Abstract
Objectives:
To evaluate the cytotoxic effects of a bleaching agent composed of 0.01% carbamide peroxide (CP; 2.21μg/ml H2O2) on the MDPC-23 odontoblastic cell line, and to determine whether sodium ascorbate (SA) is capable of reducing, or even eliminating, the toxic effects caused by this bleaching agent.
Methods:
The cells were seeded in wells and incubated for 48 hours. CP and SA were dissolved in a culture medium (DMEM) in order to obtain experimental extracts. Six groups of cells (n=10) were treated as follows: G1: no treatment (control); G2: 0.25 mM SA/60 min; G3: 0.5 mM SA/60 min; G4: 0.25 mM SA+0.01% CP/60 min; G5: 0.5 mM SA+0.01% CP/60 min; and G6: 0.01% CP/60 min. The cell metabolism was evaluated by MTT assay, and the cell morphology was assessed by scanning electron microscopy. The data obtained were analyzed by 2-way ANOVA and post-hoc Tukey’s test (α=5%).
Results:
The percentages of cell metabolism were as follows: G1 (control)=100%; G2=110.06%, G3=108.57%, G4=90.35%, G5=97.63%, and G6=66.88%. Group 6 presented a statistically lower cell metabolism than did the other groups, and the cells that remained on the substrate exhibited changes in their morphology. SA decreased the cytotoxic effects caused by CP, demonstrating its protective effect against the toxic components of this dental product.
Conclusions:
It was concluded that CP gel has cytopathic effects on MDPC-23 odontoblastic cells, even at low concentrations such as 0.01%. SA at 0.25 mM, and that 0.5 mM is able to protect these cultured cells against the cytotoxic effects of CP.
Keywords: Bleaching agent, Carbamide peroxide, Odontoblasts, Sodium ascorbate, Cytotoxicity
INTRODUCTION
Bleaching treatments employ procedures that attenuate or remove dyes from teeth and have, recently, been widely used, mainly by patients seeking an attractive and apparently healthy smile.1 However, such aesthetic procedures may cause side effects, such as morphological changes in the hard dental tissues1–3 and decreases in the bond strength of resin composites to the bleached dental surface.4,5 Dentin hypersensitivity is another side effect caused by the diffusion of bleaching agents through the tooth structure to the pulp tissue,6–10 resulting in pulp inflammation.6 Such side effects are attributed to the generation of reactive oxygen species (ROS), which play an important role in the tooth-bleaching therapy, but may also have deleterious effects on cells due to the lipid peroxidation process.11
In order to reverse the effects of bleaching agents on composite bond strength to the bleached tooth surface, the use of 10% sodium ascorbate (SA) has been proposed.12 Sodium ascorbate is considered a powerful hydro-soluble antioxidant capable of deoxidizing the reactions of oxygen and nitrogen free radical species. Therefore, SA is able to prevent important deleterious oxidative effects on biological macromolecules, such as DNA, lipids, and proteins.13,14
Dental materials, or their components, that are capable of trans-dentin diffusion can cause irreversible pulp injuries or even induce a death process and tissue necrosis.15 Consequently, the use of materials that can reduce or even eliminate the injuries caused by toxic components diffusing through the dentin tubules to the pulp may be of great value, since the restorative procedures may become not only effective, but also safe. Therefore, the aims of the current study were these: a) to evaluate the cytotoxicity of a bleaching agent when applied to the immortalized MDPC-23 odontoblastic cell line; and b) to determine whether SA can reduce or eliminate the toxic effects caused by a bleaching agent on such cells. The null hypotheses tested were that the bleaching agent does not exert any toxic effects on cultured odontoblast-like cells and that SA has no protective effect against the potential cytotoxicity of the bleaching agent.
MATERIALS AND METHODS
Cell culture
Immortalized cells of the MDPC-23 cell line were cultured (30,000 cells/cm2) on sterilized 24-well acrylic dishes (Costar Corp., Cambridge, MA, USA) and were then incubated for 48 hours in a humidified incubator with 5% CO2 and 95% air at 37°C. Dulbecco's Modified Eagle's Medium (DMEM, SIGMA Chemical Co., St. Louis, MO, USA) with 10% fetal calf serum (FBS, Cultilab, Campinas, SP, Brazil), supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L glutamine (GIBCO, Grand Island, NY, USA), was used as the culture medium.
Preparation of the solutions used in the study
One bleaching agent composed of 10% CP (Whiteness, FGM, Joinvile, SC, Brazil) was used in the present in vitro study. The bleaching agent was diluted in culture medium with no serum fetal bovine (DMEM- SFB) until it reached a final concentration of 0.01% (2.21 μg/ml of H2O2). In order to prepare the antioxidant solution, sodium ascorbate (Sigma Chemical Co., St. Louis, MO, USA) was dissolved in DMEM-SFB to obtain concentrations of 0.25 mM/mL and 0.5 mM/mL.14 Therefore, the following five control and experimental groups (n=10) were created: G1=no treatment (control); G2=0.25 mM/mL SA; G3=0.5 mM/mL SA; G4=0.25 mM/mL SA + 0.01% PC; and G5=0.5 mM/mL SA + 0.01% CP; G6: 0.01% CP. The experimental extracts (1ml) were applied to cultured MDPC-23 cells and maintained in an incubator at 37°C for 60 minutes.
Cytotoxicity test (MTT assay)
The control extracts (G1) and those obtained from both CP and SA dilutions in DMEM (G2-G6) were applied to MDPC-23 cells (1 ml). The cells in contact with the extracts were maintained in a humidified incubator at 37°C with 5% CO2 and 95% for 60 minutes. Afterwards, the cells were rinsed carefully in phosphate-buffered saline (PBS) and were submitted to cell metabolic activity analysis by methyltetrazolium assay (MTT assay).16 This test determines the activity of succinate dehydrogenase enzyme (SDH) produced by the mitochondria of viable cells. In order to prepare the MTT stock solution, 5 mg of methyltetrazolium salt (Sigma Chemical Co., St. Louis, MO, USA) was diluted in 1 mL of PBS. Eight wells from each of the experimental and control cell cultures were aspirated in a vertical laminar flow cabinet, and the medium was replaced by a solution composed of 900 μL DMEM and 100 μL of MTT stock solution. The cells in contact with this solution were then incubated for 4 hours. After this period, the DMEM + MTT solution was aspirated and was replaced by 600 μL of acidified isopropanol solution (0.04 N HCl) to dissolve the blue crystals of formazan present in the cells, resulting from the cleavage of methyltetrazolium salt by the succinate dehydrogenase (SDH) enzyme produced in viable cell mitochondria. After agitation and confirmation of the homogeneity of the solutions, three 100 μL aliquots were transferred from each well to the wells of a 96-well dish (Costar Corp., Cambridge, MA, USA). Cell viability was determined as being proportional to the absorbance measured at a 570 nm wavelength with an ELISA plate reader (Multiskan, Ascent 354, Labsystems CE, Lês Ulis, France).
The data obtained from the MTT assay were analyzed statistically by 2-way ANOVA (SA and CP), and statistical differences among groups were detected by Tukey’s post-hoc test at a pre-set alpha of 5%. The group means were used to determine cell viability (%) in comparison to the control group (G1), which was considered as 100%.
Analysis of cell morphology by scanning electron microscopy
The other two specimens were selected for analysis of cell morphology by SEM. For this, sterile 12-mm-diameter cover glasses (Fisher Scientific, Pittsburgh, PA, USA) were placed on the bottom of the wells of 24-well dishes immediately before seeding of the MDPC-23 cells (30,000 cells/cm2). The extracts were applied to the cells, according to each group, in a manner similar to the protocol for the analysis of cell metabolism. Thereafter, the extracts were aspirated and the viable cells that remained attached to the glass substrate were fixed in 1 ml of buffered 2.5% glutaraldehyde for 120 min. Next, the cells were submitted to three 5-minute rinses with 1 mL PBS and post-fixed in 1% osmium tetroxide for 60 min. Afterwards, the cover glasses with cells were dehydrated in increasing concentrations of ethanol solutions (30%, 50%, 70%, 90%, 100%). Finally, the cells on the discs were subjected to drying by low surface tension solvent 1, 1, 1, 3, 3, 3,-hexamethyldisilazane (98% HMDS; Acros Organics, New Jersey, USA) and kept in desiccators for 12 hours. Then, the cover glasses were fixed on metal stubs and gold sputtered. These procedures allowed the cell morphology analysis in SEM. (JEOL-JMS-T33A Scanning Microscope, JEOL-USA Inc., Peabody, MA, USA).
RESULTS
The values of SDH enzyme activity (as determined by MTT assay) are presented in Table 1, according to the presence or absence of the bleaching agent and SA concentration. In groups G2 and G3, in which SA was added to the culture medium, a discrete increase in cell metabolism was observed. As a consequence, cell viability values of higher than 100% were recorded in these experimental groups. However, this higher cell metabolism determined in groups G2 and G3 was not statistically different when compared to the control group (G1). When SA was associated with CP, a significant decrease in the cytotoxic effects of CP was observed, with higher SDH production (P<.05). The lowest metabolic values were observed in groups in which only the experimental bleaching agent was added to the culture medium. Considering the control group as 100% cell metabolism, the values obtained by the MTT assay regarding SDH production for groups 2, 3, 4, 5, and 6, were 110.06%; 108.57%; 90.35%; 97.63% and 66.88%, respectively.
Table 1.
Production of SDH enzyme (means ± standard deviation) detected by MTT assay, according to SA concentration and the presence of the bleaching agent.
| Bleaching agent | ||
|---|---|---|
| SA | Absent | Present |
| 0 | 0.309±0.029 ab | 0.207±0.019 c |
| 0.25 mM | 0.340±0.050 a | 0.279±0.027 b |
| 0.5 mM | 0.323±0.025 a | 0.302±0.036 ab |
Significant differences detected by post-hoc Tukey’s test are represented by different lower case letters at a pre-set alpha of 5% (n=10).
Scanning electron microscopy (SEM) analysis of cell morphology
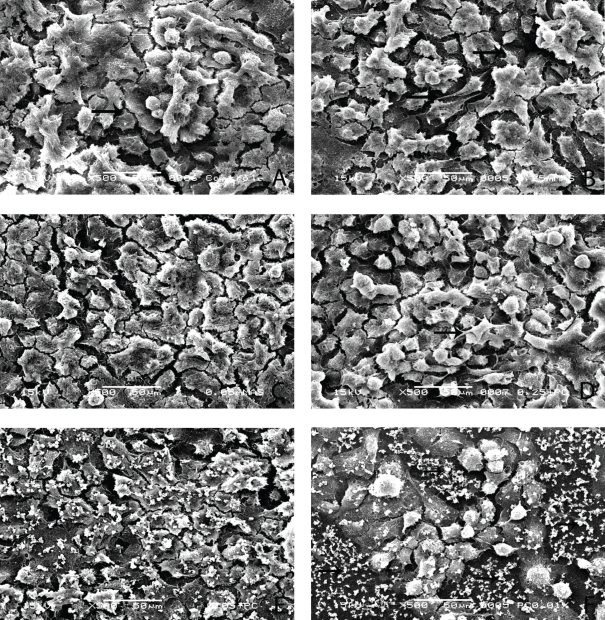
In the control group (G1) and in groups G2 and G3, a considerable amount of MDPC-23 cells, organized in epithelioid nodules, remained attached to the glass substrate. Such cells presented a large cytoplasm, and a number of cytoplasmic processes originated from their membrane (Figure 1A–C). Similar amounts of cells with the same morphological features were observed in group G4 (Figure 1D). In group G5, most of the MDPC-23 cells that remained on the substrate exhibited a few short cytoplasmic processes. These cells were also organized in epithelioid nodules and presented a smooth, round shape (Figure 1E). In group G6, a great number of cells were detached from the glass substrate. Therefore, wide areas with granular structures, similar to the residual membrane of dead cells, were seen on the glass disk. However, the small number of cells that remained attached to the substrate maintained their organization in epithelioid nodules (Figure 1F).
Figure 1.
The MDPC-23 cells, which are organized in epithelioid nodules, present large plasma membrane with several small cytoplasmic processes (arrows). Experimental groups G1(a), G2(b), G3(c), and G4(d). SEM, original magnification ×500. In group G5(e), odontoblasts with morphological changes characterized by small size and a few cytoplasmic processes are observed. In this experimental group, some residual cell membrane fragments are observed. SEM, original magnification ×500. In group G6(f), a number of MDPC-23 cells detached from the glass substrate. On the exposed glass substrate, a number of residual fragments of cytoplasmatic membrane from death cells can be seen (arrows). The cells that remained attached to the substrate are organized in epithelioid nodules. SEM, original magnification ×500.
DISCUSSION
The present in vitro study demonstrated that a solution of 0.01% of carbamide peroxide causes cytotoxic effects on the MDPC-23 odontoblastic cell line. Because of these results, the first hypothesis of this study was rejected. It has been reported that bleaching agents, placed in the aqueous medium, decompose and originate subproducts, such as hydrogen peroxide and urea.17
Hydrogen peroxide is classified as one of the many ROS; these are highly reactive molecules capable of causing injuries in many cell components, such as plasmatic membrane, organelles, and DNA.18 The imbalance between endogenous cellular antioxidant agents and ROS results in oxidative stress,19 which may cause several injuries that vary from reversible lesions to cell death.11 Therefore, the deleterious effects observed when the bleaching agent was applied to the cells (G6) may be attributed to the known cytotoxic effect of hydrogen peroxide, present at a concentration of 2.21 μg/mL in culture medium. Such toxic effects, caused by the CP dissolved in aqueous medium (DMEM), were confirmed not only by the decrease in SDH production by odontoblast-like cells (as detected by MTT assay), but also by the decrease in number and morphological changes of cells that remained adhered to the glass substrate after the bleaching agent application (Figure 1F).
It has been demonstrated that hydrogen peroxide diffuses through enamel and dentin and reaches the pulp tissue when the bleaching agent is applied to the tooth.9,20,21 Therefore, the current in vitro study used 0.01% CP, dissolved in culture medium (DMEM), in an attempt to simulate the concentration that reaches the pulp after the clinical procedure of bleaching therapy.9,20,21 Several investigations have demonstrated that hydrogen peroxide is released from the bleaching agents applied on enamel, and a defined concentration (2.21 μg/ml of H2O2) of this oxygen-derived free radical may reach the pulpal chamber after diffusion through enamel and dentin.9,20,21 Therefore, it was important in the present study to evaluate the cytotoxic effects of hydrogen peroxide, at this concentration, on cells with the odontoblast phenotype, such as MDPC-23. Since the outer pulp tissue layer is composed of odontoblasts that are organized in a monolayer to underlie the tubular dentin,22 this cell type is the first to come into contact with the toxic compounds released from the dental materials, such as enamel and dentin, that are able to diffuse through hard tissues. Consequently, odontoblast cell lines, such as the immortalized MDPC-23 cells, are appropriate to evaluate the toxic effects of dental products applied on tooth structures.23
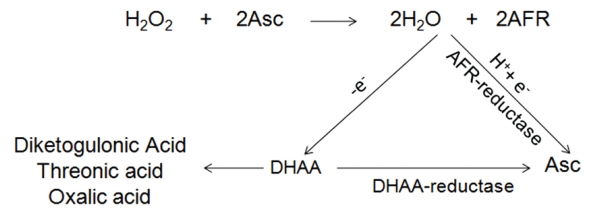
Since SA, when applied along with CP (G4 e G5), is capable of protecting the pulp cells against the toxic effects of sub-products released from the bleaching agent dissolved in aqueous medium, the second hypothesis was also rejected. Cell protection is attributed to the inactivation of ROS by antioxidant agents, which convert the highly reactive radicals into stable molecules (Figure 2).24 For this reason, as confirmed by MTT assay and SEM analysis of cell morphology, it is possible to suggest that SA at 0.25 mM (G4) or 0.5 mM (G5) actually prevented cell damage when applied to cells that were later exposed to 0.01% CP. Based on the MTT assay, the metabolism of MDPC-23 cells was decreased by 33.12% when no SA was used before the application of the extract with CP. On the other hand, when SA, at 0.25 mM (G4) or 0.5 mM (G5), was added to the culture medium, the metabolism decreased by only 9.65% and 2.37%, respectively. This protector effect of SA, at both concentrations, was also demonstrated by the SEM analysis of cell morphology. In the experimental groups with no SA (G6), a noticeable decrease in the amount of cells attached to the glass substrate was observed. On the glass surface previously occupied by MDPC-23 cells, only residual fragments of membrane from dead cells were noted. These results confirm that the imbalance between the presence of antioxidants and ROS on the cells can cause direct cell death.18 Based upon these results and, according to the statistical analysis applied to numerical data obtained from the MTT assay, the protective effectiveness of SA at both 0.25 mM (G4) and 0.5 mM (G5) concentrations was confirmed for MDPC-23 cells, with no significant difference between them. These favorable results regarding the antioxidant effects of SA were also observed when resinous materials were applied to cell cultures.14 It is important to emphasize that, in groups G2 and G3, in which culture medium with only SA was applied to the cultured MDPC-23 cells, a discrete increase in cell metabolism occurred. However, the results of cell metabolism determined for G2 and G3 were not statistically different from those of the control group (G1). Despite the lack of statistical significance, it is possible to speculate that the slight increase in cell metabolism observed in Groups G2 and G3 might be attributed to the antioxidant effect of SA, which may have neutralized the endogenous free radicals produced by the cells during normal mitochondrial oxidative phosphorylation.18 Therefore, it may be hypothesized that cell damage or death that may have occurred in group G1 (control) did not take place in groups G2 and G3, in which the cell death was prevented and, consequently, SDH production was increased.
Figure 2.
Hydrogen peroxide (H2O2) is neutralized by the transfer of a single-electron from ascorbate- (Asc)-producing water and ascorbyl free radicals (AFR). The pairs of AFR form one molecule of dehydroascorbic acid (DHAA) and one Asc. The lactone ring of DHAA breaks down forming the inert products, diketogulonic acid, threonic acid, and oxalic acid; alternatively, DHAA can be reduced to the useful Asc.
Despite the important scientific data presented in the current investigation, not only regarding the cytopathic effects of CP at low concentration but also the protective effect of SA as an antioxidant agent, it has been suggested that results of in vitro studies cannot be directly extrapolated to clinical situations.25 Therefore, further in vivo studies are required to evaluate the effects on pulp tissue of bleaching agents with carbamide peroxide in their composition. In addition, it is necessary to assess whether the in vitro pulp cell protection caused by sodium ascorbate, like that observed in the present study, also occurs in clinical situations of tooth bleaching treatment.
CONCLUSIONS
Based on the methodology used in the current in vitro study, it was possible to conclude that 0.01% CP promoted cytopathic effects on the immortalized odontoblast cell line, MDPC-23. It was also concluded that SA at concentrations of 0.25 mM and 0.5 mM was capable of reducing the cytotoxicity of CP to cultured cells.
Acknowledgments
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP (Grant: 2006-58780-8) and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (Grants: 476137/2006-3 and 301029/2007-5).
REFERENCES
- 1.Zantner C, Beheim-Schwarzbach N, Neumann K, Kielbassa AM. Surface microhardness of enamel after different home bleaching procedures. Dent Mater. 2007;23:243–250. doi: 10.1016/j.dental.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues JA, Marchi GM, Ambrosano GM, Heymann HO, Pimenta LA. Microhardness evaluation of in situ vital bleaching on human dental enamel using a novel study design. Dent Mater. 2005;21:1059–1067. doi: 10.1016/j.dental.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Kawamoto K, Tsujimoto Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J Endod. 2004;30:45–50. doi: 10.1097/00004770-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Cavalli V, Reis AF, Giannini M, Ambrosano GM. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent. 2001;26:597–602. [PubMed] [Google Scholar]
- 5.Turkun M, Kaya AD. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. J Oral Rehabil. 2004;31:1184–1191. doi: 10.1111/j.1365-2842.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 6.Robertson WD, Melfi RC. Pulpal response to vital bleaching procedures. J Endod. 1980;6:645–649. doi: 10.1016/S0099-2399(80)80166-X. [DOI] [PubMed] [Google Scholar]
- 7.Browning WD, Blalock JS, Frazier KB, Downey MC, Myers ML.Duration and timing of sensitivity related to bleaching J Esthet Restor Dent 200719256–264.; discussion 264. [DOI] [PubMed] [Google Scholar]
- 8.Hanks CT, Fat JC, Wataha JC, Corcoran JF. Cytotoxicity and dentin permeability of carbamide peroxide and hydrogen peroxide vital bleaching materials, in vitro. J Dent Res. 1993;72:931–938. doi: 10.1177/00220345930720051501. [DOI] [PubMed] [Google Scholar]
- 9.Gokay O, Tuncbilek M, Ertan R. Penetration of the pulp chamber by carbamide peroxide bleaching agents on teeth restored with a composite resin. J Oral Rehabil. 2000;27:428–431. doi: 10.1046/j.1365-2842.2000.00514.x. [DOI] [PubMed] [Google Scholar]
- 10.Gokay O, Mujdeci A, Algin E. In vitro peroxide penetration into the pulp chamber from newer bleaching products. Int Endod J. 2005;38:516–520. doi: 10.1111/j.1365-2591.2005.00979.x. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai SC, Mak YF, Cheung GS, Osorio R, Toledano M, Carvalho RM, Tay FR, Pashley DH. Reversal of compromised bonding to oxidized etched dentin. J Dent Res. 2001;80:1919–1924. doi: 10.1177/00220345010800101101. [DOI] [PubMed] [Google Scholar]
- 13.Meister A. On the antioxidant effects of ascorbic acid and glutathione. Biochem Pharmacol. 1992;44:1905–1915. doi: 10.1016/0006-2952(92)90091-v. [DOI] [PubMed] [Google Scholar]
- 14.Soheili Majd E, Goldberg M, Stanislawski L. In vitro effects of ascorbate and Trolox on the biocompatibility of dental restorative materials. Biomaterials. 2003;24:3–9. doi: 10.1016/s0142-9612(02)00221-1. [DOI] [PubMed] [Google Scholar]
- 15.Costa CA, Mesas AN, Hebling J. Pulp response to direct capping with an adhesive system. Am J Dent. 2000;13:81–87. [PubMed] [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Haywood VB, Heymann HO. Nightguard vital bleaching. Quintessence Int. 1989;20:173–176. [PubMed] [Google Scholar]
- 18.Shackelford RE, Kaufmann WK, Paules RS. Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med. 2000;28:1387–1404. doi: 10.1016/s0891-5849(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 20.Gokay O, Yilmaz F, Akin S, Tuncbilek M, Ertan R. Penetration of the pulp chamber by bleaching agents in teeth restored with various restorative materials. J Endod. 2000;26:92–94. doi: 10.1097/00004770-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Camargo SE, Valera MC, Camargo CH, Gasparoto Mancini MN, Menezes MM. Penetration of 38% hydrogen peroxide into the pulp chamber in bovine and human teeth submitted to office bleach technique. J Endod. 2007;33:1074–1077. doi: 10.1016/j.joen.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Arana-Chavez VE, Massa LF. Odontoblasts: the cells forming and maintaining dentine. Int J Biochem Cell Biol. 2004;36:1367–1373. doi: 10.1016/j.biocel.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 23.MacDougall M, Selden JK, Nydegger JR, Carnes DL.Immortalized mouse odontoblast cell line MO6-G3 application for in vitro biocompatibility testing Am J Dent 199811Spec No:S11–16. [PubMed] [Google Scholar]
- 24.Chaudiere J, Ferrari-Iliou R. Intracellular antioxidants: from chemical to biochemical mechanisms. Food Chem Toxicol. 1999;37:949–962. doi: 10.1016/s0278-6915(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 25.Costa CA, Hebling J, Hanks CT. Current status of pulp capping with dentin adhesive systems: a review. Dent Mater. 2000;16:188–197. doi: 10.1016/s0109-5641(00)00008-7. [DOI] [PubMed] [Google Scholar]