Abstract
Objective
To assess the feasibility of recruiting and retaining cancer survivors with lower limb lymphedema into an exercise intervention study. To develop preliminary estimates regarding the safety and efficacy of this intervention. We hypothesized that progressive weight training would not exacerbate leg swelling and that the intervention would improve functional mobility and quality of life.
Design
Before-after pilot study of 5 months duration.
Setting
University of Pennsylvania
Participants
Cancer survivors with a known diagnosis of lower limb lymphedema (N=10) were directly referred by University of Pennsylvania clinicians. All 10 participants completed the study.
Intervention
Twice weekly slowly progressive weight-lifting, supervised for 2 months, unsupervised for 3 months.
Main Outcome Measures
The primary outcome was interlimb volume differences as measured by optoelectronic perometry. Additional outcome measures included safety (adverse events), muscle strength, objective physical function, and quality of life.
Results
Interlimb volume differences were 44.4 and 45.3% at baseline and 5 months, respectively (pre-post comparison, p = 0.70). There were 2 unexpected incident cases of cellulitus within the first two months. Both resolved with oral antibiotics and complete decongestive therapy by 5 months. Bench and leg press strength increased by 47% and 27% over 5 months (p = 0.001 and p = 0.07, respectively). Distance walked in 6 minutes increased by 7% in 5 months (p = 0.01). No improvement was noted in self-reported quality of life.
Conclusions
Recruitment of patients with lower limb lymphedema into an exercise program is feasible. Despite some indications that the intervention may be safe (e.g., a lack of clinically significant interlimb volume increases over 5 months), the unexpected finding of two cellulitic infections among the 10 participants suggests additional study is required before concluding lower extremity lymphedema patients can safely perform weight-lifting.
Keywords: Exercise, Lymphedema, Neoplasms, Rehabilitation
It is estimated that there are over 11 million cancer survivors alive in the U.S. today.1 The increased success of cancer treatments has created the welcome challenge of addressing the long term sequelae of those treatments. One common negative effect of cancer treatment is lymphedema, which is defined as an abnormal accumulation of protein rich fluid in the affected limb, which can occur after lymph node removal, trauma or irradiation. This chronic, progressive condition has no known cure and is well documented to have negative effects on wound healing, local blood flow, and tissue oxygenation2-5, as well as physical function and quality of life.6-9
American Cancer Society estimates that approximately 480,000 adults are diagnosed annually with cancers for which treatments include irradiation and/or removal of lymph nodes from the groin or lower torso, which may lead to LLL (e.g. melanomas or gynecologic or genitourinary cancers).10 Estimates of lymphedema incidence in these patients varies by threshold for diagnosis, intensity of lymph node treatment, and length of follow-up. However, it is commonly estimated that 20-30% of these patients (up to 140,000 per year) will develop LLL secondary to cancer treatment.11-22
Across multiple cancer diagnoses with distinct etiologies, those with LLL secondary to lymph node removal or damage progress over time to an alteration in the ability to walk at a functional pace, for functional distances, as well as changes in ability to lift heavy objects, or stand for long periods.6, 7, 9 In the absence of any empirically tested interventions to guide them, cancer survivors who experience LLL limit their mobility, sometimes retiring early, quitting work, and/or limiting their social lives. The financial burden can be tremendous, including the loss of work, lymphedema treatment (up to $2000 per month), and compression garments used to control the swelling (ranging from $24 to $574, with several purchased per year).6
Strength training might assist patients with LLL to improve function, which may also improve quality of life. However, resistance exercise has historically been contraindicated for individuals with or at risk for lymphedema. There have been multiple studies that have assessed the safety and efficacy of upper body exercise, including resistance training, in breast cancer survivors with and at risk for lymphedema.23, 24 The promising results from these studies have resulted in alterations in exercise guidelines for individuals with or at risk for lymphedema.25 However, this research has focused solely on the problem of arm lymphedema in breast cancer survivors who have axillary lymph nodes removed as part of treatment. There have been no such studies for patients with LLL secondary to cancer treatment. Clinical differences between upper and lower limb lymphedema after cancer treatment preclude the assumption that the success of strength training interventions for breast cancer survivors can be translated to those at risk for LLL without careful testing. For example, anatomical and hemodynamic differences between the arms and legs are substantive: the column that makes up the leg is longer and larger than the arm, thus the leg moves a greater amount of lymph fluid a longer distance 26. Differences in the ability to use the affected limb less, preferentially using the unaffected limb. It is possible to carry things with the unaffected arm; it is not possible to walk on just one leg. Further, bilateral lymphedema is more common in lower limb cases. Survivors of cancers that may lead to LLL are generally older, thus the baseline physiologic status of the participants cannot be assumed to be the same as for breast cancer survivors from our prior studies 27, 28. Obesity is reported to be a larger issue for clinical course of lower than upper limb lymphedema because of larger volume of fat in the legs versus the arms 29, 30. This makes an exercise intervention even more attractive for this understudied population. LLL may also be confused with or co-morbid with lower limb venous disease, which makes it more difficult to know exactly how to treat this more complicated disease 7. Those who develop LLL interact with this chronic condition differently than those who develop upper limb lymphedema secondary to breast cancer. Changes in hands are recognized before changes in feet, simply because of how much more heavily we count on our hands for functional tasks. Therefore, it is commonly clinically observed that individuals who develop LLL do not notice it or seek treatment until it has progressed in a manner that makes management more complicated. For these reasons, there is a need to assess the feasibility, safety, and efficacy of resistance training exercise in patients with LLL after cancer treatment.
To address this gap in the literature, we conducted a pilot study to assess the feasibility of recruiting and retaining patients with LLL into an exercise intervention study, to determine the feasibility and acceptability of the intervention, and to develop preliminary estimates regarding the safety and efficacy of this intervention with regard to physical function and quality of life. We hypothesized that progressive weight training would not exacerbate leg swelling or lymphedema symptoms, and that the intervention would lead to improvements in measures of physical function and quality of life.
METHODS
Study Design, Recruitment, and Eligibility
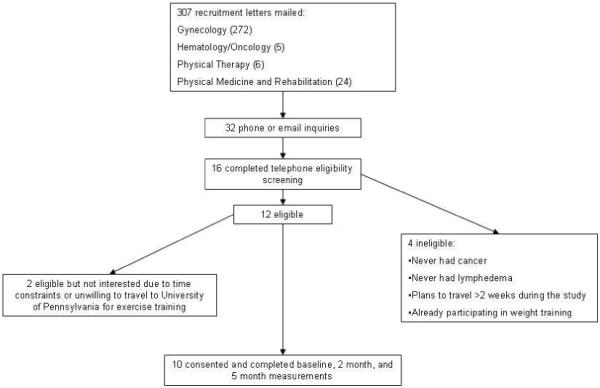
The protocol for this pre-post, non-controlled pilot and feasibility study was approved by the University of Pennsylvania institutional review board. All participants provided written informed consent and written permission from their physician for participation. The flow of participants through the study is illustrated in Figure 1. Recruitment letters were mailed between October 2007 and February 2008 to patients of the University of Pennsylvania known to have lower extremity lymphedema secondary to cancer. Eligible participants had completed cancer treatment at least one year prior to consent and had at least one lymph node surgically removed. Participants had to have lower limb lymphedema secondary to cancer treatment, defined as a ≥6% interlimb discrepancy by volume from perometry or by circumference at the point of greatest visible difference OR written confirmation of clinically diagnosed lymphedema. The threshold of ≥6% limb discrepancy was chosen to ensure the discrepancies were larger than the 5% limb discrepancy threshold for defining grade one lymphedema according to the Common Toxicity Criteria for Adverse Events version 3.0.31 The allowance of written confirmation of diagnosis allowed for the possibility of participants with bilateral lymphedema and/or well controlled lymphedema, and or lymphedema isolated to a small enough section of the lower extremities that it is clearly diagnosable but does not meet the above thresholds. The lymphedema had to be controlled, defined as having had none of the following in the three months prior to consent: 1) recorded change of leg girth of 15%, 2) a lymphedema related infection requiring antibiotics, 3) worsening of lymphedema that altered activities of daily living, or 4) therapist delivered decongestive therapy. Participants could not have any medical conditions that would prohibit participation, plans for reconstructive surgery or to be away for a week or more during the study, and could not have a body mass index ≥ 50 kg/m2). Survivors reporting strength training or more than 3 aerobic exercise sessions per week were also excluded. There was no lower age eligibility limit, the upper age limit was 90 years.
Figure 1. Flow of Participants.
Measurements
All of the following measurements were taken at baseline, 2, and 5 months by trained study personnel.
Lymphedema measurements
Leg volume was measured using an optoelectronic perometer, which uses infrared beams to calculate limb volume by measuring leg circumference every 0.5 cm (Model 350Sa). The perometer provides a measurement of limb volume that is more accurate than traditional circumferential measures.32, 33 One participant whose affected leg was too large for the perometer had leg-circumference measurements taken on both legs at 4 cm intervals. Leg volumes for this participant were calculated using a truncated cone formula.34 Because perometry measurements do not include the foot or ankle and are limited in length by the participant’s ability to abduct the leg not being measured, circumference measurements were taken on both legs at the great metatarsal phalangeal joints and the ankle using the figure-of-8 method.35 Thigh circumferences were also measured at the level of the groin. Participants were instructed to remove compression garments 20 minutes before measurements were taken. A survey validated for use in patients with arm lymphedema was adapted to measure self-report of LLL diagnosis, symptoms and treatment over the last 3 months.36 The lymphedema survey asked participants about 12 symptoms specific to LLL, including puffiness, decreased mobility, pain, and skin changes. An additional survey asked patients to self-report whether they experienced pain associated with lymphedema using a Visual Analogue Scale from 0 to 10.37-39
Anthropometry
Body weight was measured at baseline, 2, and 5 months using a digital scale and height was measured at baseline only, on a scale mounted stadiometer (Scale-tronix 5005 stand-on digital scaleb), calibrated weekly. Body fat (percent and total) and lean mass were measured by dual energy X-ray absorptiometryc.
Strength and physical function measures
Upper and lower body strength was assessed by one-repetition maximum tests, (the maximum amount of weight that can be lifted once) for the bench press and leg press. One repetition maximum tests are the standard by which increases in muscular strength are evaluated and are reported to be safe for most populations when properly supervised.40-42 Trained measurement staff verbally encouraged participants according to a standardized script.
Cardiorespiratory functional endurance was assessed using the 6-Minute Walk Test performed using American Thoracic Society Guidelines, including assessment of dyspnea at the end of the test.43 This test has been shown to be a valid and reliable measure of aerobic capacity in patients with heart failure, peripheral vascular disease and pulmonary disease.44, 45 Distance walked in the 6-Minute Walk Test has been shown to correlate with impairments in strength and power in the lower extremity.46 Time to walk 50 feet was assessed during the first 50 feet of the 6-Minute Walk Test. Dynamic balance was assessed as per the hierarchical approach used in the Short Physical Performance Battery.47 All participants were able to do the one leg balance test from this hierarchical approach. Range of motion measurements were taken with a goniometer. Ankle dorsiflexion and plantarflexion have been shown to be important indicators of functional mobility including stair climbing and walking.48
Quality of life was assessed using the Medical Outcomes Study 36-Item Short Form Health Survey.49-51 It has well-established normative values and has been shown to be both valid and reliable.52 Participants also completed a demographics survey. Medical record abstraction was performed to ensure accuracy of cancer diagnosis and treatment and to verify the number of lymph nodes removed.
Ensuring Participant Safety
Prior to starting the intervention, all participants visited with a certified lymphedema therapist for a standardized clinical lymphedema assessment and to be measured for custom fitted Jobst Elvarex compression garmentsd. The intervention did not start until custom fitted garments were received by participants. Participants also underwent a baseline Doppler ultrasound of the lower extremities to rule out deep venous thrombosis prior to study participation (none was detected). Perometer and circumference measurements were performed weekly during the 8 week supervised intervention. Participants were also asked at each session about changes in symptoms. Changes in symptoms and/or swelling (e.g., a >5% increase in affected leg volume or a greater than 2 cm increase in circumferential measures) resulted in a referral to a lymphedema therapist for evaluation of a possible flare-up.
Weight Training Intervention
For the first 8 weeks of weight training, participants met twice weekly at a physical therapy clinic associated with the University of Pennsylvania. All 16 sessions were led by a certified fitness professional who had been through extensive training with the principal investigator. Participants exercised in groups of 5 to ensure adequate instruction and monitoring. A physical therapist and/or the principal investigator were also on site and available to answer questions during most sessions. Participants received instruction in performance of warm-up, stretching, diaphragmatic breathing, weight training, and additional stretching exercises. Twelve weight-training exercises were performed using variable resistance machines, free weights, and ankle weights. The exercises included seated row, chest press, lateral raises, bicep curls, tricep pushdowns, leg press, leg extension, leg curl, hip flexion, leg abduction, prone straight leg lifts, and calf raises. One to three exercises were introduced per session. If pain, injury, or altered range of motion due to limb swelling prevented performing a specific exercise, modifications were made at the discretion of the fitness professional.
Participants started at the lowest possible resistance. Resistance was increased weekly at the lowest possible increment. Participants built up from 2 sets to 3 sets of 10 repetitions per exercise over the first 4-5 weeks of the intervention. Each exercise session lasted approximately 90 minutes. Participants kept exercise logs that were reviewed by the fitness trainers. After 8 weeks, the participants were provided with a free 3 month membership to a YMCA fitness facility within 20 minutes of the participants’ home or workplace. A certified fitness professional visited the YMCA facility with the participant for a single session to translate the intervention to the available equipment and to ensure that the participant knew how to use the equipment at the facility. Thereafter, participants kept their own logs. One call was made 6 weeks into the 12 week unsupervised portion of the intervention to ask about exercise adherence and lymphedema symptoms. Participants were instructed not to make any purposeful changes in diet or exercise habits outside of the intervention.
Statistical Analysis
Continuous variables were described using means, standard deviations, quantiles, and range; categorical variables were described with proportions. All statistical analyses were performed using Microsoft Excel Version 11.5.1. Comparisons of pre- versus post-intervention values for continuous variables were made using paired, two-sided Student’s T-tests. A p-value of 0.05 was considered statistically significant. It is acknowledged that the multiple comparisons in this analysis may inflate the type 1 error rate in this pilot study.
RESULTS
Table 1 describes baseline characteristics of the 10 participants, all of whom completed the study. The majority of the participants were Caucasian (N=9) and female (N=7) with a mean age of 60.1 years (range = 50 to 71). The majority of the participants had completed a college education.
Table 1. Baseline characteristics of Participants [mean (SD) or n (%)] (N=10).
| Variable | |
|---|---|
| Age (y) | 60.1 (7.72) |
| Ethnicity | |
| Caucasian | 9 (90) |
| African-American | 1 (10) |
| Sex | |
| Male | 3 (30) |
| Female | 7 (70) |
| Type of Cancer | |
| Bladder | 1 (10) |
| Cervical | 3 (30) |
| Endometrial | 2 (20) |
| Melanoma | 3 (30) |
| Uterine | 1 (10) |
| Time since last Cancer Treatment (y) | 13.05 (13.66) |
| Number of lymph nodes removed (N=8) | 20 (4.12) |
| Received radiation treatment | 4 (40) |
| Ever received intensive lymphedema therapy | |
| Yes | 9 (90) |
| No | 1 (10) |
| Currently seeing a lymphedema therapist | |
| Yes | 6 (60) |
| No | 4 (40) |
| Education | |
| High School Graduate | 1 (10) |
| Some College or Vocational Degree | 2 (20) |
| College degree | 3 (30) |
| Graduate or professional degree | 4 (40) |
| Work Status | |
| Work full-time | 2 (20) |
| Work part-time | 3 (30) |
| Retired | 4 (40) |
| Disability | 1 (10) |
| Persons living in home | 2.3 (0.95) |
| Currently live with children | 3(30) |
| Marital Status | |
| Never Married | 1 (10) |
| Married | 5 (50) |
| Divorced/Separated | 4 (40) |
| Mode of Transportation | |
| Personal car | 8 (80) |
| Public transit | 2 (20) |
All but one participant attended at least 81%, or 13 out of 16 supervised exercise sessions; average attendance during the supervised intervention was 91% with a range from 69-100%. Exercise adherence during the unsupervised 3 months averaged 77%, with a range of 0-100%. One participant exercised twice weekly for six weeks and then stopped. Two developed cellulitic infections during the supervised portion of the intervention and never returned to exercise. One performed three unsupervised sessions prior to being diagnosed with recurrent melanoma and never returned to exercise. Another participant found her local YMCA to be inconveniently located and never exercised after the supervised intervention. The other five had 100% adherence during the three month unsupervised intervention. No musculoskeletal injuries were incurred as a result of the intervention.
Table 2 describes the changes in body composition, strength, and function. Statistically significant improvements noted after two months of progressive strength training for bench press, time to walk 50 feet, dyspnea after the 6-minute walk test, time balancing on one leg, and dorsiflexion of the ankle on the more affected lower limb. At five months, statistically significant improvements were noted for bench press, time to walk 50 feet, and distance walked in 6 minutes.
Table 2.
Anthropometry, Strength, and Physical Function (N=10) (Mean ± SD)
| Baseline | 2 months | p-value* (2 months vs. baseline) |
5 months | p-value* (5 months vs. baseline) |
|
|---|---|---|---|---|---|
| Anthropometry | |||||
| Weight (kg) | 89.3 ± 20.1 | 89.5 ± 29.4 |
0.86 | 89.4 ± 20.6 | 0.98 |
| BMI (kg/m2) | 32.2 ± 6.4 | 32.2 ± 6.3 | 0.95 | 32.2 ± 6.4 | 0.96 |
| Body fat % | 35.3 ± 6.9 | 36.2 ± 6.8 | 0.05 | 35.5 ± 7.8 | 0.68 |
| Lean mass (kg) | 57.0 ± 16.0 | 55.8 ± 15.8 |
0.10 | 56.2 ± 15.7 | 0.26 |
| Strength | |||||
| Bench press 1 rep max (lbs) |
60 ± 42 | 72 ± 41 | 0.04 | 88 ± 45 | <0.01 |
| Leg press 1 rep max (lbs) | 211 ± 115 | 238 ± 110 | 0.10 | 271 ± 89 | 0.07 |
| Physical function | |||||
| 6 min walk test (meters) | 475 ± 63 | 491 ± 88 | 0.23 | 504 ± 66 | 0.01 |
| 50-foot walk time (secs) | 11.9 ± 1.9 | 11.0 ± 1.9 | 0.04 | 10.5 ± 1.8 | <0.01 |
| Dorsiflexion on more affected ankle (degrees) |
5.5 ± 5.1 | 12.1 ± 5.1 | 0.02 | 12.4 ± 17.8 | 0.28 |
| Plantarflexion on more affected ankle (degrees) |
37.5 ± 14.6 | 33.5 ± 7.7 | 0.42 | 60.8 ± 46.4 | 0.14 |
| Single leg stand (secs) | 15.4 ± 11.6 | 19.2 ± 10.8 |
0.02 | 15.4 ± 11.8 | 0.99 |
Abbreviation: BMI, body mass index
from t-tests were paired and two sided. A p-value of 0.05 was considered statistically significant.
No significant changes were noted for the overall scores or sub-scales of the Medical Outcomes Study 36-Item Short Form Health Survey (results not shown). Self-reported pain averaged 0.75 on a visual analog scale that ranged from 0 to 10 at all three measurement time points. Participants endorsed 5.8, 5.0, and 5.2 symptoms at baseline, 2, and 5 months, Average lymphedema symptom severity significantly improved from 1.1 to 0.8 over the first two months of the intervention (p = 0.02). This decrease in symptom severity was largely maintained at 5 months.
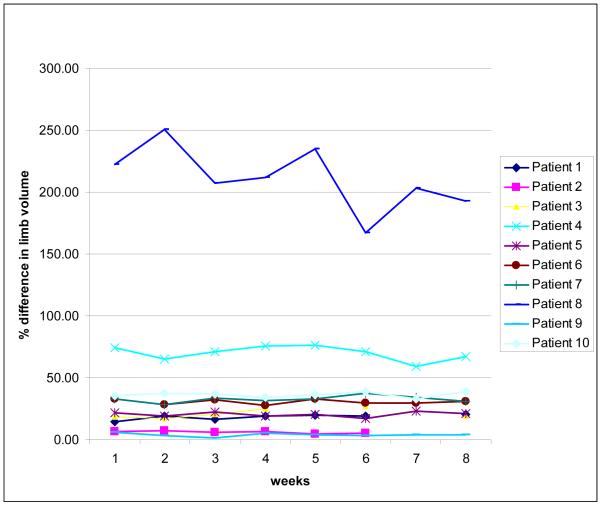
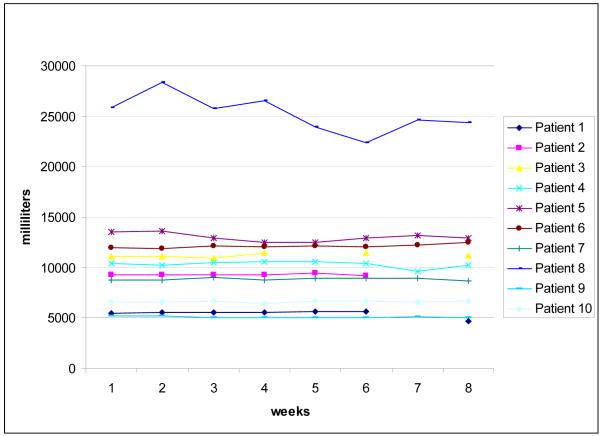
Perometer and Circumference outcome data are presented in Table 3. Figures 2 and 3 show the interlimb percent difference and affected limb volumes (respectively), as measured just prior to strength training sessions each week during the 2 month supervised intervention (baseline and two month outcome assessments are not included in figures, refer to table 3 for these values).
Table 3.
Lymphedema Outcomes: perometer (volume) and circumferences (Mean ± SD) (N=10)
| Baseline | 2 months | p-value* (2 months vs. baseline) |
5 months | p-value* (2 months vs. baseline) |
|
|---|---|---|---|---|---|
| Unaffected leg volume (ml) |
7944 ± 2226 |
8007 ± 2325 | 0.41 | 7867 ± 2390 | 0.66 |
| Affected leg volume (ml) |
11383 ± 5838 |
11358 ± 5413 | 0.90 | 11356 ± 5794 |
0.89 |
| Interlimb % difference |
44.4 ± 64.9 | 43.4 ± 55.0 | 0.78 | 45.3 ± 66.0 | 0.70 |
| Unaffected Limb: | |||||
| Great metatarsal phalangal joint (cm) |
22.8 ± 3.0 | 23.4 ± 2.9 | 0.01 | 22.9 ± 2.9 | 0.56 |
| Figure of eight (foot) (cm) |
53.9 ± 6.8 | 53.7 ± 7.2 | 0.53 | 53.7 ± 6.6 | 0.48 |
| Thigh at groin level (cm) |
65.2 ± 6.4 | 66.7 ± 7.5 | 0.21 | 64.7 ± 7.7 | 0.59 |
| Affected Limb: | |||||
| Great metatarsal phalangal joint (cm) |
23.7 ± 3.1 | 23.7 ± 2.9 | 0.47 | 23.5 ± 3.2 | 0.40 |
| Figure of eight (foot) (cm) |
56.5 ± 8.2 | 56.2 ± 7.6 | 0.47 | 23.5 ± 3.2 | 0.40 |
| Thigh at level of groin (cm) |
69.2 ± 8.5 | 67.8 ± 10.8 | 0.19 | 72.5 ± 10.5 | 0.91 |
from 2-sided, paired t-tests. A p-value of 0.05 is considered statistically significant.
Figure 2. Interlimb % differences.
Figure 3. Total volume, more affected leg.
During the 2 month supervised intervention, two participants were diagnosed with cellulitis, placed on oral antibiotics, and underwent complete decongestive therapy. These two participants discontinued the strength training intervention at the end of the two month supervised intervention. For both of these individuals, limb volume differences (overall and calf) were back to within 0.5 percentage points of baseline values at 5 months.
DISCUSSION
This uncontrolled pilot study represents the first step in the development of a research program on exercise to improve functional status among cancer survivors with LLL. We have established the feasibility to recruit and retain patients with LLL into an exercise intervention study and that the intervention is behaviorally feasible and acceptable to participants. The primary finding is that there was no clinically meaningful worsening in total leg volume in the ten participants, combined with clinically meaningful improvements in several measures of functional status. The 25% improvement in balance and the 120% improvement in dorsiflexion of the affected ankle at the end of the supervised intervention are particularly notable. Given that the clinical course of LLL often includes loss of physical function with aging and worsening of the disease, this finding, along with the relative safety of the intervention, suggest the need for further research to develop exercise programming for this often ignored population of cancer survivors. It is notable that the distance walked in 6-minutes was 30% lower among the cancer survivors with LLL in this pilot study than values reported for healthy adults of similar age.53 Clearly, there is value to pursuing methods to improve physical function in this population.
There were two cases of cellulitus diagnosed among the ten participants. This was unexpected and suggests that exercise prescription for this population should proceed with caution. Both infections were controlled with oral antibiotics and complete decongestive therapy, there were no hospitalizations or debridement required. That said, these relatively minor adverse outcomes were unexpected. The a priori assumption was that the risk associated with weight training would be increased swelling, not inflammation and infection. While the link between cellulitis and lymphedema has been extensively studied54-57, there has been no examination of a link between cellulitis and exercise. All participants in the present study wore compression garments and shoes during exercise, which prevented direct lower extremity contact with exercise equipment. Any shared measurement items that came into contact with skin were cleaned with an alcohol solution between participants. Another possible source of infection could be an unclean compression garment, although there was no examination of risk factors for cellulitis as it relates to hygiene. One risk factor for dermatomycosis is hydrosis.58 Participants in this study may have been predisposed to hydrosis as they were exerting themselves during the intervention. One of the participants diagnosed with cellulitis had a Fludeoxyglucose Positron Emission Tomography / Computed Tomography scan before enrolling in the study that showed hypermetabolic abdominal adenopathy that was increased from a previous scan. Increased activity in the lymph nodes could have caused further lymphatic dysfunction, increasing the risk for cellulitis. The other participant gardens as a hobby and had a bite mark on the affected leg at the time of diagnosis. A known site for pathogen entry is a risk factor for cellulitis with an odds ratio of 7.02.56 Neither participant any increase in interlimb discrepancy or increases in total limb volume on the affected side at the time the cellulitic infections were discovered. Both had 5% increases in affected limb volume for subset of the limb up to the knee at the time the cellulitic infections were discovered. Both of these increases had resolved by the final measurement time point.
Study Limitations
All participants wore the same type of compression garments during exercise. However, patient log reports of self-care habits differed when not exercising. Some participants wrapped, some only used compression on specific areas of the lower extremity and some chose not to use compression at all outside of the intervention. In addition, there were changes in 3 factors during the intervention: exercise, season and garments. This makes it impossible to know whether the negative clinical outcomes were due to the exercise. A large randomized-controlled trial is needed to separate out these effects.
CONCLUSIONS
It was feasible to recruit and retain patients with LLL secondary to cancer into a 5 month weight training intervention. Adherence was excellent during the supervised intervention, more varied during unsupervised intervention. Initial indications of safety with regard to swelling and efficacy with regard to improving physical function and strength are balanced with the unexpected cellulitic infections, suggesting that a randomized controlled trial of this exercise intervention should be pursued to ensure it is safe. Given recent findings of the safety of weight training for women with arm lymphedema secondary to breast cancer23, 24, it is important that clinicians know that these safety findings for arm lymphedema are not automatically transferable to patients with LLL.
Acknowledgments
Supported by the FOCUS Medical Student Fellowship in Women’s Health (a Bertha Dagan Berman Award); National Center for Research Resources (grant no. UL1RR024134); and BSN Medical supplied the custom fitted compression garments worn by participants free of charge.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
List of Abbreviations
- LLL
lower- limb lymphedema
- YMCA
Young Men’s Christian Association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. National Academies Press; Washington, D.C.: 2006. [Google Scholar]
- 2.Brorson H, Svensson H. Skin blood flow of the lymphedematous arm before and after liposuction. Lymphology. 1997;30(4):165–72. [PubMed] [Google Scholar]
- 3.Stanton AW, Levick JR, Mortimer PS. Cutaneous vascular control in the arms of women with postmastectomy oedema. Exp Physiol. 1996;81(3):447–64. doi: 10.1113/expphysiol.1996.sp003948. [DOI] [PubMed] [Google Scholar]
- 4.Daroczy J. Pathology of lymphedema. Clin Dermatol. 1995;13(5):433–44. doi: 10.1016/0738-081x(95)00086-u. [DOI] [PubMed] [Google Scholar]
- 5.Leu AJ, Leu HJ, Franzeck UK, Bollinger A. Microvascular changes in chronic venous insufficiency--a review. Cardiovasc Surg. 1995;3(3):237–45. doi: 10.1016/0967-2109(95)93871-l. [DOI] [PubMed] [Google Scholar]
- 6.Ryan M, Stainton MC, Jaconelli C, Watts S, MacKenzie P, Mansberg T. The experience of lower limb lymphedema for women after treatment for gynecologic cancer. Oncol Nurs Forum. 2003;30(3):417–23. doi: 10.1188/03.ONF.417-423. [DOI] [PubMed] [Google Scholar]
- 7.Buren J, Linton C. The other lymphedema: lower extremity lymphedema creasted a host of physical and psychosocial issues for people with this condition. Advance for Directors in Rehabilitation. 2000;9(10):52–5. [Google Scholar]
- 8.Frid M, Strang P, Friedrichsen MJ, Johansson K. Lower limb lymphedema: experiences and perceptions of cancer patients in the late palliative stage. J Palliat Care. 2006;22(1):5–11. [PubMed] [Google Scholar]
- 9.Borbasi S, Emden C, Hawes C, Addicoat R. Getting it together: men’s and their carers’ experience of lymphoedema. Australian Journal of Cancer Nursing. 2004;5(2):23–33. [Google Scholar]
- 10.American Cancer Society . Cancer Facts and Figures. Atlanta: 2008. [Google Scholar]
- 11.van Akkooi AC, Bouwhuis MG, van Geel AN, Hoedemaker R, Verhoef C, Grunhagen DJ, et al. Morbidity and prognosis after therapeutic lymph node dissections for malignant melanoma. Eur J Surg Oncol. 2007;33(1):102–8. doi: 10.1016/j.ejso.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Wrightson WR, Wong SL, Edwards MJ, Chao C, Reintgen DS, Ross MI, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10(6):676–80. doi: 10.1245/aso.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Karakousis CP, Driscoll DL. Groin dissection in malignant melanoma. Br J Surg. 1994;81(12):1771–4. doi: 10.1002/bjs.1800811221. [DOI] [PubMed] [Google Scholar]
- 14.Okeke AA, Bates DO, Gillatt DA. Lymphoedema in urological cancer. Eur Urol. 2004;45(1):18–25. doi: 10.1016/j.eururo.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Werngren-Elgstrom M, Lidman D. Lymphoedema of the lower extremities after surgery and radiotherapy for cancer of the cervix. Scand J Plast Reconstr Surg Hand Surg. 1994;28(4):289–93. doi: 10.3109/02844319409022014. [DOI] [PubMed] [Google Scholar]
- 16.Haberthur F, Almendral AC, Ritter B. Therapy of vulvar carcinoma. Eur J Gynaecol Oncol. 1993;14(3):218–27. [PubMed] [Google Scholar]
- 17.Karakousis CP, Driscoll DL, Rose B, Walsh DL. Groin dissection in malignant melanoma. Ann Surg Oncol. 1994;1(4):271–7. doi: 10.1007/BF02303564. [DOI] [PubMed] [Google Scholar]
- 18.Karakousis CP, Heiser MA, Moore RH. Lymphedema after groin dissection. Am J Surg. 1983;145(2):205–8. doi: 10.1016/0002-9610(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 19.Karakousis CP. Surgical procedures and lymphedema of the upper and lower extremity. J Surg Oncol. 2006;93(2):87–91. doi: 10.1002/jso.20349. [DOI] [PubMed] [Google Scholar]
- 20.Ingvar C, Erichsen C, Jonsson PE. Morbidity following prophylactic and therapeutic lymph node dissection for melanoma--a comparison. Tumori. 1984;70(6):529–33. doi: 10.1177/030089168407000610. [DOI] [PubMed] [Google Scholar]
- 21.Urist MM, Maddox WA, Kennedy JE, Balch CM. Patient risk factors and surgical morbidity after regional lymphadenectomy in 204 melanoma patients. Cancer. 1983;51(11):2152–6. doi: 10.1002/1097-0142(19830601)51:11<2152::aid-cncr2820511134>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.de Vries M, Vonkeman WG, van Ginkel RJ, Hoekstra HJ. Morbidity after inguinal sentinel lymph node biopsy and completion lymph node dissection in patients with cutaneous melanoma. Eur J Surg Oncol. 2006;32(7):785–9. doi: 10.1016/j.ejso.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Cheema B, Gaul CA, Lane K, Fiatarone Singh MA. Progressive resistance training in breast cancer: a systematic review of clinical trials. Breast Cancer Res Treat. 2008;109(1):9–26. doi: 10.1007/s10549-007-9638-0. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz K, Ahmed RL, Troxel A, Cheville A, Smith R, Grant LL, Bryan CJ, Williams-Smith CT, Greene QP. Weight lifting in women with breast cancer- related lymphedema. New England Journal of Medicine. 2009;361:664–73. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 25.National_Lymphedema_Network_Medical_Advisory_Committee . Topic: Exercise for Lymphedema Patients. Position Statement of the National Lymphedema Network. National Lymphedema Network Medical Advisory Committee; 2008. [Google Scholar]
- 26.Guyton A. Unit IV: The Circulation. Textboook of Medical Physiology. 8th ed. W.B. Saunders Co; Philadelphia: 1991. pp. 149–271. [Google Scholar]
- 27.Barton DP. The prevention and management of treatment related morbidity in vulval cancer. Best Pract Res Clin Obstet Gynaecol. 2003;17(4):683–701. doi: 10.1016/s1521-6934(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 28.Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER Cancer Statistics Review. 2003 [cited 2003 December 22]. Available from: URL: http://seer.cancer.gov/csr/1975_2000.
- 29.Rouzier R, Haddad B, Dubernard G, Dubois P, Paniel BJ. Inguinofemoral dissection for carcinoma of the vulva: effect of modifications of extent and technique on morbidity and survival. J Am Coll Surg. 2003;196(3):442–50. doi: 10.1016/S1072-7515(02)01895-1. [DOI] [PubMed] [Google Scholar]
- 30.Hinrichs CS, Gibbs JF, Driscoll D, Kepner JL, Wilkinson NW, Edge SB, et al. The effectiveness of complete decongestive physiotherapy for the treatment of lymphedema following groin dissection for melanoma. J Surg Oncol. 2004;85(4):187–92. doi: 10.1002/jso.20020. [DOI] [PubMed] [Google Scholar]
- 31.Cheville AL, McGarvey CL, Petrek JA, Russo SA, Thiadens SR, Taylor ME. The grading of lymphedema in oncology clinical trials. Semin Radiat Oncol. 2003;13(3):214–25. doi: 10.1016/S1053-4296(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 32.Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR. Validation of an optoelectronic limb volumeter (Perometer) Lymphology. 1997;30(2):77–97. [PubMed] [Google Scholar]
- 33.Tierney S, Aslam M, Rennie M, Grace P. Infrared optoelectronic volumetry, the ideal way to measure limb volume. European Journal Vascular Endovascular Surgery. 1997;14(5):415–6. doi: 10.1016/s1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 34.Casley-Smith JR. Measuring and representing peripheral oedema and its alterations. Lymphology. 1994;27(2):56–70. [PubMed] [Google Scholar]
- 35.Mawdsley RH, Hoy DK, Erwin PM. Criterion-related validity of the figure-of-eight method of measuring ankle edema. J Orthop Sports Phys Ther. 2000;30(3):149–53. doi: 10.2519/jospt.2000.30.3.149. [DOI] [PubMed] [Google Scholar]
- 36.Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther. 2001;81(6):1192–205. [PubMed] [Google Scholar]
- 37.Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue for evaluating pain. Anaesthesia. 1976;31(9):1191–8. doi: 10.1111/j.1365-2044.1976.tb11971.x. [DOI] [PubMed] [Google Scholar]
- 38.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37(4):378–81. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ration scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 40.Fleck S, Kraemer W. Designing resistance training programs. 2nd ed. Human Kinetics; Champaign, IL: 1997. [Google Scholar]
- 41.Shaw CE, McCully KK, Posner JD. Injuries during the one repetition maximum assessment in the elderly. J Cardiopulm Rehabil. 1995;15(4):283–7. doi: 10.1097/00008483-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Barnard KL, Adams KJ, Swank AM, Mann E, Denny DM. Injuries and muscle soreness during the one repetition maximum assessment in a cardiac rehabilitation population. J Cardiopulm Rehabil. 1999;19(1):52–8. doi: 10.1097/00008483-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Society AT. ATS Statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 44.Ingle L, Shelton R, Rigby A, Nabb S, Clark A, Cleland J. The reproducibility and sensitivity of the 6-min walk test in elderly patients with chronic heart failure. European Heart Journal. 2005;26(17):1742–51. doi: 10.1093/eurheartj/ehi259. [DOI] [PubMed] [Google Scholar]
- 45.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–70. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 46.Bean J, Kiely D, Leveille S, Herman S, Huynh C, Fielding R, et al. The 6-minute walk test in mobility-limited elders: What is being measured? J Gerontol Med Sci. 2002;57A(11):M751–M6. doi: 10.1093/gerona/57.11.m751. [DOI] [PubMed] [Google Scholar]
- 47.Guralnik J, Simonsick E, Ferrucci L, Glynn R, Berkman L, Blazer D, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 48.McGibbon CA, Krebs DE. Discriminating age and disability effects in locomotion: neuromuscular adaptations in musculoskeletal pathology. J Appl Physiol. 2004;96(1):149–60. doi: 10.1152/japplphysiol.00422.2003. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura K, Utsunomiya N, Ichioka K, Matsui Y, Terai A, Arai Y. Impact of superficial bladder cancer and transurethral resection on general health-related quality of life: an SF-36 survey. Urology. 2005;65(2):290–4. doi: 10.1016/j.urology.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 50.Ashing-Giwa KT, Kim J, Tejero JS. Measuring quality of life among cervical cancer survivors: preliminary assessment of instrumentation validity in a cross-cultural study. Qual Life Res. 2008;17(1):147–57. doi: 10.1007/s11136-007-9276-3. [DOI] [PubMed] [Google Scholar]
- 51.Cashin RP, Lui P, Machado M, Hemels ME, Corey-Lisle PK, Einarson TR. Advanced cutaneous malignant melanoma: a systematic review of economic and quality-of-life studies. Value Health. 2008;11(2):259–71. doi: 10.1111/j.1524-4733.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- 52.Ware JE, Snow KK, Kosinski MK. SF-36 Health Survey Manual and Interpretation Guide. Nimrod Press; Boston, MA: 1993. [Google Scholar]
- 53.Gibbons W, Fruchter N, Sloan S, Levy R. Reference values for a multiple repetition 6-minute walk test in health adults older than 20 years. J Cardiopulmonary Rehabil. 2001;21(2):87–93. doi: 10.1097/00008483-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol. 2008;158(6):1175–6. doi: 10.1111/j.1365-2133.2008.08590.x. [DOI] [PubMed] [Google Scholar]
- 55.Lewis SD, Peter GS, Gomez-Marin O, Bisno AL. Risk factors for recurrent lower extremity cellulitis in a U.S. Veterans Medical Center population. Am J Med Sci. 2006;332(6):304–7. doi: 10.1097/00000441-200612000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Bjornsdottir S, Gottfredsson M, Thorisdottir AS, Gunnarsson GB, Rikardsdottir H, Kristjansson M, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis. 2005;41(10):1416–22. doi: 10.1086/497127. [DOI] [PubMed] [Google Scholar]
- 57.Dupuy A, Benchikhi H, Roujeau JC, Bernard P, Vaillant L, Chosidow O, et al. Risk factors for erysipelas of the leg (cellulitis): case-control study. Bmj. 1999;318(7198):1591–4. doi: 10.1136/bmj.318.7198.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pickup TL, Adams BB. Prevalence of tinea pedis in professional and college soccer players versus non-athletes. Clin J Sport Med. 2007;17(1):52–4. doi: 10.1097/JSM.0b013e31802ed88e. [DOI] [PubMed] [Google Scholar]