Systemic arterial hypertension affects 72 million U.S. adults and additional hundreds of million persons worldwide (1, 2). Most of these are candidates for pharmacologic treatment to reduce risk of cardiovascular disease (CVD) events, based primarily on a very large body of epidemiologic and intervention research in humans. Because of this high prevalence and the cardiovascular consequences of untreated or inadequately treated hypertension, the selection of drugs for initial and continuing, long-term treatment has large public health and health economic implications. Fortunately, such decisions and the expert recommendations that seek to guide them can call upon evidence from four decades of randomized multi-center clinical trials evaluating effects of treatment on clinical CVD. We summarize that evidence in this paper, in approximate chronological order, and we comment on the related treatment guidelines. We close with some of the major clinical questions yet to be resolved.
Trials of blood pressure reduction
Before the current era beginning in the early 1990s of emphasis on positive control trials, which directly compare different drug regimens, there were three decades of trials that compared an active regimen with placebo or in a few cases “usual care”. For most of this period the mainstay of treatment was generally a thiazide-type diuretic (hereinafter called thiazides) or to a lesser extent, a beta-adrenergic blocker (termed beta-blockers). With few exceptions, these trials—especially those with high statistical power and thiazide-based regimens—showed benefit for CV outcomes (3–8). This evidence, which provided a basis for recommending thiazides or beta-blockers as first-step drugs in most editions of U.S. guidelines through 1997 (9), needs to continue to be given due weight in practice and practice guidelines.
The largest and most consistent benefits from the earlier trials were for stroke and (where reported) heart failure. Benefits for coronary heart disease (CHD) were less clear, and commentators frequently speculated that potentially adverse metabolic effects--on potassium (for diuretics), lipids, and glucose--of thiazides and beta-blockers were related to this “shortfall” in reducing CHD outcomes. Two reports in the early 1990s considerably reduced concern about the CHD shortfall. A meta-analysis of essentially all randomized antihypertensive treatment trials with clinical events outcomes—placed in an epidemiologic context so as to address the question of what effects would be expected based on risks of various events at different blood pressure (BP) levels—showed that treatment significantly reduced non-fatal myocardial infarction (MI) or CHD death (major CHD) by 14%, which represented about 2/3 of the epidemiologic expectation (10, 11). The authors attributed the shortfall to the short duration of treatment in the trials, averaging about 2–3 years to CHD events—a plausible conclusion. Shortly thereafter, the Systolic Hypertension in the Elderly Program reported that a thiazide-based regimen (using “low-dose” chlorthalidone) not only reduced fatal and non-fatal stroke by 36%, but also lowered major CHD by 27% (8).
The results of these trials have also provided a basis for guidelines on drug choice through indirect comparisons among trials and groups of trials. Subsequent to SHEP, the similarly designed Systolic Hypertension in Europe (Syst-Eur) trial reported that treatment with a dihydropyridine calcium channel blocker (CCB) as the main drug reduced BP, stroke, and all cardiac events to a similar extent as in SHEP (12). However, neither effects on CHD or heart failure were separately statistically significant. For angiotensin-converting-enzyme (ACE) inhibitors there was no large trial focused on hypertension, but several trials reported that these drugs reduced mortality and morbidity in patients with heart failure and/or CHD (13–16); the Studies of Left Ventricular Dysfunction (SOLVD) prevention trial showed that enalapril lowered the risk of MI and overt heart failure in patients with reduced ejection fraction (17). The size of the heart failure effect was, however, only 20%, in contrast with the 49% benefit in SHEP (18).
These newer trials provided important added justification for earlier recommendations concerning use of CCBs and ACE-inhibitors as first-line drugs in treating hypertension in the 1988 Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (19). However, both JNCV and JNCVI recommended thiazides and beta-blockers as preferred initial drugs for antihypertensive treatment (9, 20). As in the case of ACE-inhibitors, the trials of secondary prevention of heart disease continued to play a large role in the thinking about benefits of beta-blockers in hypertension.
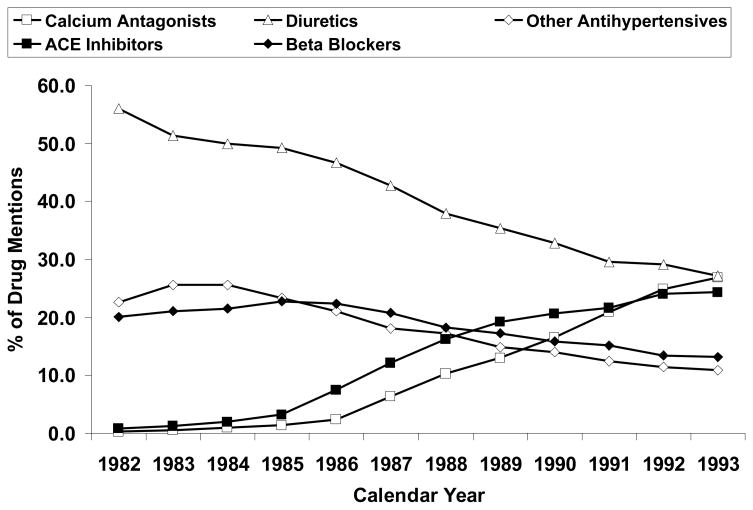
In spite of all these factors--the weight of the evidence, the consensus incorporated in JNC recommendations, and the low acquisition costs of several generic agents--, the use of thiazides and beta-blockers to treat hypertension declined dramatically after 1981-83 through the early 1990s (Figure 1) (21). These trends can be attributed to 1) concerns raised regarding metabolic effects, 2) results of trials that used doses of thiazides much higher than in general use today (22), and 3) effective marketing of newer patented drugs, in part based on their effects on intermediate markers of various disease processes. Thiazide dosing issues were eventually addressed based on the dose-response curves for BP effects versus potassium depletion, the SHEP trial results, and most persuasively by a meta-analysis that separated use of “low-dose” (actually, low-to-moderate dose) from high-dose thiazides among CV events trials (23). Nevertheless, it was increasingly recognized that large, direct comparison trials would be needed to provide a solid scientific basis for drug selection. One of the first such trials, and the largest in terms of total study population as well as patients per treatment arm, was the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Figure 1. Percentage of drug mentions by class of antihypertensive agent, 1982 through 1993. ACE indicates angiotensin-converting enzyme (21). Data from IMS America (59).
Used by permission: Archives of Internal Medicine; April 24, 1995, volume 155, pages 829–837 (Figure 1), copyright © 1995 American Medical Association.
The continuing case for thiazides
Direct comparison trials
ALLHAT: design and pre-specified outcomes
ALLHAT was designed to address the issue of which class of drugs should be used for initial therapy for hypertension. It was planned as a practice-based trial to mirror community treatment of hypertension, obtain sufficient patients, and capture the diversity of hypertensive patients (by age, sex, ethnicity, and diabetic status). More specifically, the study was a randomized, double-blind, multicenter clinical trial, designed to determine whether the incidence of CHD is reduced in high-risk patients with hypertension by a CCB (represented by amlodipine), an ACE-inhibitor (represented by lisinopril), or an alpha-blocker (represented by doxazosin), each compared with diuretic treatment (represented by chlorthalidone) (24). The overall findings of the trial showed that CHD risk was not improved for any of the three newer agents compared with chlorthalidone (25, 26) and that total mortality was similar for the four groups (Table 1 and Table 2).
Table 1. Clinical Outcomes by Antihypertensive Treatment Group (25).
Used by permission: Journal of the American Medical Association; December 18, 2002, volume 289, page 2990 (Table 5), copyright © 2002 American Medical Association.
| Chlorthalidone | Amlodipine | Lisinopril | RR (95% CI) | z score | p value | RR (95% CI) | z score | p value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Total Events | 6-Year Rate per 100 Persons (SE) | No. of Total Events | 6-Year Rate per 100 Persons (SE) | No. of Total Events | 6-Year Rate per 100 Persons (SE) | |||||||
| Primary Endpoint | ||||||||||||
| CHD† | 1362 | 11.5 (0.3) | 798 | 11.3 (0.4) | 796 | 11.4 (0.4) | 0.98 (0.90–1.07) | −0.46 | 0.65 | 0.99 (0.91 – 1.08) | −0.24 | 0.81 |
| Secondary Endpoints | ||||||||||||
| All-cause mortality | 2203 | 17.3 (0.4) | 1256 | 16.8 (0.5) | 1314 | 17.2 (0.5) | 0.96 (0.89 – 1.02) | −1.27 | 0.20 | 1.00 (0.94 – 1.08) | 0.12 | 0.90 |
| Combined CHD‡ | 2451 | 19.9 (0.4) | 1466 | 19.9 (0.5) | 1505 | 20.8 (0.5) | 1.00 (0.94 – 1.07) | 0.04 | 0.97 | 1.05 (0.98 – 1.11) | 1.35 | 0.18 |
| Stroke | 675 | 5.6 (0.2) | 377 | 5.4 (0.3) | 457 | 6.3 (0.3) | 0.93 (0.82 – 1.06) | −1.09 | 0.28 | 1.15 (1.02 – 1.30) | 2.31 | 0.02 |
| Combined CVD‡ | 3941 | 30.9 (0.5) | 2432 | 32.0 (0.6) | 2514 | 33.3 (0.6) | 1.04 (0.99 – 1.09) | 1.55 | 0.12 | 1.10 (1.05 – 1.16) | 3.78 | <0.001 |
| End-stage renal disease | 193 | 1.8 (0.1) | 129 | 2.1 (0.2) | 126 | 2.0 (0.2) | 1.12 (0.89 – 1.40) | 0.98 | 0.33 | 1.11 (0.88 – 1.38) | 0.87 | 0.38 |
| Components of secondary outcomes | ||||||||||||
| Heart failure | 870 | 7.7 (0.3) | 706 | 10.2 (0.4) | 612 | 8.7 (0.4) | 1.38 (1.25 – 1.52) | 6.29 | <0.001 | 1.19 (1.07 – 1.31)|| | 3.33 | <0.001 |
| Hospitalized/fatal heart failure | 724 | 6.5 (0.3) | 578 | 8.4 (0.4) | 471 | 6.9 (0.4) | 1.35 (1.21 – 1.50) | 5.37 | <0.001 | 1.10 (0.98 – 1.23)|| | 1.59 | 0.11 |
| Angina (hospitalized or treated) | 1567 | 12.1 (0.3) | 950 | 12.6 (0.4) | 1019 | 13.6 (0.4) | 1.02 (0.94 – 1.10) | 0.42 | 0.67 | 1.11 (1.03 – 1.20) | 2.59 | 0.01 |
| Angina (hospitalized) | 1078 | 8.6 (0.3) | 630 | 8.4 (0.4) | 693 | 9.6 (0.4) | 0.98 (0.89 – 1.08) | −0-.41 | 0.68 | 1.09 (0.99 – 1.20) | 1.85 | 0.06 |
| Coronary revascularizations | 1113 | 9.2 (0.3) | 725 | 10.0 (0.4) | 718 | 10.2 (0.4) | 1.09 (1.00 – 1.20) | 1.88 | 0.06 | 1.10 (1.00 – 1.21) | 1.95 | 0.05 |
| Peripheral arterial disease (hospitalized or treated) | 510 | 4.1 (0.2) | 265 | 3.7 (0.2) | 311 | 4.7 (0.4) | 0.87 (0.75 – 1.01) | −1.86 | 0.06 | 1.04 (0.90 – 1.19) | 0.48 | 0.63 |
RR indicates relative risk; CI, confidence interval; CHD, coronary heart disease; and CVD, cardiovascular disease. CHD includes nonfatal myocardial infarction (MI) and fatal CHD; end-stage renal disease: kidney disease death, kidney transplant, or start of chronic renal dialysis; and heart failure: fatal, nonfatal hospitalized, or treated.
Nonfatal MIs comprise 64% to 66% of the primary outcome.
Combined CHD indicates CHD death, nonfatal MI, coronary revascularization procedures, and hospitalized angina. Combined CVD indicates CHD death, nonfatal MI, stroke, coronary revascularization procedures, hospitalized or treated angina, treated or hospitalized heart failure, and peripheral arterial disease (hospitalized or outpatient revascularization).
Denominators are 11 361 chlorthalidone, 6757 amlodipine, and 6665 lisinopril.
Proportional hazards assumption violated; data are RRs from a 2 × 2 table.
Table 2. Outcomes in the Blood Pressure Component of ALLHAT by Treatment Group as of February 15, 2000 (26).
Used by permission: Hypertension, September 2003, volume 42, page 243 (Table 3), copyright © 2003 Lippincott Williams & Wilkins.
| Total No. of Patients With Outcomes | 4-Year Rate Per 100 (SE) | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes | Chlorthalidone Group | Doxazosin Group | Chlorthalidone Group (n=15,255) | Doxazosin Group (n=9,061) | RR (D/C), 95% CI | Z Score | P* |
| CHD (nonfatal MI + fatal CHD) | 818 | 499 | 7.76 (0.30) | 7.91 (0.39) | 1.03 (0.92 – 1.15) | 0.49 | 0.62 |
| All-cause mortality | 1258 | 769 | 10.51 (0.32) | 11.04 (0.43) | 1.03 (0.94 – 1.13) | 0.68 | 0.50 |
| Cardiovascular | 551 | 377 | 4.74 (0.22) | 5.60 (0.32) | 1.15 (1.01 – 1.32) | 2.15 | 0.03 |
| MI | 184 | 105 | 1.65 (0.13) | 1.76 (0.19) | 0.96 (0.76 – 1.22) | −0.32 | 0.75 |
| Definite CHD | 57 | 39 | 0.54 (0.08) | 0.54 (0.10) | 1.16 (0.77 – 1.74) | 0.70 | 0.49 |
| Possible CHD | 62 | 43 | 0.50 (0.08) | 0.63 (0.11) | 1.17 (0.79 – 1.73) | 0.79 | 0.43 |
| Stroke | 92 | 76 | 0.79 (0.10) | 1.25 (0.16) | 1.39 (1.03 – 1.89) | 2.14 | 0.03 |
| HF | 59 | 42 | 0.60 (0.09) | 0.65 (0.11) | 1.20 (0.81 – 1.78) | 0.91 | 0.36 |
| Other cardiovascular | 97 | 72 | 0.88 (0.10) | 1.12 (0.15) | 1.25 (0.92 – 1.70) | 1.44 | 0.15 |
| Non-cardiovascular | 561 | 317 | 4.82 (0.23) | 4.72 (0.30) | 0.95 (0.83 – 1.09) | −0.67 | 0.50 |
| Cancer | 314 | 162 | 2.78 (0.18) | 2.43 (0.21) | 0.87 (0.72 – 1.05) | −1.43 | 0.15 |
| Kidney disease | 12 | 12 | 0.11 (0.04) | 0.24 (0.09) | 1.69 (0.76 – 3.77) | 1.29 | 0.20 |
| Accident/suicide/homicide | 39 | 28 | 0.33 (0.06) | 0.40 (0.09) | 1.21 (0.75 – 1.97) | 0.78 | 0.44 |
| Other noncardiovascular | 196 | 115 | 1.75 (0.14) | 1.84 (0.19) | 0.99 (0.79 – 1.25) | −0.08 | 0.93 |
| Unknown | 146 | 75 | 1.37 (0.13) | 1.18 (0.16) | 0.87 (0.66 – 1.15) | −1.00 | 0.32 |
| Combined CHD† | 1642 | 1040 | 14.87 (0.39) | 16.00 (0.53) | 1.07 (0.99 – 1.16) | 1.82 | 0.07 |
| Stroke | 434 | 325 | 4.08 (0.22) | 5.49 (0.35) | 1.26 (1.10 – 1.46) | 3.20 | 0.001 |
| Combined CVD‡ | 2829 | 1947 | 25.09 (0.48) | 28.56 (0.64) | 1.20 (1.13 – 1.27) | 6.13 | <0.001 |
| HF (fatal, hospitalized, treated) | 546 | 584 | 5.35 (0.26) | 8.89 (0.42) | 1.80 (1.61 – 2.02)§ | 10.27 | <0.001 |
| HF (fatal, hospitalized) | 440 | 434 | 4.41 (0.24) | 6.63 (0.37) | 1.66 (1.46 – 1.89)§ | 7.72 | <0.001 |
| Coronary revascularization | 770 | 508 | 7.08 (0.28) | 8.02 (0.40) | 1.12 (1.00 – 1.25) | 1.97 | 0.05 |
| Hospitalized or treated angina | 1227 | 811 | 10.81 (0.33) | 11.82 (0.45) | 1.13 (1.03 – 1.23) | 2.65 | 0.01 |
| Lower extremity peripheral arterial disease | 376 | 217 | 3.68 (0.21) | 3.49 (0.27) | 0.97 (0.82 – 1.15) | −0.31 | 0.76 |
| End-stage renal disease | 104 | 64 | 1.10 (0.13) | 1.08 (0.17) | 1.04 (0.76 – 1.42) | 0.26 | 0.80 |
To adjust for multiple comparisons compare the P value to 0.018 rather than 0.05.
Combined CHD = CHD death, nonfatal MI, coronary revascularization procedures, and hospitalized angina.
Combined CVD = CHD death, nonfatal MI, stroke, coronary revascularization procedures, hospitalized or treated angina, treated or hospitalized CHF, and peripheral arterial disease (hospitalized, or outpatient revascularization).
PH assumption violated; numbers given are RRs.
However, diuretic-based therapy was superior to alpha-blocker-based, ACE-inhibitor-based, and CCB-based therapy in preventing one or more major forms of CVD, including heart failure and (in some comparisons) stroke. (25, 26). Results were consistent for all outcomes by age, sex, diabetic status, and ethnicity, except for stroke and combined CVD. For these end points, significant heterogeneity was seen in the lisinopril-chlorthalidone comparison by ethnicity: black persons assigned to chlorthalidone had a greater reduction in risk for stroke and CVD, in keeping with larger BP differences.
On the basis of these findings, the ALLHAT investigators recommended that diuretics should be the drug of choice for initial hypertension therapy and, since most hypertensive patients require more than one drug, diuretics should generally be part of any antihypertensive regimen. With regard to applicability of these conclusions, although ALLHAT was conducted in high-risk patients to ensure that enough outcome events would occur during the study to detect important treatment differences, its findings (just as those from most trials) can and should be reasonably extrapolated beyond the exact sample in which it was conducted.
BP differences in ALLHAT
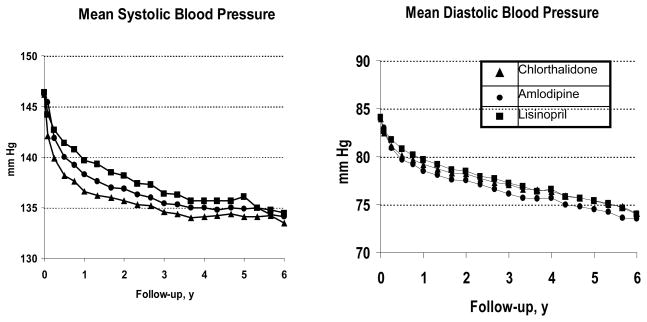
One of the main criticisms of ALLHAT was that its outcome findings (especially the subgroup findings for stroke) could be explained by observed BP differences among treatment groups. The chlorthalidone-based regimen happened to be the most effective in reducing clinical outcomes and, to a small degree, in BP lowering (Figure 2). (25)
Figure 2. Mean Systolic and Diastolic Blood Pressure by Year During Follow-up (25).
Used by permission: Journal of the American Medical Association; December 18, 2002, volume 289, page 2989 (Figure 2), copyright © 2002 American Medical Association.
Meta-regressions of effects of BP differences on trial results (27) suggest that they do offer a partial explanation, except for heart failure. In ALLHAT, the small differences in achieved mean systolic BP and diastolic BP — < 1 mm Hg—between the amlodipine and chlorthalidone groups overall and the lisinopril and chlorthalidone groups in nonblack persons should have had a negligible effect on cardiovascular event rates. In these respective comparisons, the observed rates of heart failure were higher with amlodipine (RR=1.38, 95% CI 1.25–1.52) and lisinopril (RR=1.15, 95% CI 1.01–1.30) than with chlorthalidone (25).
Extrapolating findings to drugs within a class
Could the ALLHAT results be extrapolated to other drugs of the same class? For alpha-blockers, ACE inhibitors, and dihydropyridine calcium-channel blockers, such extrapolation seems reasonable (28). Data from studies using various thiazide-type or thiazide-like diuretics (chlorthalidone, hydrochlorothiazide, indapamide, and bendrofluazide) suggest similar benefits among equivalent doses of all thiazide type diuretics tested in CVD prevention trials against placebo, usual care, or another drug class (5, 6, 8, 29, 30–34). However, there are a few studies that suggest that chlorthalidone’s longer duration of action may provide some advantage in CVD prevention over hydrochorothiazide (22, 35).
Results from other trials
After the ALLHAT results appeared, several other active comparator trials were reported. The Second Australian National Blood Pressure Study (ANBP2), a practice-based open-label trial, was the only other large trial besides ALLHAT to compare diuretic-based (hydrochlorothiazide recommended) with ACE inhibitor-based (enalapril recommended) antihypertensive treatment. (34) A total of 6083 participants, 65–84 years of age, were treated and followed for a mean of 4.1 years. The primary end point was a composite of all cardiovascular events (including recurrent events, an unusual design) plus all-cause mortality. Cardiovascular events included major coronary events, stroke and transient ischemic attacks, heart failure (not otherwise defined), acute occlusion of any other major artery, and dissecting or ruptured aortic aneurysm. The results for the primary end point favored the ACE inhibitor group (relative risk with marginal significance, 0.89 [95% CI, 0.79 to 1.00]; P = 0.05). The corresponding results using first cardiovascular event had essentially the same relative risk, (P=0.07). The relative risk for heart failure was 0.85 [95% CI, 0.62 to 1.18], p = 0.33. Frohlich (36) weighed the supposedly conflicting results of ANBP2 and ALLHAT, suggesting possible explanations such as the patients studied (many more black patients in ALLHAT) and the specific drugs used. Additionally, there were almost eight times as many cardiovascular events in the two comparable arms in ALLHAT as in ANBP2, and only ALLHAT was double-blind. ANBP2 used a prospective, randomized, open-label, blinded end-point (PROBE) design, increasing the potential for bias in the reporting of events (the rates of some outcomes might have been “expected” to be lower with the ACE-inhibitor) even though ANBP2 relied on endpoint-committee-adjudicated outcomes. Doses of agents in ANBP2 were left up to the local investigator and were not reported; thus, it is not possible to assess whether appropriate doses of hydrochlorothiazide were used. Even given the possible biases, however, the results of ANBP2 are consistent with those of ALLHAT if the upper confidence limit for the relative risks in ANBP2 is compared with the estimates of relative risk in ALLHAT (37).
The International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) was the other large trial besides ALLHAT to compare diuretic-based (co-amilozide) with CCB-based (nifedipine) antihypertensive treatment on cardiovascular mortality and morbidity in high-risk patients with hypertension (33). It was a randomized, double-blind trial in 6321 patients aged 55–80 years with hypertension. Patients had at least one additional cardiovascular risk factor and were randomly assigned patients to nifedipine (30–60 mg in a long-acting gastrointestinal-transport system (GITS) formulation) or co-amilozide (hydrochlorothiazide 25–50 mg plus amiloride). The primary outcome was cardiovascular death, myocardial infarction, heart failure, or stroke. Primary outcomes occurred in 200 (6.3%) patients in the nifedipine group and in 182 (5.8%) in the co-amilozide group (relative risk 1.10 [95% CI 0.91–1.34], p=0.35). The CCB was not superior to the diuretic in preventing cardiovascular morbidity and mortality. Non-fatal heart failure was more common in the CCB arm (relative risk = 2.20 95% CI, 1.07 to 4.49, p= 0.028). There were only 3 fatal heart failure events − 2 in the CCB arm and 1 in the diuretic arm.
Further details on heart failure in ALLHAT
Another major ALLHAT criticism concerned the heart failure findings. Specifically, were the findings real and could they be explained by withdrawal from antihypertensive medications, such as diuretics and ACE inhibitors, upon entry into ALLHAT?
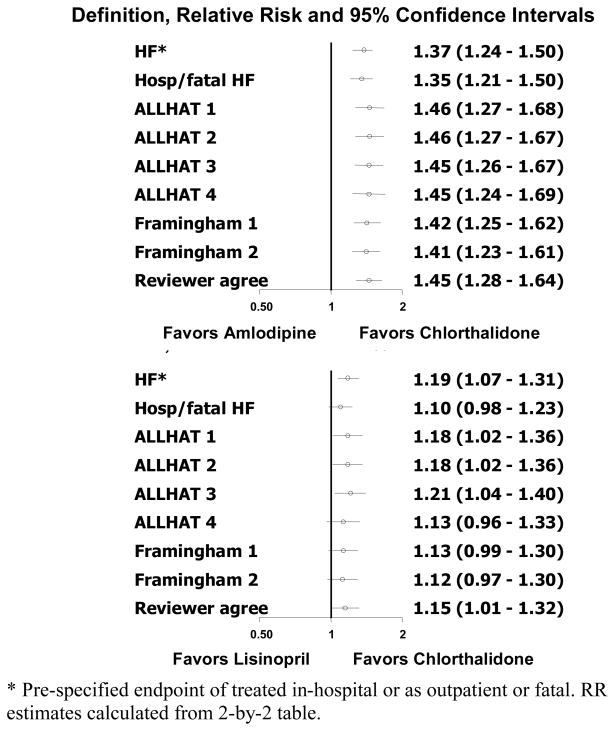
Several papers have addressed these two questions in detail (38–41). The reliability of the HF diagnosis during the trial was examined in depth via the ALLHAT Heart Failure Validation Study (HFVS) (40). This study was designed to validate and elucidate the significance of heart failure events in ALLHAT. This study involved all hospitalized heart failure events and relevant hospital records related to these events. Cardiology fellows, external to ALLHAT and blinded to treatment assignment, centrally abstracted the documentation for each heart failure hospitalization (2778 in 1935 patients; 2 independent reviews per case). ALLHAT and Framingham criteria were assigned by a computer algorithm; the reviewers also rendered a global clinical judgment. Percent agreements with site physician diagnoses were 71%, 80%, and 84% for ALLHAT, Framingham, and reviewers’ judgment, respectively. Based on these 3 criteria, relative risks (95% CI) for new-onset hospitalized heart failure compared with chlorthalidone were, respectively, 1.46 (1.27–1.68), 1.42 (1.25–1.62), and 1.45 (1.28–1.64) for amlodipine; 1.18 (1.02–1.28), 1.13 (0.99–1.30), and 1.15 (1.01–1.32) for lisinopril; and 1.79 (1.51–2.11), 1.71 (1.46–2.00), and 1.80 (1.55–2.10) for doxazosin (Figure 3).(40)
Figure 3. Incident hospitalized HF outcomes by antihypertensive treatment group (amlodipine/lisinopril vs chlorthalidone) (40).
Used by permission: This article was published in the American Heart Journal, Volume 153 - Einhorn PT, Davis BR, Massie BM, Cushman WC, Piller LB, Simpson LM, Levy D, Nwachuku CE, Black HR, ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) heart failure validation study: Diagnosis and prognosis, pages 42–53, copyright Elsevier (2007).
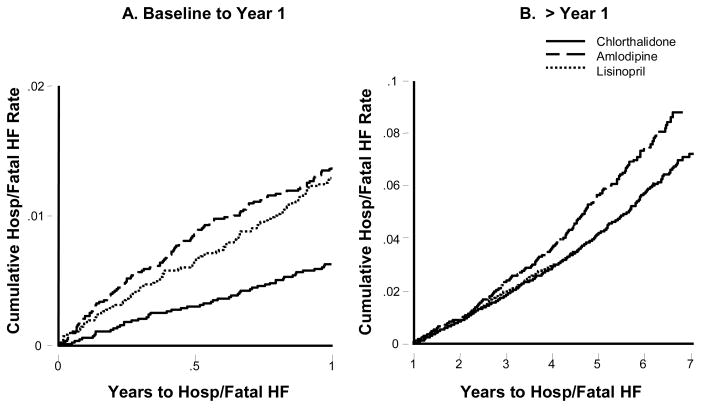
Although there was early divergence of the heart failure incidence curves in ALLHAT, it continued after the first year for doxazosin and amlodipine versus chlorthalidone. For lisinopril versus chlorthalidone, the curves also separated early but appeared to converge between years 6 and 7(25). Diagnostic analysis revealed that the proportional hazards assumption of constant relative risk over time was not valid (39). A more appropriate model showed relative risks of amlodipine or lisinopril versus chlorthalidone during year 1 were 2.22 (1.69 to 2.91; p<0.001) and 2.08 (1.58 to 2.74; P<0.001), and after year 1, 1.22 (1.08 to 1.38; p<0.001) and 0.96 (0.85 to 1.10; p = 0.58) (Figure 4). (39)
Figure 4. Cumulative event rates for hospitalized (Hosp)/fatal HF by treatment group. (39).
All comparisons are unadjusted. A, Year 1: both the amlodipine and lisinopril groups had a significantly higher risk of hospitalized/fatal HF than the chlorthalidone group (RR 2.22, 95% CI 1.69 to 2.91, P<0.001 and RR 2.08, 95% CI 1.58 to 2.74, P<0.001, respectively). No significant difference was observed for amlodipine vs lisinopril (RR 1.07, 95% CI 0.82 to 1.38, P=0.63). B, >Year 1: the amlodipine group had a 22% higher risk of hospitalized/fatal HF than the chlorthalidone group (RR 1.22, 95% CI 1.08 to 1.38, P=0.001). No significant difference was observed for lisinopril vs chlorthalidone (RR 0.96, 95% CI 0.85 to 1.10, P=0.58). The amlodipine group had a 27% higher risk of hospitalized/fatal HF than the lisinopril group (RR 1.27, 95% CI 1.10 to 1.45, P=0.001).
Also, information about previous medication use was collected on the hospitalized and fatal cases of heart failure as a follow-up to the HFVS (41). When case-only design theory was used to assess interactions (42), the analyses did not support an effect of pre-entry diuretic use on the observed heart failure differences. However, the addition of second- and third-line drugs (~30% at year one) probably contributed to the lessening of the divergence starting at 6 to 12 months after randomization. Given these additional examinations, the original conclusions remained the same. Thiazide-type diuretics should be the preferred first-step therapy for prevention of heart failure in high-risk patients with hypertension.
Diabetes in ALLHAT
As noted above, the initial ALLHAT reports showed consistent findings for those in the pre-specified sub-groups with and without a baseline history of diabetes (25, 26). A subsequent report on comparisons among the chlorthalidone, amlodipine, and lisinopril arms utilized baseline fasting glucose (FG) levels in addition to history to classify participants into those with diabetes, impaired fasting glucose (110–125 mg/dl), and fasting normglycemia. Results were similar in all three sub-groups—in particular, the superiority of chlorthalidone for heart failure and the absence of any outcome (including end-stage renal disease) for which another arm was superior (43).
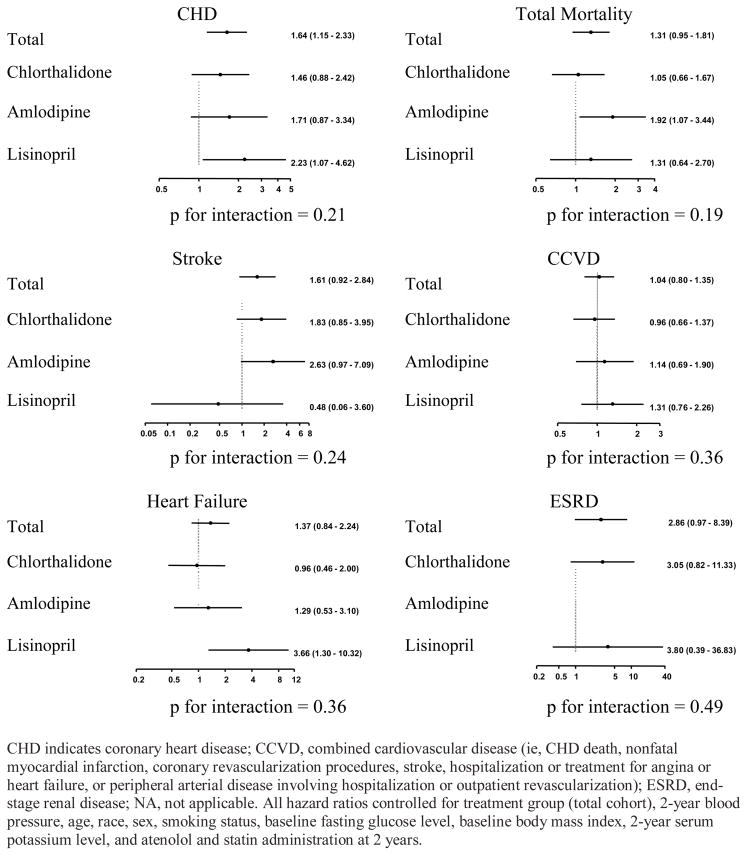
Among those without DM at baseline the mean FG was about 93.5 mg/dl. Changes in FG and percentage of incident diabetes mellitus (IDM) at 4 years, although not prespecified outcomes, were +10.8 mg/dl and 11.0% in the chlorthalidone group; +9.3mg/dl and 9.3% in the amlodipine group, and +6.8 mg/dl and 7.8% in the lisinopril group, respectively, (25). It was observed that these and other metabolic differences did not translate into any overall disadvantage for this treatment arm during the mean follow-up of 4.9 years (range 4–8 years). Nevertheless, further epidemiologic-type analyses were conducted to examine the association of glucose changes with CVD and renal outcomes (44). There was no significant association of 2-year FG change with subsequent events, overall or in the chlorthalidone arm separately. Also, among those who developed IDM by 2 years compared to those who did not, there was no significant increase in subsequent risk for any major disease outcomes except CHD, e.g. the relative risks for all CVD combined were 1.04 (95% CI 0.80–1.35) for all arms and 0.96 (0.66–1.37) for chlorthalidone, and for total mortality, 1.31 (0.96–1.81) and 1.05 (0.66–1.67), respectively. For CHD the risk associated with IDM was 1.64 (1.15–2.33) overall, but only 1.46 (0.88–2.42) in the chlorthalidone arm (Figure 5).
Figure 5. Cox regression models showing the hazard ratios (95% confidence intervals) associated with incident diabetes mellitus during the first 2 years of follow-up on subsequent cardiovascular disease and renal end points in those without diabetes mellitus at baseline. (44).
Used by permission: Archives of Internal Medicine; November 13, 2006, volume 166, page 2199 (Figure 3), copyright © 2006 American Medical Association.
So is this tendency for IDM occurring during low-to-moderate dose chlorthalidone treatment to impart less risk for adverse clinical events than during other regimens real, and if so, what could be the explanation? First, in the only other long-term follow-up data in treated hypertensive patients that are relatively uncontaminated with concomitant drugs, the findings in the SHEP 14-year extended follow-up study showed a similar phenomenon—a contrast between an increased CVD risk associated with IDM in the placebo arm, but not in the chlorthalidone arm (45). Second, it must be recognized that in a typical hypertensive populations the great majority of IDM that occurs while taking a thiazide is not drug-induced but due to typical causes of Type 2 diabetes, and therefore would be expected to carry the same risk as any such occurrence. From ALLHAT data on IDM at 4-years such a fraction can be estimated as 83%, if it is assumed that the CCB is metabolically neutral (46). If only 1 out of 5 cases of IDM in patients prescribed a thiazide is due to the drug but these cases cannot be separated from the majority, the lower risk in such patients would serve to somewhat lower but not eliminate the overall DM-associated risk.
For the reason why thiazide-induced IDM could carry less risk than “naturally-occurring” DM, one needs to consider the abundant--but mostly older and often forgotten--literature on potassium depletion and glucose disorders (46, 47). To the extent that glucose disorders are due to this mechanism, it is plausible that its natural history is quite different. Further, as pointed out in (46), “In the diuretic-treated patient hypokalemia is likely to be intermittent, due to dietary and drug adherence variation, and potassium-sparing therapeutic intervention [which] may also translate to dysglycemia that is intermittent …and thus confer little risk of diabetic complications.”
Network meta-analysis
The role of the efficacy of various antihypertensive therapies used as first-line agents in preventing major cardiovascular disease outcomes has been assessed using network meta-analysis (48). This type of analysis combines direct within-trial between-drug comparisons with indirect evidence from the other trials. The indirect comparisons, which preserve the within-trial randomized findings, were constructed from trials that had one treatment in common.
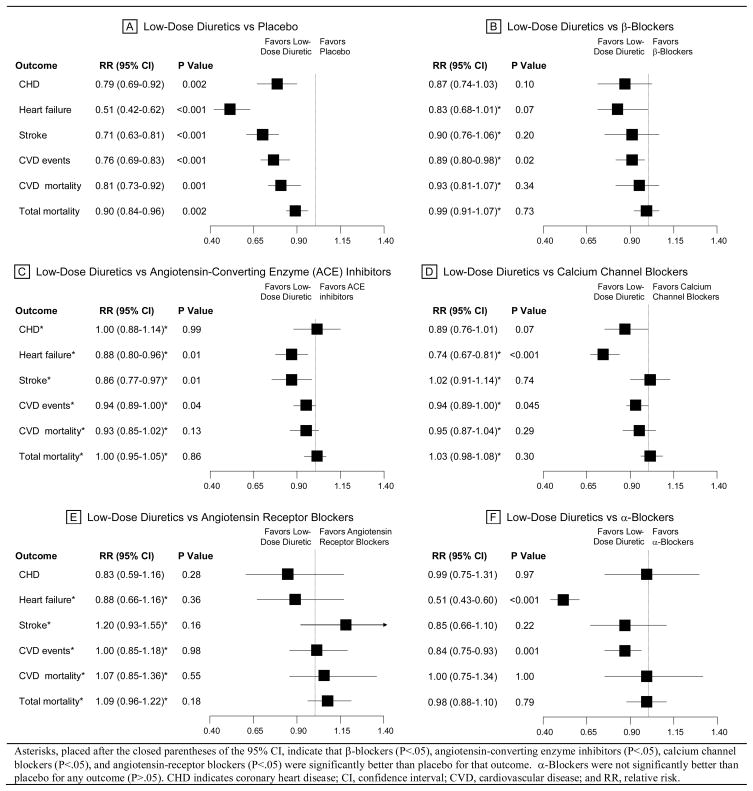
Data were combined from 42 clinical trials that included 192 478 patients randomized to 7 major treatment strategies, including placebo. For all outcomes, “low-dose” diuretics were superior to placebo (Figure 6): coronary heart disease (CHD; RR, 0.79; 95% confidence interval [CI], 0.69–0.92); congestive heart failure (CHF; RR, 0.51; 95% CI, 0.42–0.62); stroke (RR, 0.71; 0.63–0.81); cardiovascular disease events (RR, 0.76; 95% CI, 0.69–0.83); cardiovascular disease mortality (RR, 0.81; 95% CI, 0.73–0.92); and total mortality (RR, 0.90; 95% CI, 0.84–0.96). None of the other first-line treatment strategies--beta-blockers, ACE-inhibitors, CCBs, alpha-blockers, and angiotensin receptor blockers--was significantly better than “low-dose” diuretics for any outcome. Blood pressure changes were similar between comparison treatments. Based on this network meta-analysis, the authors concluded that “low-dose” diuretics were the most effective first-line treatment for preventing the occurrence of cardiovascular disease morbidity and mortality.
Figure 6. Network Meta-analysis of First-Line Treatment Strategies in Randomized Controlled Clinical Trials in Hypertension (48).
Used by permission: Journal of the American Medical Association; May 21, 2003, volume 289, page 2541 (Figure 2), copyright © 2003 American Medical Association.
JNC7 Recommendations
In 2003, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) issued its recommendations based upon the ALLHAT results and other trial evidence that was available at that time (49). Its major conclusions and recommendations were as follows.
In trials comparing thiazide-type diuretics with other classes of antihypertensive agents, they are 1) well-tolerated; 2) effective and relatively safe for the management of hypertension despite potential adverse metabolic effects; and 3) unsurpassed in preventing the cardiovascular complications of hypertension. They are also less expensive and underutilized. The doses of thiazide-type diuretics used in successful morbidity trials of low-moderate dose diuretics should be used (generally the equivalent of 25 to 50 mg of hydrochlorothiazide or 12.5 to 25 mg of chlorthalidone), although therapy may be initiated at lower doses and titrated to these doses if tolerated.
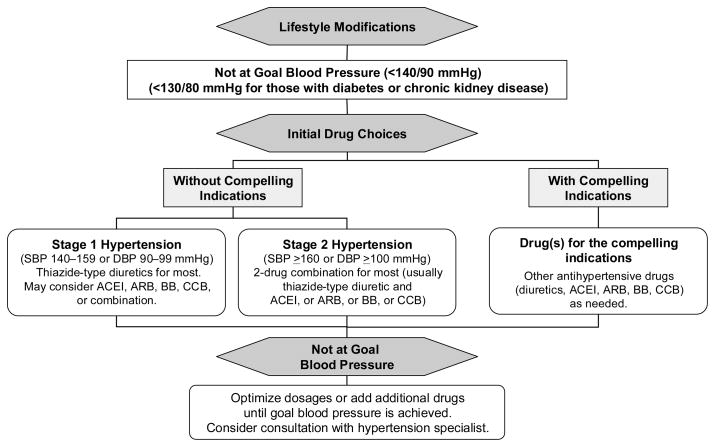
The algorithm for the treatment of hypertensive patients is to begin with lifestyle modification, and if the BP goal is not achieved, thiazide-type diuretics should be used as initial therapy for most patients, either alone or in combination with one of the other classes (ACEIs, ARBs, BBs, CCBs) that have also been shown to reduce one or more hypertensive complications in randomized controlled outcome trials (Figure 7). Selection of one of these other agents as initial therapy is recommended when a diuretic cannot be used or when a compelling indication is present that requires the use of a specific drug. If the initial drug selected is not tolerated or is contraindicated, then a drug from one of the other classes proven to reduce cardiovascular events should be substituted. Since most hypertensive patients will require 2 or more antihypertensive medications to achieve their BP goals, addition of a second drug from a different class should be initiated when use of a single agent in adequate doses fails to achieve the goal. It is further recommended that patients with Stage 2 hypertension be started on 2 drugs initially, one of which should be a thiazide.
Figure 7. JNC7 algorithm for treatment of hypertension. (49).
Used by permission: Hypertension, December 2003, volume 42, page 1221 (Figure 16), copyright © 2003 Lippincott Williams & Wilkins.
The evidence for and against beta-blockers
The British Medical Research Council (BMRC) trial of treatment of mild hypertension was the first clinical events trial with a beta-blocker-based arm (propanolol) in addition to a thiazide arm (bendrofluazide). (6) Compared with placebo, only the thiazide significantly reduced stroke, likely due to the greater BP-reduction than with the beta-blocker. Both active treatment arms showed reductions in major CV events. Along with trials that found improved survival in post-MI patients with beta-blockers, this experience was sufficient for guidelines to begin recommending thiazides and beta-blockers as relatively equivalent alternatives for initiating treatment. This remained the situation through the 1990s, in spite of publication of results from the BMRC trial of treatment of hypertension in older adults, the beta-blocker arm of which showed no significant reduction in either stroke or CHD events compared with placebo. (7) In keeping with practice trends, a cardioselective beta-blocker, atenolol, was selected in this trial; in this arm systolic BP was less well controlled for the first year than in the hydrochlorothiazide arm.
Although one observer began questioning the role of the class for first-line treatment (50), during the era of direct comparison trials the arm representing traditional classes of drugs most often offered participating clinicians a choice of thiazides or beta-blockers, or selected a beta-blocker (most commonly, atenolol) as the comparator. Generally, prior to 2005 there were few differences for major CV event rates in individual trials between such regimens and those based on newer classes; an exception was the LIFE (Losartan Intervention For Endpoint reduction in hypertension) trial, in which a composite of stroke, MI, and CV death was 13% (p=.02) less frequent in the losartan-based than the atenolol-based group, owing mostly to an advantage for stroke. (51) This small disadvantage for the beta-blocker in a special study population selected for left ventricular hypertrophy, in the light of numerous trials not showing such a disadvantage, was not sufficient for the JNC7 group to downgrade the role of beta-blockers as alternative first-line drugs.(49)
The balance of evidence changed substantially with the reporting of results of the Anglo-Scandinavian Cardiac Outcomes Trial—Blood Pressure Lowering Treatment Arm (ASCOT-BPLA).(52) This large open-label trial (PROBE design) compared atenolol-based treatment (bendroflumethiazide as an add-on) with amlodipine-based treatment (perindopril as add-on); it was stopped early because of 11% lower all-cause mortality in the amlodipine group (p=.02). When the study was terminated early, there was no advantage in this arm for the primary CHD end-point (hazard ratio 0.90, p=.11), stroke was also significantly reduced, by 23%. This may have been related to the lower mean BP—2.7/1.9 mmHg on average, with a greater difference during the first year. Both effects may have in turn been related to the one daily use of atenolol and/or the relatively low dose of the thiazide added to atenolol when needed for BP control.
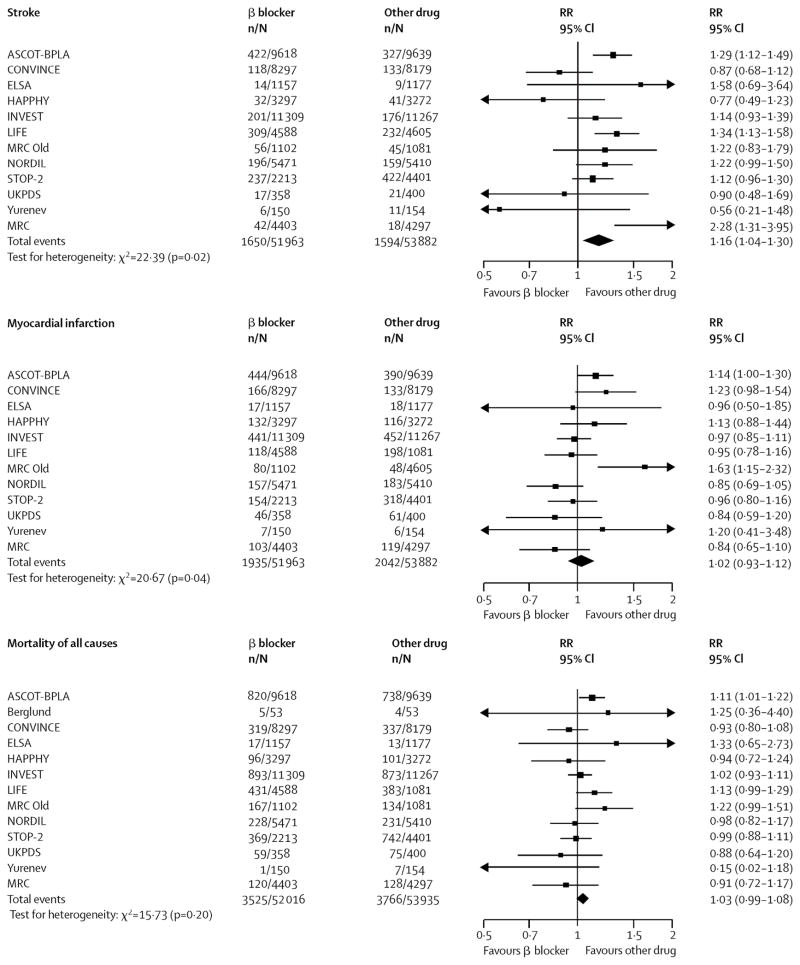
After the publication of ASCOT several meta-analyses were produced that led to recommendations unfavorable to beta-blockers (while continuing to acknowledge that evidence on non-atenolol beta-blockers in a primary prevention setting is scanty). (53, 54) Although the trials included differed somewhat between the meta-analyses and the Lindholm paper did not address heart failure, they both concluded that beta-blockers were less effective than other major antihypertensive classes in preventing stroke (Figure 8). Therefore, the recommendations were to delete beta-blockers as a first-line antihypertensive treatment, except where there are compelling indications, mainly for patients with CHD.
Figure 8. Outcome data for all β-blockers versus other antihypertensive treatment. (53).
Reprinted with permission from Elsevier (The Lancet, 2005, Volume 366, pages 1545–53).
Conclusions
We believe that the JNC7 Report came to the proper conclusions about thiazides, based on 1) results of the abundant trials aimed at the effect of BP reduction on clinical events comparing thiazide-based regimens to placebo or lesser treated controls; 2) ALLHAT results in the context of the few other trials evaluating thiazide-based treatment versus another class (trials testing a beta-blocker-and/or-thiazide protocol versus another class are not very informative); and 3) the network meta-analysis by Psaty that summarized all of this evidence. As noted in JNC7, thiazides are 1) well-tolerated—better than other classes in the double-blind setting of ALLHAT, where a heterogeneous patient population was treated in widely diverse clinical settings; 2) at least as effective as other classes for BP control in most patients, with the possible exception of younger white men (55); 3) unsurpassed in preventing CV events and improving survival, including an advantage in prevention of heart failure (in the short-term versus ACE inhibitors, and in the long-term versus CCBs); and 4) have very low acquisition costs. Although frequency of their use in antihypertensive regimens has increased substantially since the ALLHAT and JNC7 publications (56), they are still underutilized, and organized efforts to improve performance in this regard are continuing, most impressively in the Department of Veterans Affairs medical system. (personal communication, William Cushman, MD)
Some commentators have focused on the small increase in IDM associated with thiazide use, while virtually ignoring the advantage for heart failure prevention. This seems backward. Regarding diabetes, naturally-occurring disease carries appreciable long-term CV risk, but it is not clear that thiazide-induced cases do, and they are largely preventable or reversible by management of potassium balance. On the other hand, heart failure imparts high functional impact and mortality risk in the short-to-medium term. It seems that this benefit should be given considerable weight, except in patients known to be at very low risk of heart failure. Thus the British recommendation to the effect that thiazides and CCBs are equally desirable choices as first-line drugs in older patients seems ill-considered. (54)
As noted above, the role of other major drug classes (ACE-inhibitors, angiotensin receptor blockers, beta-blockers, and CCBs) in JNC7 is where a thiazide is not tolerated or contraindicated—an infrequent situation; for listed compelling indications; or in combination treatment, which is likely to be required to control BP in most patients. There is no update of JNC guidelines currently underway that might consider a change in the place of beta-blockers, but British guidelines have placed them as a lesser choice than the other classes except for compelling indications—situations in which there is another indication for their use. This conclusion seems reasonable to us, even though the data are scant for non-atenolol beta-blockers and the evidence for benefit in secondary prevention is much stronger for other beta-blockers (57). Interestingly, the parallel situation for ACE inhibitors in younger patients (lack of CV outcome data) did not deter the British report from recommending them as first choice in this demographic group. Once again, the recommendation seems to turn on effects on dysglycemia without really knowing their importance. However, to our knowledge there is no reassuring evidence regarding associated risks and mechanisms for beta-blockers, as exists for thiazides.
Although a strong evidence base exists for a consensus on preferred and other acceptable drug classes for initiating antihypertensive pharmacotherapy, there are several other major research questions for ongoing and needed trials, several of which were highlighted in a report from a 2003 NHLBI workshop (58). These include: 1) what is the optimal second drug class to add to a thiazide; and 2) what goal BP, especially systolic, should be sought, for minimizing CVD risk. From the evidence on heart failure in ALLHAT and the importance of the condition generally, we believe that heart fauilure should be included in any composite primary CVD end-point for future hypertension treatment trials.
Acknowledgments
The authors acknowledge the invaluable technical assistance of Sara L. Pressel, MS.
Footnotes
Disclosures: Dr Davis has served as a consultant for Biomarin, GlaxoSmithKline, Proctor & Gamble, and Takeda Pharmaceutical Company Limited..
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2007 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.WHO. the world health report 2002 - reducing risks, promoting healthy life [homepage on the Internet] [cited 6/25/2007]. Available from: http://www.who.int/whr/2002/en/
- 3.Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967 Dec 11;202(11):1028–34. [PubMed] [Google Scholar]
- 4.Veterans Administration Cooperative Study Group on Antihypertensive Agents. Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970 Aug 17;213(7):1143–52. [PubMed] [Google Scholar]
- 5.Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the Hypertension Detection and Follow-up Program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. JAMA. 1979 Dec 7;242(23):2562–71. [PubMed] [Google Scholar]
- 6.MRC trial of treatment of mild hypertension: Principal results. medical research council working party. Br Med J (Clin Res Ed) 1985 Jul 13;291(6488):97–104. doi: 10.1136/bmj.291.6488.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medical research council trial of treatment of hypertension in older adults: Principal results. MRC Working Party. BMJ. 1992 Feb 15;304(6824):405–12. doi: 10.1136/bmj.304.6824.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) SHEP Cooperative Research Group. JAMA. 1991 Jun 26;265(24):3255–64. [PubMed] [Google Scholar]
- 9.The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997 Nov 24;157(21):2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 10.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1. Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet. 1990 Mar 31;335(8692):765–74. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 11.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2. Short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context. Lancet. 1990 Apr 7;335(8693):827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 12.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (SYST-EUR) Trial Investigators. Lancet. 1997 Sep 13;350(9080):757–64. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 13.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991 Aug 1;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 14.Rutherford JD, Pfeffer MA, Moye LA, Davis BR, Flaker GC, Kowey PR, Lamas GA, Miller HS, Packer M, Rouleau JL. Effects of captopril on ischemic events after myocardial infarction. Results of the Survival and Ventricular Enlargement trial. SAVE Investigators. Circulation. 1994 Oct;90(4):1731–8. doi: 10.1161/01.cir.90.4.1731. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000 Jan 20;342(3):145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 16.Fox KM EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: Randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003 Sep 6;362(9386):782–8. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 17.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992 Sep 3;327(10):685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 18.Kostis JB, Davis BR, Cutler J, Grimm RH, Jr, Berge KG, Cohen JD, Lacy CR, Perry HM, Jr, Blaufox MD, Wassertheil-Smoller S, Black HR, Schron E, Berkson DM, Curb JD, Smith WM, McDonald R, Applegate WB. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA. 1997 Jul 16;278(3):212–6. [PubMed] [Google Scholar]
- 19.The 1988 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1988 May;148(5):1023–38. [PubMed] [Google Scholar]
- 20.The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V) Arch Intern Med. 1993 Jan 25;153(2):154–83. [PubMed] [Google Scholar]
- 21.Manolio TA, Cutler JA, Furberg CD, Psaty BM, Whelton PK, Applegate WB. Trends in pharmacologic management of hypertension in the United States. Arch Intern Med. 1995 Apr 24;155(8):829–37. [PubMed] [Google Scholar]
- 22.Multiple Risk Factor Intervention Trial Research Group. Mortality after 10 1/2 years for hypertensive participants in the Multiple Risk Factor Intervention Trial. Circulation. 1990 Nov;82(5):1616–28. doi: 10.1161/01.cir.82.5.1616. [DOI] [PubMed] [Google Scholar]
- 23.Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, Lemaitre RN, Wagner EH, Furberg CD. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997 Mar 5;277(9):739–45. [PubMed] [Google Scholar]
- 24.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Jr, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. American Journal of Hypertension. 1996 Apr;9(4 Pt 1):342–60. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 25.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002 Dec 18;288(23):2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 26.Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: Final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2003 Sep;42(3):239–46. doi: 10.1161/01.HYP.0000086521.95630.5A. [DOI] [PubMed] [Google Scholar]
- 27.Turnbull F Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. Lancet. 2003 Nov 8;362(9395):1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 28.Chalmers J. All hats off to ALLHAT: A massive study with clear messages. J Hypertens. 2003 Feb;21(2):225–8. doi: 10.1097/00004872-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 29.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001 Sep 29;358(9287):1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 30.Amery A, Birkenhager W, Brixko P, Bulpitt C, Clement D, Deruyttere M, De Schaepdryver A, Dollery C, Fagard R, Forette F. Mortality and morbidity results from the European Working Party on High Blood Pressure in the Elderly trial. Lancet. 1985 Jun 15;1(8442):1349–54. doi: 10.1016/s0140-6736(85)91783-0. [DOI] [PubMed] [Google Scholar]
- 31.Hansson L, Lindholm LH, Ekbom T, Dahlof B, Lanke J, Schersten B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: Cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999 Nov 20;354(9192):1751–6. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 32.Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, Neaton JD, Grimm RH, Jr, Hansson L, Lacourciere Y, Muller J, Sleight P, Weber MA, Williams G, Wittes J, Zanchetti A, Anders RJ CONVINCE Research Group. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003 Apr 23–30;289(16):2073–82. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 33.Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, Ruilope LM. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) Lancet. 2000 Jul 29;356(9227):366–72. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- 34.Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Marley JE, Morgan TO, West MJ Second Australian National Blood Pressure Study Group. A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003 Feb 13;348(7):583–92. doi: 10.1056/NEJMoa021716. [DOI] [PubMed] [Google Scholar]
- 35.Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJG, Phillips BB, Zimmerman MB, Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006 Mar;47(3):352–8. doi: 10.1161/01.HYP.0000203309.07140.d3. [DOI] [PubMed] [Google Scholar]
- 36.Frohlich ED. Treating hypertension - what are we to believe? N Engl J Med. 2003 Feb 13;348(7):639–41. doi: 10.1056/NEJMe020179. [DOI] [PubMed] [Google Scholar]
- 37.Cutler JA. The ANBP2 and ALLHAT: Conflicting or consistent? J Clin Hypertens. 2003 May–Jun;5(3):192–5. doi: 10.1111/j.1524-6175.2003.02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piller LB, Davis BR, Cutler JA, Cushman WC, Wright JT, Jr, Williamson JD, Leenen FH, Einhorn PT, Randall OS, Golden JS, Haywood LJ The ALLHAT Collaborative Research Group. Validation of heart failure events in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants assigned to doxazosin and chlorthalidone. Curr Control Trials Cardiovasc Med. 2002 Nov 14;3(1):10. doi: 10.1186/1468-6708-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis BR, Piller LB, Cutler JA, Furberg C, Dunn K, Franklin S, Goff D, Leenen F, Mohiuddin S, Papademetriou V, Proschan M, Ellsworth A, Golden J, Colon P, Crow R Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Role of diuretics in the prevention of heart failure: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Circulation. 2006 May 9;113(18):2201–10. doi: 10.1161/CIRCULATIONAHA.105.544031. [DOI] [PubMed] [Google Scholar]
- 40.Einhorn PT, Davis BR, Massie BM, Cushman WC, Piller LB, Simpson LM, Levy D, Nwachuku CE, Black HR ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) heart failure validation study: Diagnosis and prognosis. Am Heart J. 2007 Jan;153(1):42–53. doi: 10.1016/j.ahj.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Grimm RH, Davis BR, Piller L, Cutler J, Margolis KL, Barzilay J, Dart R, Graumlich J, Murden R, Randall O for the ALLHAT Collaborative Research Group. . Heart failure in ALLHAT: Did blood pressure medication at study entry influence outcome? doi: 10.1111/j.1751-7176.2009.00149.x. (submitted for review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piegorsch WW, Weinberg CR, Taylor JA. Non-hierarchical logistic models and case-only designs for assessing susceptibility in population-based case-control studies. Stat Med. 1994 Jan 30;13(2):153–62. doi: 10.1002/sim.4780130206. [DOI] [PubMed] [Google Scholar]
- 43.Whelton PK, Barzilay J, Cushman WC, Davis BR, Iiamathi E, Kostis JB, Leenen FH, Louis GT, Margolis KL, Mathis DE, Moloo J, Nwachuku C, Panebianco D, Parish DC, Pressel S, Simmons DL, Thadani U ALLHAT Collaborative Research Group. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2005 Jun 27;165(12):1401–9. doi: 10.1001/archinte.165.12.1401. [DOI] [PubMed] [Google Scholar]
- 44.Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, Margolis KL, Ong ST, Sadler LS, Summerson J ALLHAT Collaborative Research Group. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: A report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2006 Nov 13;166(20):2191–201. doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- 45.Kostis JB, Wilson AC, Freudenberger RS, Cosgrove NM, Pressel SL, Davis BR SHEP Collaborative Research Group. Long-term effect of diuretic-based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005 Jan 1;95(1):29–35. doi: 10.1016/j.amjcard.2004.08.059. [DOI] [PubMed] [Google Scholar]
- 46.Cutler JA. Thiazide-associated glucose abnormalities: Prognosis, etiology, and prevention: Is potassium balance the key? Hypertension. 2006 Aug;48(2):198–200. doi: 10.1161/01.HYP.0000231339.51310.b3. [DOI] [PubMed] [Google Scholar]
- 47.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: A quantitative review. Hypertension. 2006 Aug;48(2):219–24. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 48.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: A network meta-analysis. JAMA. 2003 May 21;289(19):2534–44. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 49.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 50.Messerli FH, Grossman E, Goldbourt U. Are beta-blockers efficacious as first-line therapy for hypertension in the elderly? A systematic review. JAMA. 1998 Jun 17;279(23):1903–7. doi: 10.1001/jama.279.23.1903. [DOI] [PubMed] [Google Scholar]
- 51.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint Reduction in Hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002 Mar 23;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 52.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomised controlled trial. Lancet. 2005 Sep 10–16;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 53.Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005 Oct 29;366(9496):1545–53. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- 54.National Collaborating Centre for Chronic Conditions. Hypertension: Management of hypertension in adults in primary care: Partial update. London: Royal College of Physicians; 2006. [Google Scholar]
- 55.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. the department of veterans affairs cooperative study group on antihypertensive agents. N Engl J Med. 1993;328:914–21. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 56.Stafford RS, Monti V, Furberg CD, Ma J. Long-term and short-term changes in antihypertensive prescribing by office-based physicians in the United States. Hypertension. 2006;48:213–8. doi: 10.1161/01.HYP.0000229653.73128.b6. [DOI] [PubMed] [Google Scholar]
- 57.Freemantle N, Cleland J, Young P, Mason J, Harrison J. β Blockade after myocardial infarction: systematic review and meta regression analysis. Brit Med J. 1999;318:1730–7. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.The National Heart, Lung, and Blood Institute Working Group on Future Directions in Hypertension Treatment Trials. Major clinical trials: what should be done? Hypertension. 2005;46:1–6. [Google Scholar]
- 59.IMS America. National Disease and Therapeutic Index. Ambler, Pa: IMS America; 1993. [Google Scholar]