Abstract
Background
This study estimates the maximum tolerated dose (MTD) and describes the toxicities of oxaliplatin combined with irinotecan in children with refractory solid tumors.
Methods
Oxaliplatin was administered on days 1 and 8 in combination with irinotecan on days 1–5 and 8–12 of a 21-day cycle. An oral cephalosporin was administered daily to ameliorate irinotecan-associated diarrhea. Pharmacokinetic studies of oxaliplatin and UGT1A1 genotyping were performed.
Results
Thirteen patients were enrolled. Dose-limiting diarrhea (n = 3), serum lipase elevation (n = 3), serum amylase elevation (n = 2), colitis, abdominal pain, and headache (n = 1 each) occurred at the first dose level (60 mg/m2/dose oxaliplatin; 20 mg/m2/dose irinotecan). Only 1 of 7 patients treated with reduced doses of both agents (40 mg/m2/dose oxaliplatin; 15 mg/m2/dose irinotecan) experienced a DLT, diarrhea. When the oxaliplatin dose was re-escalated (60 mg/m2) with irinotecan 15 mg/m2, 2 of 3 patients had DLT (1 diarrhea, 1 hypokalemia). Myelosuppression was minimal. One patient had a complete response and another had stable disease for 6 cycles of therapy. The median oxaliplatin area under the concentration versus time curve (AUC0→∞) was 5.9 µ g·h/mL (range 1.8–7.6 µg·h/mL). The frequency of 6/6, 6/7, and 7/7 UGT1A1 promoter genotypes were 5/10, 4/10, and 1/10, respectively.
Conclusion
The oxaliplatin MTD was 40 mg/m2/dose on days 1 and 8 in combination with irinotecan 15 mg/m2/dose (days 1–5 and 8–12). There was some evidence of anti-tumor activity; however, severe toxicity, expected (diarrhea) and unexpected (elevation in pancreatic enzymes), was observed.
Keywords: child, adolescent, clinical trial, phase I, oxaliplatin, irinotecan
Introduction
Based on observations of additive or synergistic anti-tumor activity in preclinical models,1–3 the combination of a platinating agent with a topoisomerase I poison has been of interest in treating pediatric tumors.4–6 A limited number of responses to the combination of cisplatin6 or carboplatin5 plus topotecan have been observed in children with rhabdomyosarcoma, Ewing sarcoma, hepatoblastoma, and ependymoma. Unfortunately, myelotoxicity limited further development of these platinum analogs in combination with topoisomerase I inhibitors.
Oxaliplatin, trans-l-1,2-diaminocyclohexane (DACH) oxalatoplatinum, is a novel platinum agent more potent than cisplatin in vitro7–11 that has demonstrated efficacy in preclinical and clinical studies against a spectrum of tumors, including cisplatin-resistant ones.8–14 DNA adducts formed by oxaliplatin are bulkier and therefore more effectively inhibit DNA synthesis than those formed by cisplatin and carboplatin.15,16 Cisplatin-resistant cell lines may be sensitive to oxaliplatin because loss of mismatch repair contributes to cisplatin but not oxaliplatin resistance and replicative bypass of DNA adducts contributes more significantly to cisplatin than to oxaliplatin resistance.17
Irinotecan, a potent inhibitor of topoisomerase I, has antitumor activity in relapsed18–20 and newly diagnosed21 rhabdomyosarcoma, neuroblastoma,20,22,23 pediatric brain tumors,24,25 non-Hodgkin lymphoma,18 and hepatoblastoma.22 In preclinical xenograft models of various childhood tumors, a protracted schedule of irinotecan administration has been associated with better disease responses.20,26,27 A variety of schedules have been used in phase I studies of irinotecan in children. A protracted dosing schedule of intravenous irinotecan administered to children with refractory solid tumors daily for 5 days of 2 consecutive weeks every 3 weeks [(qd×5)×2] produced one of the better reported objective response rates.20 The predominant reported toxicity associated with this protracted schedule of irinotecan has been diarrhea with minimal dose-limiting myelosuppression.18,20 Irinotecan has been administered to children with rhabdomyosarcoma in several studies. At a dose of 20 mg/m2/day administered (qd×5)×2, 8 of 19 patients (42%) with newly diagnosed rhabdomyosarcoma had a partial response,21 whereas 3 of 4 patients (75%) with recurrent rhabdomyosarcoma demonstrated a complete or partial response.18 In contrast, in patients with recurrent rhabdomyosarcoma, only 4 of 35 (11%) had an objective response to a 600 mg/m2/dose of irinotecan administered every 3 weeks19 and 1 of 18 (5.6%) had an objective response to 50 mg/m2/day for 5 days.28 These clinical results supported the preclinical studies that indicate that irinotecan may be more effective when administered on a protracted schedule in childhood malignancies.
The combination of oxaliplatin and irinotecan has additive or synergistic effects in colon carcinoma cell lines and xenografts.1,29 In adult colorectal carcinoma trials that employ weekly to once every 2–3-week dosing schedules, the combination has been tolerable and effective.30–34 Pharmacokinetic studies from two adult trials of the combination showed no significant alterations in the pharmacokinetics of either drug.32,35 The major toxicities include diarrhea, myelosuppression, and peripheral sensory neuropathy.
Herein we report the results of a pediatric phase 1 trial of the combination of irinotecan and oxaliplatin. The irinotecan schedule of (qd×5)×2 was selected due to its promising activity noted in preclinical and early clinical trials. To maximize simultaneous drug exposure, thereby potentially increasing synergy, and because weekly oxaliplatin was tolerable in pediatric patients,36 oxaliplatin was administered at the start of each week of irinotecan. Since the pharmacokinetics of irinotecan are well described in the pediatric population20,23,28,37–41 and there is no evidence of a pharmacokinetic interaction when oxaliplatin is administered with irinotecan,32,33,42,43 only oxaliplatin pharmacokinetic studies were obtained during this trial. Because the UGT1A1*28 genotype is associated with increased irinotecan-associated toxicity in adults receiving irinotecan every 3 weeks,44 UGT1A1 promoter genotyping was performed.
Materials and Methods
Patient eligibility
Patients aged 1 to 22 years with a histologically verified solid tumor refractory to conventional therapy, a weight >10 kg, and a Karnofsky or Lansky score ≥50% were eligible. Patients must have recovered from the acute toxic effects of prior therapy, with no evidence of active graft versus host disease, and not have received (1) myelosuppressive therapy within 3 weeks (nitrosourea within 6 weeks); (2) hematopoietic growth factors or biologic (antineoplastic) agents within 1 week; (3) small-port palliative radiation therapy within 2 weeks; (4) total body, craniospinal, whole spinal, whole lung/abdomen, or >50% pelvic radiation within 6 months; (5) other substantial bone marrow radiation within 6 weeks; and (6) stem cell transplantation within 3 months. No previous oxaliplatin exposure was allowed. Organ function requirements included (1) bone marrow [peripheral ANC ≥1000/µl, platelet count ≥100,000/µl (transfusion independent), and hemoglobin ≥8 g/dL]; (2) renal [normal serum creatinine for age or a glomerular filtration rate ≥70 mL/min/1.73m2, and? ≤grade 1 serum electrolyte abnormalities (supplementation allowed)]; (3) liver [≤grade 1 hyperbilirubinemia, ≤ grade 2 hypoalbuminemia, and ≤grade 2 elevation in ALT (SGPT)]; (4) cardiac (no arrhythmia on ECG); and (5) pulmonary (no dyspnea at rest, no exercise intolerance, no radiographic evidence of pulmonary fibrosis, and pulse oximetry >94% on room air if history of pulmonary abnormalities). Pregnant or breastfeeding patients were excluded. Patients of reproductive age had to agree to use an effective contraceptive method. Patients receiving other investigational or anticancer agents or drugs that interact with CYP3A (phenytoin, carbamazepine, oxcarbazepine, barbiturates, rifampicin, phenobarbital, azole antifungal agents, aprepitant, and St. John’s wort) were excluded. Patients on corticosteroids had to receive a stable or decreasing dose for ≥ 7 days prior to enrollment. Other exclusions included (1) uncontrolled infection, (2) inability to comply with protocol monitoring, (3) life-threatening allergy to protocol-required agents, (4) >grade 1 peripheral neuropathy, and (5) inability to tolerate enteral medications. Following the observation of dose limiting elevations in serum lipase and amylase in the first patient cohort, the study was amended to exclude patients with > grade 1 elevation in serum amylase or lipase.
Informed consent was obtained from patients, parents, or legal guardians, and assent as appropriate. The protocol was approved by institutional review boards of participating institutions.
Drug administration and study design
Oxaliplatin (Eloxatin™, Sanofi-aventis, Bridgewater, NJ) was supplied by the Cancer Therapy Evaluation Program (CTEP), National Cancer Institute (NCI), Bethesda, MD. The drug was reconstituted in water with 5% dextrose and infused intravenously over 2 h after administering an antiemetic. The infusion duration was increased to 6 h in patients experiencing pharyngolaryngeal dysesthesia.
Irinotecan (Camptosar™, Pfizer, New York, NY) was obtained from commercial suppliers, diluted in water with 5% dextrose, and infused intravenously over 1 h. Oral cefixime [8 mg/kg/d (maximum dose 400 mg)] or cefpodoxime [10 mg/kg/d (maximum dose 400 mg/d) divided BID] was administered for 21 days starting on day 1 of each course to decrease irinotecan-associated diarrhea.45 Guidelines were provided for treating acute irinotecan-associated diarrhea with atropine and late diarrhea with loperamide.
The starting dose for oxaliplatin was 60 mg/m2/dose on days 1 and 8, and for irinotecan 20 mg/m2/dose on days 1–5 and 8–12 of a 21-day cycle, with planned escalation of oxaliplatin to 85 mg/m2/dose. Since all patients in the first cohort experienced DLT, the next cohort received reduced doses of both irinotecan (15 mg/m2/dose) and oxaliplatin (40 mg/m2/dose). Oxaliplatin was then escalated as described above with the irinotecan dose remaining 15 mg/m2. Irinotecan was administered immediately after oxaliplatin on days 1 and 8. Intrapatient dose escalation was not allowed. Up to 17 courses of therapy were allowed in the absence of disease progression if adequate organ function was maintained.
At least 3 evaluable patients were treated at each dose level. If 1 of 3 patients at a given level experienced a dose-limiting toxicity (DLT), 3 more were accrued at that level. If ≥2 patients experienced DLT, the maximum tolerated dose (MTD) was exceeded and 3 more patients were treated at the next lower dose level. The MTD was the dose level at which ≤1 patient experienced DLT, with ≥2 of 3–6 patients experiencing a DLT at the next higher level.
Patient evaluation
Patient histories and physical examinations were obtained before enrollment, weekly during course 1 of therapy, and before each subsequent course. Routine CBCs, serum electrolytes, renal and liver function tests, and pregnancy tests (if applicable) were obtained. Serum amylase and lipase were monitored after the first cohort.
Adverse events were assessed using the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.46 Nonhematologic DLT was defined as any grade 3 or 4 nonhematologic toxicity attributable to the investigational drug, with the exclusion of (1) grade 3 nausea, vomiting, dehydration, or anorexia requiring <7 days of IV fluids, tube feedings, or TPN; (2) grade 3 liver enzyme elevation returning to baseline before the next course; (3) grade 3 fever or infection; (4) grade 3 electrolyte abnormality; (5) grade 3 diarrhea persisting for <24 h; and (6) grade 3 diarrhea without protocol-defined supportive care (cefixime, loperamide, and atropine). Grade 2 peripheral neurotoxicity persisting to day 21 of course 1 was defined as DLT. Hematologic DLT was defined as grade 4 neutropenia or thrombocytopenia for >7 days or myelosuppression causing a delay of >14 days between treatment courses. Patients with bone marrow involvement by tumor were not evaluable for hematologic DLT.
Patients underwent disease-appropriate evaluations within 2 weeks prior to study entry, after course 1 of therapy, and every other course thereafter. If a patient had a documented response, studies were repeated after the next consecutive course. Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST).47
Oxaliplatin pharmacokinetic studies
In consenting patients, blood samples (5 mL) were collected before oxaliplatin infusion on day 8 of course 1, and then 3, 4, and 48 h postinfusion. An additional level was drawn before the next oxaliplatin infusion on day 1 of course 2 (~336 h post infusion). Samples were processed as previously described48 and plasma ultrafiltrate (PUF) platinum (Pt) concentration was measured by inductively coupled plasma mass spectrometry at ABC Laboratories (Columbia, MO). The lower limit of quantitation was 1 ng/mL; within-run and between-run precision (CV%) was less than 10%.
Pharmacokinetic analyses were performed by nonlinear mixed effects modeling with S-ADAPT,49 and data described by a two-compartment pharmacokinetic model with first-order elimination. Model parameters estimated were elimination rate constant (ke), volume of distribution of the central compartment (V), and intercompartmental rate constants (k12, k21). Parameter distribution was assumed log-normal; thus, intersubject (IIV) was modeled using an exponential error model (Equation 1):
| (Equation 1) |
where θi is the estimated pharmacokinetic parameter for the ith individual, θpop the population parameter estimate, and ηi the individual deviation of θi from the population estimate. Intraindividual variability or residual error was evaluated by a mixed proportional and additive error model (Equation 2):
| (Equation 2) |
where Cpik and Ĉpik represent the kth actual and predicted PUF Pt concentrations, respectively, in individual i. Error terms εrel and εabs are components of the proportional (relative error) and additive error (absolute error), respectively. All error model parameters were assumed to be normally distributed. Residual error components were obtained in terms of standard deviation. Area under the curve (AUC0→∞) was calculated with the simulated concentrations obtained by the empirical Bayesian estimates parameters using ADAPT II.
Pharmacogenetic studies
In consenting patients, 5 mL of whole blood collected before drug administration was shipped on ice to the reference laboratory at St. Jude Children’s Research Hospital. Genomic DNA (10 ng) extracted from peripheral blood mononuclear cells was used to genotype the UGT1A1*28 promoter by PCR amplification followed by fragment size analysis as described previously.50
Results
From April 2005 to February 2006, 14 patients were enrolled. One patient had grade 3 neutropenia prior to the first dose of therapy and was therefore ineligible. Table 1 summarizes the characteristics of the 13 eligible patients who received 27 courses of therapy (median 2; range 1–6 courses).
Table 1.
Characteristics of Eligible Patients (n=13)
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| Median | 16 |
| Range | 5–21 |
| Sex | |
| Male | 4 (30.8) |
| Female | 9 (69.2) |
| Race | |
| White | 11 (84.6) |
| Black or African American | 1 (7.7) |
| Unknown | 1 (7.7) |
| Ethnicity | |
| Non-Hispanic | 13 (100) |
| Diagnosis | |
| Osteosarcoma | 3 (23.0) |
| Astrocytoma | 2 (15.4) |
| Renal cell carcinoma | 2 (15.4) |
| Rhabdomyosarcoma | 2 (15.4) |
| Ewing sarcoma | 1 (7.7) |
| Ganglioglioma | 1 (7.7) |
| Medulloblastoma | 1 (7.7) |
| Neuroblastoma | 1 (7.7) |
| Prior Therapy | |
| Chemotherapy Regimens | |
| Median | 1 |
| Range | 1–3 |
| Number of Patients with Prior Radiation Therapy | 5 |
Toxicity
All 3 patients at the first dose level (60 mg/m2/dose oxaliplatin, 20 mg/m2/dose irinotecan) experienced similar DLTs and all required admission to the hospital during the third week of course 1 (Table 2). Two of the three were admitted with grade 3 diarrhea and subsequently developed elevation in serum lipase, one with elevation in serum amylase and grade 2 abdominal pain, as well. The third was admitted with nausea, vomiting, dehydration, and subsequent severe diarrhea during the third week. Grade 3 colitis and abdominal pain along with elevation of serum amylase and lipase developed during the fourth week of course 1. Due to the length and severity of these toxicities, all of these patients were removed from protocol therapy after the first course. None of them had a history of pancreatitis, diabetes mellitus or the use of concomitant medications that would be associated with pancreatitis (such as corticosteroids or asparaginase). Two of the three had received cefixime as prescribed in the protocol. One missed more than 50% of the prescribed doses due to nausea.
Table 2.
Summary of Dose-Limiting Toxicities
| Dose Level | |||||
|---|---|---|---|---|---|
| No. Entered |
No. Evaluable |
No. DLTs |
DLTs (# patients) |
||
| Oxaliplatin (mg/m2/dose) |
Irinotecan (mg/m2/dose) |
||||
| Diarrhea (3) | |||||
| Lipase (3) | |||||
| 60 | 20 | 3 | 3 | 3 | Amylase (2) |
| Colitis (1) | |||||
| Abdominal Pain (1) | |||||
| Headache (1) | |||||
| 40 | 15 | 7* | 6 | 1 | Diarrhea (1) |
| 60 | 15 | 3 | 3 | 2 | Diarrhea (1) |
| Hypokalemia (1) | |||||
One patient received an incorrect irinotecan dose of 20 mg/m2/d
Due to severe toxicity and unexpected dose-limiting pancreatitis, the next cohort received reduced doses of oxaliplatin (40 mg/m2/dose) and irinotecan (15 mg/m2/dose). No DLTs occurred in the initial 3-patient cohort at this dose. Re-escalation of the oxaliplatin dose to 60 mg/m2 with the reduced irinotecan dose of 15 mg/m2 resulted in 2 of the 3 patients experiencing DLT (diarrhea, hypokalemia). The oxaliplatin 40 mg/m2 - irinotecan 15 mg/m2 dose level was therefore expanded to six patients; one patient received an incorrect irinotecan dose of 20 mg/m2/dose and was replaced. One patient in this expanded cohort experienced dose-limiting diarrhea, another had grade 3 diarrhea not considered dose-limiting because loperamide was not used; the patient who received the incorrect irinotecan dose did not experience DLT. Four of the 7 patients in this expanded cohort received 2 or more courses. None of these patients discontinued protocol therapy due to toxicity.
Therapy was discontinued secondary to toxicity in 6 of the 13 evaluable patients. Table 2, Table 3, and Table 4 summarize DLTs, hematologic adverse events, and nonhematologic adverse events, respectively. One patient had non-dose-limiting grade 4 neutropenia of two days duration.
Table 3.
Non-Dose Limiting Hematologic toxicities observed in 13 evaluable patients.
| Course 1 (n=13) |
Courses 2 to 6 (n=14) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Hemoglobin | 1 | 2 | 1 | 4 | ||||
| Leukocytes | 4 | 2 | 2 | 2 | 1 | 1 | ||
| Lymphopenia | 1 | 2 | 3 | 1 | 1 | 1 | ||
| Neutrophils | 1 | 3 | 1 | 1 | 2 | 4 | ||
| Platelets | 1 | 1 | 1 | 2 | 1 | |||
Note: This table consists of non-dose limiting hematologic toxicities independent of frequency and attribution
Table 4.
Non-Dose Limiting Non-Hematologic toxicities related to protocol therapy and observed in more than 10 percent of 13 evaluable patients.
| Course 1 (n=13) |
Courses 2 to 6 (n=14) |
|||||
|---|---|---|---|---|---|---|
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 |
| Fatigue | 2 | 1 | ||||
| Weight loss | 4 | 1 | ||||
| Anorexia | 1 | 3 | 1 | 3 | ||
| Dehydration | 5 | 1 | ||||
| Diarrhea | 4 | 2 | 1 | 4 | 2 | |
| Nausea | 1 | 2 | 1 | 1 | 1 | 1 |
| Vomiting | 2 | 2 | 1 | 1 | 2 | 1 |
| Paresthesia/dysesthesia | 2 | |||||
| Pancreatitis | 1 | 1 | 1 | |||
| Hypoalbuminemia | 1 | 2 | 2 | |||
| ALT, SGPT | 3 | 1 | 1 | 2 | 1 | |
| AST, SGOT | 2 | 1 | 2 | |||
| Hypocalcemia | 2 | 1 | 2 | 2 | ||
| GGT | 1 | 1 | 1 | 1 | ||
| Lipase | 2 | 2 | ||||
| Hypomagnesemia | 2 | 1 | 1 | |||
| Hypokalemia | 2 | 1 | ||||
| Pain Abdomen NOS | 2 | 2 | 2 | |||
Antitumor activity
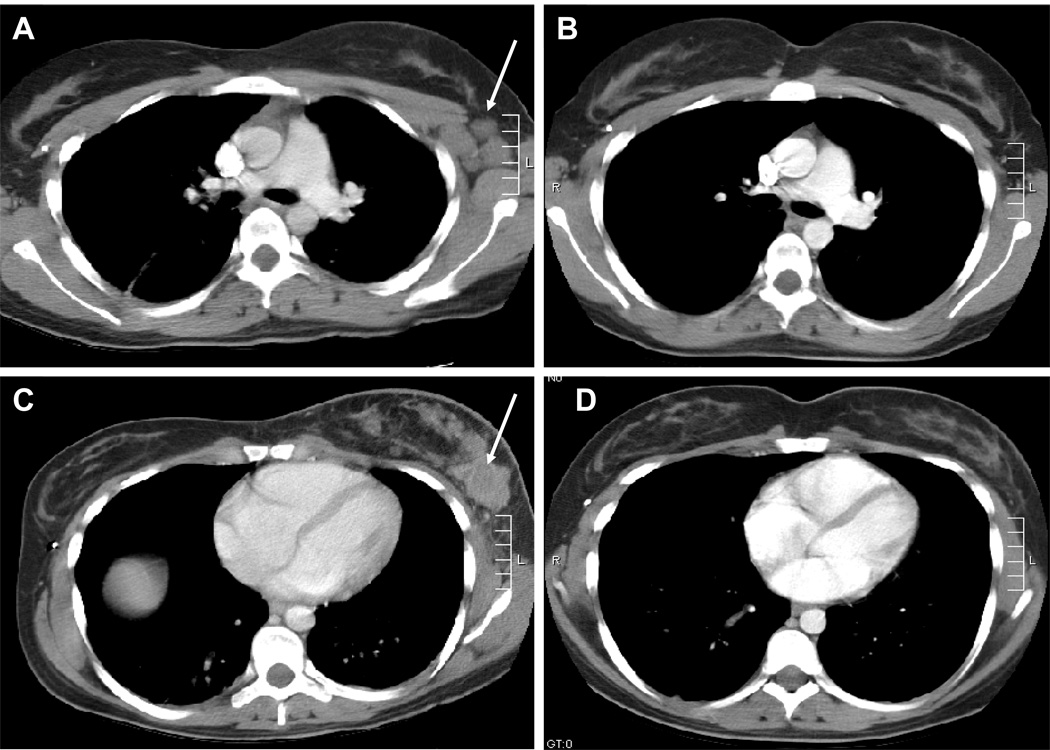
Five patients were removed from treatment prior to response assessment. Another 4 patients were removed for progressive disease after 1–3 courses. One patient with recurrent metastatic rhabdomyosarcoma, previously treated adjuvantly with irinotecan, had a complete response after 1 course, which was sustained through the fourth course (see Figure 1). She voluntarily withdrew at that point to pursue other therapy. Another patient with refractory neuroblastoma had disease stabilization through 6 courses of therapy. Both of these patients were treated at the MTD.
Figure 1.
Response of metastatic alveolar rhabdomyosarcoma in left axillary lymph nodes (A and B) and left breast (C and D) to protocol therapy. The arrows indicate pre-therapy disease (A and C) with response shown in B and D.
Pharmacokinetics
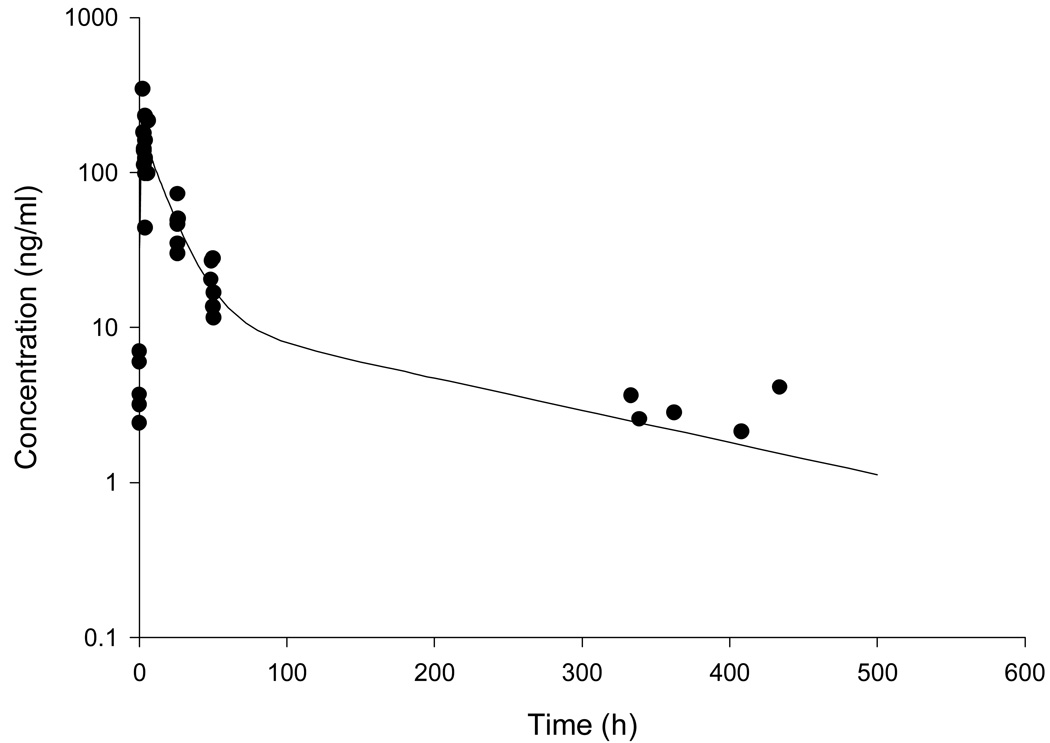
Seven patients consented to pharmacokinetic studies. PUF Pt data were evaluable for pharmacokinetic modeling from six patients. Samples from one patient could not be used due to preparation issues (i.e., plasma not PUF). Population pharmacokinetics of PUF Pt were described by a two-compartment model (Figure 2). Population pharmacokinetic parameters (SE) obtained were for ke 0.035 (0.002) h−1, V 320.8 (57.9) L/m2, k12 0.024 (0.004) h−1, and k21 0.0086 (0.002) h−1. Interindividual variabilities for PUF Pt ke and V were 11% and 42%, respectively. The proportional component of residual variability was 17%, in accordance to the analytical assay error, whereas the additive error was fixed considering the assay’s quantitation limit. The median (range) AUC calculated to the last data point and AUC0→∞ were 5.8 µg·h/mL (1.6–7.5 µg·h/mL) and 5.9 µg·h/mL (1.8–7.6 µg·h/mL), respectively. The median (range) terminal half-life was 142 hours (132–166 hours).
Figure 2.
PUF Pt (oxaliplatin) concentration vs. time profile. Observed plasma concentrations are plotted(●) and the solid line represents the model predicted concentrations (considerig the base model).
Pharmacogenetics
Ten patients consented to pharmacogenetic studies. The frequencies of 6/6, 6/7, and 7/7 UGT1A1 promoter genotypes were 5/10, 4/10, and 1/10, respectively. Genotype and toxicity showed no clear correlation.
Discussion
The MTD of oxaliplatin and irinotecan plus an oral cephalosporin was oxaliplatin 40 mg/m2/dose on days 1 and 8 with irinotecan 15 mg/m2/dose on days 1–5 and 8–12. The most common DLT was diarrhea. However, at higher doses, the combination unexpectedly resulted in significant elevations in serum amylase and lipase. Myelosuppression was not significant and no patient experienced dose-limiting hematologic toxicity. Pharyngolaryngeal dysesthesia was not common, but this was expected given the low single dose of oxaliplatin used. Peripheral neuropathy observed in adults receiving higher cumulative doses of oxaliplatin was not observed in this trial; however, only one patient received a significant cumulative dose of oxaliplatin (480 mg/m2).
The combination of irinotecan (175–200 mg/m2/dose) with oxaliplatin (85–130 mg/m2/dose) every 3 weeks51,52 or of irinotecan (50 mg/m2/dose) with oxaliplatin (60 mg/m2/dose) weekly33 has been well-tolerated in adults. The novel schedule studied here in children did not prove feasible. The basis for this decreased tolerance is unclear, but a drug interaction resulting in unexpectedly high irinotecan exposures is unlikely to be the cause. The lack of pharmacokinetic interactions observed in previous studies in adults32,33,42,43 coupled with the differences in metabolism and elimination mechanism of oxaliplatin53 and irinotecan54 do not support such a hypothesis, even though this was not directly studied in the current trial. The most likely basis is a schedule dependent pharmacodynamic difference in drug tolerance of the combination on a protracted schedule.
The finding of clinically mild pancreatitis with marked elevations in serum amylase and lipase was unexpected, as pancreatitis or elevation in serum lipase or amylase have not been reported in published trials of adults receiving the combination of oxaliplatin and irinotecan either with42,43.or without.32,34,55 fluorouracil/leucovorin. Interestingly, a study on the use of irinotecan with carboplatin56 reported mild pancreatitis in 1 of 9 patients with non-small-cell lung cancer. There is also a report of pancreatitis in a patient with hepatic metastases from colorectal carcinoma receiving oxaliplatin via hepatic arterial infusion in combination with folinic acid and 5-fluorouracil.57 However, it was unclear whether the patient’s underlying disease contributed to the pancreatitis. Thirty-eight cases of pancreatitis or elevated pancreatic enzymes have occurred in clinical trials during Sanofi-aventis’ (Bridgewater, NJ) postmarketing experience (personal communication, Paul E. Juniewicz, PhD). Most cases had alternative possible explanations for the pancreatitis, including concomitant medications or relevant medical history. It is thus unclear whether the combination of oxaliplatin with irinotecan on this schedule directly caused pancreatitis or if elevated serum amylase and lipase are a result of severe diarrhea as described in patients with infectious diarrhea.58
The pharmacokinetic limited sampling model used for oxaliplatin pharmacokinetic studies consisted of only 4 plasma samples. Because data were sparse, we used a nonlinear mixed effects modeling analysis that uses the Monte Carlo Parametric Expectation Maximization (MC-PEM) algorithm as implemented in S-ADAPT. With this population approach, PUF Pt pharmacokinetics after a 2-h infusion of oxaliplatin was adequately described by a two-compartment model. Population estimates of PUF Pt pharmacokinetic parameters are similar to those reported in pediatric and adult patients.48,59 We found the mean clearance to be 11.2 L/h/m2, which is within the BSA-normalized clearance ranges of 5.3–18.2 L/h/m2 observed in adult patients.53,60
Current U.S. Food and Drug Administration (FDA) guidelines recommend that patients’ UGT1A1 promoter genotype be considered when prescribing irinotecan, because adult patients with the 7/7 genotype have increased incidence of neutropenia following every 21 day dosing of irinotecan.61 However, recent studies report no correlation of toxicity with UGT1A1 genotype when irinotecan is administered on a low-dose protracted schedule in pediatric patients.50,62 We too could not find a relationship between UGT1A1 genotype and irinotecan-induced toxicity, but our patient numbers were small.
One complete response to the oxaliplatin and irinotecan combination was observed in a patient with metastatic alveolar rhabdomyosarcoma. Even with a reduced dose of irinotecan, the combination was effective, consistent with preclinical reports of synergy when these two agents are combined.1,29 Despite signs of clinical activity, the combination of oxaliplatin with irinotecan administered on a protracted schedule was poorly tolerated. Even at the MTD, 2 patients had grade 3 diarrhea, although one was not considered dose-limiting due to noncompliance with loperamide therapy. Alternative schedules in pediatric patients would need to be evaluated should additional data emerge that would support further clinical evaluation of this combination.
Acknowledgments
We thank Elizabeth O’Connor, Shanila Faghfoor, Dori Triplett, and Mark Ingle for their assistance conducting this study, Dr. Frederick Hoffer for reviewing the diagnostic imaging, Vani Shanker for editing assistance and the patients and their parents who agreed to participate.
Supported by National Cancer Center Institute grants No. CA97452, CA21765 and NCRR M01-RR-00037
Footnotes
All patients or their guardians, as appropriate, provided written informed consent to participate in this trial.
Reference List
- 1.Guichard S, Arnould S, Hennebelle I, Bugat R, Canal P. Combination of oxaliplatin and irinotecan on human colon cancer cell lines: activity in vitro and in vivo. Anticancer Drugs. 2001;12:741–751. doi: 10.1097/00001813-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 2.van Waardenburg RC, de Jong LA, van Eijndhoven MA, et al. Platinated DNA adducts enhance poisoning of DNA topoisomerase I by camptothecin. J.Biol.Chem. 2004;279:54502–54509. doi: 10.1074/jbc.M410103200. [DOI] [PubMed] [Google Scholar]
- 3.Tortora G, Ciardiello F, Damiano V, et al. Preclinical and phase I study of oxaliplatin and topotecan in combination in human cancer. Ann.Oncol. 2002;13:392–398. doi: 10.1093/annonc/mdf030. [DOI] [PubMed] [Google Scholar]
- 4.Souid AK, Dubowy RL, Blaney SM, et al. Phase I clinical and pharmacologic study of weekly cisplatin and irinotecan combined with amifostine for refractory solid tumors. Clin.Cancer Res. 2003;9:703–710. [PubMed] [Google Scholar]
- 5.Athale UH, Stewart C, Kuttesch JF, et al. Phase I study of combination topotecan and carboplatin in pediatric solid tumors. Journal of Clinical Oncology. 2002;20:88–95. doi: 10.1200/JCO.2002.20.1.88. [DOI] [PubMed] [Google Scholar]
- 6.Wells RJ, Reid JM, Ames MM, et al. Phase I trial of cisplatin and topotecan in children with recurrent solid tumors: Children's Cancer Group Study 0942. J.Pediatr.Hematol.Oncol. 2002;24:89–93. doi: 10.1097/00043426-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Cvitkovic E. Ongoing and unsaid on oxaliplatin: the hope. Br.J.Cancer. 1998;77 Suppl 4:8–11. doi: 10.1038/bjc.1998.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn TA, Schmoll HJ, Grunwald V, Bokemeyer C, Casper J. Comparative cytotoxicity of oxaliplatin and cisplatin in non-seminomatous germ cell cancer cell lines. Invest New Drugs. 1997;15:109–114. doi: 10.1023/a:1005800520747. [DOI] [PubMed] [Google Scholar]
- 9.Pendyala L, Creaven PJ. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res. 1993;53:5970–5976. [PubMed] [Google Scholar]
- 10.Riccardi A, Ferlini C, Meco D, Mastrangelo R, Scambia G, Riccardi R. Antitumour activity of oxaliplatin in neuroblastoma cell lines. Eur.J.Cancer. 1999;35:86–90. doi: 10.1016/s0959-8049(98)00342-6. [DOI] [PubMed] [Google Scholar]
- 11.Rixe O, Ortuzar W, Alvarez M, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem.Pharmacol. 1996;52:1855–1865. doi: 10.1016/s0006-2952(97)81490-6. [DOI] [PubMed] [Google Scholar]
- 12.Silvestro L, Anal H, Sommer F, et al. Comparative effects of a new platinum analogue (trans-1-diamine-cyclohexane oxalato-platinum; L-OHP) with CDDP on various cells: correlation with intracellular accumulation. Anticancer Res. 1990;10:A115. [Google Scholar]
- 13.Fukuda M, Ohe Y, Kanzawa F, Oka M, Hara K, Saijo N. Evaluation of novel platinum complexes, inhibitors of topoisomerase I and II in non-small cell lung cancer (NSCLC) sublines resistant to cisplatin. Anticancer Res. 1995;15:393–398. [PubMed] [Google Scholar]
- 14.Kraker AJ, Moore CW. Accumulation of cis-diamminedichloroplatinum(II) and platinum analogues by platinum-resistant murine leukemia cells in vitro. Cancer Res. 1988;48:9–13. [PubMed] [Google Scholar]
- 15.Woynarowski JM, Chapman WG, Napier C, Herzig MC, Juniewicz P. Sequence- and region-specificity of oxaliplatin adducts in naked and cellular DNA. Mol.Pharmacol. 1998;54:770–777. doi: 10.1124/mol.54.5.770. [DOI] [PubMed] [Google Scholar]
- 16.Mamenta EL, Poma EE, Kaufmann WK, Delmastro DA, Grady HL, Chaney SG. Enhanced replicative bypass of platinum-DNA adducts in cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 1994;54:3500–3505. [PubMed] [Google Scholar]
- 17.Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin.Oncol. 1998;25:4–12. [PubMed] [Google Scholar]
- 18.Cosetti M, Wexler LH, Calleja E, et al. Irinotecan for pediatric solid tumors: the Memorial Sloan-Kettering experience. J.Pediatr.Hematol.Oncol. 2002;24:101–105. doi: 10.1097/00043426-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Vassal G, Couanet D, Stockdale E, et al. Phase II trial of irinotecan in children with relapsed or refractory rhabdomyosarcoma: a joint study of the French Society of Pediatric Oncology and the United Kingdom Children's Cancer Study Group. J.Clin.Oncol. 2007;25:356–361. doi: 10.1200/JCO.2006.06.1960. [DOI] [PubMed] [Google Scholar]
- 20.Furman WL, Stewart CF, Poquette CA, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J.Clin.Oncol. 1999;17:1815–1824. doi: 10.1200/JCO.1999.17.6.1815. [DOI] [PubMed] [Google Scholar]
- 21.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children's Oncology Group. J.Clin.Oncol. 2007;25:362–369. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 22.Blaney S, Berg SL, Pratt C, et al. A phase I study of irinotecan in pediatric patients: a pediatric oncology group study. Clin.Cancer Res. 2001;7:32–37. [PubMed] [Google Scholar]
- 23.Mugishima H, Matsunaga T, Yagi K, et al. Phase I study of irinotecan in pediatric patients with malignant solid tumors. J.Pediatr.Hematol.Oncol. 2002;24:94–100. doi: 10.1097/00043426-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Turner CD, Gururangan S, Eastwood J, et al. Phase II study of irinotecan (CPT-11) in children with high-risk malignant brain tumors: the Duke experience. Neuro.-oncol. 2002;4:102–108. doi: 10.1093/neuonc/4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vassal G, Chastagner P, Doz F, et al. A phase II study of irinotecan (IRI) in children with relapsed or refractory CNS tumors (medulloblastoma and PNET) Program/Proc Am.Soc.Clin.Oncol. 2003;22:805. [Google Scholar]
- 26.Vassal G, Boland I, Santos A, et al. Potent therapeutic activity of irinotecan (CPT-11) and its schedule dependency in medulloblastoma xenografts in nude mice. Int.J.Cancer. 1997;73:156–163. doi: 10.1002/(sici)1097-0215(19970926)73:1<156::aid-ijc24>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.Houghton PJ, Cheshire PJ, Hallman JD, et al. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother.Pharmacol. 1995;36:393–403. doi: 10.1007/BF00686188. [DOI] [PubMed] [Google Scholar]
- 28.Bomgaars LR, Bernstein M, Krailo M, et al. Phase II trial of irinotecan in children with refractory solid tumors: a Children's Oncology Group Study. J.Clin.Oncol. 2007;25:4622–4627. doi: 10.1200/JCO.2007.11.6103. [DOI] [PubMed] [Google Scholar]
- 29.Zeghari-Squalli N, Raymond E, Cvitkovic E, Goldwasser F. Cellular pharmacology of the combination of the DNA topoisomerase I inhibitor SN-38 and the diaminocyclohexane platinum derivative oxaliplatin. Clin.Cancer Res. 1999;5:1189–1196. [PubMed] [Google Scholar]
- 30.Becouarn Y, Gamelin E, Coudert B, et al. Randomized multicenter phase II study comparing a combination of fluorouracil and folinic acid and alternating irinotecan and oxaliplatin with oxaliplatin and irinotecan in fluorouracil-pretreated metastatic colorectal cancer patients. J.Clin.Oncol. 2001;19:4195–4201. doi: 10.1200/JCO.2001.19.22.4195. [DOI] [PubMed] [Google Scholar]
- 31.Scheithauer W, Kornek GV, Raderer M, et al. Randomized multicenter phase II trial of oxaliplatin plus irinotecan versus raltitrexed as first-line treatment in advanced colorectal cancer. J.Clin.Oncol. 2002;20:165–172. doi: 10.1200/JCO.2002.20.1.165. [DOI] [PubMed] [Google Scholar]
- 32.Wasserman E, Cuvier C, Lokiec F, et al. Combination of oxaliplatin plus irinotecan in patients with gastrointestinal tumors: results of two independent phase I studies with pharmacokinetics. J.Clin.Oncol. 1999;17:1751–1759. doi: 10.1200/JCO.1999.17.6.1751. [DOI] [PubMed] [Google Scholar]
- 33.Kemeny N, Tong W, Gonen M, et al. Phase I study of weekly oxaliplatin plus irinotecan in previously treated patients with metastatic colorectal cancer. Ann.Oncol. 2002;13:1490–1496. doi: 10.1093/annonc/mdf247. [DOI] [PubMed] [Google Scholar]
- 34.Scheithauer W, Kornek GV, Raderer M, et al. Combined irinotecan and oxaliplatin plus granulocyte colony-stimulating factor in patients with advanced fluoropyrimidine/leucovorin-pretreated colorectal cancer. J.Clin.Oncol. 1999;17:902–906. doi: 10.1200/JCO.1999.17.3.902. [DOI] [PubMed] [Google Scholar]
- 35.Kemeny N, Garay CA, Gurtler J, et al. Randomized multicenter phase II trial of bolus plus infusional fluorouracil/leucovorin compared with fluorouracil/leucovorin plus oxaliplatin as third-line treatment of patients with advanced colorectal cancer. J.Clin Oncol. 2004;22:4701–4709. doi: 10.1200/JCO.2004.03.119. [DOI] [PubMed] [Google Scholar]
- 36.Geoerger B, Doz F, Mayer M, et al. Dose-finding and pharmacokinetic study of weekly oxaliplatin in pediatric solid malignancies. Program/Proc Am.Soc.Clin.Oncol. 2003;22 doi: 10.1200/JCO.2008.16.7585. [DOI] [PubMed] [Google Scholar]
- 37.Bomgaars L, Kerr J, Berg S, Kuttesch J, Klenke R, Blaney SM. A phase I study of irinotecan administered on a weekly schedule in pediatric patients. Pediatr.Blood Cancer. 2006;46:50–55. doi: 10.1002/pbc.20355. [DOI] [PubMed] [Google Scholar]
- 38.Crews KR, Stewart CF, Jones-Wallace D, et al. Altered irinotecan pharmacokinetics in pediatric high-grade glioma patients receiving enzyme-inducing anticonvulsant therapy. Clin.Cancer Res. 2002;8:2202–2209. [PubMed] [Google Scholar]
- 39.Thompson PA, Gupta M, Rosner GL, et al. Pharmacokinetics of irinotecan and its metabolites in pediatric cancer patients: a report from the children's oncology group. Cancer Chemother.Pharmacol. 2008 doi: 10.1007/s00280-008-0692-z. [DOI] [PubMed] [Google Scholar]
- 40.Vassal G, Doz F, Frappaz D, et al. A phase I study of irinotecan as a 3-week schedule in children with refractory or recurrent solid tumors. J.Clin Oncol. 2003;21:3844–3852. doi: 10.1200/JCO.2003.08.175. [DOI] [PubMed] [Google Scholar]
- 41.Ma MK, Zamboni WC, Radomski KM, et al. Pharmacokinetics of irinotecan and its metabolites SN-38 and APC in children with recurrent solid tumors after protracted low-dose irinotecan. Clin.Cancer Res. 2000;6:813–819. [PubMed] [Google Scholar]
- 42.Falcone A, Masi G, Allegrini G, et al. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional Fluorouracil, and leucovorin: a pilot study in patients with metastatic colorectal cancer. J.Clin Oncol. 2002;20:4006–4014. doi: 10.1200/JCO.2002.12.075. [DOI] [PubMed] [Google Scholar]
- 43.Gil-Delgado MA, Bastian G, Guinet F, et al. Oxaliplatin plus irinotecan and FU-FOL combination and pharmacokinetic analysis in advanced colorectal cancer patients. Am.J.Clin Oncol. 2004;27:294–298. doi: 10.1097/01.coc.0000071383.39986.a4. [DOI] [PubMed] [Google Scholar]
- 44.Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J.Clin.Oncol. 2004;22:1382–1388. doi: 10.1200/JCO.2004.07.173. [DOI] [PubMed] [Google Scholar]
- 45.Furman WL, Crews KR, Billups C, et al. Cefixime allows greater dose escalation of oral irinotecan: a phase I study in pediatric patients with refractory solid tumors. J.Clin.Oncol. 2006;24:563–570. doi: 10.1200/JCO.2005.03.2847. [DOI] [PubMed] [Google Scholar]
- 46.Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2006 Sep 3; NIH Publication No.03-5410.
- 47.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl.Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 48.Spunt SL, Freeman BB, III, Billups CA, et al. Phase I clinical trial of oxaliplatin in children and adolescents with refractory solid tumors. J.Clin.Oncol. 2007;25:2274–2280. doi: 10.1200/JCO.2006.08.2388. [DOI] [PubMed] [Google Scholar]
- 49.Guzy S, Bauer RJ. Use of the Monte-Carlo Parametric Expectation Maximization (MC-PEM) Estimation Method with Important Sampling for Very Sparse Data Settings. American Society for Clinical Pharmacology and Therapeutics. 2003;73(2):P51. [Google Scholar]
- 50.Stewart CF, Panetta JC, O'Shaughnessy MA, et al. UGT1A1 promoter genotype correlates with SN-38 pharmacokinetics, but not severe toxicity in patients receiving low-dose irinotecan. J.Clin.Oncol. 2007;25:2594–2600. doi: 10.1200/JCO.2006.10.2301. [DOI] [PubMed] [Google Scholar]
- 51.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J.Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 52.Hoff PM, Saad ED, Pazdur R, et al. Phase I trial of combined irinotecan and oxaliplatin given every three weeks to patients with metastatic colorectal cancer. Invest New Drugs. 2004;22:307–313. doi: 10.1023/B:DRUG.0000026257.31142.41. [DOI] [PubMed] [Google Scholar]
- 53.Graham MA, Lockwood GF, Greenslade D, Brienza S, Bayssas M, Gamelin E. Clinical pharmacokinetics of oxaliplatin: a critical review. Clin.Cancer Res. 2000;6:1205–1218. [PubMed] [Google Scholar]
- 54.Kuhn JG. Pharmacology of irinotecan. Oncology (Huntingt) 1998;12:39–42. [PubMed] [Google Scholar]
- 55.Kemeny N, TongKern W, GonenBeckert B, et al. Phase I and pharmacokinetic study of weekly hepatic arterial infusion with oxaliplatin plus irinotecan in previously treated patients with metastaticin combination with folinic acid and 5-fluorouracil in patients with hepatic metastases from colorectal cancer. Ann.Oncol. 2002;1312:1490599–1496603. [Google Scholar]
- 56.Govindan R, Read W, Faust J, Mc LH. Irinotecan and carboplatin in metastatic or recurrent non-small-cell lung cancer. Oncology (Williston.Park) 2003;17:27–29. [PubMed] [Google Scholar]
- 57.Kern W, Beckert B, Lang N, et al. Phase I and pharmacokinetic study of hepatic arterial infusion with oxaliplatin in combination with folinic acid and 5-fluorouracil in patients with hepatic metastases from colorectal cancer. Ann.Oncol. 2001;12:599–603. doi: 10.1023/a:1011186708754. [DOI] [PubMed] [Google Scholar]
- 58.Reimund JM, Muller CD, Finck G, Escalin G, Duclos B, Baumann R. Factors contributing to infectious diarrhea-associated pancreatic enzyme alterations. Gastroenterol.Clin.Biol. 2005;29:247–253. doi: 10.1016/s0399-8320(05)80757-2. [DOI] [PubMed] [Google Scholar]
- 59.Fouladi M, Blaney SM, Poussaint TY, et al. Phase II study of oxaliplatin in children with recurrent or refractory medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors: a pediatric brain tumor consortium study. Cancer. 2006;107:2291–2297. doi: 10.1002/cncr.22241. [DOI] [PubMed] [Google Scholar]
- 60.Ehrsson H, Wallin I, Yachnin J. Pharmacokinetics of oxaliplatin in humans. Med.Oncol. 2002;19:261–265. doi: 10.1385/MO:19:4:261. [DOI] [PubMed] [Google Scholar]
- 61.Ramchandani RP, Wang Y, Booth BP, et al. The role of SN-38 exposure, UGT1A1*28 polymorphism, and baseline bilirubin level in predicting severe irinotecan toxicity. J.Clin.Pharmacol. 2007;47:78–86. doi: 10.1177/0091270006295060. [DOI] [PubMed] [Google Scholar]
- 62.Bomgaars L, Kuttesch N, Bernstein M, Blaney S. Correlation of UGT1A1 promoter genotype with pharmacokinetics and toxicity in pediatric patients receiving irinotecan (CPT-11) Proc Am.Soc.Clin.Oncol. 2003;22 [Google Scholar]