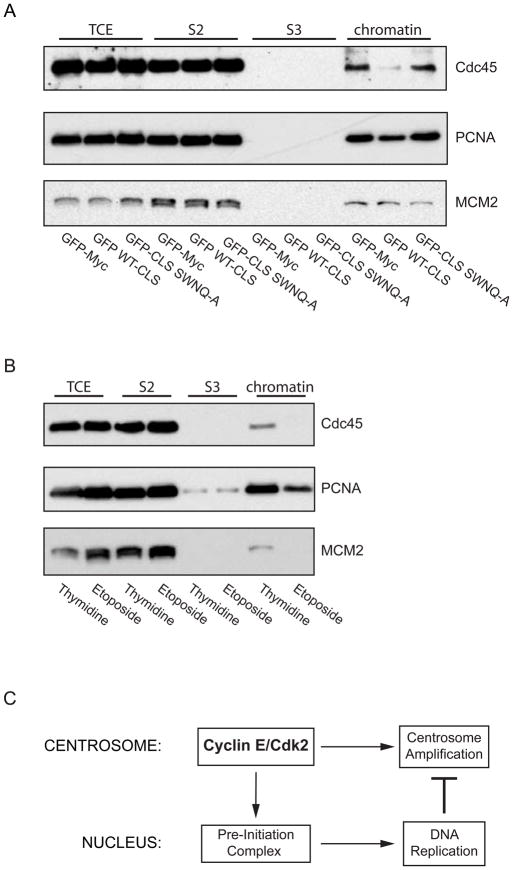
It was previously reported that MCM2 is loaded onto chromatin even when the CLS is expressed [8]. Chromatin loading of MCM proteins occurs during late M phase to early G1, when Cdk activity is low, and is widely regarded as the last step in pre-replication complex formation [15]. Since the CLS influences DNA synthesis later, at the G1-S transition, it is evident that expression of the CLS does not disrupt formation of the pre-replication complex and hence licensing of chromatin for replication. Therefore, inhibiting either the formation of the pre-initiation complex at the G1-S phase transition, activation of the pre-initiation complex during origin firing, or elongation could explain the loss of BrdU incorporation. Using a biochemical fractionation scheme described by Mendez and Stillman [16], we isolated the chromatin fraction from asynchronous CHO cells expressing GFP, the GFP WT-CLS, or the GFP CLS-SWNQ-A. To assess initiation of DNA synthesis, chromatin loading of Cdc45, an essential pre-initiation factor [17, 18], was examined, and for DNA elongation, chromatin loading of PCNA, a processivity factor [19, 20], was investigated. Since expression of the CLS does not influence pre-RC formation, MCM2 serves as a loading control. In asynchronous cells expressing the wild-type CLS, we observed a significant reduction in chromatin-bound Cdc45 and PCNA compared to the control population, signifying inhibition of pre-initiation complex formation (Fig. 4A). This shows that initiation of DNA replication and origin firing is inhibited when cyclin E has been mislocalized from centrosomes. Additionally, since chromatin loading of MCM2, Cdc45, and PCNA are known to be cell cycle regulated, chromatin fractionation was also carried out on cells in known phases of the cell cycle (Fig. 4B). As expected, cells synchronized at G1-S by double thymidine treatment have high chromatin binding of MCM2, Cdc45 and PCNA compared to cells synchronized with etoposide in late S-G2, a point in the cell cycle when DNA replication factors have been removed from chromatin. As a further control, one chromatin sample from cells expressing GFP alone was digested with DNase I. While the vast majority of MCM2 was released from chromatin, Cdc45 and PCNA are tightly chromatin bound and largely DNase-insoluble during S phase and were only partially released (data not shown).
Figure 4. Expression of the cyclin E CLS prevents chromatin loading of pre-initiation complex proteins.
(A) Western blots of chromatin fractions from asynchronous CHO-K1 cells for Cdc45, PCNA, and MCM2 (loading control) in cells expressing GFP-Myc (control), the wild-type cyclin E CLS, or the mutant SWNQ-A CLS. The proteins from the total cell extract (TCE), in the soluble fraction (S2), the solubilized chromatin supernatant fraction (S3), and the chromatin-bound fraction (chromatin) are shown.
(B) Western blots of chromatin fractions as in Panel A from CHO-K1 cells synchronized at the G1-S boundary by double thymidine treatment or in G2 phase by etoposide treatment. The distribution of proteins from the total cell extract (TCE), in the soluble fraction (S2), solubilized nuclear protein fraction (S3) and chromatin-bound fraction (chromatin) are shown.
(C) A model for communication between the centrosome and the nucleus at the G1-S transition. The model depicts cyclin E-Cdk2 localization on the centrosome as contributing to Pre-Initiation complex formation on chromatin in the nucleus and to centrosome amplification. Once DNA synthesis commences, replication factors released from chromatin are recruited to centrosomes and inhibit over-duplication of centrosomes.
DNA replication and centrosome duplication are fundamentally similar in that they are the only cellular components to undergo once-and-only-once, semi-conservative duplication during every cell cycle. Both replication events are initiated at the G1-S transition, and this essential coordination is achieved at least in part by the activation of Cdk2 coupled to cyclin E. The data presented here show that initiation of DNA replication is dependent on Cdk activity specifically at the centrosome and may help explain why some cells arrest in late G1 when centrosomes are ablated, microsurgically removed, disrupted by antibody microinjection, or impaired by siRNA knockdown of centrosomal proteins [5–7, 14].
The mechanism of DNA synthesis inhibition after CLS expression clearly involves regulation of Cdc45 loading onto chromatin by centrosomally-localized cyclin E-Cdk2. The binding of Cdc45 to chromatin defines the transformation from a pre-replication complex to a pre-initiation complex during which the replisome is loaded onto the origin, and chromatin loading of Cdc45 is essential for association of polymerase -primase with chromatin [21, 22]. Recent work in both budding and fission yeast has identified phosphorylation of two replication proteins, Sld2 and Sld3, as the minimal Cdk-dependent steps required for initiation of DNA replication [23, 24]. Of particular interest is Sld3, as it has genetic and physical interactions with Cdc45 and is essential for functional chromatin binding [25]. While it appears that the molecular mechanisms regulating the initiation of DNA replication are highly conserved throughout eukaryotes, and orthologs of all major DNA replication proteins have been identified in animal cells, it is important to note that S1d3 has only been described in yeast. However, it has been postulated that a homolog of S1d3 does exist and functions during DNA replication in higher eukaryotes but has likely undergone rapid evolution, making identification difficult [24, 26].
Centrosomal localization of cyclin E has previously been implicated in the control of DNA synthesis in two different paradigms. In one, the acceleration of entry into S phase from G1 by cyclin E over-expression requires a wild type CLS [8]. In the other paradigm, cyclin E promotes MCM loading, but not origin firing, in Cdk2 knockout fibroblasts without a need for centrosomal localization [27]. Neither of these two functions requires associated Cdk kinase activity, unlike the regulation of DNA synthesis reported here (Fig. 3), suggesting a centrosomal Cdk substrate is involved. A candidate substrate is nucleophosmin (NPM/B23), a multi-functional protein implicated in multiple cellular activities including both centrosome duplication and DNA replication [28, 29]. It is intriguing that NPM/B23 has DNA binding activity and stimulates DNA polymerase -primase enzymatic activity [29]. However, because NPM/B23 affects centrosome duplication, DNA synthesis, and many other cellular processes, it is difficult to directly evaluate a potential role in coordination of DNA replication with centrosomal function by over-expression or knockdown approaches.
Recent work by this laboratory and others has provided evidence that DNA replication proteins create negative feedback mechanisms to prevent inappropriate centrosome re-duplication [11, 30, 31]. For example, both MCM5 and ORC1 are on centrosomes, and over-expression of either blocks centrosome over-duplication in S phase-arrested cells. However, centrosomal localization of MCM5 is not evident when the PACT-Myc-cyclin E SWNQ-A is expressed [11], despite the ability of this construct to restore DNA replication (Fig. 3). MCM5 is also not a substrate for cyclin E-Cdk2 (ref. 22 and data not shown). Therefore MCM5 is unlikely to be involved in S phase inhibition due to CLS expression. Orc1 is tightly chromatin-bound during G1 phase and in mammalian cells is released from chromatin only after initiation of replication [32], making it also unlikely to be a key cyclin E-Cdk2 centrosomal target. Previous evidence indicated that centrosomal-nuclear communication was important for the G2-M transition [33]. Our results demonstrate that cyclin E-Cdk2-dependent communication between the centrosome and the nucleus is also essential for initiation of DNA synthesis. This suggests there is communication at the G1-S boundary in both directions by the centrosome (regulating cyclin E-dependent initiation of nuclear DNA synthesis) and the nucleus (regulating centrosome duplication) (Fig. 4C).
Experimental Procedures
Cell culture, synchronization and transfection
CHO-K1 cells were cultured and transfected as previously described [11]. Cells were synchronized at the G1-S boundary by double thymidine treatment (2mM). Cells were initially blocked for 16 hrs, washed, released for 8 hrs, and blocked again for an additional 16 hrs. For time course BrdU assays, cells were washed, released, and harvested at the indicated times. Synchronized populations were transfected between thymidine treatments, two hrs after release.
Immunofluorescence and BrdU Incorporation Assays
Immunofluorescent localization studies were carried out as previously described [11] using primary antibodies to Myc (Invitrogen) and GFP (Invitrogen). Microscopic observations were made on a Nikon Eclipse TE 300, PCM 2000 inverted microscope with a 100X oil-immersion objective (NA 1.4). Images were obtained with an air-cooled charge-coupled device (CCD) camera (SenSys Photometrics) attached to a 0.76X coupler (Diagnostic Instruments). For microscopic analysis, Simple PCI (Compix) acquisition software was used. To visualize BrdU incorporation, cells were incubated for 20 min with 10 μM BrdU (Roche) followed by a 15 min fixation with 3.7% paraformaldehyde in PBS. Cells were permeabilized with 0.25% Triton X-100 in PBS for 10 min and stained with anti-GFP for one hr. Cells were fixed a second time for 15 min with 3.7% paraformaldehyde in PBS, treated with 4N HCl- 1% Triton X-100 for 10 min, and stained with antibody to BrdU (Sigma). Microscopic observation was made on a Nikon Eclipse TE 300, PCM 2000 inverted microscope with a 60X water-immersion objective (NA 1.2).
Chromatin Isolation
The method described by Mendez and Stillman was employed {Mendez, 2000 #207}. Briefly, twenty hr after transfection, cells were trypsinized and collected by low-speed centrifugation (1800 rpm, 3 min at 4°), and resuspended in buffer A (10 mM HEPES [pH 7.5], 1mM EDTA, 0.4M NaCl, 10% sucrose) supplemented with 1mM DTT, 1X complete protease inhibitor (Roche), 1mM AEBSF (Sigma) and 0.1% Triton X-100. Cells were incubated on ice for 5 minutes and nuclei pelleted by low-speed centrifugation (1,300 × g, 4 min at 4°). Nuclei were washed once in buffer A and then lysed in buffer B (3mM EDTAm 0.2 mM EGTA, 1mM DTT, 1X complete protease inhibitor mix, and 1mM AEBSF). Cells were incubated on ice for 15 min and insoluble chromatin was collected by centrifugation (1,700 × g, 4 min at 4°). The chromatin pellet was resuspended in Laemmli buffer and sonicated for 30 sec. To release chromatin-bound proteins by nuclease treatment, cell nuclei were resuspended in buffer A plus 1mM CaCl2 and 2 U of Turbo DNase (Ambion). After incubation at 37° for 10 min, the reaction was stopped by addition of 1mM EGTA, and nuclei were lysed as described above. Samples were electrophoresed on 10% SDS-PAGE, transferred to a PVDF membrane, and proteins of interest detected by immunoblot analysis. The following primary antibodies were used: DNA polymerase alpha (Abcam); Cdc45 (Abnova); PCNA (Abcam); and MCM2 (BD Biosciences). Appropriate HRP-conjugated goat anti-rabbit or goat-anti mouse secondary antibodies (Pierce) were used.
Acknowledgments
We are grateful to Dr. Sean Munro (Cambridge, UK) for providing cDNA encoding the human PACT domain. We thank Drs. Bob Sclafani, Frank Eckerdt, and Gaetan Pascreau for helpful discussions. This work was supported by The Howard Hughes Medical Institute, where JLM is an Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto Y, Hayashi K, Nishida E. Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr Biol. 1999;9:429–432. doi: 10.1016/s0960-9822(99)80191-2. [DOI] [PubMed] [Google Scholar]
- 3.Coverley D, Laman H, Laskey RA. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat Cell Biol. 2002;4:523–528. doi: 10.1038/ncb813. [DOI] [PubMed] [Google Scholar]
- 4.Furstenthal L, Kaiser BK, Swanson C, Jackson PK. Cyclin E uses Cdc6 as a chromatin-associated receptor required for DNA replication. J Cell Biol. 2001;152:1267–1278. doi: 10.1083/jcb.152.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 2001;291:1547–1550. doi: 10.1126/science.1056866. [DOI] [PubMed] [Google Scholar]
- 6.Khodjakov A, Rieder CL. Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J Cell Biol. 2001;153:237–242. doi: 10.1083/jcb.153.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikule K, Delaval B, Kaldis P, Jurcyzk A, Hergert P, Doxsey S. Loss of centrosome integrity induces p38-p53-p21-dependent G1-S arrest. Nat Cell Biol. 2007;9:160–170. doi: 10.1038/ncb1529. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto Y, Maller JL. A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science. 2004;306:885–888. doi: 10.1126/science.1103544. [DOI] [PubMed] [Google Scholar]
- 9.Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol. 2007;176:173–182. doi: 10.1083/jcb.200607073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doza YN, Cuenda A, Thomas GM, Cohen P, Nebreda AR. Activation of the MAP kinase homologue RK requires the phosphorylation of Thr-180 and Tyr-182 and both residues are phosphorylated in chemically stressed KB cells. FEBS Lett. 1995;364:223–228. doi: 10.1016/0014-5793(95)00346-b. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson RL, Maller JL. Cyclin E-dependent localization of MCM5 regulates centrosome duplication. J Cell Sci. 2008;121:3224–3232. doi: 10.1242/jcs.034702. [DOI] [PubMed] [Google Scholar]
- 12.Gillingham AK, Munro S. The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW. Flies without centrioles. Cell. 2006;125:1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Edde B, Bornens M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DePamphilis ML, Blow JJ, Ghosh S, Saha T, Noguchi K, Vassilev A. Regulating the licensing of DNA replication origins in metazoa. Curr Opin Cell Biol. 2006;18:231–239. doi: 10.1016/j.ceb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Mendez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda T, Mimura S, Takisawa H. CDK- and Cdc45-dependent priming of the MCM complex on chromatin during S-phase in Xenopus egg extracts: possible activation of MCM helicase by association with Cdc45. Genes Cells. 2003;8:145–161. doi: 10.1046/j.1365-2443.2003.00621.x. [DOI] [PubMed] [Google Scholar]
- 18.Walter J, Newport J. Initiation of eukaryotic DNA replication: origin unwinding and sequential chromatin association of Cdc45, RPA, and DNA polymerase alpha. Mol Cell. 2000;5:617–627. doi: 10.1016/s1097-2765(00)80241-5. [DOI] [PubMed] [Google Scholar]
- 19.Langston LD, O’Donnell M. DNA polymerase delta is highly processive with proliferating cell nuclear antigen and undergoes collision release upon completing DNA. J Biol Chem. 2008;283:29522–29531. doi: 10.1074/jbc.M804488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda Y, Suzuki M, Piao J, Gu Y, Tsurimoto T, Kamiya K. Dynamics of human replication factors in the elongation phase of DNA replication. Nucleic acids research. 2007;35:6904–6916. doi: 10.1093/nar/gkm822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mimura S, Takisawa H. Xenopus Cdc45-dependent loading of DNA polymerase alpha onto chromatin under the control of S-phase Cdk. Embo J. 1998;17:5699–5707. doi: 10.1093/emboj/17.19.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sclafani RA, Holzen TM. Cell cycle regulation of DNA replication. Annual review of genetics. 2007;41:237–280. doi: 10.1146/annurev.genet.41.110306.130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka S, Umemori T, Hirai K, Muramatsu S, Kamimura Y, Araki H. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature. 2007;445:328–332. doi: 10.1038/nature05465. [DOI] [PubMed] [Google Scholar]
- 24.Zegerman P, Diffley JF. Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature. 2007;445:281–285. doi: 10.1038/nature05432. [DOI] [PubMed] [Google Scholar]
- 25.Kamimura Y, Tak YS, Sugino A, Araki H. Sld3, which interacts with Cdc45 (Sld4), functions for chromosomal DNA replication in Saccharomyces cerevisiae. Embo J. 2001;20:2097–2107. doi: 10.1093/emboj/20.8.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka S, Tak YS, Araki H. The role of CDK in the initiation step of DNA replication in eukaryotes. Cell Div. 2007;2:16. doi: 10.1186/1747-1028-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng Y, Lee YM, Welcker M, Swanger J, Zagozdzon A, Winer JD, Roberts JM, Kaldis P, Clurman BE, Sicinski P. Kinase-independent function of cyclin E. Mol Cell. 2007;25:127–139. doi: 10.1016/j.molcel.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 28.Okuda M, Horn HF, Tarapore P, Tokuyama Y, Smulian AG, Chan PK, Knudsen ES, Hofmann IA, Snyder JD, Bove KE, et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell. 2000;103:127–140. doi: 10.1016/s0092-8674(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 29.Umekawa H, Sato K, Takemura M, Watanabe Y, Usui S, Takahashi T, Yoshida S, Olson MO, Furuichi Y. The carboxyl terminal sequence of nucleolar protein B23.1 is important in its DNA polymerase alpha-stimulatory activity. Journal of biochemistry. 2001;130:199–205. doi: 10.1093/oxfordjournals.jbchem.a002973. [DOI] [PubMed] [Google Scholar]
- 30.Hemerly AS, Prasanth SG, Siddiqui K, Stillman B. Orc1 controls centriole and centrosome copy number in human cells. Science. 2009;323:789–793. doi: 10.1126/science.1166745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu F, Lan R, Zhang H, Jiang Q, Zhang C. Geminin is partially localized to the centrosome and plays a role in proper centrosome duplication. Biol Cell. 2009;101:273–285. doi: 10.1042/BC20080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li CJ, Vassilev A, DePamphilis ML. Role for Cdk1 (Cdc2)/cyclin A in preventing the mammalian origin recognition complex’s largest subunit (Orc1) from binding to chromatin during mitosis. Mol Cell Biol. 2004;24:5875–5886. doi: 10.1128/MCB.24.13.5875-5886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]