Abstract
Background
Tamoxifen use has been associated with increased risk of thromboembolic events (TEs) in women with breast cancer and women at high risk for the disease. Factor V Leiden (FVL) is the most common inherited clotting factor mutation and also confers increased thrombosis risk. We investigated whether FVL was associated with TE risk in women with early-stage breast cancer who took adjuvant tamoxifen.
Methods
A case–control study was conducted among 34 Cancer and Leukemia Group B (CALGB) institutions. We matched each of 124 women who had experienced a documented TE while taking adjuvant tamoxifen for breast cancer (but who were not necessarily on a CALGB treatment trial) to two control subjects (women who took adjuvant tamoxifen but did not experience TE) by age at diagnosis (±5 years). DNA from blood was analyzed for FVL mutations. Conditional logistic regression was used to estimate odds ratios (ORs) and to evaluate other potential factors associated with TE and tamoxifen use. All P values are based on two-sided tests.
Results
FVL mutations were identified in 23 (18.5%) case and 12 (4.8%) control subjects (OR = 4.66, 95% confidence interval = 2.14 to 10.14, P < .001). In the multivariable model, FVL mutation was associated with TE (OR = 4.73, 95% confidence interval = 2.10 to 10.68, P < .001). Other statistically significant factors associated with TE risk were personal history of TE and smoking.
Conclusions
Among women taking adjuvant tamoxifen for early-stage breast cancer, those who had a TE were nearly five times more likely to carry a FVL mutation than those who did not have a TE. Postmenopausal women should be evaluated for the FVL mutation before prescription of adjuvant tamoxifen if a positive test would alter therapeutic decision making.
CONTEXT AND CAVEATS
Prior knowledge
Although both use of tamoxifen and factor V Leiden (FVL) mutation are independent risk factors for thromboembolic events (TEs), it was not known whether women who take adjuvant tamoxifen for breast cancer are at increased risk of TE if they have the FVL mutation.
Study design
A case–control study was designed to match 124 women who took tamoxifen for breast cancer and developed TE to 248 women who took tamoxifen but did not develop TE. All women were tested for the FVL mutation.
Contribution
FVL mutations were found in 23 (18.5%) case subjects vs 12 (4.8%) control subjects so in this setting, FVL mutations are associated with greater risk of TE.
Implications
Clinicians should consider testing breast cancer patients for FVL mutations before prescribing adjuvant tamoxifen if it would alter management.
Limitations
Family histories of TE were not confirmed, data were not available for every parameter that might confer risk of TE, and tamoxifen in combination with anticoagulants was not studied.
From the Editors
More people receive tamoxifen for cancer than any other drug or therapy (1). Tamoxifen improves disease-free and overall survival as an adjuvant treatment for pre- and postmenopausal patients with hormone receptor–positive breast cancer (2), induces prolonged responses in patients with hormonally responsive metastatic disease, and substantially reduces the incidence of breast cancer in women at increased risk for the disease (3). However, thromboembolic events (TEs) are among the most serious complications associated with tamoxifen use. In a meta-analysis of four primary prevention trials that compared tamoxifen vs a placebo, tamoxifen use was estimated to increase overall TE risk in healthy women by about twofold (relative risk = 1.9, 95% confidence interval [CI] = 1.4 to 2.6) and to be associated with even higher risks in women aged 50 years or older (4). In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 trial, in which 5 years of adjuvant tamoxifen use was compared with use of a placebo in women with estrogen receptor–positive lymph node–negative breast cancer, the incidence of TE was fourfold greater overall in the tamoxifen-treated group (1.7%) than in the group receiving placebo (0.4%), with the majority of events in women aged 50 years and older (5). The prothrombotic effect of 6 months of adjuvant chemotherapy plus tamoxifen was assessed in the NSABP B-20 trial, in which women who received adjuvant chemotherapy plus tamoxifen had approximately threefold more TE (6.5%–7.0%) than women who took tamoxifen alone (1.8%). The increased risk was limited to the treatment period (6,7). Although the thrombogenic effect of tamoxifen is well documented, the effect of underlying breast cancer is less well quantified. In the B-14 trial, women with breast cancer in the placebo group had a higher rate of breast cancer recurrence than did women in the tamoxifen-treated group. However, the cumulative TE rate in the placebo group was about 0.4% over 5 years, the same rate as among women who took a placebo in the NSABP P-1 Breast Cancer Prevention Trial, in which participants had elevated breast cancer risk but no breast cancer history.
It is important to determine the baseline risk for development of TE to assess the risk–benefit profile of tamoxifen for a given patient. Patients with breast cancer may be at increased risk for TE because of their malignancy, surgery, central vascular access devices, chemotherapy, and/or inherited or acquired hypercoagulable states. The most common cause of an inherited hypercoagulable state is the factor V Leiden (FVL) mutation, a single G → A transition at position 1691 of the gene for factor V (8). The FVL mutation results in a loss of one of the three activated protein C cleavage sites in factor V, which renders the protein resistant to the anticoagulant activity of activated protein C (9). The FVL mutation is a dominant trait, with a 2%–5% incidence in the non-Hispanic white population and lower prevalence in other ethnic groups (10,11).
Presence of the FVL mutation is associated with an increased risk of thrombosis in patients with other extrinsic risk factors for TE, including hormone replacement therapy (HRT), high-dose oral contraceptives, and pregnancy (12–14). We hypothesized that a similar association might exist between FVL and tamoxifen-associated TE because the estrogen agonist activity of tamoxifen is thought to confer additional risk of TE. In addition, a 1997 case report described three patients (two on adjuvant treatment for breast cancer and one with metastatic melanoma) who developed TE while on tamoxifen and were found to have the FVL mutation (15). This finding suggested to us that a pharmacogenetic study might help to determine the relationship between the FVL mutation and TE risk to patients taking tamoxifen. Therefore, we designed a case–control study under the auspices of the Cancer and Leukemia Group B (CALGB). Our goals were 1) to estimate the prevalence of the FVL mutation among patients with early-stage breast cancer who had experienced thrombosis while taking adjuvant tamoxifen, 2) to compare the frequency of the FVL mutation among patients who took adjuvant tamoxifen and experienced thrombosis with that among age-matched patients who took tamoxifen without thrombosis, and 3) to evaluate other factors that might contribute to the occurrence of TE during use of adjuvant tamoxifen for early-stage breast cancer.
Subjects and Methods
Subjects
The study population consisted of 412 women who received tamoxifen as adjuvant treatment for stage I, II, or IIIA breast cancer at one of the 34 CALGB main member or Community Clinical Oncology Program institutions. This study was approved by the Executive Committee of the CALGB; participating institutions and practices obtained protocol approval from their local Institutional Review Boards. Eligible women, who were aged 80 years or younger and had received adjuvant tamoxifen either as the sole adjuvant systemic therapy or concurrent with or following adjuvant chemotherapy, were enrolled between January 15, 1999, and April 1, 2005. Patients were accrued by physicians affiliated with CALGB; however, patients were not required to have been on a CALGB treatment trial. Patients were registered by the CALGB Statistical Center.
Case patients were women who developed a venous or arterial embolism or deep venous thrombosis while taking adjuvant tamoxifen for stage I, II or IIIA breast cancer. Superficial phlebitis was not considered a thrombotic event. Catheter-associated thrombosis sufficient to require removal of the catheter was considered a TE for the purpose of case eligibility, but catheter-associated clots that resolved with thrombolytic therapy alone were not sufficient for case status. Use of heparin or warfarin during adjuvant therapy before the TE rendered potential subjects ineligible. Medical record documentation of the reported thromboembolic event and its therapy were provided for central review in 96% of case subjects.
Control subjects were women who did not develop a venous or arterial embolism while taking tamoxifen as adjuvant treatment for stage I, II, or IIIA breast cancer at a CALGB institution, though not necessarily on a CALGB treatment trial. Women who used heparin or warfarin during adjuvant therapy were ineligible. Patients who experienced a thrombotic or embolic event more than 2 months after completion of adjuvant tamoxifen therapy were eligible to participate as control subjects, although none was enrolled. Two control subjects were matched to each case patient by age at breast cancer diagnosis (±5 years) and enrolling institution but not by treatment with adjuvant chemotherapy. Enrollment of the triplet (one case subject plus two control subjects from the case institution) was successful for 85% of case subjects; the final control subjects, who were matched to each case subject by age at breast cancer diagnosis (±5 years), were enrolled from the institutions that accrued the largest number of case–control triplets (Figure 1).
Figure 1.
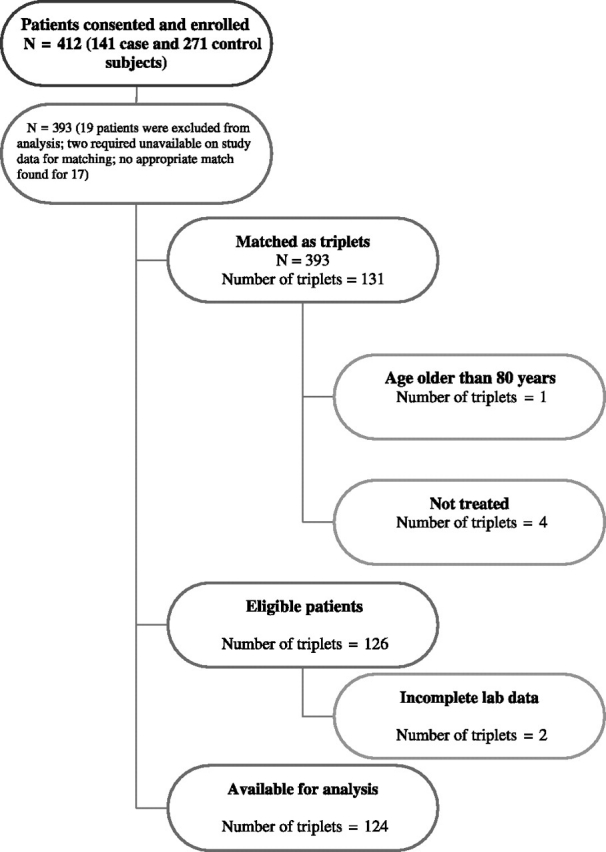
CONSORT diagram of all patients enrolled on CALGB 9872.
Consenting case and control subjects were interviewed regarding the number of their pregnancies, their menopause status (clinical definition, documentation not required), the number of months they took postmenopausal HRT before their breast cancer diagnosis, their smoking status while on adjuvant therapy, and their history of TE (personal and in first-degree relatives). Other data were collected from medical records: age at breast cancer diagnosis, cancer stage, date of diagnosis, adjuvant chemotherapy use, and personal history of prior TE. Reported family histories of TE were not validated from medical records.
Determination of FVL Mutation Status
Blood specimens were obtained from eligible women after appropriate informed consent. All specimens were centrally processed and aliquoted at the DNA Processing Laboratory at the University of North Carolina. DNA was extracted from whole blood, and polymerase chain reaction to detect the FVL mutation was performed in the research laboratory of N. Berliner at Yale Medical School. A 221-base pair fragment that spanned the 3′ end of exon 10 and the 5′ end of intron 10 of the Factor V gene (GenBank accession number: NG_011806; exon 10 nt: 36635–36878) was amplified using primers PR-6967 (sense: 5′ TGC CAA GTG CTT AAC AAG ACC A 3′ nt: 36611–36632) and PR-990 (antisense 5′ CTT GAA GGA AAT GCC CCA TAA 3′ nt: 36811–36832). This fragment contains nucleotides 1690–1695, which encode the Arg 506-Gly507–activated protein C cleavage site of factor V. Amplified DNA fragments were digested with Mnl1 and HindIII to find restriction fragment length polymorphisms characteristic of the FVL mutation (G1691A). All reactions were run with the appropriate controls including a water-only negative control for all polymerase chain reaction reactions and a sample bearing a known FLV mutation as a positive control for the restriction enzyme digests. The laboratory was not Clinical Laboratory Improvement Amendments (CLIA)–certified: per protocol, FVL results were not returned to subjects or their treating physicians. Data were collected and analyzed at the CALGB Statistical Center at Duke University.
Statistical Analysis
Assuming a 1:2 case–control ratio, the proportion of discordant sets among all sets was 10%, based on the excess risk of TE on adjuvant therapy for breast cancer from a large Eastern Cooperative Oncology Study (16). A Mantel–Haenszel test required a total of 120 triplets to achieve a power approximating 80% to detect a relative risk of 4 or greater, with a one-sided type I error rate of 0.05. However, in accordance with the policy of the Journal, only two-sided P values are reported. Population attributable risk was calculated using a standard formula (17).
Case and control subjects were compared with regard to baseline characteristics using the χ2 statistic for categorical variables (race, stage, menopausal status, adjuvant chemotherapy, clotting episode, family history of thromboembolism, and smoking history) and the Wilcoxon test for continuous variables (age, HRT use, and number of pregnancies). Conditional logistic regression analysis was performed to estimate the odds ratio (OR) and the 95% confidence interval for the relationship between FVL mutation and thromboembolism. Analyses of prior history of clot, family history of TE, and smoking history are based on bivariate analysis. These parameters were also included in the final multivariable logistic regression model to evaluate potential predictors of TE risk in women who took adjuvant tamoxifen. All P values were based on two-sided tests.
Results
Participant, tumor, and treatment characteristics were distributed similarly among case and control subjects except for past hormone use, blood clot history, and stage, with more women diagnosed at stage I among case subjects (Table 1). The study included 412 women who took adjuvant tamoxifen for breast cancer: 141 case and 271 control subjects. The analysis is based on 124 triplets matched by age at breast cancer diagnosis (±5 years) because 19 patients were excluded for lack of matching and 21 patients were either ineligible or had incomplete laboratory data (Figure 1). Most women were postmenopausal, with a median age of 64 years among both case and control subjects. Almost all patients had early-stage breast cancer: 91% of case and 95% of control subjects had confirmed stage I or II disease. Among the case subjects, 96 women had deep vein thrombosis, 12 had a pulmonary thromboembolism, 12 had both deep vein thrombosis and pulmonary thromboembolism, three had an arterial clot, and one patient had an intravenous line–related thrombus. Subjects in the control group had received postmenopausal HRT for a statistically significantly longer period of time before breast cancer diagnosis than case subjects (median 4 vs 0 months, P = .014). Also, a statistically significantly greater proportion of case subjects reported having smoked while on tamoxifen therapy compared with control subjects (15% vs 6%, P = .019). Proportionally more case than control subjects had experienced a prior episode of TE (14% vs 5%, P = .002). Although there were more case than control subjects with a family history of TE (20% vs 12%, P = .055), this difference was not statistically significant.
Table 1.
Patient and clinical characteristics at study enrollment for the 124 triplets*
| Demographics | Case subjects (N = 124) | Control subjects (N = 248) | P† |
| Median age, y (interquartile range) | 64 (55.5–71) | 64 (54.5–70) | .612‡ |
| Median months HRT (interquartile range) | 0 (0–48) | 4 (0–120) | .014‡ |
| Median number pregnancies (interquartile range) | 2 (1–3) | 2 (1–3) | .317‡ |
| Race, No. (%) | |||
| White | 110 (89) | 230 (93) | .171 |
| Other | 13 (10) | 16 (6) | |
| Missing | 1 (1) | 2 (1) | |
| Stage, No (%) | |||
| I | 62 (50) | 96 (39) | .041 |
| II | 51 (41) | 140 (56) | |
| IIIA | 6 (5) | 10 (4) | |
| Missing | 5 (4) | 2 (1) | |
| Menopausal status at diagnosis§, No. (%) | |||
| Pre | 12 (10) | 36 (14) | .413 |
| Peri | 7 (5) | 12 (5) | |
| Post | 105 (85) | 200 (81) | |
| Adjuvant chemotherapy, No. (%) | |||
| Yes | 57 (46) | 130 (52) | .241 |
| No | 67 (54) | 118 (48) | |
| Type of clot, No (%) | |||
| DVT | 96 (77) | ||
| PE | 12 (10) | NA | |
| DVT and PE | 12 (10) | ||
| Arterial embolism | 3 (2) | ||
| Clotted line | 1 (1) | ||
| Clotting episode before tamoxifen treatment, No. (%) | |||
| Yes | 17 (14) | 12 (5) | .002 |
| No | 105 (85) | 236 (95) | |
| Missing | 2 (1) | 0 (0) | |
| Family history of thromboembolism, No. (%) | |||
| Yes | 25 (20) | 29 (12) | .055 |
| No | 76 (61) | 179 (72) | |
| Missing | 23 (19) | 40 (16) | |
| Smoked on treatment§, No. (%) | |||
| Yes | 19 (15) | 16 (6) | .019 |
| No | 101 (82) | 220 (89) | |
| Missing | 4 (3) | 12 (5) | |
DVT = deep vein thrombosis; HRT = hormone replacement therapy; NA = not applicable; PE = pulmonary thromboembolism.
All tests except where otherwise indicated were based on the χ2 statistic and were two-sided.
Wilcoxon P values based on two-sided tests.
Menopause and smoking status were provided by patient at enrollment.
A statistically significantly greater proportion of case subjects had FVL mutations compared with control subjects (18.5% vs 4.8%, all heterozygotes; unadjusted OR = 4.66, 95% CI = 2.14 to 10.14, P < .001). A statistically significantly higher percentage of women with the FVL mutation had a personal history of TE compared with those without, and a family history of clot was more frequent among those with the mutation (Table 2).
Table 2.
Frequency of factor V Leiden heterozygotes by case–control, prior clotting, and smoking history*
| Patient characteristic | Total | Factor V Leiden, No. (%) |
OR (95% CI) | P† | |
| Yes | No | ||||
| Case subjects | 124 | 23 (18.5) | 101 (81.5) | ||
| Control subjects | 248 | 12 (4.8) | 236 (95.2) | 4.66 (2.14 to 10.14) | <.001 |
| Total | 372 | 35 (9.4) | 337 (90.6) | ||
| Prior history of clot | |||||
| Case subjects | 17 | 7 (41.2) | 10 (58.8) | <.001 | |
| Control subjects | 12 | 0 (0) | 12 (100) | 4.38 (1.97 to 9.72) | |
| No prior history of clot | |||||
| Case subjects | 107 | 16 (15.0) | 91 (85.0) | ||
| Control subjects | 236 | 12 (5.1) | 224 (95.9) | ||
| Family history of clot‡ | |||||
| Case subjects | 25 | 5 (20.0) | 20 (80.0) | 5.27 (2.17 to 12.82) | <.001 |
| Control subjects | 29 | 1 (3.5) | 28 (96.5) | ||
| No family history of clot | |||||
| Case subjects | 76 | 15 (19.7) | 61 (80.3) | ||
| Control subjects | 179 | 11 (6.2) | 168 (93.8) | ||
| Smoked‡ | |||||
| Case subjects | 19 | 3 (15.8) | 16 (84.2) | <.001 | |
| Control subjects | 16 | 0 (0) | 16 (100) | 5.01 (2.21 to 11.35) | |
| Did not smoke | |||||
| Case subjects | 101 | 19 (18.8) | 82 (81.2) | ||
| Control subjects | 220 | 10 (4.6) | 210 (95.5) | ||
CI = confidence interval; OR = odds ratio.
χ2 tests based on conditional logistic regression were two-sided.
Women with missing family history or smoking data were excluded from the bivariate analyses.
There was not a statistically significant difference in the median time from diagnosis of breast cancer to TE among women with the FVL mutation compared with those without (13 vs 12 months, P = .830, Table 3). The median time from tamoxifen initiation to clot was similar in both groups (9 vs 9 months, P = .517).
Table 3.
Time to thromboembolic event (TE) in 124 case patients by factor V Leiden mutation status
| Median time to thromboembolic event | Factor V Leiden mutation |
P‡ | |
| Yes* | No† | ||
| From breast cancer diagnosis to TE, mo (interquartile range) | 13 (7–24) (n = 22) | 12 (6–28) (n = 94) | .830 |
| From tamoxifen initiation to TE, mo (interquartile range) | 9 (2–23) (n = 23) | 9 (3–22) (n = 101) | .517 |
There were 22 patients with the factor V Leiden mutation who were evaluated for median time from breast cancer diagnosis to TE and 23 for time from tamoxifen initiation to TE.
There were 94 patients without the factor V Leiden mutation who were evaluated for median time from breast cancer diagnosis to TE and 101 for time from tamoxifen initiation to TE.
Wilcoxon P values based on two-sided tests.
In the multivariable logistic regression model, the likelihood of having had a TE while taking adjuvant tamoxifen (ie, becoming a case subject) was statistically significantly increased among women who carried the FVL mutation (OR = 4.73, 95% CI = 2.10 to 10.68, P < .001; Table 4). Other statistically significant factors for risk of TE were a personal history of TE, reported smoking during adjuvant therapy, and a family history of TE (Table 4).
Table 4.
Logistic regression modeling the probability of the risk of thromboembolism among breast cancer patients taking adjuvant tamoxifen*
| Variable | OR (95% CI) | P† |
| Factor V Leiden mutation | ||
| Yes vs no | 4.73 (2.10 to 10.68) | <.001 |
| Prior history of clot | ||
| Yes vs no | 3.05 (1.18 to 7.87) | .021 |
| Family history of clot | ||
| Yes vs no | 2.06 (1.04 to 4.11) | .040 |
| Unknown vs no | 1.34 (0.67 to 2.66) | .411 |
| Smoking status | ||
| Yes vs no | 2.97 (1.34 to 6.56) | .007 |
| Unknown vs no | 0.37 (0.07 to 1.87) | .230 |
CI = confidence ratio; OR = odds ratio.
All P values are based on two-sided tests.
The population attributable risk percent of TE among women with breast cancer taking adjuvant tamoxifen accounted for by FVL mutations was 14%, that is, 14% of TE among women taking tamoxifen occurred among women with the FVL mutation.
Discussion
This case–control study found an increased risk of a FVL mutation among women taking adjuvant tamoxifen for early-stage breast cancer who experienced a TE compared with those who did not. Risk of TE was also increased among women who had had a previous TE and those who smoked during breast cancer treatment.
Tamoxifen has been an important and effective agent in the treatment of patients with hormone receptor–positive breast cancer for more than three decades. Its ability to substantially reduce the incidence of new hormone receptor–positive invasive and in situ breast cancers has been established for women who are at increased risk for breast cancer based on family history and other risk factors, including prior breast cancer (3,18–20). The duration of benefit for both adjuvant and risk-reducing indications extends years beyond the initial use (21).
Despite these advantages, both patients and their physicians have become increasingly concerned about using tamoxifen because of real and perceived adverse effects over the years of its recommended use (22–25). A simple, inexpensive, and widely available assay that would identify the subset of women at higher risk for one of the most serious adverse effects of tamoxifen would likely be attractive to patients and providers. Because of this prospect, we examined the potential association between tamoxifen-associated TE and the FVL mutation, using a pharmacogenetic approach to toxicity assessment. This study demonstrated that women who experienced a TE while taking adjuvant tamoxifen for early-stage breast cancer were nearly five times more likely to carry a FVL mutation compared with women who took adjuvant tamoxifen but did not develop a TE.
In the decade after the thrombotic effects of tamoxifen were recognized and quantified (26), the FVL mutation was identified, and its epidemiology and implications were defined (8,27). A germline FVL mutation is associated with an annualized rate of TE of approximately 2.8%–7.5%; among individuals without FVL, annualized event rates range from 1.1% to 3.1% (28). In particular, the FVL mutation confers increased risk of TE, and the risk is elevated further with female steroid hormone exposures. The increased risk of TE among persons heterozygous for the FVL mutation has been observed in the presence of both endogenous hormonal states like pregnancy (14) and exogenous agents such as oral contraceptives (15-fold in a recent study) (13) or HRT (OR = 6.7 in the Women’s Health Initiative) (12). The risks are even greater for persons who are homozygous for the FVL mutation. The prevalence of the FVL mutation among ethnic groups in the United States is approximately 5% in non-Hispanic whites, but only 2.2% among Hispanics, 1.23% among African Americans, and less than 1% among Asian Americans (29). Despite the increased risks of TE associated with the FVL mutation and hormonal medications, there are currently no recommendations for screening asymptomatic populations to identify individuals for anticoagulation or to preclude the introduction of oral contraceptive or hormone replacement therapies (30). The identification of the FVL mutation in patients with recent clotting events or with a history of TE during pregnancy may result in prolongation of anticoagulation therapy or initiation of antithrombotic therapy during pregnancy or the postpartum period (31).
The potential interaction between the FVL mutation and tamoxifen, a serum estrogen receptor modulator with known thrombogenic effects that have been attributed to its estrogen agonist activity, has been systematically explored in the large tamoxifen risk-reduction trials, which should provide the setting in which the effects are easiest to discern. The International Breast Cancer Intervention Study (IBIS-1) was a nested case–control study that assessed intrinsic and acquired risk factors for TE, in which women with elevated breast cancer risk based on family history were randomized to 5 years of tamoxifen or placebo use. That analysis identified surgery, immobilization, and fracture as factors associated with increased risk of TE. Both the FVL mutation and the thrombophilic prothrombin G20210A mutation were paradoxically confined to the control group, so no association between FVL mutation and tamoxifen was demonstrated (32). A nested case–cohort study from the NSABP Breast Cancer Prevention Trial (P-1), in which tamoxifen was compared with placebo in women whose increased breast cancer risk was based on multiple risk factors (33), found no association between either FVL or the prothrombin G20210A mutation and risk of TE. A substantially higher body mass index was observed in women with TE in the trial cohort (33).
What might account for the difference between the nearly fivefold excess of the FVL mutation among women who experienced TE while taking tamoxifen in this study and the lack of a relationship between the FVL mutation, tamoxifen use, and risk of TE in the IBIS and NSABP P-1 prevention trials? The obvious difference in the subject populations—women with early-stage breast cancer in this study and women at risk but without breast cancer in the risk-reduction trials—suggests that effects of the breast cancer are important. The risk of recurrence among women with early-stage hormone receptor–positive breast cancer is low during adjuvant hormonal therapy and rises after tamoxifen use is stopped (34,35). The TE events in this study typically occurred within the first 2 years of tamoxifen therapy when the burden of occult breast cancer should be small. In a recent analysis of women aged 50 years or older on therapeutic tamoxifen, the overall risk of breast cancer recurrence within the first 2.5 years of use was 7.9% (36). Similarly, in Trial 1-98, a large randomized controlled trial of adjuvant hormonal therapies in postmenopausal women with hormone receptor–positive breast cancer (37), the Breast International Group investigators cited an 8.1% risk of recurrence in the tamoxifen arm by 3 years after random assignment. These data suggest that it is unlikely that the occurrence of TE in case subjects in our study resulted from occult metastatic disease.
A particularly important difference between the tamoxifen-treated women in our study and those in the large randomized tamoxifen prevention trials is that our cohort did not exclude the 29 women who had a personal history of prior TE from eligibility as case subjects (see Table 1). It was certainly more common to use tamoxifen, despite a history of TE, in the treatment of postmenopausal women with hormone receptor–positive breast cancer before aromatase inhibitors became an established therapeutic alternative. The Food and Drug Administration approved aromatase inhibitors for adjuvant use only 9 years ago, in 2001. Previous history of a clot was an exclusion criterion for the NSABP P-1 trial but was not for other adjuvant trials begun in the early 1990s. However, in our study, women with a personal history of TE (29 total) plus those with a family history of TE (54 total) accounted for fewer than half of the FVL mutation carriers who had a TE on tamoxifen treatment (five and seven case subjects, respectively, vs 31 case subjects among 598 with no prior personal or family history of a clot; see Table 2). If the 29 women with a personal history of TE (who were reported as part of 25 triplets) had been excluded, women who experienced a TE would still be more likely to have the FVL mutation (based on the analysis of 99 triplets, unadjusted OR = 2.77, 95% CI = 1.13 to 6.80, P = .026; OR adjusted for family history and smoking history = 2.71, 95% CI = 1.08 to 6.81, P = .034).
The prevalence of current smoking among case subjects in our study was 15%, very similar to the prevalence of current smokers in the IBIS prevention trial (19%–21% in case and control subjects) and the prevalence of ever-smokers in NSABP-P1 (13.2% in case and 12.9% in control subjects). Among our control subjects, only 6% reported current smoking. Current smoking, but not former smoking, has been shown to further increase risk of TE among women who carry thrombophilic mutations alone (38) and in the setting of hormone exposure (39), which should provide another impetus for women to stop smoking if they are expected to survive their breast cancers.
The etiology of increased risk of TE among patients with cancer is not precisely known, although it is thought that cancer generally induces a hypercoagulable state by stimulating circulating endothelial and mononuclear cells to express procoagulant molecules, such as tissue factor (40). The volume of cancer necessary to induce a clinically significant effect is unknown. Management of cancer patients involves the use of prothrombotic measures, such as the placement of central venous catheters and the administration of chemotherapy, which could potentially have long-lasting effects on endothelium or clotting proteins. About half of the patients in our study had received adjuvant chemotherapy, which is thought to increase the risk of TE by damaging the vascular endothelium and decreasing plasma levels of coagulation inhibitors (41). However, we found no statistically significant difference in chemotherapy exposure between case and control subjects in univariate or multivariable analyses. Thus, the difference in chemotherapy administration is unlikely to explain the prevalence of the FVL mutation among women with a tamoxifen-associated blood clot in this study. It is most likely that other differences in the study populations account for the disparate observations between the women in our cohort and those without a history of breast cancer in the primary prevention trials.
There are several limitations to this study. Family history of TE was more prevalent among the women with TE in this study but was not limited to women with the FVL mutation. We did not confirm family history of TE with medical records nor were potential subjects evaluated for other underlying hereditary coagulopathies, such as deficiencies of protein C, protein S, or antithrombin III, or presence of the prothrombin G20210A mutation, though persons with these conditions are rare. We did not collect data on parameters that have been shown to mediate risk of TE in other cohorts taking tamoxifen, including body mass index, recent surgery, or other immobilization (19,33). Because of increased awareness of tamoxifen's procoagulant properties, women with a history of TE would not be likely to receive tamoxifen for adjuvant therapy in current practice without prophylactic full anticoagulation.
Our data show that women who experience TE events after taking tamoxifen have a 4.66-fold chance of carrying the FVL mutation. These data may prove useful to women who must decide between tamoxifen and an effective, essentially nonthrombogenic, alternative adjuvant therapy for breast cancer, such as aromatase inhibitors for postmenopausal women and gonadotropin-releasing hormone analogs or oophorectomy for premenopausal women. A frail woman, for whom the challenge of a TE would be particularly dangerous, can eliminate up to 10% of the risk of TE attributable to tamoxifen by testing to exclude the possibility that she is carrying an FVL mutation. For the population of women with estrogen receptor–positive breast cancers, if adjuvant tamoxifen were not given to those with FVL mutations, only 14% of TE events would be avoided. However, because ongoing trials in postmenopausal women with hormone receptor–positive breast cancer have shown some therapeutic value from sequential administration of tamoxifen and an aromatase inhibitor, the issue may arise more often in the future (37).
Funding
The research for CALGB 9872 was supported, in part, by grants from the National Cancer Institute (CA31946 and CA37447) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601) and from the Donaghue Foundation and Susan G. Komen for the Cure Foundation (9634) to N.B.
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. The study sponsor had no role in the design of the study; the collection, analysis or interpretation of the data; the writing of the article, or the decision to submit the article for publication.
G. Kimmick reports associations with Astra Zeneca and Pfizer and support from Astra Zeneca.
The following institutions participated in this study: Christiana Care Health Services, Inc. CCOP, Wilmington, DE (Stephen Grubbs, MD, supported by CA45418); Dana-Farber Cancer Institute, Boston, MA (Eric P. Winer, MD, supported by CA32291); Duke University Medical Center, Durham, NC (Jeffrey Crawford, MD, supported by CA47577); Georgetown University Medical Center, Washington, DC (Minetta C. Liu, MD, supported by CA77597); Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY (Jeffrey Kirshner, MD, supported by CA45389); Illinois Oncology Research Association, Peoria, IL (John W. Kugler, MD, supported by CA35113); Massachusetts General Hospital, Boston, MA (Jeffrey W. Clark, MD, supported by CA12449); Medical University of South Carolina, Charleston, SC (Mark Green, MD, supported by CA03927); Mount Sinai School of Medicine, New York, NY (Lewis R. Silverman, MD, supported by CA04457); Roswell Park Cancer Institute, Buffalo, NY (Ellis Levine, MD, supported by CA02599); Southeast Cancer Control Consortium, Inc. CCOP, Goldsboro, NC (James N. Atkins, MD, supported by CA45808); State University of New York Upstate Medical University, Syracuse, NY (Stephen L. Graziano, MD, supported by CA21060); The Ohio State University Medical Center, Columbus, OH (Clara D Bloomfield, MD, supported by CA77658); University of California at San Francisco, San Francisco, CA (Alan P. Venook, MD, supported by CA60138); University of Chicago, Chicago, IL (Gini Fleming, MD, supported by CA41287); University of Iowa, Iowa City, IA (Daniel A. Vaena, MD, supported by CA47642); University of Missouri/Ellis Fischel Cancer Center, Columbia, MO (Michael C Perry, MD, supported by CA12046); University of Vermont, Burlington, VT (Hyman B. Muss, MD, supported by CA77406); Wake Forest University School of Medicine, Winston-Salem, NC (David D Hurd, MD, supported by CA03927).
References
- 1.Fricker J. End of the road for tamoxifen? Lancet Oncol. 2004;5(1):2. doi: 10.1016/s1470-2045(03)01305-6. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88(21):1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 6.Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107(23) suppl 1:I17–I21. doi: 10.1161/01.CIR.0000078466.72504.AC. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89(22):1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 8.Dahlback B. The protein C anticoagulant system: inherited defects as basis for venous thrombosis. Thromb Res. 1995;77(1):1–43. doi: 10.1016/0049-3848(94)00138-4. [DOI] [PubMed] [Google Scholar]
- 9.Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369(6475):64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 10.Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet. 1995;346(8983):1133–1134. doi: 10.1016/s0140-6736(95)91803-5. [DOI] [PubMed] [Google Scholar]
- 11.Svensson PJ, Dahlback B. Resistance to activated protein C as a basis for venous thrombosis. N Engl J Med. 1994;330(8):517–522. doi: 10.1056/NEJM199402243300801. [DOI] [PubMed] [Google Scholar]
- 12.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Spannagl M, Heinemann LA, Schramm W. Are factor V Leiden carriers who use oral contraceptives at extreme risk for venous thromboembolism? Eur J Contracept Reprod Health Care. 2000;5(2):105–112. doi: 10.1080/13625180008500383. [DOI] [PubMed] [Google Scholar]
- 14.Griffin JH, Evatt B, Wideman C, Fernandez JA. Anticoagulant protein C pathway defective in majority of thrombophilic patients. Blood. 1993;82(7):1989–1993. [PubMed] [Google Scholar]
- 15.Weitz IC, Israel VK, Liebman HA. Tamoxifen-associated venous thrombosis and activated protein C resistance due to factor V Leiden. Cancer. 1997;79(10):2024–2027. [PubMed] [Google Scholar]
- 16.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 17.Rothman KJ, Greenland S. Modern Epidemiology. 2nd edn. Philadelphia, PA: Lippincott, Williams and Wilkins; 1998. [Google Scholar]
- 18.Powles T, Eeles R, Ashley S, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet. 1998;352(9122):98–101. doi: 10.1016/S0140-6736(98)85012-5. [DOI] [PubMed] [Google Scholar]
- 19.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360(9336):817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451–1467. [PubMed] [Google Scholar]
- 21.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99(4):283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 22.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 23.Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor—positive breast cancer. J Clin Oncol. 2004;22(16):3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 24.Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project's Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615–1623. doi: 10.1093/jnci/93.21.1615. [DOI] [PubMed] [Google Scholar]
- 25.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17(9):2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 26.Lipton A, Harvey HA, Hamilton RW. Venous thrombosis as a side effect of tamoxifen treatment. Cancer Treat Rep. 1984;68(6):887–889. [PubMed] [Google Scholar]
- 27.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88(10):3698–3703. [PubMed] [Google Scholar]
- 28.Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–2485. doi: 10.1001/jama.2009.853. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA. 1997;277(16):1305–1307. [PubMed] [Google Scholar]
- 30.Wu O, Robertson L, Twaddle S, et al. Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study. Health Technol Assess. 2006;10(11):1–110. doi: 10.3310/hta10110. [DOI] [PubMed] [Google Scholar]
- 31.De Stefano V, Rossi E, Paciaroni K, Leone G. Screening for inherited thrombophilia: indications and therapeutic implications. Haematologica. 2002;87(10):1095–1108. [PubMed] [Google Scholar]
- 32.Duggan C, Marriott K, Edwards R, Cuzick J. Inherited and acquired risk factors for venous thromboembolic disease among women taking tamoxifen to prevent breast cancer. J Clin Oncol. 2003;21(19):3588–3593. doi: 10.1200/JCO.2003.10.111. [DOI] [PubMed] [Google Scholar]
- 33.Abramson N, Costantino JP, Garber JE, Berliner N, Wickerham DL, Wolmark N. Effect of factor V Leiden and prothrombin G20210–>A mutations on thromboembolic risk in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 2006;98(13):904–910. doi: 10.1093/jnci/djj262. [DOI] [PubMed] [Google Scholar]
- 34.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 35.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97(17):1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 36.Kennecke H, McArthur H, Olivotto IA, et al. Risk of early recurrence among postmenopausal women with estrogen receptor-positive early breast cancer treated with adjuvant tamoxifen. Cancer. 2008;112(7):1437–1444. doi: 10.1002/cncr.23320. [DOI] [PubMed] [Google Scholar]
- 37.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25(5):486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 38.Severinsen MT, Kristensen SR, Johnsen SP, Dethlefsen C, Tjonneland A, Overvad K. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7(8):1297–1303. doi: 10.1111/j.1538-7836.2009.03490.x. [DOI] [PubMed] [Google Scholar]
- 39.Pomp ER, le Cessie S, Rosendaal FR, Doggen CJ. Risk of venous thrombosis: obesity and its joint effect with oral contraceptive use and prothrombotic mutations. Br J Haematol. 2007;139(2):289–296. doi: 10.1111/j.1365-2141.2007.06780.x. [DOI] [PubMed] [Google Scholar]
- 40.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6(6):401–410. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 41.Mandala M, Curigliano G, Bucciarelli P, et al. Factor V Leiden and G20210A prothrombin mutation and the risk of subclavian vein thrombosis in patients with breast cancer and a central venous catheter. Ann Oncol. 2004;15(4):590–593. doi: 10.1093/annonc/mdh146. [DOI] [PubMed] [Google Scholar]


